- 1Department of Anesthesia and Intensive Care, “Vito Fazzi” Hospital, Lecce, Italy
- 2Department of Women, Child, General and Specialist Surgery, University of Campania “L. Vanvitelli”, Naples, Italy
- 3Department of Experimental Medicine, University of Salento, Lecce, Italy
Bickerstaff brainstem encephalitis (BBE) is a rare autoimmune disease characterized by the subacute onset of bilateral external ophthalmoplegia, ataxia, and decreased level of consciousness. BBE is part of a group of rare autoimmune diseases in children that can affect the nervous system at any level. The onset of neurological deficits is often sudden and nonspecific. The diagnosis is based on clinical findings and abnormal findings on cerebrospinal fluid (CSF), electroencephalography (EEG), electromyography (EMG), and magnetic resonance imaging (MRI). BBE is associated with the presence of the antiganglioside antibody, anti-GQ1b and anti-GM1. Intravenous immunoglobulin (IVIg) and plasma exchange are often used as treatments for these patients. We conducted a review on clinical presentation, diagnosis, treatment and outcome of reported cases of BBE. 74 cases are reported in the literature from the first cases described in 1951 to today. The prevalence is unknown while the incidence is higher in males. In 50% of cases, BBE occurs following respiratory or gastrointestinal tract infections. The most frequent initial symptoms were consciousness disturbance, headache, vomiting, diplopia, gait disturbance, dysarthria and fever. During illness course, almost all the patients developed consciousness disturbance, external ophthalmoplegia, and ataxia. Lumbar puncture showed pleocytosis or cytoalbuminological dissociation. Abnormal EEG and MRI studies revealed abnormalities in most cases. Anti-GQ1b antibodies were detected in more than half of the patients; anti-GM1 antibodies were detected in almost 40% of patients. Treatment guidelines are missing. In our analysis, steroids and IVIg were administered alone or in combination; as last option, plasmapheresis was used. BBE has a good prognosis and recovery in childhood is faster than in adulthood; 70% of patients reported no sequelae in our analysis. Future studies need to investigate pathogenesis and possible triggers, and therapeutic possibilities.
Introduction
Bickerstaff brainstem encephalitis (BBE) was first described by Bickerstaff and Cloake in 1951 under the title “Mesencephalitis and rhombencephalitis” (1). A few years later, Bickerstaff named this condition “brainstem encephalitis” (2).
BBE is a rare autoimmune disease characterized by the subacute onset of bilateral external ophthalmoplegia, ataxia, and decreased level of consciousness (3). Pupillary abnormalities, bilateral facial paralysis, Babinski’s sign, and bulbar paralysis are commonly present (4). Presence of limb weakness indicates overlap with Guillain–Barré syndrome (GBS) (3, 4).
The prevalence is unknown. According to a Japanese nationwide survey, the annual incidence of BBE is estimated to be approximately 0.078 per 100,000 inhabitants (5). The incidence of BBE is higher in males (male/female ratio 1.3) with an average age at onset of 39 years (5).
BBE has been reported to occur often after upper respiratory or gastrointestinal tract infections (6, 7). Although the exact pathological mechanism is not completely understood, BBE is associated with the presence of the antiganglioside antibody, anti-GQ1b. These antibodies are highly specific for patients with BBE and also GBS, Miller Fisher syndrome (MFS), and external ophthalmoplegia (8); anti-GQ1b antibodies are present in 68% of patients with BBE (9). Anti-GQ1b antibody testing are not necessary for a definitive diagnosis of BEE (3). The detection of these antibodies, however, is useful to confirm the diagnosis of BBE when incomplete syndromes or atypical symptoms are present, or when an altered mental status does not allow the evaluation of ataxia.
Clinical features include a classic triad of ataxia, ophthalmoplegia and altered consciousness (10). Other common features include hyperreflexia, Babinski’s sign, deep sensory impairment, facial weakness, bulbar palsy and nystagmus (10).
Despite the diagnosis being based on clinical findings, abnormal findings on cerebrospinal fluid (CSF), electroencephalography (EEG), electromyography (EMG), and magnetic resonance imaging (MRI) are common. CSF analysis often shows evidence of albumin-cytologic dissociation and pleocytosis (9, 10). At first, albumin-cytologic dissociation occurred in 25% of the BBE and pleocytosis in 32% of the BBE; during the second week, albumin-cytologic dissociation of CSF occurred in 46% of the BBE patients and pleocytosis in 31% of the BBE (9). EEG and EMG are indicative of central nervous system (CNS) impairment and predominantly in the brainstem (9). Patients with BBE showed a characteristic “unarousable sleep-like” EEG (11). In about one-third of the BBE patients, MRI shows high-intensity areas on T2-weighted images of the brainstem, thalamus, cerebellum and cerebrum (12).
There is a lack of consensus on the management of the BBE (13). Intravenous immunoglobulin (IVIg) and plasma exchange are often used as treatments for these patients (13).
The BBE course is generally monophasic with complete remission of symptoms within 6 months in over half of the patients.
Very few studies investigated pediatric BBE and its incidence rate is unknown (14–16). BBE is part of a group of rare autoimmune diseases in children that can affect the central or peripheral nervous system at any level (17, 18).
Aims
We conducted a review on clinical presentation, diagnosis, treatment and outcome of reported cases of Bickerstaff brainsteam encephalitis. To the best of our knowledge, this review is one of the first to address the BBE in childhood. We believe that clinical cases and case series could contribute relevant knowledge that should be considered in this review, especially when data from randomized and observational studies are not available or insufficient.
Materials and methods
Protocol and literature search strategy
We conducted a narrative review. Narrative reviews are useful educational articles because they provide a broad perspective on a topic and often describe the history or development of a problem or its management (19).
The main electronic databases (PubMed, Embase, Scopus, Google Scholar and Cochrane Library) were screened to identify studies reporting Bickerstaff brainstem encephalitis cases in children and adolescents. We used a combination of keywords (MESH terms) including “Bickerstaff brainstem encephalitis,” “Bickerstaff’s encephalitis,” “Bickerstaff’s syndrome,” “children” or “pediatric.” Only English articles were included.
The initial search was performed on January 15, 2024. All articles were included from the first described case made by Bickerstaff in 1951 to the end of December 2023.
Eligibility criteria
The population, intervention, comparison, and outcome (PICO) criteria were applied to the research question (see Table 1). Patients younger than 18 years diagnosed with Bickerstaff brainstem encephalitis were considered as the population (P); the intervention (I) was the diagnostic and therapeutic management; the comparison (C) concept was not applicable to the research question; length of hospitalization and the presence of sequelae were considered the outcomes (O) for this systematic review.
Diagnostic criteria were based on those published by Wakerley et al. for BBE and BBE with overlapping GBS (3).
Studies with incomplete outcomes were excluded. Studies written not in English were excluded from this review.
Outcomes
The outcome was to analyze BEE in childhood focusing on clinical presentation, diagnosis, treatment and outcome. We wanted to evaluated the length of hospitalization and the incidence of sequelae.
Selection of studies
Titles and abstracts were screened by two researchers (LGG and DM) to identify keywords. We eliminated studies that clearly did not satisfy PICO criteria and obtained full copies of the remaining studies. The selected articles were read in full by the two independent reviewers and, in case of disagreement, a third reviewer (LM) was consulted.
Data extraction and management
Data extracted included the following: age and sex of participants; number of participants enrolled and completing the study; preinfectious history; symptoms and signs of BBE; diagnostic management including CSF analysis, computed tomography (CT) and/or MRI examination, EEG, EMG, and serum antibody testing; therapy management; outcomes in terms of length of hospitalization and sequelae.
Data analysis
Data were analyzed using standard computer program (Excel, 2016). Symptoms and signs are reported as a ratio between the number of patients in which the variable was present (n) and the total number of patients (N): n/N (%). We assumed that they were absent rather than missing if they were not cited in the manuscript, to account for reporting bias, and therefore described as zero (n) out of the total number of reported cases (N). Diagnostic studies are reported as a ratio between the number of positive studies (n) and the total number of performed studies (N): n/N (%). Other data are reported as mean ± standard deviation (SD).
Results
We included 41 studies (20–60), for a total of 74 cases. 73% of cases were among males (54 males, 20 females). The mean age at onset was 8.65 ± 4.93 years (7 months – 18 years).
Preinfection history
In half of the cases, a previous illness was reported 10.12 ± 6.99 days before the onset of neurological symptoms. As shown in Table 2, upper respiratory tract infections (URIs) occurred in 17 cases and lower respiratory tract infections (LRIs) in 10 cases. Gastroenteritis and diarrhea were reported in 8 patients, while other unspecified infections were reported in 4 patients. Twenty-three cases were not associated with previous disease.
When a microbiological study was performed, the most frequently diagnosed microbiological agents were M. pneumoniae (n = 5), C. jejuni (n = 3) and H. influenzae (n = 2). One case of BBE occurred 2 weeks after COVID-19 vaccination (40).
Symptoms and signs
At the onset of BBE, patients presented an altered state of consciousness in 46% of cases, 5 of which were due to seizures (see Table 3). Headache and vomiting were present in 21 and 15 patients, respectively. Sixteen patients had diplopia and 12 were dysarthric. Limb weakness was found in 9 patients; gait disturbances were found in 23 patients. A febrile state was present in 40.5% of cases at initial presentation.
As shown in Table 2 at initial evaluation, disturbance of consciousness affected more than 50% of patients. Fifty-one cases of ophthalmoplegia were detected. Neurological examination of the cranial nerves showed ocular motor weakness and ptosis (n = 36), facial weakness (n = 42), and bulbar weakness (n = 39). Twenty-seven patients presented with limb weakness; 62.2% of patients were ataxic. Muscle reflex abnormalities were present in 56.8% of patients: in 28 cases they were absent or decreased, while in 14 cases they were brisk. Babinski sign was elicited in 18 patients. 8.1% of patients showed dysautomia, while 5.4% had sensory disturbances.
Diagnostics
In more than 90% of cases the diagnostic assessment included performing a lumbar puncture. As shown in Table 4, 55.1% of all were pathological: pleocytosis was reported 15 times and cytoalbuminological dissociation 23 times. Abnormal EEG was reported in more than 85% of those performed. Eleven patients had abnormal EMG. MRI studies were performed in 78.4% of patients, showing abnormalities in 24 cases. When CT studies were performed, no abnormalities were revealed.
Serum antibody samples were collected in 46 cases. In 54.5% of these cases, anti-GQ1b antibodies were found positive. Anti-GM1 antibodies were positive in 3 of 8 cases.
