
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 11 June 2024
Sec. Headache and Neurogenic Pain
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1365445
Purpose: This systematic review and meta-analysis aimed to evaluate the efficacy of repetitive transcranial magnetic stimulation (rTMS) in postherpetic neuralgia (PHN).
Methods: Through an extensive search in four databases until October 2023, we selected five randomized controlled trials adhering to our specific criteria, involving 257 patients in total. For continuous outcomes, the standardized mean difference (SMD) was calculated. Heterogeneity among the studies was assessed using Cochran’s I2 and Q statistics, adopting a random-effects model for I2 values over 50%. For assessing potential publication bias, we utilized both funnel plot and Egger’s test.
Results: Our analysis found that rTMS reduced the overall visual analogue scale (VAS) (SMD: −1.52, 95% CI: −2.81 to −0.23, p = 0.02), VAS at 1 month post-treatment (SMD: −2.21, 95% CI: −4.31 to −0.10, p = 0.04), VAS at 3 months post-treatment (SMD: −1.51, 95% CI: −2.81 to −0.22, p = 0.02), as well as patients’ global impression of change scale (PGIC) (SMD: −1.48, 95% CI: −2.87 to −0.09, p = 0.04) and short-form McGill pain questionnaire (SF-MPQ) (SMD: −1.25, 95% CI: −2.41 to −0.09, p = 0.03) compared to the sham-rTMS group.
Conclusion: Our study suggests that rTMS might have a potential alleviating effect on PHN symptoms. However, due to the limited number of studies and variations in rTMS parameters, larger sample studies involving more diverse populations, as well as further clarification of the most appropriate stimulation protocol, are still needed.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, Identifier ID: CRD42023488420.
Postherpetic neuralgia (PHN) is a common complication of herpes zoster. It generally refers to pain that persists for more than 1 month after the healing of herpes zoster rash (1), and is also defined as pain lasting more than 90 days (2). The incidence rate of herpes zoster is 10 to 20%, with 9 to 13% of patients developing PHN (3), and the incidence of this disease has been gradually increasing in recent years (4). The pain of PHN is characterized by spontaneous pain, pain hypersensitivity, allodynia, and abnormal sensations, with a long-lasting course, leading to negative emotions like anxiety, despair, and depression in patients, significantly affecting their quality of life (1, 5). Early and effective treatment is very important for PHN patients, as it can have a profound impact on their quality of life, including physical, emotional, and social aspects.
Currently, pharmacotherapy remains the primary method for treating PHN. However, the effectiveness of pharmacotherapy is not satisfactory. For most patients, medication only partially relieves pain. This is mainly because the adverse reactions of the drugs limit the achievable doses (6). Other measures, such as botulinum toxin, nerve blocks, spinal cord stimulation, and radiofrequency, also make significant contributions to the treatment of PHN. However, these treatments are invasive, and their effectiveness, safety, and tolerability still need to be monitored (7). Considering the limitations of the current treatments, seeking alternative, effective, and safer therapeutic methods is of great importance.
Transcranial magnetic stimulation (TMS) is based on the principle of electromagnetic induction, utilizing the magnetic field pulses generated when a strong varying current passes through a coil placed over the head, and the resulting induced current that initiates action potentials (8), affecting the entire brain functional network (9). Repetitive transcranial magnetic stimulation (rTMS), as a non-invasive brain stimulation technique, has emerged as a promising intervention in the treatment of neuropathic pain (10). However, there is limited research on rTMS for treating PHN, and there is controversy over its effectiveness (11–16). The improvement of short-form McGill pain questionnaire (SF-MPQ) (11, 14, 15, 17) and patients’ global impression of change scale (PGIC) (14, 15, 17) post-treatment is also a subject of debate.
Therefore, it is necessary to summarize and analyze the data from published studies. The purpose of this meta-analysis is to integrate the current evidence from randomized controlled trials to evaluate the efficacy of rTMS in the treatment of PHN.
The meta-analysis rigorously followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines. We registered the protocol for this meta-analysis with PROSPERO under the identifier CRD42023488420.
A comprehensive search was conducted in databases including PubMed, Embase, China National Knowledge Infrastructure (CNKI), and WANFANG DATA, for relevant publications up to October 2023. Search terms are listed in the Supplementary Table S1. Additionally, we manually examined the reference lists of selected articles to identify additional relevant research.
This meta-analysis includes randomized controlled trials evaluating the efficacy of rTMS in PHN, requiring a minimum sample size of 10 participants.
The exclusion criteria covered duplicate articles, letters, case reports, reviews, meta-analyses, and irrelevant titles or abstracts. Studies offering incomplete or equivocal data, impeding accurate outcome assessment, were also excluded. Furthermore, we only included studies in English in patient overlap situation (articles with patient overlap that have both Chinese and English versions).
