- 1Department of Neurology, The Fourth Affiliated Hospital of Soochow University, Suzhou, China
- 2Department of Neurology, Xinqiao Hospital and the Second Affiliated Hospital, Army Medical University (Third Military Medical University), Chongqing, China
- 3Department of Pharmacy, The Fourth Affiliated Hospital of Soochow University, Suzhou, China
Introduction: Symptomatic intracranial hemorrhage (sICH) is a serious complication of acute ischemic stroke (AIS) after endovascular treatment (EVT). Limited data exist regarding predictors and clinical implications of sICH after EVT, underscoring the significance of identifying risk factors to enhance prevention strategies. Therefore, the main objective of this study was to evaluate the incidence of sICH and identify its predictors after EVT in patients with large infarct core-AIS in the pre-circulation stage.
Methods: Using data from the EVT for the Pre-circulation Large Infarct Core-AIS Study, we enrolled patients who were treated with EVT from the Prospective Multicenter Cohort Study of Early Treatment in Acute Stroke (MAGIC) registry. Baseline demographics, medical history, vascular risk factors, blood pressure, stroke severity, radiographic features, and EVT details were collected. The patients were classified into three groups: without intracranial hemorrhage (ICH), with asymptomatic intracranial hemorrhage (aICH), and sICH, based upon the occurrence of sICH. The main outcomes were the occurrence of sICH according to the Heidelberg Bleeding Classification and functional condition at 90 days. Multivariate logistic regression analysis and receiver operating characteristic (ROC) curves were used to identify independent predictors of sICH after EVT.
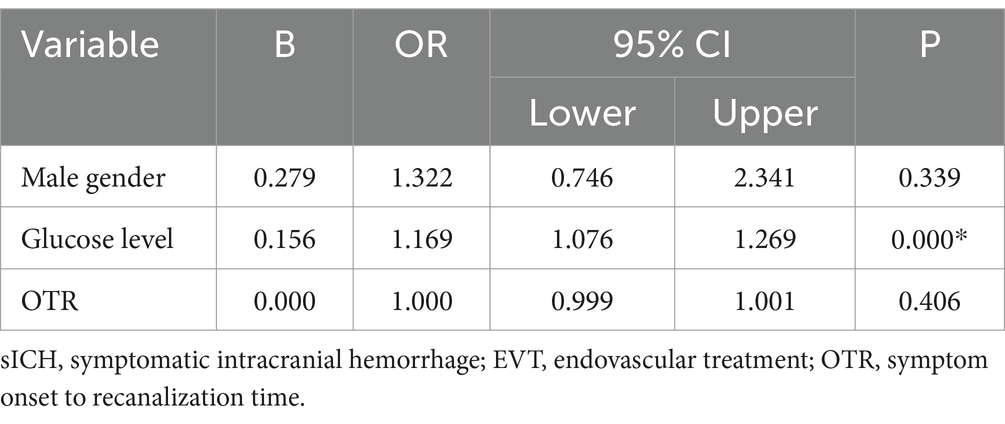
Results: The study recruited a total of 490 patients, of whom 13.3% (n = 65) developed sICH. Patients with sICH had less favorable outcomes than those without intracranial hemorrhage (ICH) and those with aICH (13.8% vs. 43.5% vs. 32.2%, respectively; p < 0.001). The overall mortality was 41.8% (n = 205) at 90 days post-EVT. The univariate analysis revealed significant differences among the three groups in terms of blood glucose levels at admission, probability of favorable outcomes, incidence of brain herniation, and 90-day mortality. The multifactorial logistic regression analysis revealed that the blood glucose level at admission [odds ratio (OR) 1.169, p < 0.001, confidence interval (CI) 1.076–1.269] was an independent predictor of sICH. A blood glucose level of 6.95 mmol/L at admission was the best predictor of sICH, with an area under the ROC curve (AUC) of 0.685 (95% CI: 0.616–0.754).
Discussion: The study findings demonstrated that the probability of sICH after EVT was 13.3% in patients with pre-circulation large infarct core-AIS, and sICH increased the risk of an unfavorable prognosis. Higher blood glucose levels at admission were associated with sICH after EVT in patients with pre-circulation large infarct core AIS. These findings underscore the importance of early management strategies to mitigate this risk.
1 Introduction
As the second most lethal and disabling disease worldwide, acute ischemic stroke (AIS) has a serious impact on patient survival and quality of life (1). Approximately 20% of patients with AIS arrive at the hospital with a large infarct core, characterized by high mortality and poor prognosis, posing a global challenge in clinical management for this population (2). Endovascular therapy (EVT) has emerged as the preferred treatment option for AIS due to large vessel occlusion.
A previous study reported that, compared with patients treated with medication alone, those with large infarct-core AIS treated with EVT plus medication had a higher likelihood of reduced disability, independent ambulation, and favorable functional outcomes at 90 days (3). The Chinese Guidelines for Endovascular Treatment of Acute Ischemic Stroke 2023 recommend EVT for patients with large-vessel occlusion-induced AIS in the pre-circulation stage among those who meet the enrolment criteria of the ANGEL-ASPECT (4), RESCUE-Japan LIMIT (5), or SELECT 2 (6) studies (7).
Despite the success associated with EVT, intracranial hemorrhage (ICH) is a common complication, with a prevalence of 46.0–49.5% (8). In particular, symptomatic ICH (sICH) can reduce the benefit–risk ratio of treatment and increase the risk of mortality (9). However, limited data are available regarding the predictors and clinical relevance of sICH after EVT. Therefore, risk factors for sICH after EVT should be identified for the prevention of sICH and improvement in the efficacy of this new treatment strategy. Utilizing the database from the Prospective Multicenter Cohort Study of Early Treatment in Acute Stroke (MAGIC) study, the present study aimed to analyze potential predictors of sICH after EVT in patients with pre-circulation large infarct-core AIS.
2 Materials and methods
2.1 Patients
Patients were enrolled from the database of the MAGIC study, a nationwide prospective registry of consecutive patients who presented with acute, symptomatic, and radiologically confirmed pre-circulation large infarct core AIS. The MAGIC registry included patients with AIS with a large infarct core due to pre-circulation large-vessel occlusion who underwent EVT from November 2021 to February 2023. This prospective observational study was approved by the ethics committees of the participating centers.
The eligibility criteria for EVT were as follows: (1) age 18–80 years; (2) AIS due to anterior circulation large vessel occlusion, defined as occlusion of the internal carotid artery (ICA) or the M1 segment or M2 segment of the middle cerebral artery (MCA); (3) large ischemic core on non-contrast computed tomography (CT) findings [defined as Alberta stroke programme early CT score (ASPECTS) score of 0–5]; (4) pre-stroke score of 0 or 1 on the modified Rankin scale (mRS), assessed retrospectively (scores ranging from 0 to 6, with higher scores indicating greater disability and a score of 6 indicating death); and (5) symptom presentation within 24 h (the time metric of time last known well within 24 h was used if the presentation time was unavailable). The exclusion criteria were as follows: (1) no follow-up brain imaging data (CT or magnetic resonance imaging) at 24 h or when neurological deterioration occurred; (2) serious, advanced, or terminal illness; and (3) no mRS data at 90 days.
2.2 Clinical data collection
The collected data included baseline demographic data (age and sex), medical history, vascular risk factors (smoking, hypertension, hyperlipidemia, diabetes, and atrial fibrillation), blood pressure at admission (systolic and diastolic), stroke severity [National Institutes of Health Stroke Score (NIHSS)], radiographic features (ASPECTS, site of the occluded arteries), and EVT-related data (procedure process time, treatment methods, and recanalization). Additional data analyzed included blood glucose level at admission, triglyceride level, low-density cholesterol level, liver function, kidney function, and intravenous thrombolytic therapy (IVT).
The site of the occluded arteries was identified using CT angiography (CTA), magnetic resonance angiography (MRA), and/or cerebral digital subtraction angiography (DSA) reports and included the ICA and MCA. Anterior circulation lesions were defined using the ASPECTS score, which involved symptom onset to puncture (OTP) time and symptom onset to recanalization (OTR) time.
The patients in our study received EVT, which included intra-arterial thrombolysis, thrombectomy with stent retrievers, thromboaspiration, intracranial angioplasty, stent implantation, or a combination of these approaches at the discretion of the treatment surgeon.
2.3 Post-procedure evaluation
CT was usually performed 24 h after the procedure or whenever ICH was indicated by clinical symptoms. Successful recanalization was defined as a modified treatment in cerebral infarction (mTICI) with a score of 2b, 2c, or 3. The mRS score was evaluated at 90 days by a stroke neurologist during a scheduled post-stroke follow-up visit or via phone interview. Functional outcomes were evaluated according to the mRS as follows: complete recovery (mRS = 0–1), partial recovery, independence (mRS = 2), dependence (mRS = 3–5), and death (mRS = 6). Favorable and unfavorable outcomes were defined as an mRS score of 0–3 and > 3 (4–6), respectively.
2.4 Evaluation of ICH
According to the Heidelberg Bleeding Classification, ICH is diagnosed within 48 h after EVT. sICH and asymptomatic intracranial hemorrhage (aICH) were classified based on the presence or absence of neurological deficit exacerbations. The diagnosis of sICH was based on the association of ICH with any of the following conditions: (1) increase in NIHSS score by >4 points compared to the score prior to ICH; (2) increase in NIHSS score by >2 points in one category; and (3) deterioration leading to intubation, hemicraniectomy, external ventricular drain placement, or any other major intervention. Symptom deteriorations were required to be unexplainable by causes other than the observed ICH (10).
2.5 Statistical analysis
Measures with normal distribution are expressed as mean ± standard deviation of variance (ANOVA) with Bonferroni correction used for multiple comparisons. Continuous variables are presented as medians [interquartile range (IQR)] according to the type of non-normal distribution, and categorical variables are presented as frequencies (percentages). The Wilcoxon rank-sum test was used for comparison between two groups, and the Kruskal–Wallis test was used for multiple comparisons. Categorical variables were analyzed using the chi-squared test or Fisher’s exact test. A multivariate logistic regression analysis was performed to evaluate independent predictors for sICH, with the adjusted odds ratio (OR) and corresponding 95% confidence interval (CI) reported. Receiver operating characteristic (ROC) curve analysis was used to evaluate the optimal cutoff value for predicting sICH and to establish optimal cutoff points for the specificity and sensitivity values. Entered factors were those with at least marginal significance (p < 0.1) in a univariate analysis. p-values of <0.05 were considered significant. Statistical analyses were performed using SPSS 23.0. (IBM, Armonk, NY, United States).
3 Results
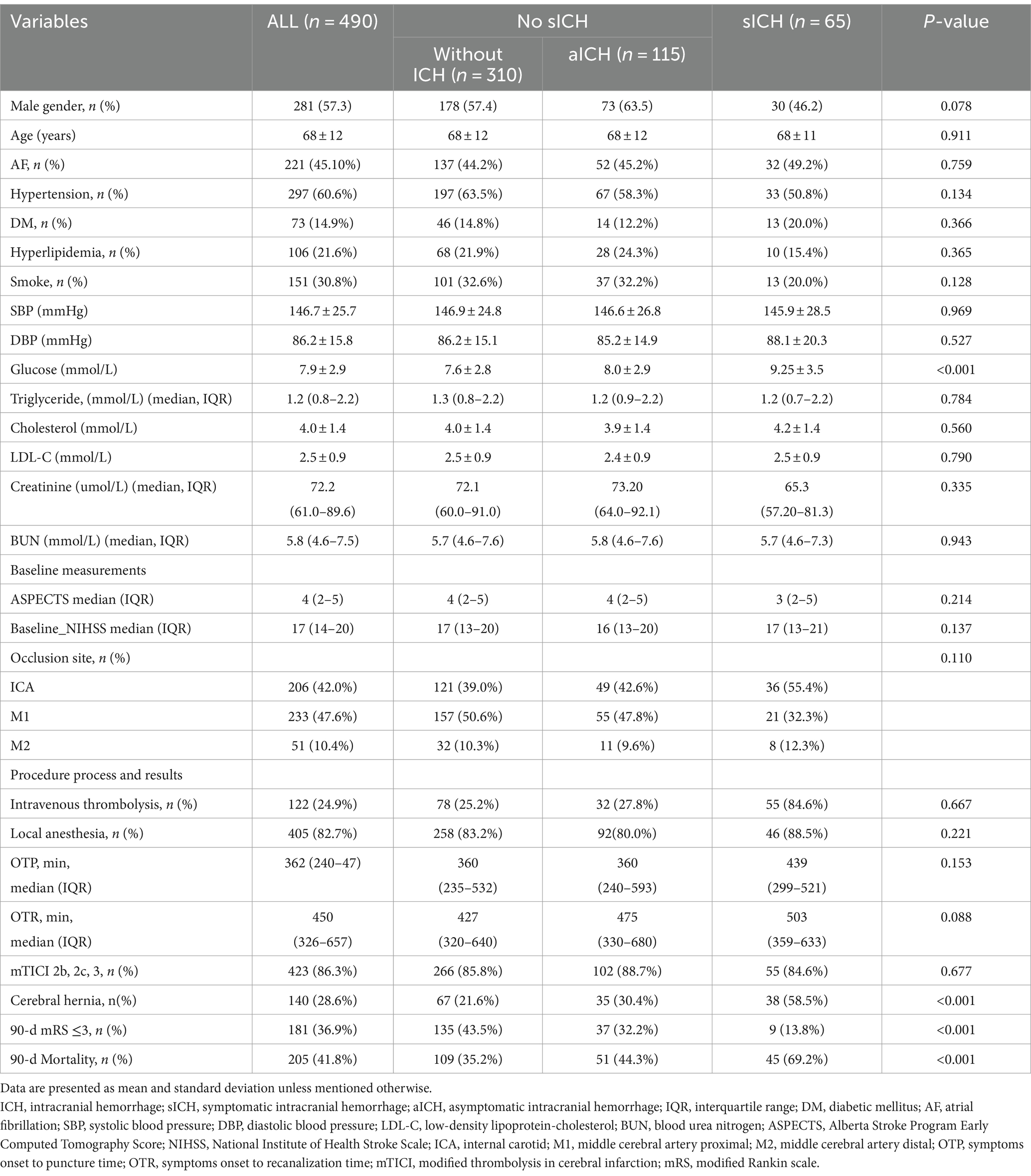
A total of 490 patients with pre-circulation large infarct-core AIS who were treated with EVT were enrolled in this study. They were divided into three groups: without ICH, with aICH, and with sICH. Overall, 115 patients (23.5%) had aICH after EVT within 24 h, whereas 65 patients (13.3%) had sICH. The proportions of reaching favorable functional outcomes in the three groups were 43.5, 32.2, and 13.8%, respectively (p < 0.001) (Figure 1 and Table 1). Significant differences were noted in the probability of developing brain herniation (21.6% vs. 30.4% vs. 58.5%, p < 0.001) and mortality at 90 days (35.2% vs. 44.3% vs. 69.2%, p < 0.001) (Table 1).
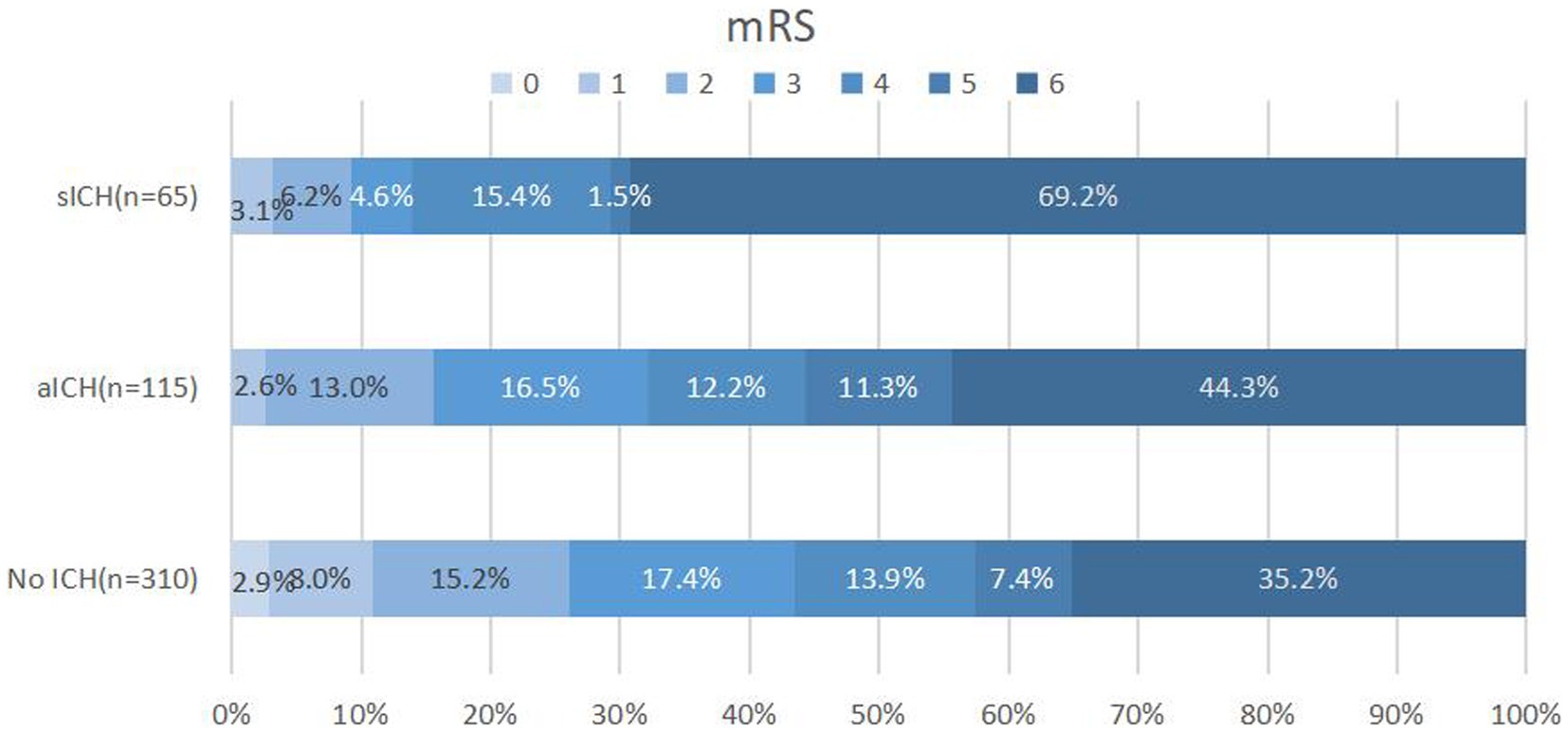
Figure 1. Distribution of 90-day mRS scores in patients without ICH, aICH, and sICH. Patients with sICH demonstrated the least favorable outcomes (mRS 0–3) 90 days post-index stroke, with a higher proportion experiencing mortality. The distribution of modified Rankin scale (mRS) scores underscores the impact of sICH on long-term prognosis. mRS, modified Rankin scale; ICH: intracranial hemorrhage; aICH, asymptomatic intracranial hemorrhage; sICH, symptomatic intracranial hemorrhage.
When comparing sICH based on the level of blood glucose at admission, a significant intergroup difference was noted (7.6 ± 2.8 vs. 8.0 ± 2.9 vs. 9.25 ± 3.5 mmol/L, respectively, p < 0.001) (Table 1). The one-way ANOVA revealed that the levels of blood glucose at admission in the sICH group were significantly different from those in the without ICH and with aICH groups (p < 0.001; p = 0.008, respectively). The count showed that the levels of blood glucose at admission in the sICH group were significantly different from those in the without ICH and with aICH groups (p < 0.001; p = 0.024, respectively, by the Bonferroni correction) (Figure 2). In multivariate analysis, higher blood glucose levels at admission (OR 1.169, p < 0.001, CI 1.076–1.269) were associated with sICH after EVT in patients with pre-circulation large infarct-core AIS (Table 2).
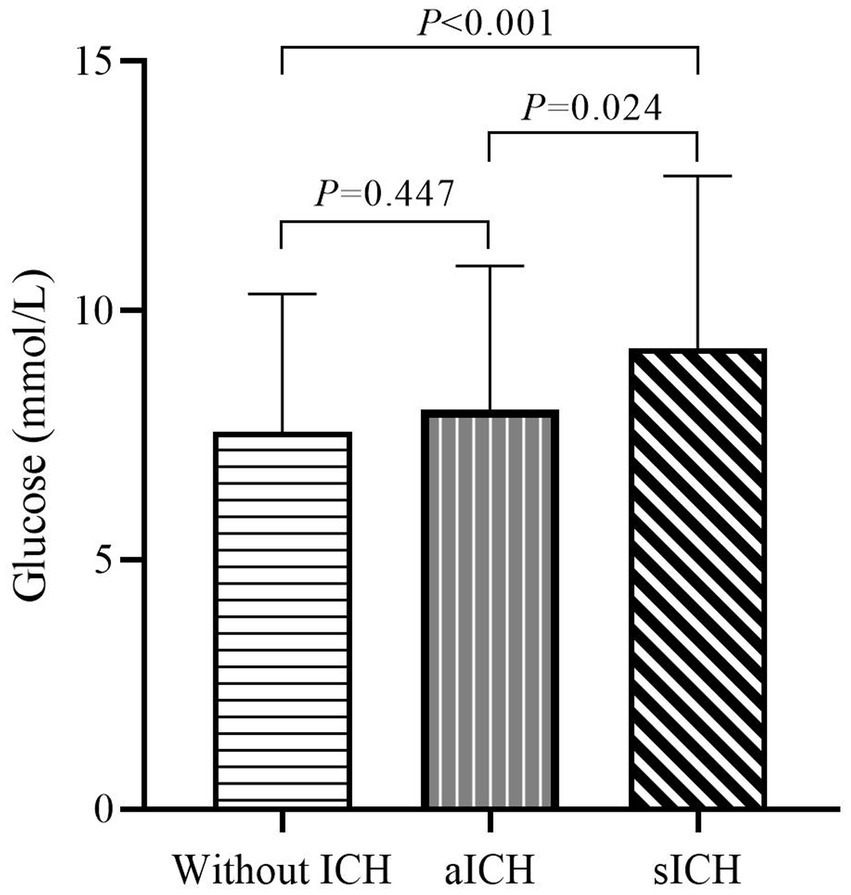
Figure 2. Admission glucose levels in patients without ICH, aICH, and sICH. Significant differences in admission glucose levels were observed between groups without ICH, aICH, and sICH. The sICH group exhibited elevated blood glucose levels compared to the other two groups, emphasizing the association between admission hyperglycemia and symptomatic intracranial hemorrhage (after the Bonferroni correction).