Therapy
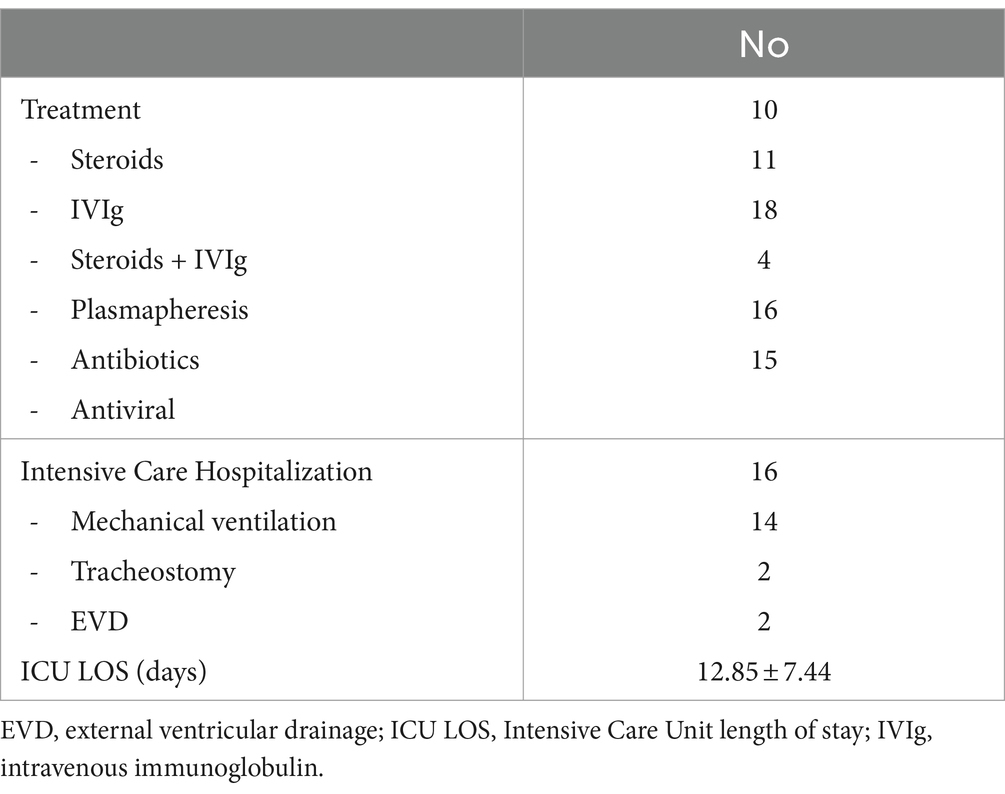
In more than 83% of patients, one or more treatments were started. Steroids were administered 10 times, IVIG 11 times, and a combination of steroids and IVIG 18 times. Plasmapheresis was performed for 4 patients. Antibiotics (n = 16) and antivirals (n = 15) were also administered.
Intensive Care hospitalizations was necessary for 16 patients. Fourteen required mechanical ventilation.
The median length of stay in ICU was 12.85 ± 7.44 days.
This is shown in Table 5.
Outcome
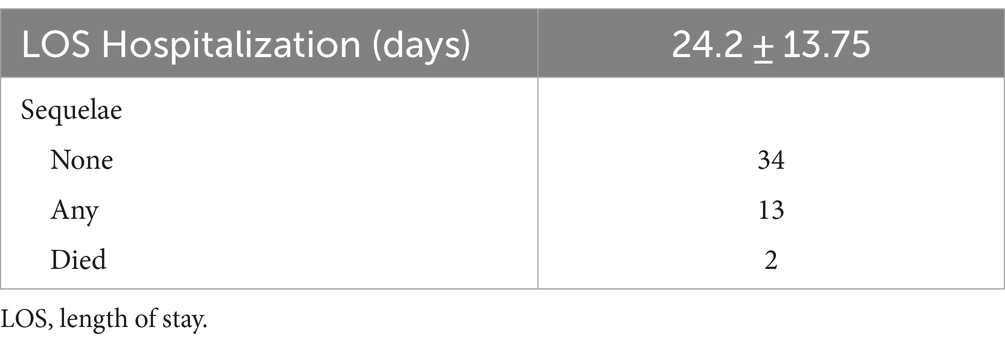
As shown in Table 6, the length of hospitalization was 24.2 ± 13.75 days. For 66.2% of patients, outcome was reported. After 4.2 ± 2.56 months, 34 patients had no sequelae, 13 had sequelae and 2 died.
Discussion
According to the literature (14–18), BBE is quite uncommon especially in children. The onset of neurologic deficits is often sudden, although nonspecific symptoms may precede neurologic manifestations by several weeks. Diagnosis by MRI and CSF analysis is essential in children when autoimmune encephalitis is suspected. The central diagnostic test is the search for neural antibodies. There is no difference in the therapeutic approach to children/adolescents and adults.
Our review provides a case analysis of BEE in childhood focusing on clinical presentation, diagnosis, treatment and outcome.
The prevalence is unknown while the incidence is higher in males. Our analysis showed a 3:1 male to female ratio, as in previous studies (15, 16). This is in contrast to general data that females are more susceptible than males to a variety of autoimmune diseases (61).
In 50% of cases an infection is reported in the 2 weeks before the onset of neurological symptoms. BBE occurs following respiratory or gastrointestinal tract infections, most frequently caused by M. pneumoniae, H. influenzae, and C. jejuni. A case study reported on an episode of BBE following COVID-19 vaccination (41). SARS-CoV-2 infection is associated with other neurological condition, such as Guillain-Barrè syndrome (62) and Miller Fisher syndrome (63). The etiology is unfortunately unknown, as for other neurological syndromes (64). A transient autoimmune response to myelin or other self-antigens through molecular mimicry during the infectious episode preceding symptoms, leading to destruction of the cerebral white matter, spinal cord, and optic nerves causing demyelination (64).