Two researchers independently evaluated article titles and abstracts based on predefined inclusion and exclusion criteria. Following this, they thoroughly inspected the full text to ascertain the studies’ eligibility. Disagreements were addressed and resolved by discussion, leading to a consensus.
Using the Cochrane Risk of Bias Tool for randomized trials, a pair of independent researchers assessed the quality levels of the selected studies. Factors such as random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete data (attrition bias), selective reporting (reporting bias), and other biases were examined by both reviewers. If there were any discrepancies, a third researcher would be consulted to resolve the issue.
Two researchers independently extracted data from each included article, including author, year, country, study design, outcome, comparison, mean age ± SD, male/female, number of patients, pain laterality (right), as well as parameters and dosage. The disagreements among researchers were settled through discussions, eventually leading to a consensus.
The primary outcome was the overall visual analogue scale (VAS) score, a straightforward tool for pain assessment. Increased VAS scores denote higher levels of pain (18, 19). The secondary outcome measures also include VAS at 1 month post-treatment, VAS at 3 months post-treatment, PGIC, and SF-MPQ. PGIC indicates the comprehensive changes in pain relief, functional improvement, emotional state, and quality of life after therapeutic interventions, also eliminating the misconception that pain reduction alone indicates successful treatment. The lower the PGIC score, the more effective the treatment is deemed (20, 21). SF-MPQ is a sensitive and dependable pain assessment tool that not only evaluates the characteristics of pain but also precisely measures the patient’s emotional and sensory experiences, encompassing fatigue, discomfort, fear, and torment. The higher the SF-MPQ score, the more severe the pain, and the worse the emotional and sensory experiences (15, 20).
In this analysis, we utilized the standardized mean difference (SMD) to evaluate continuous outcomes. Corresponding 95% confidence intervals (CIs) were also calculated to estimate the range of the effect sizes. Heterogeneity among the studies was assessed using Cochran’s I2 and Q statistics. Heterogeneity, based on I2 values, was classified as low (25%), moderate (50%), or high (75%). A fixed-effects model was applied for I2 values below 50%, above this threshold, a random-effects model was used. In instances of significant heterogeneity (I2 ≥ 50%), leave-one-out sensitivity analyses were performed to find out the heterogeneity sources and assess the stability of the results.
Publication bias, which is the tendency to favor publishing studies with positive or significant outcomes, was assessed through funnel plot analysis and Egger’s test. Analyses were performed using Stata 17 software.
The initial search identified 103 publications, of which 32 were duplicates and 59 were ineligible, leading to their exclusion. Further scrutiny of the full texts of the remaining 12 articles led to the exclusion of 7 more studies due to dates that could not be extracted (n = 2), non-randomized control trials (n = 3) and patient overlap (n = 2). This process resulted in the selection of 5 randomized controlled trials for the analysis of the efficacy of rTMS in PHN (11, 14–17). Figure 1 displays the PRISMA flow diagram depicting this selection process.
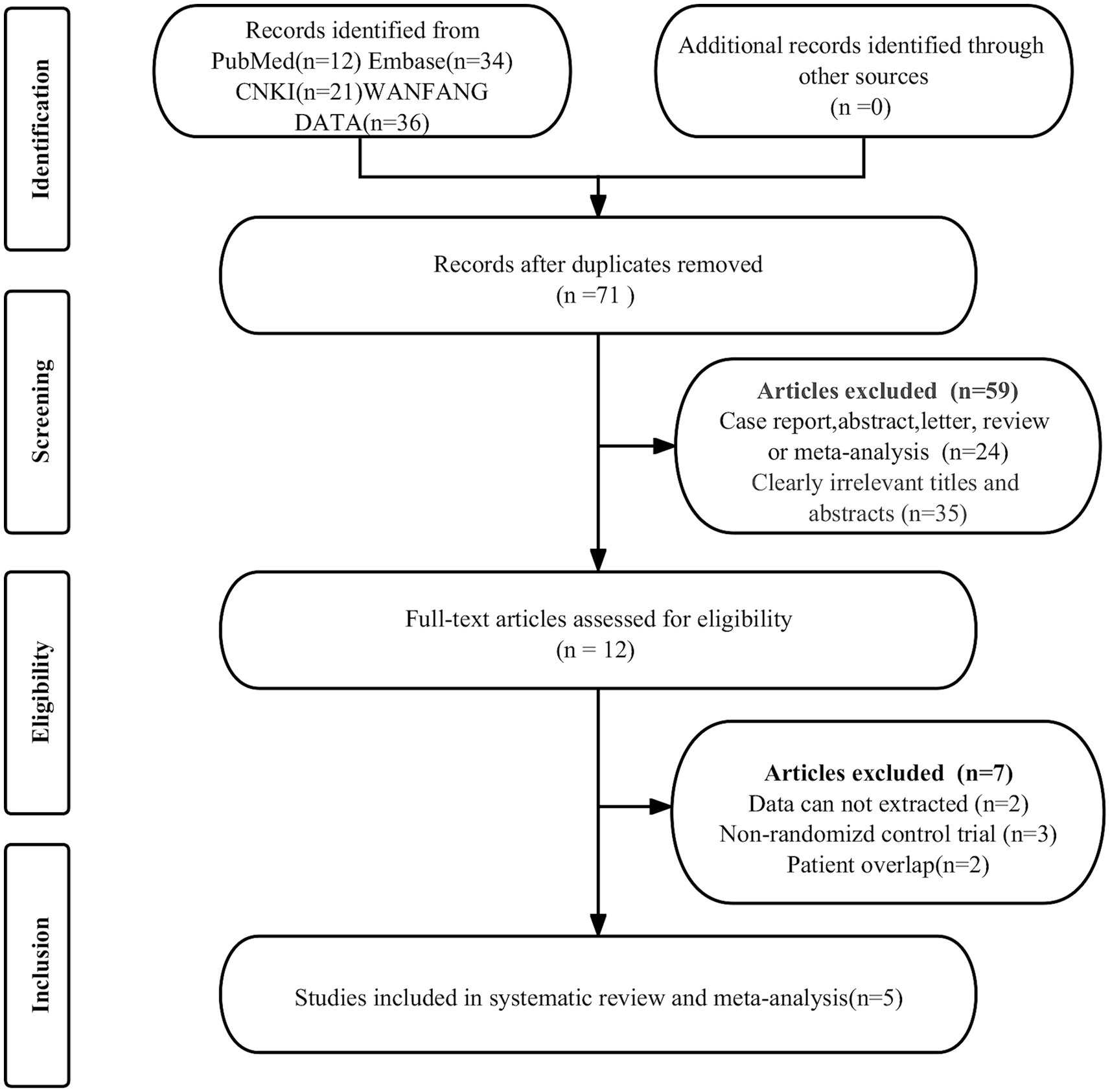
Figure 1. PRISMA flow diagram illustrating the study selection process. PRISMA, preferred reportingitems for systematic reviews and meta-analyses.
In these eligible studies, five randomized controlled trials from China, encompassing a total of 257 patients, were evaluated. Their characteristics are succinctly summarized in Table 1. The patient count in each study varied from 33 to 64, with their mean age ranging from 61.3 to 70.62 years. The stimulation frequency was 5 Hz or 10 Hz. The stimulation site was either the motor cortex (M1) or the dorsolateral prefrontal cortex (DLPFC). The intensity of stimulation was 80–100% motor threshold. Patients were treated for 10 or 15 sessions and each rTMS session delivered 1,200 to 3,000 pulses with intervals of 2.5 s, 3 s, or 25 s.
Figure 2 illustrates the risk of bias in each study, as assessed using the Cochrane Risk of Bias Tool. High risk is primarily concentrated in allocation concealment (selection bias), as the operators administering rTMS treatment must know the allocation plan to provide the corresponding treatment to patients. Overall, the included studies displayed acceptable quality.
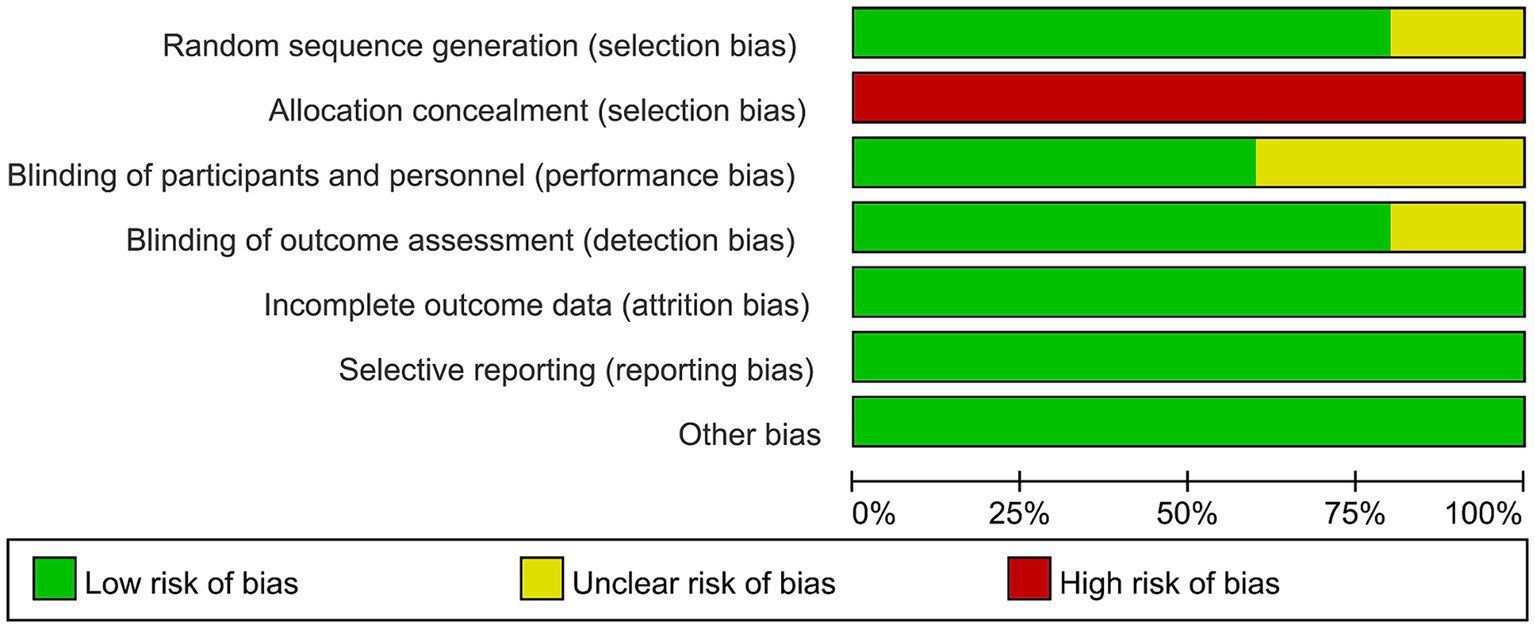
Figure 2. Risk of bias of the included studies using the Cochrane risk of bias tool for randomized trials.
Five studies, including a total of 257 patients, evaluated the overall VAS. In light of the high heterogeneity (I2 = 94.57%, p = 0.02), we applied a random-effect model. The meta-analysis revealed that rTMS significantly decreased the overall VAS (SMD: −1.52, 95% CI: −2.81 to −0.23, p = 0.02) compared to the sham rTMS group (Figure 3). The sensitivity analysis found no potential sources of heterogeneity (Supplementary Table S2). There was no detectable publication bias as indicated by both funnel plot and Egger’s test (p = 0.13) (Figure 4).
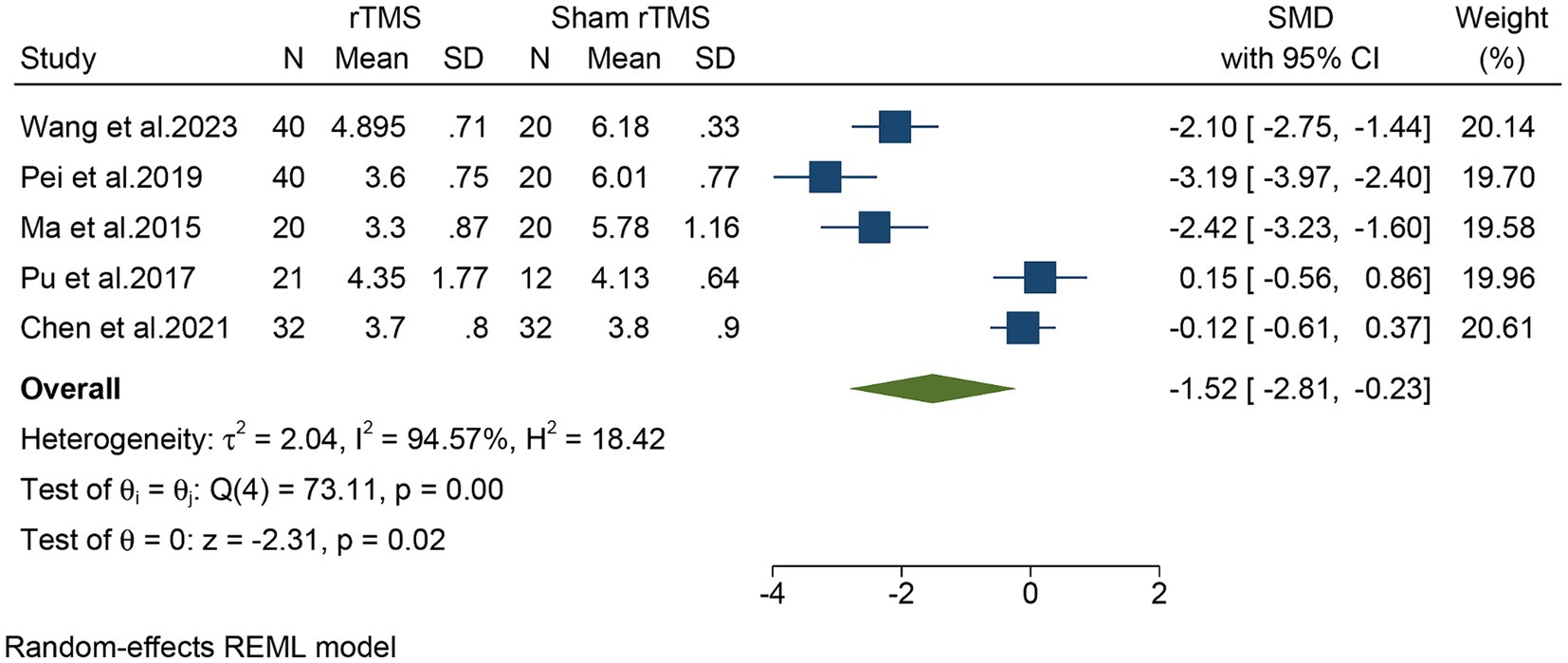
Figure 3. Forest plot of the overall VAS for the rTMS group vs. the Sham rTMS group. VAS, visual analogue scale; rTMS, repetitive transcranial magnetic stimulation.
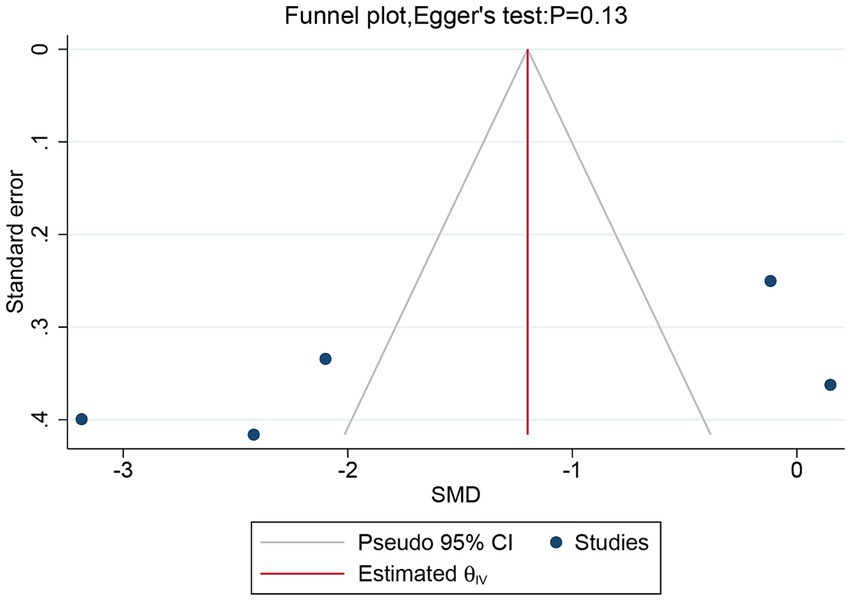
Figure 4. Funnel plot and Egger’s test of the overall VAS for the rTMS group vs. the Sham rTMS group. VAS, visual analogue scale; rTMS, repetitive transcranial magnetic stimulation.
Subgroup analyses in different regions (DLPFC vs. M1) were conducted. For DLPFC, one study, including a total of 40 patients, evaluated the overall VAS. The meta-analysis revealed that rTMS significantly decreased the overall VAS (SMD: −2.28, 95% CI: −3.08 to −1.49) compared to the sham rTMS group (Figure 5). For M1, five studies, including a total of 237 patients, evaluated the overall VAS. In light of the high heterogeneity (I2 = 97.49%, p = 0.00), we applied a random-effect model. The meta-analysis revealed that rTMS also significantly decreased the overall VAS (SMD: −2.25, 95% CI: −4.39 to −0.11) compared to the sham rTMS group (Figure 5). Although both DLPFC and M1 can reduce the overall VAS, there was no statistical difference between the two groups (p = 0.98).
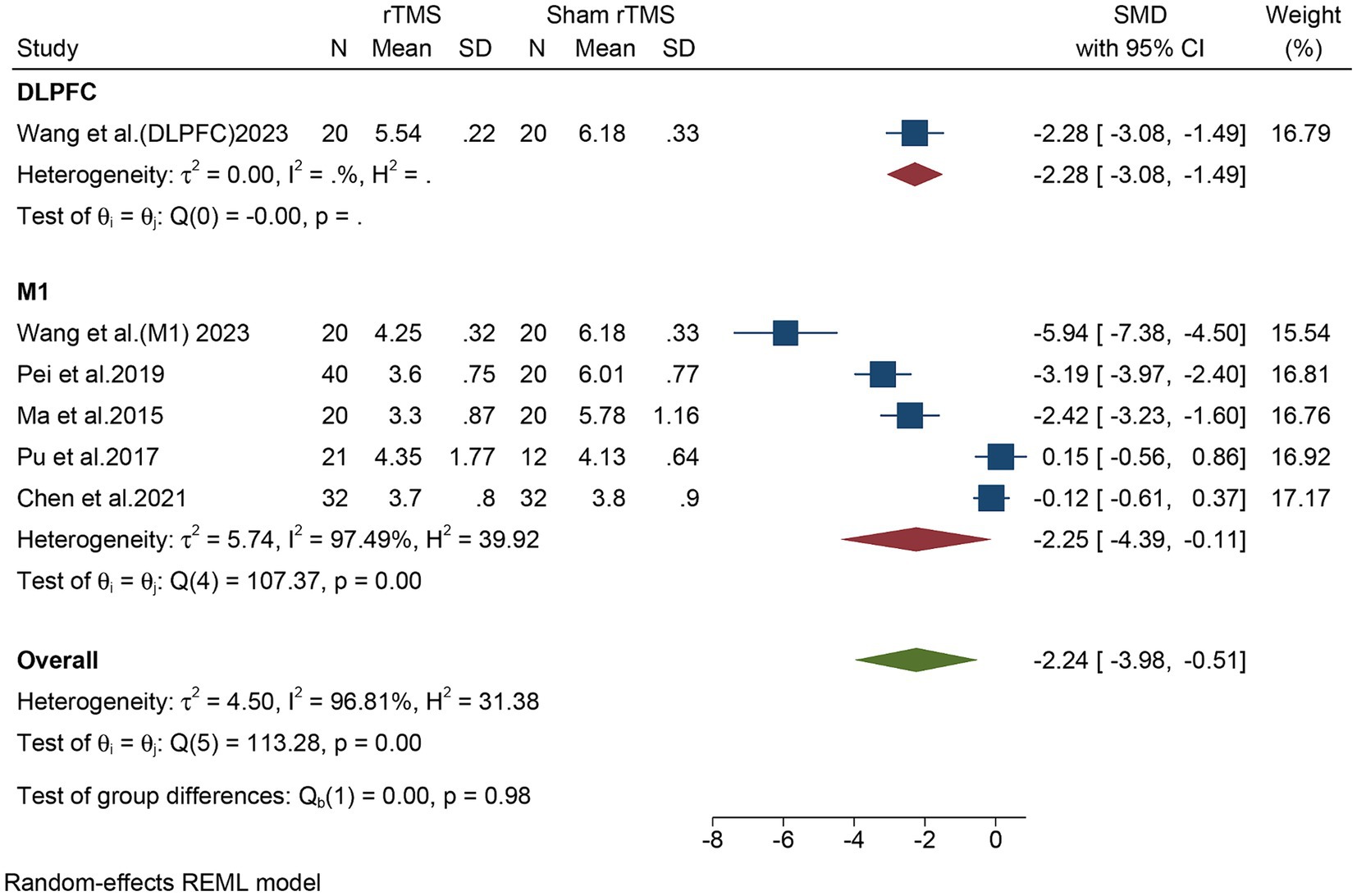
Figure 5. Subgroup analysis in different stimulate regions (M1 or DLPFC) of the overall VAS for the rTMS group vs. the Sham rTMS group. M1, motor cortex; DLPFC, dorsolateral prefrontal cortex; VAS, visual analogue scale; rTMS, repetitive transcranial magnetic stimulation.
Five studies, including a total of 257 patients, evaluated the VAS at 1 month post-treatment. In light of the high heterogeneity (I2 = 97.69%, p = 0.04), we applied a random-effect model. The meta-analysis revealed that rTMS significantly decreased the VAS at 1 month post-treatment (SMD: −2.21, 95% CI: −4.31 to −0.10, p = 0.04) compared to the sham rTMS group (Supplementary Figure S1). The sensitivity analysis found no potential sources of heterogeneity (Supplementary Table S3).
Five studies, including a total of 257 patients, evaluated the VAS at 3 months post-treatment. In light of the high heterogeneity (I2 = 94.63%, p = 0.02), we applied a random-effect model. The meta-analysis revealed that rTMS significantly decreased the VAS at 3 months post-treatment (SMD: −1.51, 95% CI: −2.81 to −0.22, p = 0.02) compared to the sham rTMS group (Supplementary Figure S2). The sensitivity analysis found no potential sources of heterogeneity (Supplementary Table S4).
Three studies, including a total of 133 patients, evaluated the PGIC. In light of the high heterogeneity (I2 = 91.27%, p = 0.04), we applied a random-effect model. The meta-analysis revealed that rTMS significantly decreased the PGIC (SMD: −1.48, 95% CI: −2.87 to −0.09, p = 0.04) compared to the sham rTMS group (Supplementary Figure S3). The sensitivity analysis found no potential sources of heterogeneity (Supplementary Table S5).
Four studies, including a total of 193 patients, evaluated the SF-MPQ. In light of the high heterogeneity (I2 = 91.63%, p = 0.03), we applied a random-effect model. The meta-analysis revealed that rTMS significantly decreased the SF-MPQ (SMD: −1.25, 95% CI: −2.41 to −0.09, p = 0.03) compared to the sham rTMS group (Supplementary Figure S4). The sensitivity analysis found no potential sources of heterogeneity (Supplementary Table S6).
Our meta-analysis indicates that compared to the sham rTMS group, rTMS significantly reduced the VAS scores in PHN patients, thereby alleviating the pain. At present, the pain-relieving mechanism of rTMS is unclear. There are some studies that suggest rTMS of the motor cortex may activate brain regions associated with descending pain modulation (22–25). This pattern of distant and diffuse brain activation is consistent with the diffuse and non-somatotopic analgesic effect induced by rTMS in the motor cortex, as many studies have shown, where localized stimulation leads to pain reduction in different body areas (26–28). Additionally, Goto et al. (29) observed that the integrity of the corticospinal tract and thalamocortical tract is significant for the pain alleviation induced by rTMS. rTMS can also regulate local cerebral blood flow and metabolism (30), and promote the release of cerebral beta-endorphin, recognized as a pain-relieving factor in the nervous system (31). Additionally, studies have shown that enhancing neuroplasticity (32), reducing the levels of neuronal nitric oxide synthase overexpressed in dorsal root ganglia, and inhibiting astrocyte activity (33) could be the pain-relieving mechanisms of rTMS.
It is noteworthy that our study observed that rTMS in both the DLPFC and M1 regions led to a significant reduction in VAS, but no statistical difference was detected between them (p = 0.98). This finding differs from Wang et al. (11), who suggested that stimulating the M1 region has a more pronounced analgesic effect. This discrepancy may be due to the smaller number of patients included in the M1 group in their experiment, as they only included 20 patients in the M1 group compared to the DLPFC group. However, due to the overall limited number of studies on DLPFC stimulation, further research is still needed. Additionally, based on multiple studies, M1 remains the most commonly used stimulation target.
Furthermore, our analysis also shows that VAS scores significantly decreased at both 1- and 3 months post-treatment, indicating that the therapeutic effect of rTMS is lasting and stable. The improvements in PGIC and SF-MPQ further confirm the treatment’s effectiveness from the viewpoint of patient-centered care.
Jiang et al. (34) included a broader range of neuropathic pain cases in their meta-analysis, demonstrating a significant benefit of rTMS over sham rTMS. Although their results are consistent with the direction of our study, they did not specifically focus on PHN patients as our study did. Our study is the first to exclusively include PHN patients, providing a more targeted assessment of the efficacy of rTMS in this group. Additionally, our study’s inclusion of PGIC and SF-MPQ assessments offers a more comprehensive view of patient outcomes, a dimension that Jiang et al. did not explore. In another meta-analysis, Jin et al. (35), found that high-frequency rTMS stimulation of the primary motor cortex can effectively alleviate neuropathic pain. They primarily focused on the optimal treatment parameters of rTMS, including stimulation frequency and number of treatments, which differ from our outcome measures.
In the current meta-analysis, significant heterogeneity was observed in overall VAS, VAS at 1- or 3 months post-treatment, PGIC, and SF-MPQ, but the sensitivity analysis was unable to clarify the exact sources of this heterogeneity. This high level of heterogeneity might be attributed to differences in patient groups, research designs, or stimulation parameters in various studies. Future studies should aim to reduce these differences to better evaluate the effects of rTMS on treatment efficacy and to more effectively devise rTMS treatment plans. Furthermore, considering publication bias, we performed funnel plot and Egger’s test on the primary outcome measure, overall VAS, and detected no publication bias.
rTMS stands out for its rapid analgesic effects, non-invasiveness, and minimal side effects, being recognized as one of the significant brain science technologies of the 21st century (36, 37). Under strict adherence to treatment indications, the adverse effects are mainly headaches, occasional hearing loss, and very rarely, the induction of seizures. The treatment has a high safety profile (38). For elderly patients and those with severe comorbidities, cautious use of pharmacotherapy for PHN is advised, making rTMS treatment even more crucial (7). However, patients must undergo rTMS treatment in medical institutions, and the lack of standardized treatment protocols poses a challenge at present (39). Especially compared to traditional treatments, further cost-benefit research is needed in the future. Clinicians should weigh these factors and the individual circumstances of the patient when considering rTMS treatment for PHN.
Currently, our research still has limitations. The first limitation is that all study patients were from China. This means we cannot assume rTMS works the same for other races. More studies with different racial groups are needed. Secondly, despite conducting sensitivity analyses, the sources of heterogeneity were not identified, suggesting that the overall estimate effects of the meta-analysis should be interpreted with caution. At the same time, we observe that due to the studies originating from different institutions, there are variations in the parameters used for rTMS. Consequently, the most appropriate stimulation protocol remains to be clearly defined. Furthermore, the relatively small sample size of rTMS in PHN studies may affect the robustness of our conclusions. We look forward to future research, preferably with larger sample sizes and more diverse patient populations, to strengthen the evidence base for rTMS in the treatment of PHN.
Our study suggests that rTMS might have a potential alleviating effect on PHN symptoms. However, due to the limited number of studies and variations in rTMS parameters, larger sample studies involving more diverse populations, as well as further clarification of the most appropriate stimulation protocol, are still needed.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
QD: Conceptualization, Data curation, Resources, Software, Visualization, Writing – original draft. AX: Conceptualization, Data curation, Investigation, Software, Supervision, Visualization, Writing – original draft. KW: Conceptualization, Investigation, Methodology, Resources, Software, Visualization, Writing – original draft. YY: Conceptualization, Investigation, Software, Supervision, Validation, Visualization, Writing – original draft. YaS: Conceptualization, Data curation, Software, Validation, Visualization, Writing – review & editing. YoS: Conceptualization, Data curation, Funding acquisition, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The article was funded by the Basic Scientific Research Project of Universities in 2023 carried out by the Department of Education of Liaoning Province (JYTMS20230075).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1365445/full#supplementary-material
rTMS, repetitive transcranial magnetic stimulation; PHN, postherpetic neuralgia; SMD, standardized mean difference; VAS, visual analogue scale; PGIC, patients’ global impression of change scale; SF-MPQ, short-form McGill pain questionnaire; TMS, transcranial magnetic stimulation; M1, motor cortex; DLPFC, dorsolateral prefrontal cortex; PRISMA, preferred reporting items for systematic reviews and meta-analyses
1. Yu, S, Wan, Y, Wan, Q, Ma, K, Wang, J, Lu, Z, et al. Chinese expert consensus on the diagnosis and treatment of postherpetic neuralgia (in Chinese). Chin J Pain Med. (2016) 22:161–7.
2. Werner, RN, Nikkels, AF, Marinović, B, Schäfer, M, Czarnecka-Operacz, M, Agius, AM, et al. European consensus-based (S2k) guideline on the Management of Herpes Zoster - guided by the European dermatology forum (EDF) in cooperation with the European academy of dermatology and venereology (EADV), part 1: diagnosis. J Eur Acad Dermatol Venereol. (2017) 31:9–19. doi: 10.1111/jdv.13995
3. Lai, Y, Chen, J, Shen, F, Song, X, Tang, S, Yang, Y, et al. Clinical observation of aseptic acupressure in the treatment of postherpetic neuralgia (in Chinese). Liaoning J Trad Chin Med. (2019) 46:1278–80. doi: 10.13192/j.issn.1000-1719.2019.06.050
4. Xu, W, and Wang, Q. Advances in the epidemiology and economic burden of herpes zoster and postherpetic neuralgia (in Chinese). Tianjin Med. (2018) 46:552–6. doi: 10.11958/20171248
5. Xu, G, Xú, G, Feng, Y, Tang, WZ, and Lv, ZW. Transcutaneous electrical nerve stimulation in combination with cobalamin injection for postherpetic neuralgia: a single-center randomized controlled trial. Am J Phys Med Rehabil. (2014) 93:287–98. doi: 10.1097/phm.0000000000000002
6. Argoff, CE, Katz, N, and Backonja, M. Treatment of postherpetic neuralgia: a review of therapeutic options. J Pain Symptom Manag. (2004) 28:396–411. doi: 10.1016/j.jpainsymman.2004.01.014
7. Shrestha, M, and Chen, A. Modalities in managing postherpetic neuralgia. Korean J Pain. (2018) 31:235–43. doi: 10.3344/kjp.2018.31.4.235
8. Lefaucheur, JP . Transcranial magnetic stimulation. Handb Clin Neurol. (2019) 160:559–80. doi: 10.1016/b978-0-444-64032-1.00037-0
9. Pei, Q, Zhuo, Z, Jing, B, Meng, Q, Ma, X, Mo, X, et al. The effects of repetitive transcranial magnetic stimulation on the whole-brain functional network of postherpetic neuralgia patients. Medicine. (2019) 98:e16105. doi: 10.1097/md.0000000000016105
10. Ma, S, and Ni, J. Clinical application progress of repetitive transcranial magnetic stimulation in the treatment of neuropathic pain (in Chinese). Chin J Rehabil Med. (2015) 30:1310–3. doi: 10.3969/j.issn.1001-1242.2015.12.027
11. Wang, H, Hu, Y, Deng, J, Ye, Y, Huang, M, Che, X, et al. A randomised sham-controlled study evaluating rTMS analgesic efficacy for postherpetic neuralgia. