- 1Department of Neurological Surgery, University of Kansas Medical Center, Kansas City, KS, United States
- 2Division of Neurosurgery, University of Vermont Medical Center, Burlington, VT, United States
Introduction: Nontraumatic subarachnoid hemorrhage (ntSAH) often results from a ruptured aneurysm and correlates with significant morbidity and mortality, particularly among the older population. Despite its impact, limited comprehensive studies evaluate the longitudinal trends in ntSAH-related mortality in older adults in the United States (US).
Methods: The authors conducted a retrospective analysis using the CDC WONDER database from 1999 to 2020, analyzing Multiple Cause-of-Death Public Use death certificates to identify ntSAH as a contributing factor in the death of adults aged 65 years and older. We calculated age-adjusted mortality rates (AAMR) and annual percent change (APC) to examine trends across demographic variables such as sex, race/ethnicity, urbanization, and states/census region.
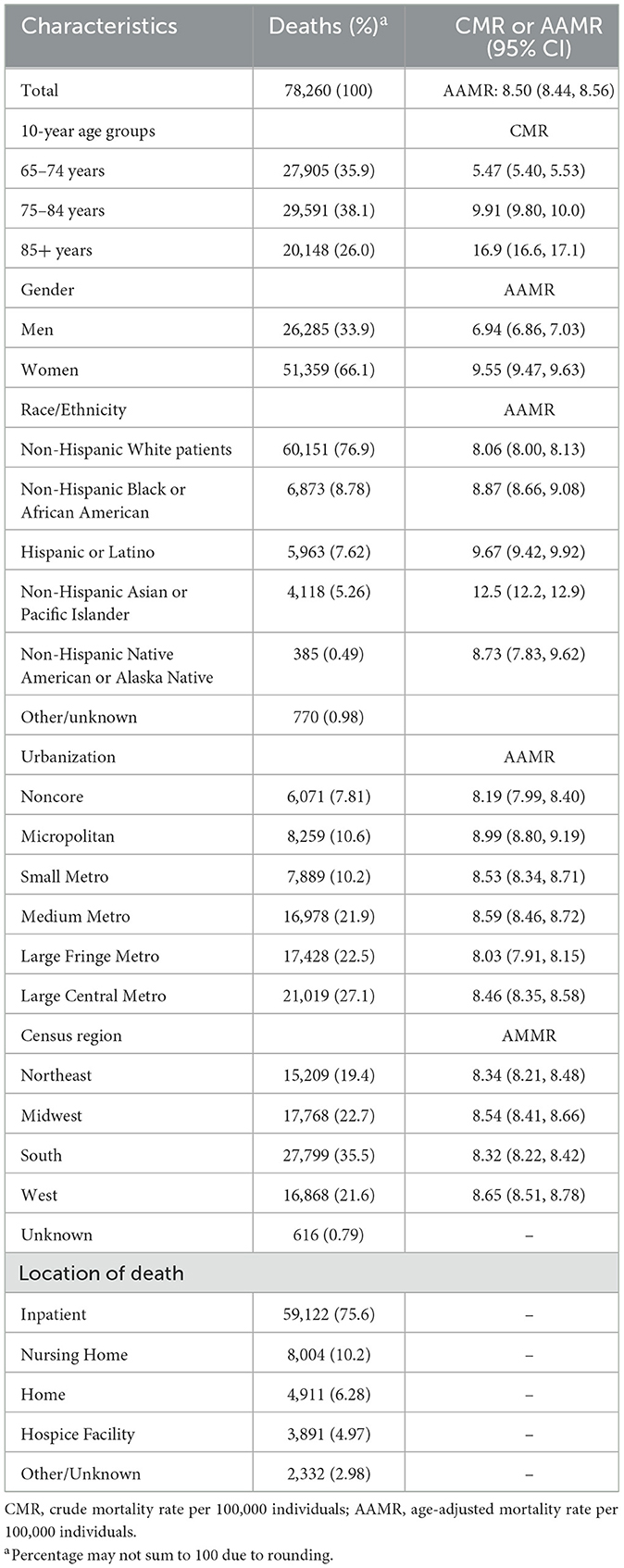
Results: A total of 78,260 ntSAH-related deaths (AAMR 8.50 per 100,000 individuals) occurred among older adults in the US from 1999 to 2020. The overall AAMR for ntSAH decreased from 9.98 in 1999 to 8.67 in 2020 with an APC of −0.7% [95% CI (−1.0, −0.3)]. However, the authors observed a noticeable rise from 2013 to 2020 with an APC of 1.7% [95% CI (0.8, 2.6)]. Sex, racial, and regional disparities were evident with higher mortality rates for ages 85 or greater (crude mortality rate 16.6), women (AAMR 9.55), non-Hispanic Asian or Pacific Islander (AAMR 12.5), and micropolitan areas (AAMR 8.99), and Western US (AAMR 8.65).