Figure 3 presents the results of the ROC analysis determining the prognostic value of blood glucose levels at admission to predict sICH. The area under the curve for the model was 0.685, with a 95% CI of 0.616–0.754, indicating that blood glucose levels at admission have a good discriminative ability. The optimal threshold was 6.95 mmol/L, corresponding to a sensitivity and specificity of 82.0 and 49.8%, respectively, with Youden’s index of 0.312. The positive predictive value was 19.2%, and the negative predictive value was 84.1%.
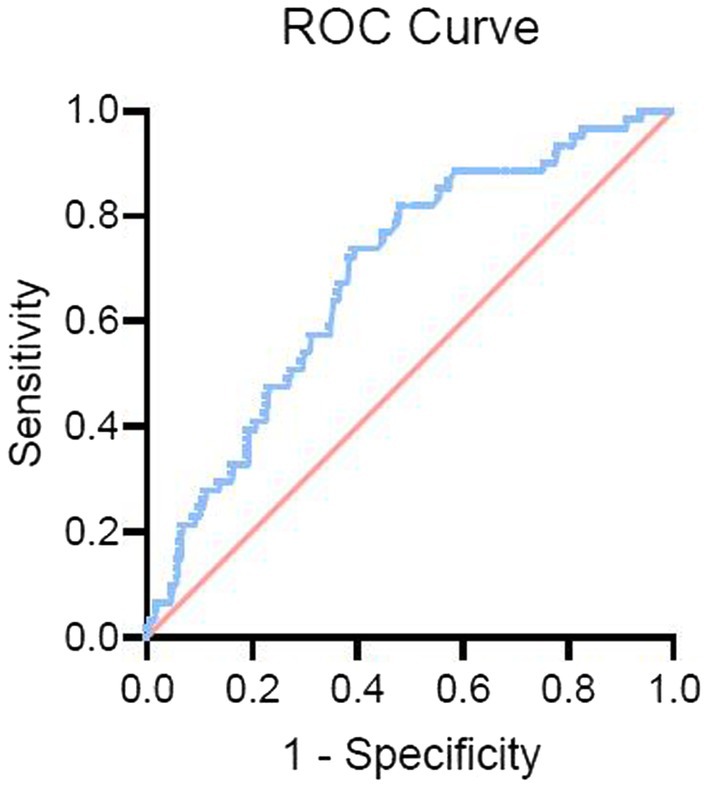
Figure 3. Receiver operating characteristic (ROC) curve for blood glucose levels predicting sICH. The ROC curve illustrates the predictive value of admission blood glucose levels for symptomatic intracranial hemorrhage (sICH). The curve highlights the discriminative ability of blood glucose levels, with an optimal threshold identified for predicting the occurrence of sICH after endovascular therapy (EVT).
4 Discussion
In our study, we explored potential predictors of sICH after EVT in patients with pre-circulation large infarct-core AIS and observed that the probability of sICH was 13.3% in patients who underwent EVT. Furthermore, sICH greatly reduced the probability of patients having a 90-day favorable prognosis and increased the risk of brain herniation and mortality. In addition, we demonstrated that the blood glucose level at admission was an independent predictor of sICH after EVT in AIS patients with large infarct cores, and the risk of sICH increased with higher blood glucose levels.
In our study, the occurrence of sICH was similar to that in the SELECT trial, which reported an increased probability of sICH in patients with large-vessel occlusion of large-core infarcts who were treated with EVT with an onset greater than 24 h (11). However, our results showed a higher value (13.3%) than that reported in a previous randomized controlled trial (4.4%) (12) and in the North American Solitaire Acute Stroke Study (9.9%) (13). This discrepancy may stem from the inclusion of patients in our study with large infarct cores in the anterior circulation and an ASPECTS score of ≤5, whereas previous EVT studies have largely excluded patients who had large infarct cores already present at the time of preoperative imaging. Several studies have demonstrated an association between larger cores on imaging and an increased risk of sICH after EVT (14, 15). In most studies, an ASPECTS score of ≤5 was reported to be associated with an approximately 2-fold risk of ICH (16, 17). Patients with large infarct cores have a high degree of blood–brain barrier disruption, and the development of ICH is closely related to blood–brain barrier damage. In previous models of cerebral ischemia in rats, loss of integrity of the matrix and basement membrane connecting the endothelial cells was observed by electron microscopy, and in some cases, glial cell protrusions were observed to be swollen or degenerative. These alterations affect the structure and function of the blood–brain barrier, which induces ICH by increasing its permeability (18).
Experimental results have shown that the ischemia–reperfusion-induced release and activation of metalloproteinases can cause rupture of the basement membrane, leading to ICH (19, 20). The results of a previous meta-analysis demonstrated that, compared to pharmacological treatment, EVT did not increase the risk of sICH but improved the functional outcome in patients with large infarct cores (21, 22). Based on the above observations, it is reasonable to attribute the higher incidence of sICH in our study to the larger infarct core rather than the EVT procedure itself.
Similar to the findings of Shen et al. (23), our results showed that patients with sICH had a higher risk of brain herniation and mortality and a greater likelihood of unfavorable outcomes than patients without ICH and with aICH. This emphasizes the critical nature of sICH as a potentially fatal complication that significantly influences patient functional outcomes, warranting emphasis on preventive measures.
In our study, blood glucose was identified as a predictor of sICH after EVT in AIS patients with large infarct cores in the anterior circulation. The blood glucose levels >6.95 mmol/L at admission were associated with an increased risk of sICH after EVT, comparable to a level of 6.6 [5.7–7.7] mmol/L reported in a previous study (24). Several previous studies have suggested that patients with hyperglycemia are at a high risk of ICH from different perspectives, such as a meta-analysis that retrospectively analyzed the clinical data of patients undergoing EVT and found that higher blood glucose levels at admission were associated with a higher incidence of sICH (25, 26). Previous studies have shown that acute hyperglycemia increases blood–brain barrier disruption and ICH incidence in rat models (27). The results of Shen et al.’s (23) study suggest that high blood glucose levels are the strongest predictors of sICH after EVT. Another study showed that an elevated glycosylated hemoglobin level was significantly associated with an unfavorable prognosis and mortality in 90 days with AIS treated with EVT (28). The results of these studies are consistent with our findings; however, the mechanisms underlying how hyperglycemia enhances ICH remain unclear. The possible causes of ICH are as follows: (1) hyperglycemia exacerbates vascular wall dystrophy and hypoxia, resulting in more susceptible degeneration and necrosis of the vascular wall (29, 30); (2) hyperglycemia leads to disruption of cellular metabolism, resulting in increased plasma osmolality and intracellular lactic acid buildup, ultimately leading to endothelial cell injury and acidosis (31); and (3) hyperglycemia can reportedly increase the activity of the matrix metalloproteinases (MMP-9 and MMP-3) in ischemic areas and aggravate blood–brain barrier dysfunction and post-reperfusion ICH (32–34). While it is hypothesized that maintaining stable blood glucose levels post-successful EVT may be crucial in preventing sICH (35), further studies are needed to confirm this.
This study has some limitations that should be considered when interpreting the results. First, as the study adopted a prospective observational design, we did not evaluate other potentially relevant variables, such as changes in perioperative blood pressure, use of heparin during EVT, and the effect of antiplatelet regimens on hemorrhagic conversion, which may influence the risk of ICH. Second, an incomplete evaluation index may not fully determine a causal connection, and the practical implications of these unresolved findings require future validation. Despite these limitations, the findings of this study provide insights into the predictive value of blood glucose levels at admission for sICH after EVT in pre-circulation large infarct core-AIS to facilitate early management.
5 Conclusion
In this multicenter study, the incidence of sICH after EVT was higher than that previously reported. Patients with pre-circulation large infarct core AIS who developed sICH after EVT had a higher incidence of unfavorable prognosis and mortality than those who did not develop sICH. Higher blood glucose levels at admission increased the risk of sICH after EVT in AIS patients with large infarct cores in the anterior circulation. It remains unclear whether controlling blood glucose levels prior to EVT can reduce the risk of sICH, and further investigation is required.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the ethics committee of Suzhou Dushu Lake Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YY: Writing – original draft, Data curation, Formal analysis, Investigation. LY: Writing – original draft, Data curation, Methodology. XS: Writing – original draft, Formal analysis, Investigation, Software. XN: Formal analysis, Writing – original draft, Data curation, Funding acquisition, Methodology. SF: Data curation, Writing – original draft, Conceptualization, Investigation, Supervision. XX: Investigation, Supervision, Data curation, Writing – original draft, Formal analysis, Methodology. JM: Methodology, Data curation, Writing – original draft, Software, Validation. SY: Methodology, Writing – original draft, Conceptualization, Project administration, Resources, Supervision. ZW: Methodology, Writing – original draft, Formal analysis, Software, Validation. WZ: Validation, Writing – review & editing, Data curation, Funding acquisition, Project administration, Resources. DY: Funding acquisition, Project administration, Resources, Writing – review & editing, Data curation, Investigation, Formal analysis. YH: Investigation, Project administration, Writing – review & editing, Supervision, Visualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Science Fund for Distinguished Young Scholars (No. 81525008), the National Natural Science Foundation of China (NSFC) (82000237), and the Suzhou Science and Technology Plan Project (SZM2023017).
Acknowledgments
The authors would like to thank all the participants and their relatives in the study and the members of the survey teams.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hasan, TF, Todnem, N, Gopal, N, Miller, DA, Sandhu, SS, Huang, JF, et al. Endovascular thrombectomy for acute ischemic stroke. Curr Cardiol Rep. (2019) 21:112. doi: 10.1007/s11886-019-1217-6
2. Wu, C, and Ji, X. Angel-ASPECT: large infarct core acute Chinese protocol for endovascular treatment of ischaemic stroke. Chin J Stroke. (2023):247–9. doi: 10.3969/j.issn.1673-5765.2023.03.002
3. Palaiodimou, L, Sarraj, A, Safouris, A, Magoufis, G, Lemmens, R, Sandset, EC, et al. Endovascular treatment for large-core ischaemic stroke: a meta-analysis of randomised controlled clinical trials. J Neurol Neurosurg Psychiatry. (2023) 94:781–5. doi: 10.1136/jnnp-2023-331513
4. Huo, X, Ma, G, Tong, X, Zhang, X, Pan, Y, Nguyen, TN, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. (2023) 388:1272–83. doi: 10.1056/NEJMoa2213379
5. Uchida, K, Shindo, S, Yoshimura, S, Toyoda, K, Sakai, N, Yamagami, H, et al. Association between Alberta stroke program early computed tomography score and efficacy and safety outcomes with endovascular therapy in patients with stroke from large-vessel occlusion: a secondary analysis of the recovery by endovascular salvage for cerebral ultra-acute embolism-Japan large ischemic core trial (RESCUE-Japan LIMIT). JAMA Neurol. (2022) 79:1260–6. doi: 10.1001/jamaneurol.2022.3285
6. Sarraj, A, Hassan, AE, Abraham, MG, Ortega-Gutierrez, S, Kasner, SE, Hussain, MS, et al. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med. (2023) 388:1259–71. doi: 10.1056/NEJMoa2214403
7. Chinese Stroke Association, Neurological Intervention Branch of the Chinese Stroke Association, Interventional Group of the Stroke Prevention and Control Committee of the Chinese Preventive Medical Association . Chinese guidelines on endovascular treatment of acute Ischaemic stroke 2023. Chin J Stroke. (2023) 18:684–711. doi: 10.3969/j.issn.1673-5765.2023.06.010
8. Chinese Society of Neurology, Chinese Stroke Society . Consensus on diagnosis and treatment of hemorrhagic transformation after acute ischemic stroke in China 2019. Chin. J Neurol. (2019) 52:252–65. doi: 10.3760/cma.j.issn.1006-7876.2019.04.003
9. Darkhabani, Z, Nguyen, T, Lazzaro, MA, Zaidat, OO, Lynch, JR, Fitzsimmons, BF, et al. Complications of endovascular therapy for acute ischemic stroke and proposed management approach. Neurology. (2012) 79:S192–8. doi: 10.1212/WNL.0b013e31826958e3
10. von Kummer, R, Broderick, JP, Campbell, BC, Demchuk, A, Goyal, M, Hill, MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46:2981–6. doi: 10.1161/STROKEAHA.115.010049
11. Sarraj, A, Kleinig, TJ, Hassan, AE, Portela, PC, Ortega-Gutierrez, S, Abraham, MG, et al. Association of endovascular thrombectomy vs medical management with functional and safety outcomes in patients treated beyond 24 hours of last known well: the SELECT late study. JAMA Neurol. (2023) 80:172–82. doi: 10.1001/jamaneurol.2022.4714
12. Goyal, M, Menon, BK, van Zwam, WH, Dippel, DW, Mitchell, PJ, Demchuk, AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
13. Zaidat, OO, Castonguay, AC, Gupta, R, Sun, CJ, Martin, C, Holloway, WE, et al. North American solitaire stent retriever acute stroke registry: post-marketing revascularization and clinical outcome results. J NeuroIntervent Surg. (2014) 6:584–8. doi: 10.1136/neurintsurg-2013-010895
14. Phan, K, Saleh, S, Dmytriw, AA, Maingard, J, Barras, C, Hirsch, JA, et al. Influence of ASPECTS and endovascular thrombectomy in acute ischemic stroke: a meta-analysis. J Neurointerv Surg. (2019) 11:664–9. doi: 10.1136/neurintsurg-2018-014250
15. Seners, P, Wouters, A, Maïer, B, Boisseau, W, Gory, B, Heit, JJ, et al. Role of brain imaging in the prediction of intracerebral hemorrhage following endovascular therapy for acute stroke. Stroke. (2023) 54:2192–203. doi: 10.1161/STROKEAHA.123.040806
16. Winkelmeier, L, Heit, JJ, Adusumilli, G, Geest, V, Christensen, S, Kniep, H, et al. Hypoperfusion intensity ratio is correlated with the risk of parenchymal hematoma after endovascular stroke treatment. Stroke. (2023) 54:135–43. doi: 10.1161/STROKEAHA.122.040540
17. Janvier, P, Kerleroux, B, Turc, G, Pasi, M, Farhat, W, Bricout, N, et al. TAGE score for symptomatic intracranial hemorrhage prediction after successful endovascular treatment in acute ischemic stroke. Stroke. (2022) 202:2809–17. doi: 10.1161/STROKEAHA.121.038088
18. Haley, MJ, and Lawrence, CB. The blood-brain barrier after stroke: structural studies and the role of transcytotic vesicles. J Cereb Blood Flow Metab. (2017) 37:456–70. doi: 10.1177/0271678X16629976
19. Fang, Z, He, QW, Li, Q, Chen, XL, Baral, S, Jin, HJ, et al. MicroRNA-150 regulates blood-brain barrier permeability via Tie-2 after permanent middle cerebral artery occlusion in rats. FASEB J. (2016) 30:2097–107. doi: 10.1096/fj.201500126
20. Fujimura, M, Gasche, Y, Morita-Fujimura, Y, Massengale, J, Kawase, M, and Chan, PH. Early appearance of activated matrix metalloproteinase-9 and blood-brain barrier disruption in mice after focal cerebral ischemia and reperfusion. Brain Res. (1999) 842:92–100. doi: 10.1016/s0006-8993(99)01843-0
21. Li, Q, Abdalkader, M, Siegler, JE, Yaghi, S, Sarraj, A, Campbell, BCV, et al. Mechanical thrombectomy for large ischemic stroke: a systematic review and meta-analysis. Neurology. (2023) 101:e922–32. doi: 10.1212/WNL.0000000000207536
22. Kobeissi, H, Adusumilli, G, Ghozy, S, Kadirvel, R, Brinjikji, W, Albers, GW, et al. Endovascular thrombectomy for ischemic stroke with large core volume: an updated, post-TESLA systematic review and meta-analysis of the randomized trials. Interv Neuroradiol. (2023) 28. doi: 10.1177/15910199231185738
23. Shen, H, Ma, Q, Jiao, L, Chen, F, Xue, S, Li, J, et al. Prognosis and predictors of symptomatic intracranial hemorrhage after endovascular treatment of large vessel occlusion stroke. Front Neurol. (2022) 202:730940. doi: 10.3389/fneur.2021.730940
24. Chamorro, Á, Brown, S, Amaro, S, Hill, MD, Muir, KW, Dippel, DWJ, et al. Glucose modifies the effect of endovascular thrombectomy in patients with acute stroke. Stroke. (2019) 50:690–6. doi: 10.1161/STROKEAHA.118.023769
25. Neuberger, U, Kickingereder, P, Schönenberger, S, Schieber, S, Ringleb, PA, Bendszus, M, et al. Risk factors of intracranial hemorrhage after mechanical thrombectomy of anterior circulation ischemic stroke. Neuroradiology. (2019) 61:461–9. doi: 10.1007/s00234-019-02180-6
26. Neuberger, U, Seker, F, Schönenberger, S, Nagel, S, Ringleb, PA, Bendszus, M, et al. Prediction of intracranial hemorrhages after mechanical thrombectomy of basilar artery occlusion. J Neurointerv Surg. (2019) 11:1181–6. doi: 10.1136/neurintsurg-2019-014939
27. Ennis, SR, and Keep, RF. Effect of sustained-mild and transient-severe hyperglycemia on ischemia-induced blood-brain barrier opening. J Cereb Blood Flow Metab. (2007) 27:1573–82. doi: 10.1038/sj.jcbfm.9600454
28. Wang, A, Cui, T, Wang, C, Zhu, Q, Zhang, X, Li, S, et al. Prognostic significance of admission glucose combined with hemoglobin A1c in acute ischemic stroke patients with reperfusion therapy. Brain Sci. (2022) 12:294. doi: 10.3390/brainsci12020294
29. Marik, PE, and Bellomo, R. Stress hyperglycemia: an essential survival response! Crit Care Med. (2013) 41:e93–4. doi: 10.1097/CCM.0b013e318283d124
30. Zhang, J, Li, X, Kwansa, H, Kim, YT, Yi, L, Hong, G, et al. Augmentation of poly(ADP ribose) polymerase-dependent neuronal cell death by acidosis. J Cereb Blood Flow Metab. (2017) 37:1982–93. doi: 10.1177/0271678X16658491
31. Desilles, JP, Syvannarath, V, Ollivier, V, Journé, C, Delbosc, S, Ducroux, C, et al. Exacerbation of thromboinflammation by hyperglycemia precipitates cerebral infarct growth and hemorrhagic transformation. Stroke. (2017) 48:1932–40. doi: 10.1161/STROKEAHA.117.017080
32. Hafez, S, Abdelsaid, M, El-Shafey, S, Johnson, MH, Fagan, SC, and Ergul, A. Matrix metalloprotease 3 exacerbates hemorrhagic transformation and worsens functional outcomes in hyperglycemic stroke. Stroke. (2016) 47:843–51. doi: 10.1161/STROKEAHA.115.011258
33. Kamada, H, Yu, F, Nito, C, and Chan, PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke Soci Theatre Res. (2007) 38:1044–9. doi: 10.1161/01.STR.0000258041.75739.cb
34. Ironside, N, Chen, CJ, Chalhoub, RM, Wludyka, P, Kellogg, RT, Al Kasab, S, et al. Risk factors and predictors of intracranial hemorrhage after mechanical thrombectomy in acute ischemic stroke: insights from the stroke Thrombectomy and aneurysm registry (STAR). J Neurointerv Surg. (2023) 15:e312–22. doi: 10.1136/jnis-2022-019513
Keywords: large infarct core, symptomatic intracranial hemorrhage, endovascular treatment, acute ischemic stroke, glucose
Citation: Yang Y, Yang L, Shi X, Ni X, Fan S, Xu X, Ma J, Yang S, Wang Z, Zi W, Yang D and Hao Y (2024) Blood glucose to predict symptomatic intracranial hemorrhage after endovascular treatment of acute ischemic stroke with large infarct core: a prospective observational study. Front. Neurol. 15:1367177. doi: 10.3389/fneur.2024.1367177
Edited by:
Giovanni Merlino, Stroke Unit and Clinical Neurology, Udine University Hospital, ItalyReviewed by:
Ahmed Y. Azzam, October 6 University, EgyptZusen Ye, First Affiliated Hospital of Wenzhou Medical University, China
Copyright © 2024 Yang, Yang, Shi, Ni, Fan, Xu, Ma, Yang, Wang, Zi, Yang and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonggang Hao, aHlnMzYyNUAxMjYuY29t; Dahong Yang, eWRoYWp5MTk5M0AxMjYuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors share senior authorship
 Yujie Yang
Yujie Yang Lihui Yang1†
Lihui Yang1† Xuan Ni
Xuan Ni Wenjie Zi
Wenjie Zi Dahong Yang
Dahong Yang Yonggang Hao
Yonggang Hao