BBE is usually a monophasic disease; only one case of recurrent BBE is reported (24). The most frequent initial symptoms were consciousness disturbance, headache, vomiting, diplopia, gait disturbance, dysarthria and fever. During illness course, almost all the patients developed consciousness disturbance, external ophthalmoplegia, and ataxia. Ocular motor weakness and ptosis, facial weakness, and bulbar weakness were common manifestations. Extensor plantar response (Babinki’s sign) was present. Deep tendon reflexes were usually absent or decreased, but can be normal or brisk. Sensory disturbance and autonomic instability may occur.
When atypical initial symptoms such as symmetrical flaccid limb weakness and dysesthesias occur, these cases were classified as BBE with overlapping/coexisting GBS (25, 31, 40, 47).
Lumbar puncture is a high-priority test in cases of suspected central nervous system disease (65). In our analysis, an abnormal lumbar puncture was found in more than half of the patients; the main CSF alterations found were albuminocytological dissociation (33.3%) and pleocytosis (21.7%). Albuminocytological dissociation occurs during the first week of illness in 25% of cases and increases up to 46% of cases in the second week; 32% of the BBE patients show CSF pleocytosis (9). These CSF findings prove an inflammatory origin of neurological disturbances compatible with autoimmune encephalitis, such as BBE (66).
EEG abnormalities were reported in more than 85% of cases in which the exam was performed. The frequency of abnormal EEG findings indicated CNS involvement, consistent with disturbance of consciousness. EEG changes correlate with the level of consciousness in patients with BBE and often show predominant N1 and/or N2 sleep patterns, even with external stimuli (“unarousable sleep-like” EEG), including the fusiform coma pattern (11). The deterioration of consciousness in BBE may be caused by a dysfunction of the brainstem reticular formation (11).
Abnormal EMG was found in more than 50% of patients in this analysis. Electrophysiologically studies showed reduced or non-recordable compound muscle action potentials (CMAPs) and sensory nerve action potentials (SNAPs). This is evidence of demyelination or axonal degeneration and is found in half of cases (9, 12).
CT scans showed no abnormalities. MRI is the gold standard technique for brain imaging in encephalitis (67). Abnormal MRI lesions, such as high-intensity areas on T2-weighted images of the brainstem, thalamus, cerebellum and cerebrum, were present in BBE patients (12). This alteration corresponds to vasogenic edema and may be reversible and not visible on MRI. When performed, MRI showed abnormalities in 41.4% of cases.
Anti-GQ1b antibodies were detected in more than half of the patients. The oculomotor (III), trochlear (IV), and abducens (VI) nerves, muscle spindles in the limbs, and the reticular formation in the brainstem have significantly higher percentages of GQ1b (8, 68). Infection by microorganisms bearing the GQ1b epitope can induce the production of anti-GQ1b immunoglobulin G (IgG) antibodies in susceptible patients. Anti-GQ1b antibodies bind to GQ1b antigens expressed on cranial nerves and muscle spindles inducing FS. It is possible that in some cases the antiGQ1b antibodies reach the brainstem and bind to GQ1b, inducing BBE (8, 68).
The presence of anti-GM1 antibodies was detected in almost 40% of patients. The role of antiganglioside antibodies in the pathogenesis of BBE as well as other diseases is not completely understood. They could participate in direct damage to the structure they bind to, be a consequence of several types of infectious diseases, or facilitate many immune-mediated pathological mechanisms (7).
In the included studies, the diagnosis of BBE was not based only on the symptoms, which were not always typical (external ophthalmoplegia, ataxia, disturbance of consciousness). In our analysis, typical MRI images (high signal lesion on T-2-weighted images located in the brainstem) and high titers of anti-GQ1b antibodies may contribute to the diagnosis of BBE.
There are no established guidelines for treatment and currently different regimens are used based on the patient’s clinical status and the doctor’s opinion. Autoimmune encephalitis, including BBE, responds to immunomodulatory therapy such as IVIG and plasmapheresis (69). This treatment is accompanied by the administration of intravenous corticosteroids (69). In our analysis, steroids and intravenous immunoglobulins were administered alone or in combination. As last option, plasmapheresis was used.
Among corticosteroids, methylprednisolone is commonly used at a dose of 1 g per day for 3–5 days followed by oral prednisone 1 mg/kg/day for 1–4 weeks (64, 69). IVIG has many immunomodulatory and anti-inflammatory effects; it is used for many neurological disorders. The IVIG doses range is between 400 mg/kg/day for 5 days or a more rapid course of 1–2 g/kg given over 1–2 days (64, 70). Plasmapheresis is a reasonable option to consider in BBE patients with no or limited improvement after corticosteroids or IVIG (71). Normally 1 session is performed every other day for 5–7 cycles (30, 64). Patients included in this review did not require the use of second-line agents, such as rituximab or cyclophosphamide.
The length of hospitalization was approximately 24 days. More than 20% of patients required ICU admission for an average of approximately 13 days, mainly due to depressed level of consciousness and respiratory insufficiency. Almost all of them required mechanical ventilation. These rates are lower compared to the adult population with autoimmune encephalitis; in an observational study, 57% of patients were intubated for an average of approximately 1 month and 68% of them required tracheostomy (72). No particular characteristics were identified in BBE cases requiring ICU admission.
BBE has a good prognosis and recovery in childhood is faster than in adulthood, usually resolving within 4 to 6 weeks after starting treatment (14, 16). Concerning the outcome, 70% of patients reported no sequelae in our analysis. The death rate was 2.7% (24, 59). Death is a very rare outcome in patients with BBE receiving optimal treatment.
Limitations
This review presents some limitations. First, it was based on case series and case reports. They are often excluded from systematic reviews due to the greater potential for bias, especially severity bias and selection bias. In this report, these studies contribute to the available evidence base, and their results supplement the limited evidence available from other studies. Second, a meta-analysis was not performed, due to the design of most of the studies (case report, case series) and the lack of a comparator.
Conclusion
BBE is a rare autoimmune disease, even in childhood. Its knowledge is essential for the early recognition of the patient suffering from BBE and therefore for its management. The pathogenesis is unclear. The combination of clinical, radiological and laboratory data is the basis of a correct diagnosis. There are few indications for correct treatment which is mainly based on immunomodulatory therapy including IVIG and in the most serious cases on plasmapheresis. Fortunately, the prognosis is good, with a good recovery rate. Future studies need to investigate the pathogenesis, possible triggers and therapeutic possibilities.
Author contributions
LG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. DM: Supervision, Writing – review & editing. RB: Data curation, Formal analysis, Methodology, Writing – review & editing. RM: Data curation, Formal analysis, Methodology, Writing – review & editing. FM: Data curation, Formal analysis, Methodology, Writing – review & editing. GPa: Data curation, Formal analysis, Methodology, Writing – review & editing. MP: Data curation, Formal analysis, Methodology, Writing – review & editing. PS: Data curation, Formal analysis, Methodology, Writing – review & editing. GPu: Supervision, Validation, Writing – review & editing. LM: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bickerstaff, ER, and Cloake, PC. Mesencephalitis and rhombencephalitis. Br Med J. (1951) 2:77–81. doi: 10.1136/bmj.2.4723.77
2. Bickerstaff, ER. Brain-stem encephalitis. Br Med J. (1957) 1:1384–90. doi: 10.1136/bmj.1.5032.1384
3. Wakerley, BR, Uncini, A, and Yuki, N. Guillain-Barré and Miller Fisher syndromes--new diagnostic classification. Nat Rev Neurol. (2014) 10:537–44. doi: 10.1038/nrneurol.2014.138
4. Graus, F, Titulaer, MJ, Balu, R, Benseler, S, Bien, CG, Cellucci, T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
5. Koga, M, Kusunoki, S, Kaida, K, Uehara, R, Nakamura, Y, Kohriyama, T, et al. Nationwide survey of patients in Japan with Bickerstaff brainstem encephalitis: epidemiological and clinical characteristics. J Neurol Neurosurg Psychiatry. (2012) 83:1210–5. doi: 10.1136/jnnp-2012-303060
6. Odaka, M, Yuki, N, and Hirata, K. Anti-GQ1b IgG antibody syndrome: clinical and immunological range. J Neurol Neurosurg Psychiatry. (2001) 70:50–5. doi: 10.1136/jnnp.70.1.50
7. Cutillo, G, Saariaho, AH, and Meri, S. Physiology of gangliosides and the role of antiganglioside antibodies in human diseases. Rev Cell Mol Immunol. (2020) 17:313–22. doi: 10.1038/s41423-020-0388-9
8. Shahrizaila, N, and Yuki, N. Bickerstaff brainstem encephalitis and Fisher syndrome: anti-GQ1b antibody syndrome. J Neurol Neurosurg Psychiatry. (2013) 84:576–83. doi: 10.1136/jnnp-2012-302824
9. Ito, M, Kuwabara, S, Odaka, M, Misawa, S, Koga, M, Hirata, K, et al. Bickerstaff’s brainstem encephalitis and Fisher syndrome form a continuous spectrum: clinical analysis of 581 cases. J Neurol. (2008) 255:674–82. doi: 10.1007/s00415-008-0775-0
10. Horton, E, Krishnamoorthy, S, and Reynolds, L. Bickerstaff’s encephalitis. BMJ Case Rep. (2014) 2014:bcr2014205336. doi: 10.1136/bcr-2014-205336
11. Yoshimura, H, Togo, M, Ishii, J, Ishiyama, H, Tamura, R, Kimura, M, et al. Electroencephalographic findings in Bickerstaff’s brainstem encephalitis: a possible reflection of the dysfunction of the ascending reticular activating system. Clin Neurophysiol Pract. (2020) 6:29–35. doi: 10.1016/j.cnp.2020.11.004
12. Odaka, M, Yuki, N, Yamada, M, Koga, M, Takemi, T, Hirata, K, et al. Bickerstaff’s brainstem encephalitis: clinical features of 62 cases and a subgroup associated with Guillain-Barré syndrome. Brain. (2003) 126:2279–90. doi: 10.1093/brain/awg233
13. Overell, JR, Hsieh, ST, Odaka, M, Yuki, N, and Willison, HJ. Treatment for fisher syndrome, Bickerstaff’s brainstem encephalitis and related disorders. Cochrane Database Syst Rev. (2007) 2010:CD004761. doi: 10.1002/14651858.CD004761.pub2
14. Marino, S, Marino, L, Greco, F, Venti, V, Fontana, A, Timpanaro, T, et al. Bickerstaff’s brainstem encephalitis in childhood: a literature overview. Eur Rev Med Pharmacol Sci. (2020) 24:12802–7. doi: 10.26355/eurrev_202012_24181
15. Santoro, JD, Lazzareschi, DV, Campen, CJ, and Van Haren, KP. Pediatric Bickerstaff brainstem encephalitis: a systematic review of literature and case series. J Neurol. (2018) 265:141–50. doi: 10.1007/s00415-017-8684-8
16. Messina, G, Sciuto, S, Fontana, A, Greco, F, Oliva, CF, Pappalardo, MG, et al. On clinical findings of Bickerstaff’s brainstem encephalitis in childhood. J Integr Neurosci. (2021) 20:509–13. doi: 10.31083/j.jin2002054
17. Sweeney, M. Autoimmune neurologic diseases in children. Semin Neurol. (2018) 38:355–70. doi: 10.1055/s-0038-1660520
18. Wells, E, Hacohen, Y, Waldman, A, Tillema, JM, Soldatos, A, Ances, B, et al. Neuroimmune disorders of the central nervous system in children in the molecular era. Nat Rev Neurol. (2018) 14:433–45. doi: 10.1038/s41582-018-0024-9
19. Green, BN, Johnson, CD, and Adams, A. Writing narrative literature reviews for peer-reviewed journals: secrets of the trade. J Chiropr Med. (2006) 5:101–17. doi: 10.1016/S0899-3467(07)60142-6
20. Abide, Z, Sif Nasr, K, Kaddouri, S, Edderai, M, Elfenni, J, and Salaheddine, T. Bickerstaff brainstem encephalitis: a case report. Radiol Case Rep. (2023) 18:2704–6. doi: 10.1016/j.radcr.2023.04.038
21. Ahmed, M, Jawaid, H, Ali, F, Saleem, A, and Ejaz, MS. Paediatric Bickerstaff brainstem encephalitis: a rare case report. J Pak Med Assoc. (2020) 70:1–2056. doi: 10.5455/JPMA.51328
22. Al-Din, AN, Anderson, M, Bickerstaff, ER, and Harvey, I. Brainstem encephalitis and the syndrome of miller fisher: a clinical study. Brain. (1982) 105:481–95. doi: 10.1093/brain/105.3.481
23. Aslan, N. A case of brainstem encephalitis treated with plasmapheresis. J Clin Anal Med. (2018):235–8. doi: 10.4328/jcam.5390
24. Chamsi Basha, A, Kolko, N, Santoro, JD, and Alomani, H. A probable case of recurrent Bickerstaff brainstem encephalitis with fulminant course in a pediatric patient. Neurologist. (2020) 25:14–6. doi: 10.1097/NRL.0000000000000255
25. Chowdhry, M, Agrawal, S, and S, ML. A case of Bickerstaff encephalitis with overlapping Gullian Barre syndrome in a pediatric patient treated with therapeutic plasma exchange. Transfus Apher Sci. (2021) 60:103260. doi: 10.1016/j.transci.2021.103260
26. Christo, PP, Silva, JS, Werneck, IV, and Dias, SL. Rhombencephalitis possibly caused by Mycoplasma pneumoniae. Arq Neuropsiquiatr. (2010) 68:656–8. doi: 10.1590/s0004-282x2010000400035
27. Coriolani, G, Ferranti, S, Squarci, G, and Grosso, S. A case of Bickerstaff encephalitis associated with Mycoplasma pneumoniae infection. Neurol Sci. (2020) 41:1605–6. doi: 10.1007/s10072-019-04223-5
28. Damasceno, A, França, MC Jr, Pimenta, DS, Deus-Silva, L, Nucci, A, and Damasceno, BP. Bickerstaff’s encephalitis, Guillain-Barré syndrome and idiopathic intracranial hypertension: are they related conditions? Arq Neuropsiquiatr. (2008) 66:744–6. doi: 10.1590/s0004-282x2008000500027
29. Ding, Y, Yu, L, Zhou, S, and Zhang, L. Clinical characteristic analysis of seven children with Bickerstaff brainstem encephalitis in China. Front Neurol. (2020) 11:557. doi: 10.3389/fneur.2020.00557
30. Fargas, A, Roig, M, Vazquez, E, and Fitó, A. Brainstem involvement in a child with ophthalmoplegia, ataxia, areflexia syndrome. Pediatr Neurol. (1998) 18:73–5. doi: 10.1016/s0887-8994(97)00141-0
31. Fong, CY, Aung, HWW, Khairani, A, Gan, CS, Shahrizaila, N, and Goh, KJ. Bickerstaff’s brainstem encephalitis with overlapping Guillain-Barré syndrome: usefulness of sequential nerve conduction studies. Brain and Development. (2018) 40:507–11. doi: 10.1016/j.braindev.2018.02.001
32. Gologorsky, RC, Barakos, JA, and Sahebkar, F. Rhomb-and bickerstaff encephalitis: two clinical phenotypes? Pediatr Neurol. (2013) 48:244–8. doi: 10.1016/j.pediatrneurol.2012.11.002
33. Hacohen, Y, Nishimoto, Y, Fukami, Y, Lang, B, Waters, P, Lim, MJ, et al. Paediatric brainstem encephalitis associated with glial and neuronal autoantibodies. Dev Med Child Neurol. (2016) 58:836–41. doi: 10.1111/dmcn.13090
34. Manjunath, MA, Kariyappa, P, Ramabhatta, S, Kittagaly, PA, and Shankar, K. Bickerstaff brainstem encephalitis - a serious disease with a favourable outcome. Pediatr Oncall. (2019) 16:86–7. doi: 10.7199/ped.oncall.2019.46
35. Maqsood, MH, Kinza, R, and Muhammad, ZM. Misdiagnosed case of bicker staff brainstem encephalitis. Professional Med J. (2019) 26:1386–8. doi: 10.29309/TPMJ/2019.26.08.3135
36. Matsumoto, H, Kobayashi, O, Tamura, K, Ohkawa, T, and Sekine, I. Miller fisher syndrome with transient coma: comparison with Bickerstaff brainstem encephalitis. Brain Dev. (2002) 24:98–101. doi: 10.1016/s0387-7604(01)00409-0
37. Matsuo, M, Odaka, M, Koga, M, Tsuchiya, K, Hamasaki, Y, and Yuki, N. Bickerstaff’s brainstem encephalitis associated with IgM antibodies to GM1b and GalNAc-GD1a. J Neurol Sci. (2004) 217:225–8. doi: 10.1016/j.jns.2003.09.008
38. McLeod, SA, Wee, W, Jacob, FD, Chapados, I, and Bolduc, FV. Recurrent diplopia in a pediatric patient with Bickerstaff brainstem encephalitis. Case Rep Neurol Med. (2016) 2016:5240274–4. doi: 10.1155/2016/5240274
39. Meyer Sauteur, PM, Roodbol, J, Hackenberg, A, de Wit, MC, Vink, C, Berger, C, et al. Severe childhood Guillain-Barré syndrome associated with Mycoplasma pneumoniae infection: a case series. J Peripher Nerv Syst. (2015) 20:72–8. doi: 10.1111/jns.12121
40. Michev, A, Musso, P, Foiadelli, T, Trabatti, C, Lozza, A, Franciotta, D, et al. Bickerstaff brainstem encephalitis and overlapping Guillain-Barré syndrome in children: report of two cases and review of the literature. Eur J Paediatr Neurol. (2019) 23:43–52. doi: 10.1016/j.ejpn.2018.11.008
41. Monte, G, Pro, S, Ursitti, F, Ferilli, MAN, Moavero, R, Papetti, L, et al. Case report: a pediatric case of Bickerstaff brainstem encephalitis after COVID-19 vaccination and Mycoplasma pneumoniae infection: looking for the culprit. Front Immunol. (2022) 13:987968. doi: 10.3389/fimmu.2022.987968
42. Natsui, H, Takahashi, M, Nanatsue, K, Itaya, S, Abe, K, Inaba, A, et al. Mother and son cases of Bickerstaff’s brainstem encephalitis and fisher syndrome with serum anti-GQ1b IgG antibodies: a case report. BMC Neurol. (2021) 21:130. doi: 10.1186/s12883-021-02159-y
43. Park, JY, Ko, KO, Lim, JW, Cheon, EJ, Yoon, JM, and Kim, HJ. A pediatric case of Bickerstaff’s brainstem encephalitis. Korean J Pediatr. (2014) 57:542–5. doi: 10.3345/kjp.2014.57.12.542
44. Pavone, P, Le Pira, A, Greco, F, Vitaliti, G, Smilari, PL, Parano, E, et al. Bickerstaff’s brainstem encephalitis (BBE) in childhood: rapid resolution after intravenous immunoglobulins treatment. Eur Rev Med Pharmacol Sci. (2014) 18:2496–9.
45. Venkateshwara Prasad, KN, Venkatesh, KS, and Devi, NG. Bickerstaff brainstem encephalitis in pediatrics - a case report. J Pediatr Neurosci. (2013) 8:263–4. doi: 10.4103/1817-1745.123718
46. Meyer Sauteur, PM, Relly, C, Hackenberg, A, Stahr, N, Berger, C, Bloemberg, GV, et al. Mycoplasma pneumoniae intrathecal antibody responses in Bickerstaff brain stem encephalitis. Neuropediatrics. (2014) 45:61–3. doi: 10.1055/s-0033-1348150
47. Rho, YI. Overlapping Guillain-Barré syndrome and Bickerstaff’s brainstem encephalitis associated with Epstein Barr virus. Korean J Pediatr. (2014) 57:457–60. doi: 10.3345/kjp.2014.57.10.457
48. Saeed, M, and Shah, MTA. A rare entity in children. Nat J Health Sci. (2018) 3:27–9. doi: 10.21089/njhs.31.0027
49. Salih, KH. A case of Bickerstaff’s rainstem encephalitis in childhood. J Sulaimani Med Coll. (2015) 5:155–9. doi: 10.17656/jsmc.10080
50. Santoro, JD. Long-term sequelae of pediatric Bickerstaff brainstem encephalitis includes autonomic and sleep dysregulation. J Child Neurol. (2019) 34:153–60. doi: 10.1177/0883073818820488
51. Steer, AC, Starr, M, and Kornberg, AJ. Bickerstaff brainstem encephalitis associated with Mycoplasma pneumoniae infection. J Child Neurol. (2006) 21:533–4. doi: 10.1177/08830738060210061401
52. Tekin, E, Çokyaman, T, Taşdemir, HA, and Özyürek, H. Silence after the storm; a case of Bickerstaff’s brainstem encephalitis. Turkish J Pediatr Dis. (2021) 15:1–344. doi: 10.12956/tchd.772099
53. Tsapis, M, Laugel, V, Koob, M, de Saint, MA, and Fischbach, M. A pediatric case of fisher-Bickerstaff spectrum. Pediatr Neurol. (2010) 42:147–50. doi: 10.1016/j.pediatrneurol.2009.09.009
54. Kamate, M, Chetal, V, Patil, P, Reddy, SL, and Hattehdi, V. Bickerstaff brain-stem encephalitis. Pediatr Oncall J. (2011) 8:100–1.
55. Kanzaki, A, Yabuki, S, and Yuki, N. Bickerstaff’s brainstem encephalitis associated with cytomegalovirus infection. J Neurol Neurosurg Psychiatry. (1995) 58:260–1. doi: 10.1136/jnnp.58.2.260
56. Kim, J, Kim, Y, Son, Y, and Woo, Y. A case of Bickerstaff’s brainstem encephalitis in childhood. Korean J Pediatr. (2010) 53:607. doi: 10.3345/kjp.2010.53.4.607
57. Kikuchi, M, Tagawa, Y, Iwamoto, H, Hoshino, H, and Yuki, N. Bickerstaff’s brainstem encephalitis associated with IgG anti-GQ1b antibody subsequent to Mycoplasma pneumoniae infection: favorable response to immunoadsorption therapy. J Child Neurol. (1997) 12:403–5. doi: 10.1177/088307389701200612
58. Wali, GM. Bickerstaff’s brainstem encephalitis associated with typhoid fever. Postgrad Med J. (1991) 67:1011–2. doi: 10.1136/pgmj.67.793.1011
59. Yalaz, K, and Tinaztepe, K. Brain stem encephalitis. Acta Paediatr Scand. (1974) 63:235–40. doi: 10.1111/j.1651-2227.1974.tb04790.x
60. Yuki, N, Odaka, M, and Hirata, K. Bickerstaff’s brainstem encephalitis subsequent to Campylobacter jejuni enteritis. J Neurol Neurosurg Psychiatry. (2000) 68:680–1. doi: 10.1136/jnnp.68.5.680
61. Voskuhl, R. Sex differences in autoimmune diseases. Biol Sex Differ. (2011) 2:1. doi: 10.1186/2042-6410-2-1
62. Sansone, P, Giaccari, LG, Aurilio, C, Coppolino, F, Esposito, V, Fiore, M, et al. Post-infectious Guillain-Barré syndrome related to SARS-CoV-2 infection: a systematic review. Life. (2021) 11:167. doi: 10.3390/life11020167
63. Li, Z, Li, X, Shen, J, Chan, MTV, and Wu, WKK. Miller fisher syndrome associated with COVID-19: an up-to-date systematic review. Environ Sci Pollut Res Int. (2021) 28:20939–44. doi: 10.1007/s11356-021-13233-w
64. Bozzola, E, Spina, G, Valeriani, M, Papetti, L, Ursitti, F, Agostiniani, R, et al. Management of pediatric post-infectious neurological syndromes. Ital J Pediatr. (2021) 47:17. doi: 10.1186/s13052-021-00968-y
65. Baunbæk Egelund, G, Ertner, G, Langholz Kristensen, K, Vestergaard Jensen, A, Benfield, TL, and Brandt, CT. Cerebrospinal fluid pleocytosis in infectious and noninfectious central nervous system disease: a retrospective cohort study. Medicine. (2017) 96:e6686. doi: 10.1097/MD.0000000000006686
66. Blinder, T, and Lewerenz, J. Cerebrospinal fluid findings in patients with autoimmune encephalitis-a systematic analysis. Front Neurol. (2019) 10:804. doi: 10.3389/fneur.2019.00804
67. Bertrand, A, Leclercq, D, Martinez-Almoyna, L, Girard, N, Stahl, JP, and de-Broucker, T. MR imaging of adult acute infectious encephalitis. Med Mal Infect. (2017) 47:195–205. doi: 10.1016/j.medmal.2017.01.002
68. Yuki, N. Fisher syndrome and Bickerstaff brainstem encephalitis (Fisher-Bickerstaff syndrome). J Neuroimmunol. (2009) 215:1–9. doi: 10.1016/j.jneuroim.2009.05.020
69. Shin, YW, Lee, ST, Park, KI, Jung, KH, Jung, KY, Lee, SK, et al. Treatment strategies for autoimmune encephalitis. Ther Adv Neurol Disord. (2017) 11:1756285617722347. doi: 10.1177/1756285617722347
70. Prasad, AN, and Chaudhary, S. Intravenous immunoglobulin in pediatrics: a review. Med J Armed Forces India. (2014) 70:277–80. doi: 10.1016/j.mjafi.2013.05.011
71. Zhang, Y, Huang, HJ, Chen, WB, Liu, G, Liu, F, and Su, YY. Clinical efficacy of plasma exchange in patients with autoimmune encephalitis. Ann Clin Transl Neurol. (2021) 8:763–73. doi: 10.1002/acn3.51313
Keywords: Bickerstaff brainstem encephalitis, Bickerstaff’s encephalitis, Bickerstaff’s syndrome, children, pediatric
Citation: Giaccari LG, Mastria D, Barbieri R, De Maglio R, Madaro F, Paiano G, Pace MC, Sansone P, Pulito G and Mascia L (2024) Bickerstaff encephalitis in childhood: a review of 74 cases in the literature from 1951 to today. Front. Neurol. 15:1387505. doi: 10.3389/fneur.2024.1387505
Edited by:
Piero Pavone, University of Catania, ItalyReviewed by:
Jonathan Douglas Santoro, Children’s Hospital of Los Angeles, United StatesKei Funakoshi, Dokkyo Medical University, Japan
Copyright © 2024 Giaccari, Mastria, Barbieri, De Maglio, Madaro, Paiano, Pace, Sansone, Pulito and Mascia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Gregorio Giaccari, bHVjYWdyZWdvcmlvLmdpYWNjYXJpQGdtYWlsLmNvbQ==
 Luca Gregorio Giaccari
Luca Gregorio Giaccari Donatella Mastria1
Donatella Mastria1 Pasquale Sansone
Pasquale Sansone