Conclusion: Mortality from ntSAH increases with age, affects women disproportionately, and occurs more often in an inpatient setting. These findings necessitate targeted, multi-dimensional health policies and clinical interventions. Specialties beyond neurosurgery can utilize this data for improved risk stratification and early treatment. Policymakers should focus on equitable resource allocation and community-level interventions to mitigate these trends effectively.
Introduction
The risk of nontraumatic subarachnoid hemorrhage (ntSAH) rises sharply with age (1–3). Due to aging demographics in the United States (US), ntSAH warrants a more focused epidemiological evaluation by American neurosurgeons. Especially among adults aged 65 years and older, the morbidity and mortality associated with ntSAH represent a considerable clinical and economic burden (4, 5). Age-specific data on ntSAH-related mortality among the older population in the US remains minimal (6). The lack of comprehensive analysis could impede the formation of targeted health policy measures aimed at reducing mortality and improving the quality of life in this vulnerable group (7).
Given the demographic shifts and the aging population's susceptibility to ntSAH, we investigate how trends in ntSAH-related mortality continue to evolve among adults aged 65 and above. To address this question, this study performs an in-depth analysis of data from the Centers for Disease Control and Prevention's Wide-ranging Online Data for Epidemiologic Research (CDC WONDER) database. By dissecting these trends, we aim to contribute to existing knowledge on ntSAH epidemiology, thereby informing clinical guidelines and health policy initiatives to mitigate the impact of ntSAH-related mortality among the older population.
Methods
Data source
This study investigated the number of deaths associated with nontraumatic subarachnoid hemorrhages (ntSAH) from ages 65 years and older in the United States from 1999 to 2020. The study used the CDC WONDER database (8). This study was exempt from institutional review board approval due to the public and anonymous nature of the database and was compliant with STROBE guidelines.
This study analyzed Multiple Cause-of-Death Public Use death certificates to identify ntSAH as a contributing factor in the death. The authors obtained the number of annual deaths related to ntSAH and the corresponding population sizes. The data were separated into categories based on sex, race/ethnicity, 10-year age groups, level of urbanization, and state. The study used the International Statistical Classifications of Diseases, Tenth Revision (ICD-10) code for ntSAH (I60.x) (9).
The sex, ethnicity, and race were recorded on the death certificates in compliance with the US Office of Management and Budget (10). Ethnicity and race were divided into separate categories, including Hispanic or Latino, non-Hispanic Black or African American, non-Hispanic Asian or Pacific Islander, or Non-Hispanic White patients. Although we included the individuals who did not fall into the categories of Hispanic or Latino or non-Hispanic in the overall analysis, we omitted these from the subgroup analysis due to the suppression of data with too few individuals in CDC WONDER.
Urbanization was defined in compliance with the 2013 National Center for Health Statistics Urban-Rural Classification Scheme for Counties (11). Metropolitan was subdivided into large central metros (population >1 million and completely contain or are entirely contained by the largest city or contain at least 250,000 residents of any city within the statistical area), large fringe metros (population >1 million and not central metro), medium metros (population 250,000–999,999), and small metros (population < 250,000). Nonmetropolitan counties are subdivided into micropolitan (population 10,000–49,999) and noncore (population < 10,000). The data for each state excluded the District of Columbia. The place of death was subdivided into inpatient, nursing home, home, hospice facility, and other/unknown.
Statistical analysis
The crude mortality rate (CMR) and age-adjusted mortality rates per 100,000 (AAMR) were calculated using the US 2000 standard population (12). To evaluate mortality trends, Joinpoint Regression Program (v4.9.1.0; National Cancer Institute, 2022) (13) was employed to analyze the overall population and subgroup segments. The Monte Carlo permutation test was employed to determine the annual percent change (APC) in mortality with 95% confidence intervals at identified segments linking join points. The slopes of APCs were then calculated and assessed using a two-sided t-test against a null value of 0. Statistical significance was determined by a P-value < 0.05. Analysis was conducted in October 2023.
Results
A total of 78,260 ntSAH-related deaths (AAMR 8.50 per 100,000 individuals) occurred among older adults in the United States from 1999 to 2020. Of the total deaths, 26,285 (33.9%) were men, 51 359 (66.1%) were women. Of the known ethnic and racial groups 60,151 (76.9%) were Non-Hispanic White patients, 6 873 (8.8%) were non-Hispanic Black or African American, 4,118 (5.3%) were non-Hispanic Asian or Pacific Islander, and 5,963 (7.6%) were Hispanic or Latino. Of the 75,928 known locations of death, 59,122 (75.6%) were inpatient facilities, 8,004 (10.2%) were nursing homes, 4,911 (6.3%) were in the decedent's home, and 3,891 (5.0%) were in hospice facilities. Among diagnosis, age, sex, race and ethnicity, and urbanization, the highest mortality rates were seen in those 85 or older (CMR 16.9), women (AAMR 9.55), non-Hispanic Asian or Pacific Islander adults (AAMR 12.5), micropolitan populations (AAMR 8.99), and in the Western US (AAMR 8.65; Table 1, Supplementary Table 1).
The overall AAMR for ntSAH in older adults decreased from 9.98 in 1999 to 8.67 in 2020 with an average annual percent change (AAPC) of −0.7%, 95% CI (−1.0, −0.3). The AAMR decreased from from 1999 to 2013 [APC, −1.8% 95% CI (−2.2, −1.5)] followed by a rise from 2013 to 2020 [APC, 1.7% 95% CI (0.8, 2.6)]. NtSAH-related mortality remained stable for men but decreased for women. Specifically, the AAMR in men changed from 7.40 in 1999 to 7.89 in 2020, while the AAMR for women decreased from 11.7 in 1999 to 9.27 in 2020. Although the AAMR for both men and women decreased from 1999 to 2014 and 1999 to 2011, respectively, the AAMR for men increased from 2014 to 2020 [APC, 2.9% 95% CI (1.7, 4.2)] while the AAMR for women had a statistically insignificant rise from 2011 to 2020 [APC, 0.6% 95% CI (−0.1, 1.3); Figure 1]
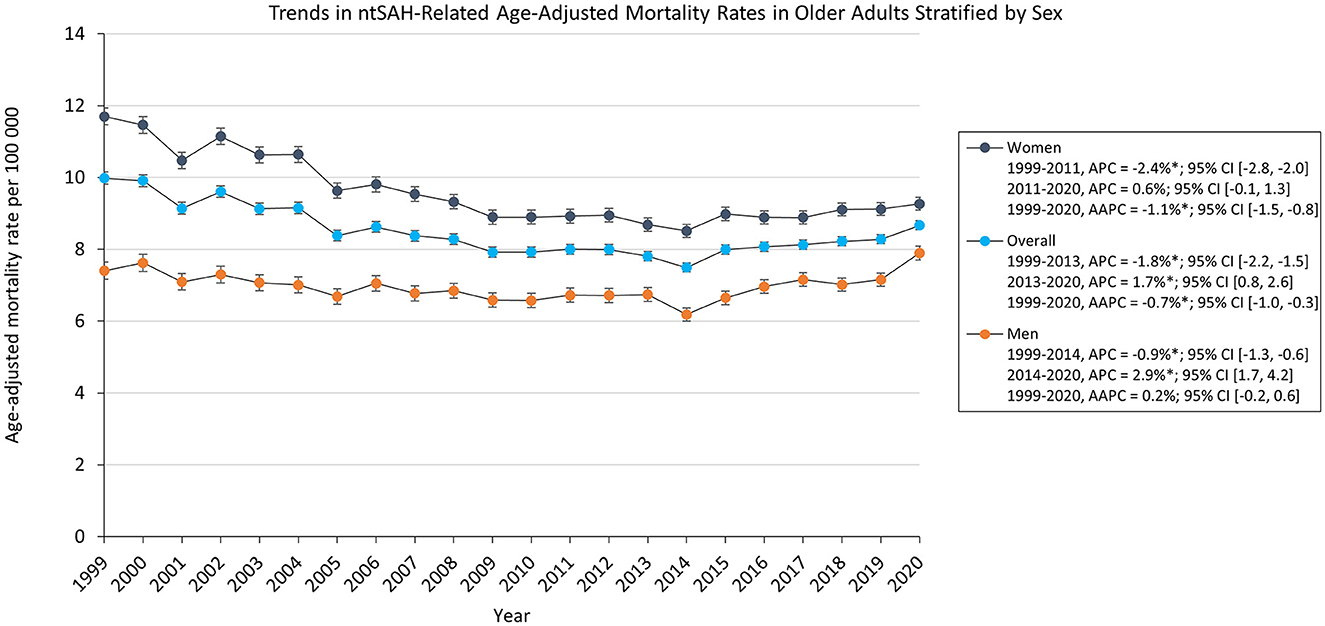
Figure 1. Trends in ntSAH-related age-adjusted mortality rates in older adults stratified by sex. *P < 0.05.
AAMR decreased for all ethnic and racial groups. Asian or Pacific Islander adults had the highest AAMR which decreased from 17.2 in 1999 to 12.0 in 2020. The AAMR for White, Black, and Asian or Pacific Islander adults decreased from 1999 to 2012, 2013, and 2014, respectively, followed by a statistically significant increase to 2020 for White and Black adults. The AAMR for Asian or Pacific Islander adults had a statistically insignificant increase [APC, 1.1% 95% CI (−1.4, 3.7)], while the AAMR for Hispanic or Latino adults decreased from 13.7 in 1999 to 9.30 in 2020 (Figure 2).
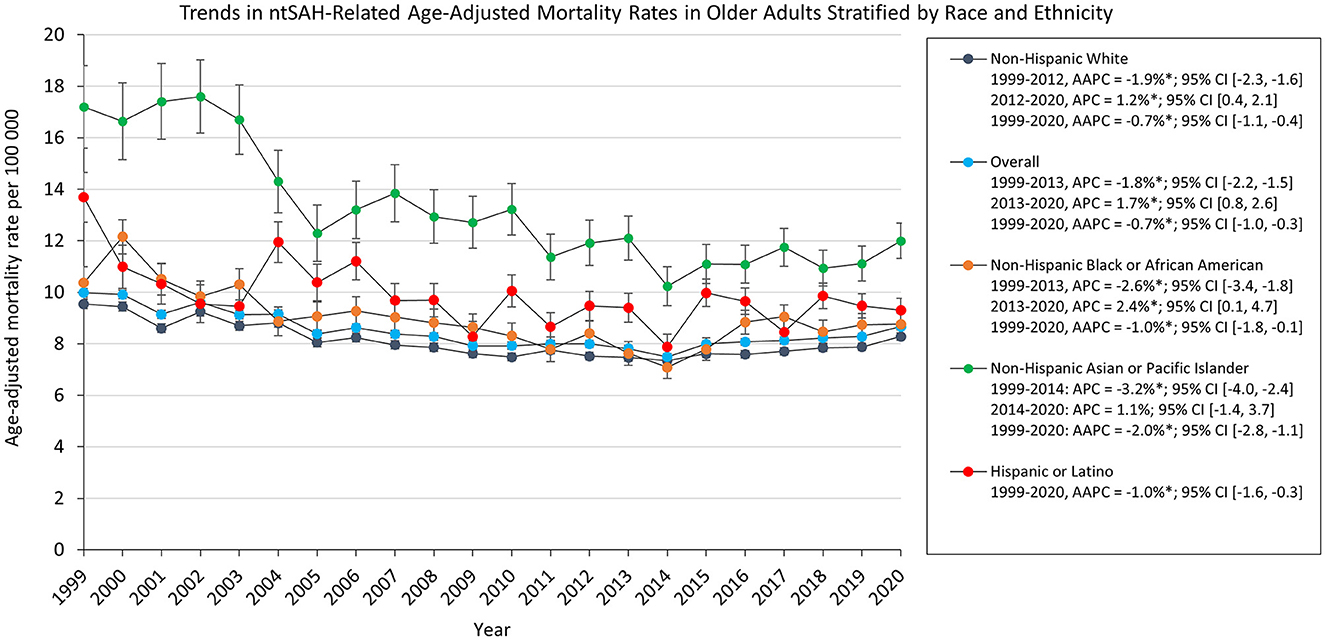
Figure 2. Trends in ntSAH-related age-adjusted mortality rates in older adults stratified by race and ethnicity. *P < 0.05.
Subgroup analysis showed that CMR in those 65 and older decreased from 9.93 in 1999 to 8.32 in 2020. The CMR for adults 65–74 and 75–84 years old decreased from 1999 through 2020 while the CMR in adults 85 or older increased from 16.0 in 1999 to 19.3 in 2020 (Supplementary Figure 1). From 1999 through 2020, the proportions of ntSAH-related deaths decreased in inpatient facilities (86.0%−66.7%). In contrast, the proportion of ntSAH-related deaths increased in homes (3.7%−11.4%), nursing homes (9.3%−9.9%), and hospice facilities (0.4% in 2003 to 9.6% in 2020; Supplementary Table 2, Supplementary Figure 2). The AAMR of ntSAH as the underlying cause of death decreased from 1999 to 2013 [APC, −2.5% 95% CI (−2.7, −2.2)] before stabilizing from 2013 to 2020 [APC, 0.2% 95% CI (−0.4, 0.9); Supplementary Table 3, Supplementary Figure 3].
Mortality rates for all urbanization groups decreased from 1999 to 2020 except for micropolitan and noncore areas which remained stable. Micropolitan areas had the highest AAMR which decreased from 11.2 in 1999 to 9.93 in 2020. All urbanization groups decreased from 1999 to the 2010s. The AAMR for noncore, micropolitan, and medium metropolitan areas increased from 2008, 2011, and 2012 through 2020, respectively, while medium metropolitan, large fringe and large central areas remained stabilized from 2012 and 2014 through 2020, respectively (Figure 3).
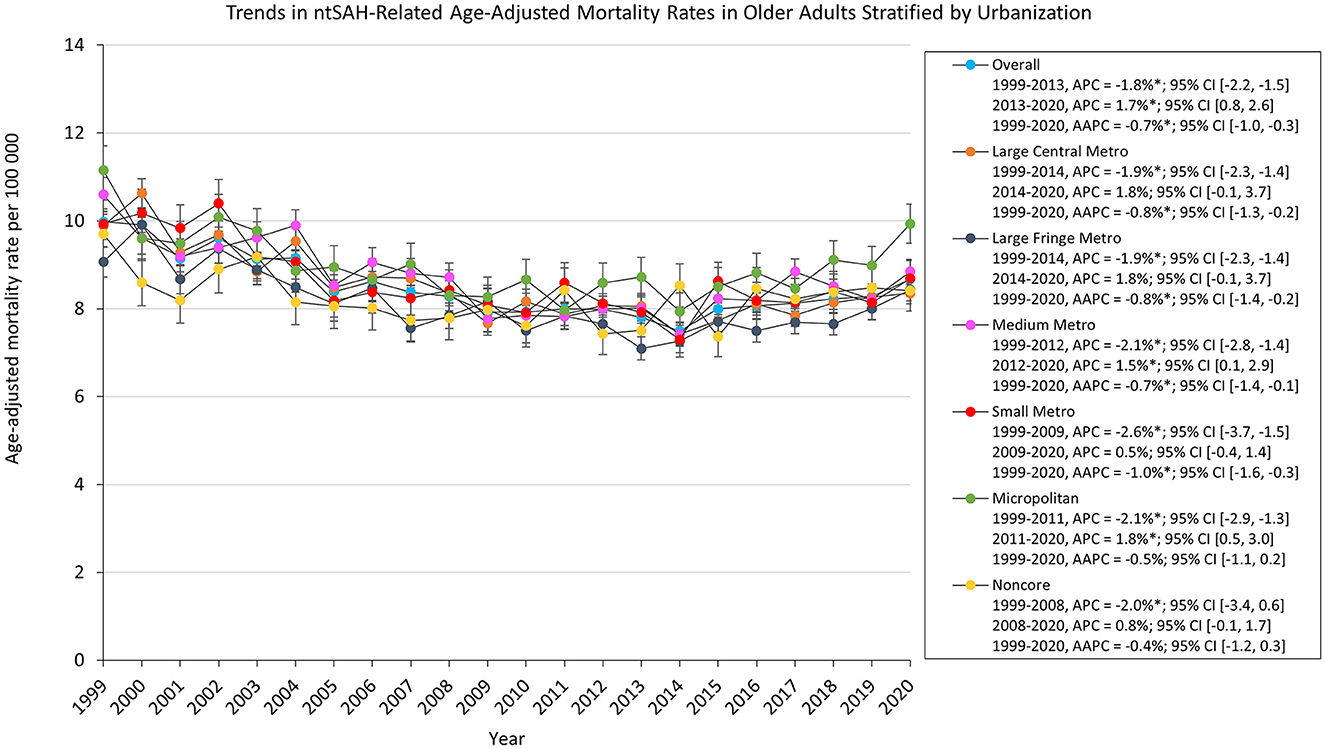
Figure 3. Trends in ntSAH-related age-adjusted mortality rates in older adults stratified by urbanization. *P < 0.05.
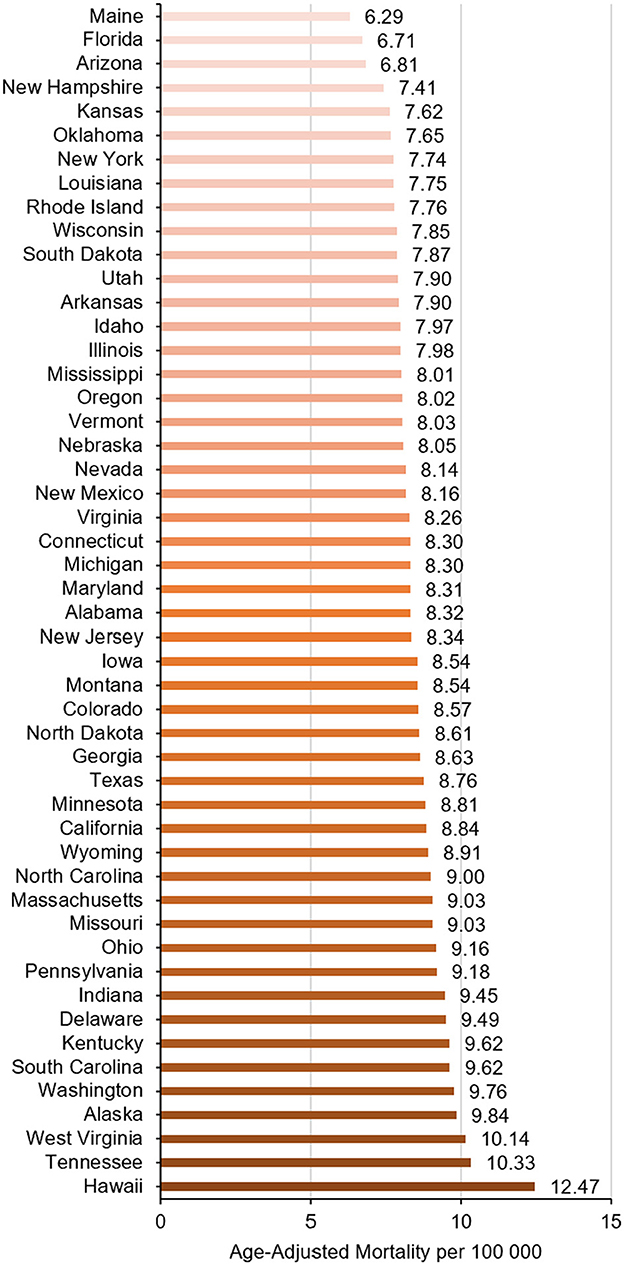
There were significant regional differences in the burden of ntSAH-related mortality with the Western US having a significantly higher mortality burden compared to the remaining census regions (Table 1, Supplementary Table 4). The top five states (Hawaii, Tennessee, West Virginia, Alaska, and Washington) with the highest ntSAH-related mortality had much higher mortality than those in the bottom five states (Kansas, New Hampshire, Arizona, Florida, and Maine; Figure 4).
Discussion
This comprehensive analysis of ntSAH-related mortality among adults aged 65 years and older from the CDC WONDER database from 1999 to 2020 demonstrates a complex trajectory. While the overall AAMR exhibited an annual decline of −0.7%, the overarching figure conceals dynamic changes over the span of 2 decades. An initial decline in mortality was observed from 1999 to 2013, followed by a concerning rise from 2013 to 2020. This resurgence in mortality rates, despite advancements in neurosurgical interventions and medical management, signals a need for further investigation into evolving risk factors and healthcare disparities in this vulnerable population.
The increase in mortality related to ntSAH in elder men observed in our study is relevant for several reasons. The results confirm that this increase is a significant trend. This suggests that understanding trends in ntSAH mortality in elder men may help target public health interventions including increasing awareness and potentially different treatment strategies for this high-risk population. This trend also encourages further research to explore underlying causes, such as biological differences, lifestyle factors, comorbidities, or access to healthcare, which might contribute to higher mortality (14–16).
As patient demographics have shifted toward an older age with overall improvements in health care and longer life expectancies, clinical practice has also evolved with more elderly ntSAH patients referred to neurosurgical centers than ever before. Nearly two decades after the publication of the International Subarachnoid Aneurysm Trial (ISAT), which highlighted the utility of endovascular interventions over traditional microsurgical clipping and craniotomy for ruptured cerebral aneurysms, the treatment of ntSAH has changed dramatically (17). In support of aggressive management of cerebral aneurysms and accommodation of elderly patients unable to tolerate surgery, the ISAT results have led to the explosive growth of endovascular coiling in the elderly SAH population. Although increasing technological sophistication has led to increased device costs and healthcare expenditures, we are now better equipped to treat patients with ntSAH (18).
Several studies have cited advancing age, along with the severity of clinical presentation on admission, as two of the strongest prognostic factors for predicting poor functional outcomes (19–23). Age negatively impacts survival and recovery as older patients have a greater number of co-morbidities and systemic complications. Moreover, ntSAH has a unique pathophysiology in elderly patients. Elderly patients have greater degrees of brain atrophy, vessel calcification or stenosis, and hypertension, which may affect the pattern and extension of blood after a rupture. Additionally, elderly patients have lower cerebral blood flow and lower cardiac output. While less elasticity of the vessels may lower the probability of vasospasm after ntSAH, elderly patients have a high likelihood of mortality and cardiovascular events compared with the younger healthy population (24–26). Although these age-related pathophysiologic differences may account for greater mortality in elderly patients, the increasing mortality rate since 2013 within the cohort is alarming. A greater number of treatments, especially endovascular interventions enhanced by rapidly advancing technologies, are now offered to elderly patient more than at any previous time. The authors suspect that with improved access to tertiary care centers and greater number of elderly patients considered for treatment, more deaths are now coded appropriately as ntSAH. This is compounded by the number of elderly individuals or family members who may choose to withdraw care and clinicians who forgo treatment understanding the poor functional outcomes within this cohort.
With respect to racial and ethnic stratification, all groups exhibited a decrease in AAMR, albeit to varying extents. Xia's article, which spanned between 2007 and 2017, showed an unchanged incidence of spontaneous SAH in Asian, Hispanic, and Non-Hispanic White patients but an increased incidence in Black patients. This possibly suggests that either incidence has particularly increased in the Asian cohort since 2017, or that there is greater mortality in this cohort after hemorrhage recently.
The findings in this study illuminate geographical discrepancies related to urbanization. While all urbanization groups witnessed a decrease in mortality rates from 1999 to 2020, micropolitan areas remained persistently higher, and along with noncore areas stabilized instead of decreasing. Regional variances further complicate the landscape of ntSAH mortality, with the Western US exhibiting significantly higher mortality. Interestingly, significant differences were noted at the state level, highlighting the influence of local healthcare infrastructure, policy, and possibly environmental factors.
Regional and urban differences in mortality may reflect different levels of access to healthcare resources. Micropolitan areas have a greater incidence of vascular risk factors, such as cigarette smoking, diabetes, and hypertension, which have been associated with greater predisposition to, and worse outcomes following ntSAH (27). Mariajoseph et al. (28) contend that this may explain the higher rates of mechanical ventilation seen in non-metropolitan location transfers after aneurysmal SAH, which yields greater rates of rebleeding due to delays in transfer. Furthermore, ntSAH care requires unique expertise, which has led to greater regionalization among higher-volume centers. Changes in mortality may suggest delayed transport to comprehensive stroke centers, which perform highly specialized endovascular and surgical procedures and have neuro-intensive care units. Care at high-volume teaching hospitals is more likely to reduce mortality and readmissions with multidisciplinary team-based interventions involving neurointensivists, neurosurgeons, endovascular specialists, nursing care, social work, and rehabilitation services. Older patients with ntSAH may require prompt consideration for referral to high-volume teaching hospitals to reduce this mortality.
Limitations
There are inherent limitations to this study that warrant consideration. The employment of the ICD codes and the dependency on death certificates for data collection may engender some level of misclassification of ntSAH as a cause of death. Additionally, advancement in diagnostic capabilities and the increasing use of electronic health records may contribute to changes in the recording of ntSAH on death certificates, potentially influencing trends without necessarily reflecting actual changes in ntSAH-related mortality rates among the older population. The study has limitations in identifying the primary driver(s) of the U-shaped trends in mortality from 2009 to 2020. We believe that reporting the cause of death was affected by the adoption of the electronic medical records due to the enactment of the Health Information Technology for Economic and Clinical Health Act (29). Additionally, CDC WONDER does not capture subgroups with smaller populations as described by Tiwari et al. (30). The CDC WONDER database from which this study draws its data lacks granularity on disease characteristics such as clinical symptoms, laboratory markers, imaging studies, or other prognostic indicators. These limitations collectively circumscribe the ability to draw definitive conclusions and suggest that the findings should be interpreted cautiously.
Conclusions
The study reveals that after an initial period of decline, the AAMR for ntSAH-related mortality among older adults has discernibly increased from 2013 to 2020 in the United States. This rise is nuanced by demographic variables such as sex, race and ethnicity, and geographic location, necessitating a multi-dimensional approach for its interpretation and management. Targeted health policy measures are imperative to address the escalating burden of ntSAH in the older population, with an emphasis on refined risk stratification, timely intervention, and the mitigation of persistent disparities.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
MM: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. AD: Writing – original draft, Writing – review & editing. EW: Writing – original draft, Writing – review & editing. PC: Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1385128/full#supplementary-material
References
1. Korja M, Kaprio J. Controversies in epidemiology of intracranial aneurysms and SAH. Nat Rev Neurol. (2016) 12:50–5. doi: 10.1038/nrneurol.2015.228
2. Nieuwkamp DJ, Setz LE, Algra A, Linn FHH, de Rooij NK, Rinkel GJE. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. (2009) 8:635–42. doi: 10.1016/S1474-4422(09)70126-7
3. Linn FH, Rinkel GJ, Algra A, van Gijn J. Incidence of subarachnoid hemorrhage: role of region, year, and rate of computed tomography: a meta-analysis. Stroke. (1996) 27:625–9. doi: 10.1161/01.STR.27.4.625
4. Ogilvy CS, Carter BS. Stratification of outcome for surgically treated unruptured intracranial aneurysms. Neurosurgery. (2003) 52:82–7; discussion 87–88. doi: 10.1227/00006123-200301000-00010
5. Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. (2012) 43:1711–37. doi: 10.1161/STR.0b013e3182587839
6. Wiebers DO, Whisnant JP, Huston J, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet Lond Engl. (2003) 362:103–10. doi: 10.1016/S0140-6736(03)13860-3
7. Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. (2019) 76:588–97. doi: 10.1001/jamaneurol.2019.0006
8. Centers for Disease Control and Prevention National Center for Health Statistics. National Vital Statistics System, Mortality 1999-2020 on CDC WONDER Online Database, released in 2021. Data are from the Multiple Cause of Death Files, 1999-2020, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Available at: http://wonder.cdc.gov/mcd-icd10.html (accessed October 1, 2023).
9. World Health Organization. ICD-10 : International Statistical Classification of Diseases and Related Health Problems : Tenth Revision, 2nd Edn. Geneva: World Health Organization (2004).
10. Office of Management and Budget. Available at: https://www.govinfo.gov/content/pkg/FR-2016-09-30/pdf/2016-23672.pdf (accessed December 12, 2023).
11. Ingram DD, Franco SJ. 2013 NCHS urban-rural classification scheme for counties. Vital Health Stat 2. (2014) 1−73.
12. Anderson RN, Rosenberg HM. Age standardization of death rates: implementation of the year 2000 standard. Natl Vital Stat Rep Cent Dis Control Prev Natl Cent Health Stat Natl Vital Stat Syst. (1998) 47:1–16, 20.
13. Joinpoint Regression Program Version 4.9.1.0 – April. Statistical Methodology and Applications Branch, Surveillance Research Program. Bethesda, MD: National Cancer Institute (2022).
14. Virta JJ, Satopää J, Luostarinen T, Raj R. One-year outcome after aneurysmal subarachnoid hemorrhage in elderly patients. World Neurosurg. (2020) 143:e334–43. doi: 10.1016/j.wneu.2020.07.127
15. Kanamaru H, Kawakita F, Asada R, Miura Y, Shiba M, Toma N, et al. Prognostic factors varying with age in patients with aneurysmal subarachnoid hemorrhage. J Clin Neurosci. (2020) 76:118–25. doi: 10.1016/j.jocn.2020.04.022
16. Schöller K, Massmann M, Markl G, Kunz M, Fesl G, Brückmann H, et al. Aneurysmal subarachnoid hemorrhage in elderly patients: long-term outcome and prognostic factors in an interdisciplinary treatment approach. J Neurol. (2013) 260:1052–60. doi: 10.1007/s00415-012-6758-1
17. Molyneux AJ, Kerr RSC, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet Lond Engl. (2005) 366:809–17. doi: 10.1016/S0140-6736(05)67214-5
18. Bekelis K, Gottlieb DJ, Su Y, Lanzino G, Lawton MT, MacKenzie TA. Medicare expenditures for elderly patients undergoing surgical clipping or endovascular intervention for subarachnoid hemorrhage. J Neurosurg. (2017) 126:805–10. doi: 10.3171/2016.2.JNS152994
19. Nieuwkamp DJ, Rinkel GJE, Silva R, Greebe P, Schokking DA, Ferro JM. Subarachnoid haemorrhage in patients > or = 75 years: clinical course, treatment and outcome. J Neurol Neurosurg Psychiatry. (2006) 77:933–7. doi: 10.1136/jnnp.2005.084350
20. Shimamura N, Naraoka M, Katagai T, Katayama K, Kakuta K, Matsuda N, et al. Analysis of factors that influence long-term independent living for elderly subarachnoid hemorrhage patients. World Neurosurg. (2016) 90:504–10. doi: 10.1016/j.wneu.2016.03.057
21. Ido K, Kurogi R, Kurogi A, Nishimura K, Arimura K, Nishimura A, et al. Effect of treatment modality and cerebral vasospasm agent on patient outcomes after aneurysmal subarachnoid hemorrhage in the elderly aged 75 years and older. PLoS ONE. (2020) 15:e0230953. doi: 10.1371/journal.pone.0230953
22. Sadamasa N, Koyanagi M, Fukuda H, Chin M, Handa A, Yamagata S. Is aneurysm repair justified for the patients aged 80 or older after aneurysmal subarachnoid hemorrhage? J Neurointerventional Surg. (2014) 6:664–6. doi: 10.1136/neurintsurg-2013-010951
23. Ohkuma H, Shimamura N, Naraoka M, Katagai T. Aneurysmal subarachnoid hemorrhage in the elderly over age 75: a systematic review. Neurol Med Chir. (2017) 57:575–83. doi: 10.2176/nmc.ra.2017-0057
24. Brawanski N, Kunze F, Bruder M, Tritt S, Senft C, Berkefeld J, et al. Subarachnoid hemorrhage in advanced age: comparison of patients aged 70-79 years and 80 years and older. World Neurosurg. (2017) 106:139–44. doi: 10.1016/j.wneu.2017.06.056
25. Malinova V, Schatlo B, Voit M, Suntheim P, Rohde V, Mielke D. Identification of specific age groups with a high risk for developing cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurg Rev. (2016) 39:429–36. doi: 10.1007/s10143-016-0701-3
26. Goldberg J, Schoeni D, Mordasini P, Z'Graggen W, Gralla J, Raabe A, et al. Survival and outcome after poor-grade aneurysmal subarachnoid hemorrhage in elderly patients. Stroke. (2018) 49:2883–9. doi: 10.1161/STROKEAHA.118.022869
27. Hammond G, Luke AA, Elson L, Towfighi A, Joynt Maddox KE. Urban-rural inequities in acute stroke care and in-hospital mortality. Stroke. (2020) 51:2131–8. doi: 10.1161/STROKEAHA.120.029318
28. Mariajoseph FP, Huang H, Lai LT. Influence of socioeconomic status on the incidence of aneurysmal subarachnoid haemorrhage and clinical recovery. J Clin Neurosci Off J Neurosurg Soc Australas. (2022) 95:70–4. doi: 10.1016/j.jocn.2021.11.033
29. Health Information Technology for Economic and Clinical Health Act. (2009). Available at: https://www.govinfo.gov/content/pkg/PLAW-111publ5/pdf/PLAW-111publ5.pdf (accessed February 3, 2023).
Keywords: epidemiology, subarachanoid hemorrhage, elderly, mortality, disparities
Citation: McCandless MG, Dharia AA, Wicks EE and Camarata PJ (2024) Geo-demographic trends in nontraumatic subarachnoid hemorrhage-related mortality among older adults in the United States, 1999–2020. Front. Neurol. 15:1385128. doi: 10.3389/fneur.2024.1385128
Received: 14 February 2024; Accepted: 31 July 2024;
Published: 14 August 2024.
Edited by:
Maurizio A. Leone, IRCCS Casa Sollievo della Sofferenza Hospital, ItalyReviewed by:
Maria Isabel Chamorro Muñoz, Andusian Health Service, SpainLuis Alberto Camputaro, Specialized Institute “Hospital El Salvador”, El Salvador
Copyright © 2024 McCandless, Dharia, Wicks and Camarata. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin G. McCandless, bW1jY2FuZGxlc3MyQGt1bWMuZWR1
 Martin G. McCandless
Martin G. McCandless Anand A. Dharia1
Anand A. Dharia1