
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Neurol. , 28 March 2024
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1376216
This article is part of the Research Topic Intracranial aneurysms, AVM and other vascular malformations, and connective tissue disorders as potential causes of stroke: Advances in diagnosis and therapeutics including novel neurosurgical techniques View all 15 articles
 Yawen Cheng1†
Yawen Cheng1† Xiangning Han1†
Xiangning Han1† Wanfu Xie2
Wanfu Xie2 Gaofeng Xu2
Gaofeng Xu2 Xiaobin Bai2
Xiaobin Bai2 Lei Qi2
Lei Qi2 Linjuan Zhang3
Linjuan Zhang3 Rui Liu1
Rui Liu1 Weihua Dong4
Weihua Dong4 Weiyi Feng4
Weiyi Feng4 Chengsen Pang4
Chengsen Pang4 Wei Zhang2
Wei Zhang2 Fude Liu1
Fude Liu1 Xiangqi Cao1
Xiangqi Cao1 Yue Xu1
Yue Xu1 Guogang Luo1*
Guogang Luo1*Objectives: This study aimed to investigate the efficacy of using a newly formulated magnesium-rich artificial cerebrospinal fluid (MACSF) as an alternative to normal saline (NS) for intraoperative irrigation during aneurysm clipping in improving the prognosis of patients with Aneurysmal subarachnoid hemorrhage (aSAH).
Methods: Patients with aSAH who underwent intraoperative irrigation with MACSF or NS during the clipping in the First Affiliated Hospital of Xi ‘an Jiaotong University from March 2019 to March 2022 were selected as MACSF group and NS group, respectively. The primary prognostic indicators were the incidence of favorable outcomes (mRS 0–2). The secondary outcome measures included cerebral vasospasm (CVS), mortality, total hospital stay, and intensive care unit (ICU) stay. Safety was evaluated based on the occurrence rates of hypermagnesemia, meningitis, and hydrocephalus.
Results: Overall, 34 and 37 patients were enrolled in the MACSF and NS groups, respectively. At 90 days after aSAH onset, the proportion of favorable prognosis in the MACSF group was significantly higher than that in the NS group (p = 0.035). The incidence of CVS within 14 days after surgery was significantly lower in the MACSF group than that in the NS group (p = 0.026). The mortality rate in the MACSF group was significantly lower than in the NS group (p = 0.048). The median lengths of hospital stay (p = 0.008) and ICU stay (p = 0.018) were significantly shorter in the MACSF group than in the NS group. No significant differences were observed in safety measures.
Conclusion: Using MACSF as an irrigation fluid for aneurysm clipping can significantly improve the 90-day prognosis of patients with aSAH, which may be related to the reduced incidence of CVS.
Clinical trial registration: https://www.clinicaltrials.gov, identifier NCT04358445.
Aneurysmal subarachnoid hemorrhage (aSAH) is a kind of stroke with high morbidity and mortality. Cerebral vasospasm (CVS) is a common complication of aSAH and is closely associated with patient death and disability of the patients (1, 2). Blood and its metabolites, being initiators of CVS, should be removed as soon as possible as they are initiators of CVS. Normal saline (NS), the most commonly used irrigation fluid in aneurysm clipping, can cause secondary brain injuries because its chemical properties of ion composition, osmolarity, and potential of hydrogen (pH) differ from those of cerebrospinal fluid (CSF) (3). Artificial cerebrospinal fluid (ACSF) has similar ionic salts, pH, and osmolality concentrations as CSF. Its safety and efficacy in neural tissues have been demonstrated (4–6). Using ACSF to flush nervous tissues did not alter the cerebrovascular reactivity or physicochemical properties of the CSF (3).
Magnesium (Mg), a vasodilator and natural calcium channel blocker, exerts unique protective functions in the brain. Intravenous magnesium administration is a potential therapeutic strategy for CVS. However, two recent randomized controlled trials (IMASH and MASH-2) failed to confirm the effectiveness of Mg in improving the prognosis of patients with aSAH patients (7, 8). Theoretically, direct intracisternal administration of Mg solution may be more effective.
However, no study has investigated whether using ACSF containing Mg as a flushing fluid during surgery is advantageous for relieving CVS and improving prognosis in patients with aSAH. Therefore, we formulated a new type of ACSF, magnesium-rich artificial cerebrospinal fluid (MACSF), which resembles physiological CSF and is enriched in Mg (9). We have verified that MACSF can maintain the normal physiological activity of rat basilar arteries in vitro and alleviate arterial hyper-responsiveness (10). In this study, MACSF was used as the irrigation fluid during aneurysm clipping and was compared with NS. Therefore, its impact on the 90-day prognosis and the CVS within 14 days postoperatively of patients with aSAH can be clarified.
This was a single-center, non-randomized, non-blinded, single-arm trial conducted at the First Affiliated Hospital of Xi’an Jiaotong University (registered on ClinicalTrials.gov with NCT 04358445 on March 16, 2020) and approved by the Ethics Committee of the hospital (approval NO. XJYFY-2019 N28). All participants provided informed consent to participate in the study and shared their clinical data. All study-related documents were securely stored in the research center, and research data were collected in a limited manner. The entire research process was supervised and monitored by the clinical research center of our hospital. The study followed the SPIRIT reporting guidelines (11).
We enrolled 71 eligible patients with aSAH between March 2020 and March 2022. The MACSF group comprised patients who received MACSF as an intraoperative irrigation fluid, while the NS group consisted of patients who underwent surgery with NS as the irrigation fluid and were enrolled before March 2020. Patients were required to meet the following inclusion criteria: (1) age between 18 and 80 years, (2) aSAH diagnosed by CTA or DSA, (3) admission within 72 h after symptom onset, (4) aneurysm clipped within 36 h after admission, and (5) provided written informed consent. To avoid the influence of the modified Rankin Scale (mRS) score before aSAH onset on the prognostic evaluation, we added additional exclusion criteria after registration. These exclusion criteria included severe craniocerebral trauma, mRS score > 2 before the onset of aSAH, or severe concomitant diseases.
MACSF was prepared by trained medical personnel from the Department of Pharmacy Intravenous Admixture Services (PIAS) on a laminar flow clean bench. MACSF consists of a finished intravenous medication consisting of 0.9% sodium chloride injection, sterilized water for injection, 10% potassium chloride injection, 25% magnesium sulfate injection, 5% sodium bicarbonate injection, and 5% glucose injection. Freshly prepared MACSF was promptly transferred to the operating room in a designated container through a specific channel. The entire procedure was strictly to aseptic principles. A comparison of the compositions and properties of NS, MACSF, other ACSFs, and physiological CSF is shown in Table 1 (9).
From March 2020 to March 2022, eligible patients were recruited into the MACSF group and received MACSF as an intraoperative irrigation fluid. From March 2019 to March 2020, patients with aSAH who used NS as an irrigation fluid in surgery met the aforementioned criteria, and had complete case data, were recruited in the NS group as historical controls. Patients in the MACSF group were irrigated with MACSF after opening the skull and cerebral dura mater, whereas patients in the NS group were irrigated with NS throughout the surgery. All the patients underwent surgery and were managed by the same neurosurgical team. The remaining treatments in both groups strictly adhered to the clinical guidelines.
Demographic data, including sex, age, smoking and alcohol consumption history, and medical history, were recorded in detail. Vital signs, Hunt-Hess Scale scores, modified Fisher grades, and World Federation of Neurosurgical Societies (WFNS) scores were evaluated by a neurologist at admission. Cranial computed tomography was performed on admission and the day after surgery. The blood flow velocity of the intracranial arteries was dynamically evaluated using TCD performed by an experienced technician to determine the occurrence and severity of CVS every alternate day until 14 days after surgery. The Acute Physiology and Chronic Health Evaluation II (APACHE II) score of 15 was used as the criteria for admission and discharge from the ICU. Prognosis was assessed 90 days after disease onset using the mRS (12–14). All TCD examinations and clinical assessments were performed free of charge.
Initially, we compared the mRS scores between the MACSF and NS groups at 1, 3, and 6 months after aSAH onset. Considering the representativeness and universality of the 90-day mRS evaluation in short-term prognosis, combined with the results of this study, we only chose 90 days as the final time point for prognostic evaluation. The prognosis was evaluated 90 days after ictus using the mRS, a 7-point scale ranging from 0 (no symptoms) to 6 (death). The mRS was dichotomized into favorable (mRS ≤ 2) and unfavorable (mRS > 2) prognosis (12, 15). Follow-up was conducted via telephone interviews by a trained neurologist blinded to the treatment assignments. If a patient was unavailable, a proxy was interviewed (16).
Ideally, cerebral angiography should be used as a diagnostic criterion for cerebral vasospasm. However, based on practical feasibility considerations, CVS was diagnosed using TCD in this study (17). The Lindeggard Index (LI) and mean blood flow velocity (MBF) are the main parameters used to evaluate the occurrence and severity of CVS. However, since some physiological or pathological conditions can also cause the increase of MBF without vasospasm, we eliminated the diagnostic criteria of “MBF of tested arteries is higher than 120 cm/s” to reduce the false-positive rate in diagnosing CVS. Therefore, the final criteria for diagnosing CVS by TCD are as follows (18, 19): (1) LI ≥ 3 or (2) Increase in MBF of tested arteries by more than 15 cm/s or 20% compared with the previous time. The severity of CVS is briefly described as follows (20–22): If the LI is greater than 6, severe CVS can be directly diagnosed. When LI ranges from 3 to 6, the severity of CVS can be classified by MBF as mild (120–139 cm/s), moderate (140–199 cm/s) or severe (≥200 cm/s).
The primary outcome measures included the incidence of CVS within 14 days after surgery and the mRS score at 90 days after onset. Secondary prognostic indicators included length of total hospital stay, length of intensive care unit (ICU) stay, and mortality within 90 days of onset. The safety index was defined as the incidence of hypermagnesemia, meningitis, or hydrocephalus within 14 days after surgery.
According to previous epidemiological investigations, the incidence of CVS in the NS group was 60% (1, 2). Owing to the lack of studies related to ACSF, we hypothesized that using MACSF for intraoperative irrigation during clipping would reduce the incidence of CVS by 50%. Based on this assumption, with a two-sided significance level of 0.05 and power of 90%, at least 27 patients were recruited into the MACSF group. Furthermore, 33 patients were required when the loss-to-follow-up rate was empirically assumed to be 20%. However, we set the ratio of the control group to the experimental group at more than 1:1.
All statistical analyses were performed using SPSS 23.0. Data are presented as mean ± standard deviation ( ± s) for continuous symmetric distribution variables, median (M) and interquartile range (IQR) for continuous skewed distribution variables, and percentages for categorical variables. Group comparisons were performed using Analysis of Variance or independent Student’s t-test for continuous variables and chi-squared test for categorical variables. The Mann–Whitney U non-parametric test was used for variables that did not meet the conditions of the parameter test. p < 0.05 was considered statistically significant.
Data were recorded and stored using both paper forms and electronic databases. Supporting data for the findings of this study are available from the corresponding author upon request.
Between March 2019 and March 2022, 71 eligible patients were enrolled (34 and 37 in the MACSF and NS groups, respectively). All patients completed the follow-up 90 days after onset. There were no significant differences between the two groups regarding age, sex, smoking and drinking history, comorbidities, family history of SAH, Hunt-Hess grade, modified Fisher grade, WFNS classification, or aneurysm characteristics. Vital signs and laboratory test results at admission were also included as baseline metrics, and there were no significant differences in these data between the two groups (Table 2).
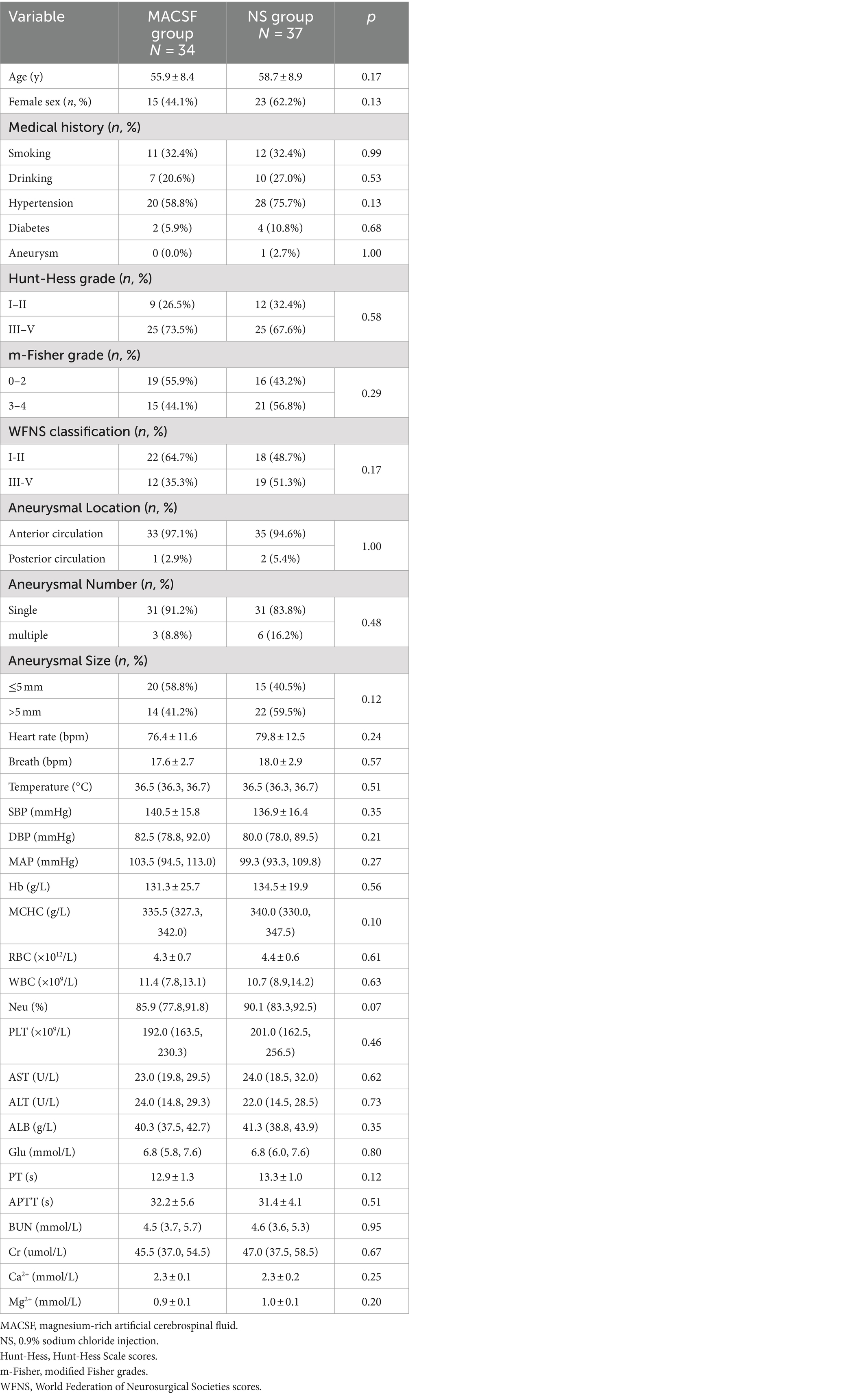
Table 2. Comparison of baseline data in patients using MACSF* and NS* as irrigating fluid during the surgery of aneurysm clipping.
All patients completed 90-day follow-up examinations. The distribution of the mRS scores 90 days after onset is presented in Figure 1. A favorable outcome (mRS ≤ 2) at 90 days after onset was significantly more common in the MACSF group (82.35%) than in the NS group (59.46%; p = 0.035). There was also a significant difference in the mortality rate 90 days after onset between the two groups (8.82% vs. 27.03%; p = 0.048) (Table 3).
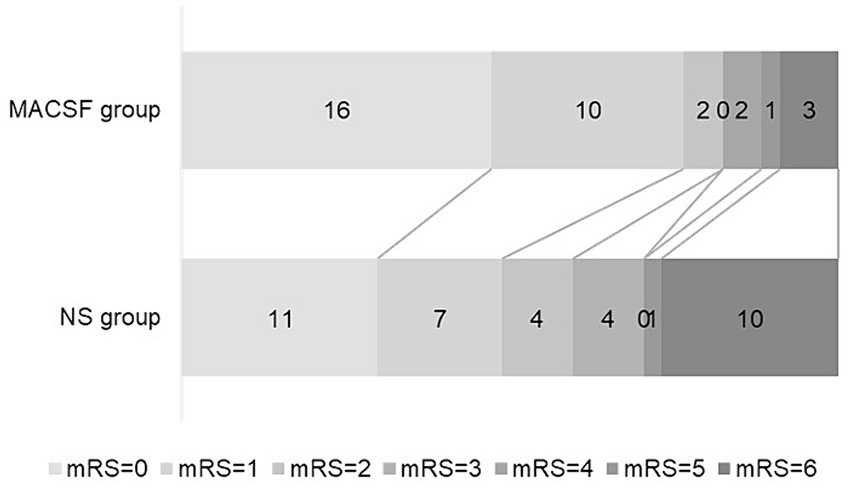
Figure 1. Outcome at 90-day after onset in patients using MACSF* and NS* as irrigating fluid during aneurysm clipping surgery. MACSF, magnesium-rich artificial cerebrospinal fluid. NS, 0.9% sodium chloride injection.
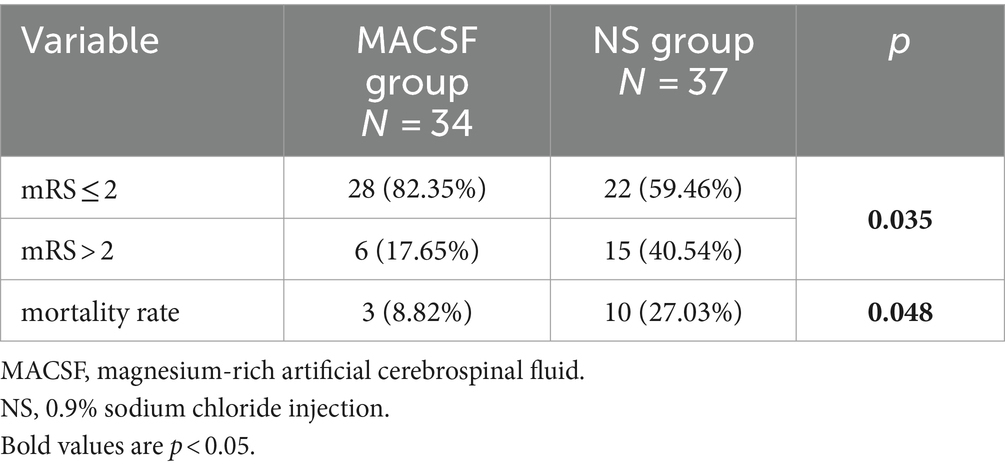
Table 3. Comparison of prognosis and mortality rate at 90 days after onset in patients using MACSF* and NS* as irrigating fluid during the surgery of aneurysm clipping.
When analyzing the incidence of CVS, six patients were excluded because of TCD examinations less than 3 times (two cases in the MACSF group and four cases in the NS group). The overall incidence of CVS was 80.00%. There was no significant difference in the duration of anti-CVS drug use (days) between the MACSF (12.63 ± 6.54) and NS groups (14.15 ± 7.94; p = 0.402). The incidence and severity of CVS in the two groups are shown in Figure 2. Statistical analysis revealed that the incidence of CVS was significantly lower in the MACSF group (22 out of 32, 68.75%) than in the NS group (30 out of 33, 90.91%; p = 0.026), and the incidence of moderate-to-severe CVS was also significantly lower in the MACSF group (5 out of 32, 15.63%) than in the NS group (13 out of 33, 39.39%; p = 0.032).
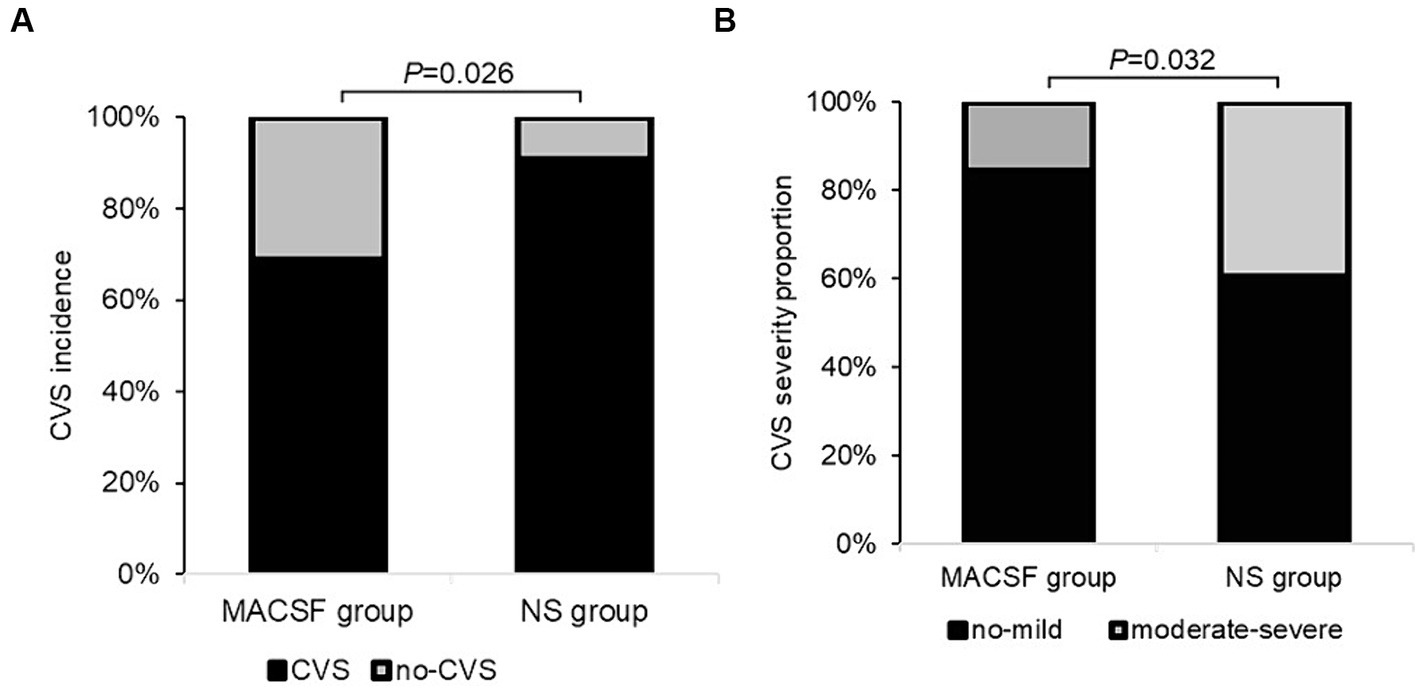
Figure 2. The incidence (A) and severity (B) of CVS within 14 days after the surgery of aneurysm clipping in patients with aSAH using MACSF* and NS* as intraoperative irrigation fluid. CVS, cerebral vasospasm. MACSF, magnesium-rich artificial cerebrospinal fluid. NS, 0.9% sodium chloride injection.
Three and 10 patients in the MACSF and NS groups, respectively, were excluded because of voluntary discharge. As is shown in Figure 3, the median length of total hospital stay was significantly shorter in the MACSF group (M 15.00, IQR 11.00–22.00) than in the NS group (M 22.00, IQR 18.00–27.00; p = 0.008), and the median length of ICU stay was also significantly shorter in the MACSF group (M 2.00, IQR 0.00–5.00) than in the NS group (M 4.00, IQR 3.00–15.00; p = 0.018).
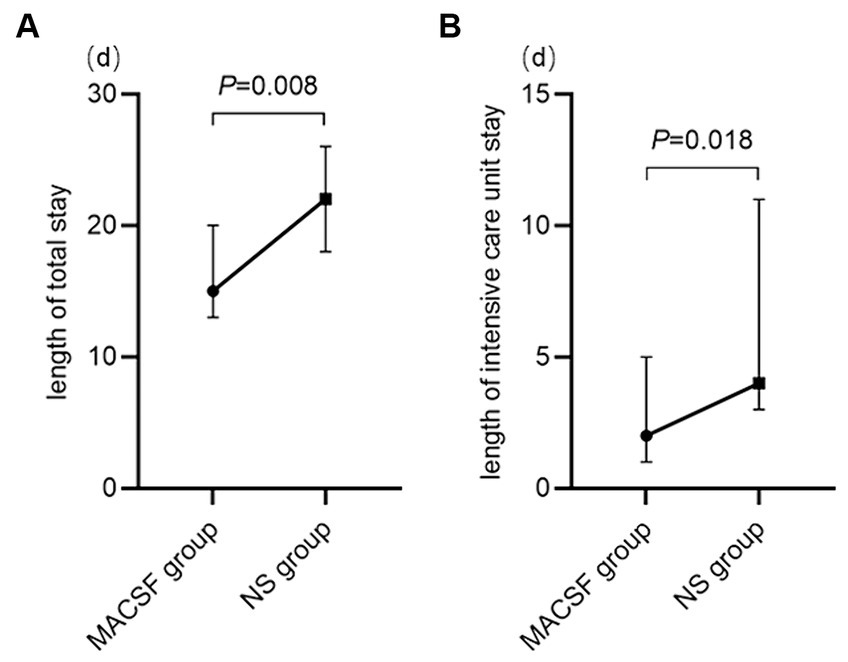
Figure 3. The length of total stay (A) and length of intensive care unit stay (B) of patients using MACSF* and NS* as irrigating fluid during the surgery of aneurysm clipping. MACSF: magnesium-rich artificial cerebrospinal fluid. NS, 0.9% sodium chloride injection.
Serum Mg2+ and Ca2+ concentrations were measured on admission and within 24 h after surgery. In the MACSF group, the mean serum concentrations of Mg2+ and Ca2+ at admission were 0.93 ± 0.11 mmol/L and 2.29 ± 0.12 mmol/L, respectively. In the NS group, they were 0.96 ± 0.10 mmol/L and 2.33 ± 0.16 mmol/L. No significant differences were observed between the groups. The postoperative serum concentrations of Mg2+ and Ca2+ compared to those at admission were not significantly different in either the MACSF or NS groups.
The incidence of hypermagnesemia was not significantly different between the MACSF (0 out of 34, 0.00%) and NS groups (2 out of 37, 5.41%; p = 0.494). Additionally, the incidence of meningitis did not differ significantly between the two groups (MACSF group [15 out of 34, 44.12%] vs. NS group [16 out of 37, 43.24%]; p = 0.941). Furthermore, the difference in the incidence of hydrocephalus was not significant (MACSF group [3 out of 34, 8.82%] vs. NS group [2 out of 37, 5.41%]; p = 0.665) (Table 4).
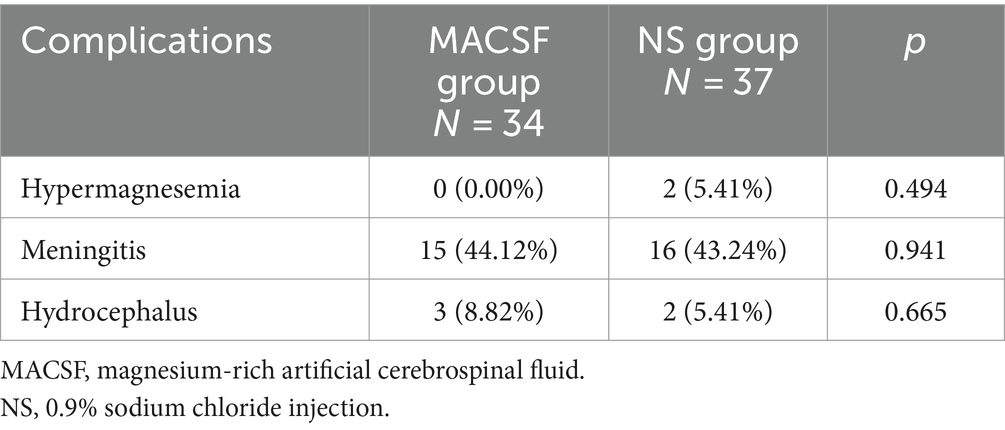
Table 4. Comparison of complications in patients using MACSF* and NS* as irrigation fluid during the surgery of aneurysm clipping.
This study confirmed that the use of MACSF as the irrigation fluid in aneurysm clipping surgery, which effectively reduced the incidence of CVS, improved the 90-day prognosis, and shortened the length of hospital stay in patients with aSAH.
MACSF is rich in magnesium ions, and irrigation with MACSF during aneurysm clamping can increase the concentration of Mg2+ in the CSF. As a natural antagonist of Ca2+, extracellular Mg2+ can reduce Ca2+–Na+ exchange on the cell membrane, preventing Ca2+ influx, vasoconstriction and CVS (23–25). Additionally, Mg2+ antagonizes the N-methyl-d-aspartate receptors in the brain, prevents glutamate stimulation, and reduces Ca2+ influx during ischemic injury (26, 27). Studies have demonstrated that Mg2+ is involved in the composition of various coenzymes and is related to cellular energy metabolism by reducing ATP consumption, damaging the cell membrane, and causing neuronal edema (25). However, two previous randomized controlled trials (IMASH and MASH-2) failed to confirm the effects of intravenous MgSO4 infusion on improving functional outcomes in patients with aSAH patients (7, 8). The most plausible reason for this outcome is that the increased serum Mg2+ concentration did not deliver an effective Mg2+ concentration in the CSF before inducing side effects. In our study, intraoperative irrigation with MACSF directly increased the concentration of Mg2+ in the CSF and better utilized the role of Mg2+ in combating CVS as well as protecting the neural tissue.
An animal study found that the CSF concentration of Mg2+ should be more than 3 mEq/L to dilate the spastic cerebral arteries in dogs effectively (28). It has been confirmed that continuous intracranial infusion of ACSF with an increased concentration of Mg2+ can cause vasodilation of the spastic cerebral arteries in dogs after SAH (29). Therefore, researchers recommend using ACSF with an appropriate concentration of Mg2+ as flushing fluid during neurosurgery to prevent CVS. Continuous intracisternal irrigation with an Mg-related solution appears to stabilize the CSF concentration of Mg2+ at an effective level to relieve CVS. One research team suggested that continuous intracisternal irrigation with 5 mmol/L (10 mEq/L) MgSO4 solution from days 4 to 14 after surgery could inhibit CVS in patients with aSAH (30). However, the overall prognosis did not improve. Furthermore, Mg-related side effects have been reported. Recently, it was demonstrated that intracisternal infusing with 2.5 mmol/L (5 mEq/L) MgSO4 solution, which was started immediately after surgery, could reduce the incidence of CVS and improve clinical outcomes in patients with poor-grade aSAH without Mg-related complications (31). This indicates that early elevation of the Mg2+ concentration in the CSF may be the key to alleviating CVS. However, continuous intracranial infusion has several disadvantages. This is traumatic and may increase the risk of meningitis. Furthermore, long-term bed rest after surgery increases the incidences of phlebothrombosis and pneumonia. In our study, we used MACSF only intraoperatively, which shortened the use time of the Mg solution and reduced the risk of complications.
Early intracranial perfusion with MACSF removes blood from the subarachnoid space, increases the CSF Mg2+ concentration, and inhibits the occurrence and development of CVS. CVS remission increases blood flow in affected arteries, improves oxygen supply to relevant brain tissues, maintains neurological function, and effectively improves patient prognosis. Intraoperative irrigation with MACSF, with an Mg2+ concentration of 4.2 mEq/L, did not increase the serum concentration of Mg2+ or cause Mg-related adverse reactions. Additionally, using MACSF alone in surgery does not increase pain and can avoid the complications of continuous intracisternal infusion for patients with aSAH. Since MACSF has not yet been industrialized, it was prepared by staff from the Department of PIAS, strictly following aseptic principles. Pollution during preparation and transportation was still possible; therefore, the incidence of meningitis was considered one of the indicators to evaluate safety. However, the current data indicates no significant differences in secondary infection rates between the two groups. Therefore, it is safe to use intravenous drugs to prepare MACSF in compliance with aseptic principles.
As a new type of ACSF that is easy to prepare and is widely used, MACSF may replace NS, Ringer’s solution, or other ACSF products, becoming a safe, effective, and convenient succedaneum for physiological CSF in the future.
But, this study adopted a historical control instead of a randomized control, which may have led to bias and a lower level of evidence than a standard randomized controlled trial (RCT). In the future, a planned RCT trial will validate the results of this study. The study sample was small because of the aSAH incidence and study period limitations (The epidemic period of COVID-19). Therefore, a larger study is needed to determine whether our protocol can be used as a standard therapeutic strategy. Lumbar puncture or lumbar cisternal drainage was not routinely performed after surgery; therefore, postoperative CSF samples were obtained from only a few patients. Consequently, the CSF concentrations of Ca2+, Mg2+ and spasmogens were not tested and should be assessed in the future.
All in all, our results suggest that using MACSF as an intraoperative irrigation fluid for aneurysm clipping can effectively reduce the incidence and severity of CVS, improve the prognosis 90 days after onset, and shorten the length of hospital stay without increasing the risk of complications in patients with aSAH. However, these findings need to be validated in randomized controlled trials.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YC: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. XH: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. WX: Methodology, Resources, Writing – review & editing. GX: Methodology, Resources, Writing – review & editing. XB: Investigation, Writing – review & editing. LQ: Methodology, Supervision, Writing – review & editing. LZ: Supervision, Writing – review & editing. RL: Writing – review & editing, Methodology. WD: Writing – review & editing, Formal analysis. WF: Formal analysis, Writing – review & editing. CP: Writing – review & editing, Project administration. WZ: Writing – review & editing, Funding acquisition, Software. FL: Software, Writing – review & editing. XC: Writing – review & editing, Data curation. YX: Writing – review & editing, Formal analysis, Software. GL: Writing – review & editing, Conceptualization, Methodology, Project administration, Supervision, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by grants from the clinical research center of the First Affiliated Hospital of Xi’an Jiaotong University (grant no. XJTU1AF-CRF-2020-015 and no. 2021ZYTS-03).
The authors would like to sincerely thank the participants and collaborators from the Department of Neurology, Department of Neurosurgery, Department of Anesthesiology and Perioperative Medicine, Department of Pharmacy Intravenous Admixture Services, Biobank in the First Affiliated Hospital of Xi’an Jiaotong University, and all members and administrators in the study team for their great support during the entire study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dorsch, NW, and King, MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage part I: incidence and effects. J Clin Neurosci (1994) 1:19–26. doi: 10.1016/0967-5868(94)90005-1
2. Bacigaluppi, S, Zona, G, Secci, F, Spena, G, Mavilio, N, Brusa, G, et al. Diagnosis of cerebral vasospasm and risk of delayed cerebral ischemia related to aneurysmal subarachnoid haemorrhage: an overview of available tools. Neurosurg Rev (2015) 38:603–18. doi: 10.1007/s10143-015-0617-3
3. Kazim, SF, Enam, SA, and Shamim, MS. Possible detrimental effects of neurosurgical irrigation fluids on neural tissue: an evidence based analysis of various irrigants used in contemporary neurosurgical practice. Int J Surg (2010) 8:586–90. doi: 10.1016/j.ijsu.2010.07.292
4. Elliott, KAC, and Jasper, HH. Physiological salt solutions for brain surgery—stuides of local PH and pial vessel reactions to buffered and unbuffered isotonic solutions. J Neurosurg (1949) 6:140–52. doi: 10.3171/jns.1949.6.2.0140
5. Morioka, Y, Nishimura, M, Takehara, H, Doi, K, Naito, S, and Yamauchi, A. Intrathecal disposition of ARTCEREB (R) irrigation and perfusion solution for cerebrospinal surgery in rats. Biol Pharm Bull (2011) 34:688–92. doi: 10.1248/bpb.34.688
6. Milhorat, TH, Uchida, K, Yamada, M, Hayashi, T, Mine, Y, and Kawase, T. Possible harmful effects on central nervous system cells in the use of physiological saline as an irrigant during neurosurgical procedures. Surg Neurol (2004) 62:96–105. doi: 10.1016/j.surneu.2003.12.014
7. Wong, GKC, Chan, MTV, Boet, R, Poon, WS, and Gin, T. Intravenous magnesium sulfate after aneurysmal subarachnoid hemorrhage: a prospective randomized pilot study. J Neurosurg Anesthesiol (2006) 18:142–8. doi: 10.1097/00008506-200604000-00009
8. Dorhout Mees, SM, Algra, A, Vandertop, WP, van Kooten, F, Kuijsten, HA, Boiten, J, et al. Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): a randomized placebo-controlled trial. Lancet (2012) 380:44–9. doi: 10.1016/S0140-6736(12)60724-7
9. Walton, JN. Davson H-physiology of cerebrospinal fliud. Br Med J (1967) 3:356. doi: 10.1136/bmj.3.5561.356
10. Cheng, Y-W, Guo, Y-C, Li, G-L, Deng, YN, Li, WJ, Xu, GF, et al. Effects of a new magnesium-rich artificial cerebrospinal fluid on contractile 5-hydroxytryptamine and endothelin receptors in rat cerebral arteries. Neurol Res (2019) 41:1015–23. doi: 10.1080/01616412.2019.1672383
11. Chan, A-W, Tetzlaff, JM, Gotzsche, PC, Altman, DG, Mann, H, Berlin, JA, et al. Explanation and elaboration: guidance for protocols of clinical trials. BMJ (2013) 2013:346. doi: 10.1136/bmj.e7586
12. van Swieten, JC, Koudstaal, PJ, Visser, MC, Schouten, HJ, and van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke (1988) 19:604–7. doi: 10.1161/01.STR.19.5.604
13. Quinn, TJ, Dawson, J, Walters, MR, and Lees, KR. Functional outcome measures in contemporary stroke trials. Int J Stroke (2009) 4:200–5. doi: 10.1111/j.1747-4949.2009.00271.x
14. Patel, N, Rao, VA, Heilman-Espinoza, ER, Lai, R, Quesada, RA, and Flint, AC. Simple and reliable determination of the modified Rankin scale score in neurosurgical and neurological patients: the mRS-9Q. Neurosurgery (2012) 71:971–5. doi: 10.1227/NEU.0b013e31826a8a56
15. Berkhemer, OA. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med (2015) 372:11–20. doi: 10.1056/NEJMoa1411587
16. Ciccone, A, Valvassori, L, Nichelatti, M, Sgoifo, A, Ponzio, M, Sterzi, R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med (2013) 368:904–13. doi: 10.1056/NEJMoa1213701
17. Lysakowski, C, Walder, B, Costanza, MC, and Tramèr, MR. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm—a systematic review. Stroke (2001) 32:2292–8. doi: 10.1161/hs1001.097108
18. Suarez, JI, Qureshi, MI, Yahia, AB, Parekh, PD, Tamargo, RJ, Williams, MA, et al. Symptomatic vasospasm diagnosis after subarachnoid hemorrhage: evaluation of transcranial Doppler ultrasound and cerebral angiography as related to compromised vascular distribution. Crit Care Med (2002) 30:1348–55. doi: 10.1097/00003246-200206000-00035
19. Li, DD, Chang, JY, Zhou, CX, and Cui, JB. Clinical diagnosis of cerebral vasospasm after subarachnoid hemorrhage by using transcranial Doppler sonography. Eur Rev Med Pharmacol Sci (2018) 22:2029–35. doi: 10.26355/eurrev_201804_14732
20. Lindegaard, KF, Nornes, H, Bakke, SJ, Sorteberg, W, and Nakstad, P. Cerebral vasospasm diagnosis by means of angiography and blood velocity measurements. Acta Neurochir (1989) 100:12–24. doi: 10.1007/BF01405268
21. Tsivgoulis, G, Alexandrov, AV, and Sloan, MA. Advances in transcranial doppler ultrasonography. Curr Neurol Neurosci Rep (2009) 9:46–54. doi: 10.1007/s11910-009-0008-7
22. The Role of Transcranial Doppler in Cerebral Vasospasm: A Literature Review. 14th International Conference on Neurvascular Events after Subarachnoid Hemorrage (Vasospasm). CA: Los Angeles (2017).
23. Chang, JJ, Mack, WJ, Saver, JL, and Sanossian, N. Magnesium: potential roles in neurovascular disease. Front Neurol (2014) 5:52. doi: 10.3389/fneur.2014.00052
24. Okada, M, and Kaneko, S. Pharmacological interactions between magnesium ion and adenosine on monoaminergic system in the central nervous system. Magnes Res (1998) 11:289–305.
25. Regan, RF, Jasper, E, Guo, YP, and Panter, SS. The effect of magnesium on oxidative neuronal injury in vitro. J Neurochem (1998) 70:77–85. doi: 10.1046/j.1471-4159.1998.70010077.x
26. Murata, T, Dietrich, HH, Horiuchi, T, Hongo, K, and Dacey, RG Jr. Mechanisms of magnesium-induced vasodilation in cerebral penetrating arterioles. Neurosci Res (2016) 107:57–62. doi: 10.1016/j.neures.2015.12.005
27. Wong, GKC, Chan, MTV, Poon, WS, Boet, R, and Gin, T. Magnesium therapy within 48 hours of an aneurysmal subarachnoid hemorrhage: neuro-panacea. Neurol Res (2006) 28:431–5. doi: 10.1179/016164106X115035
28. Mori, K, Yamamoto, T, Miyazaki, M, Hara, Y, Aiko, Y, Koike, N, et al. Optimal cerebrospinal fluid magnesium ion concentration for vasodilatory effect and duration after intracisternal injection of magnesium sulfate solution in a canine subarachnoid hemorrhage model. J Neurosurg (2011) 114:1168–75. doi: 10.3171/2010.10.JNS10866
29. MORI, K, YAMAMOTO, T, MIYAZAKI, M, HARA, Y, KOIKE, N, and NAKAO, Y. Potential risk of artificial cerebrospinal fluid solution without magnesium ion for cerebral irrigation and perfusion in neurosurgical practice. Neurol Med Chir (Tokyo) (2013) 53:596–600. doi: 10.2176/nmc.oa2012-0295
30. Yamamoto, T, Mori, K, Esaki, T, Nakao, Y, Tokugawa, J, and Watanabe, M. Preventive effect of continuous cisternal irrigation with magnesium sulfate solution on angiographic cerebral vasospasms associated with aneurysmal subarachnoid hemorrhages: a randomized controlled trial. J Neurosurg (2016) 124:18–26. doi: 10.3171/2015.1.JNS142757
Keywords: aneurysmal subarachnoid hemorrhage, artificial cerebrospinal fluid, cerebral vasospasm, magnesium, prognosis
Citation: Cheng Y, Han X, Xie W, Xu G, Bai X, Qi L, Zhang L, Liu R, Dong W, Feng W, Pang C, Zhang W, Liu F, Cao X, Xu Y and Luo G (2024) Safety and efficacy of magnesium-rich artificial cerebrospinal fluid for subarachnoid hemorrhage. Front. Neurol. 15:1376216. doi: 10.3389/fneur.2024.1376216
Received: 25 January 2024; Accepted: 19 March 2024;
Published: 28 March 2024.
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Abbas Fadhil Abdul Hussein, University of Babylon, IraqCopyright © 2024 Cheng, Han, Xie, Xu, Bai, Qi, Zhang, Liu, Dong, Feng, Pang, Zhang, Liu, Cao, Xu and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guogang Luo, bGd1b2dhbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.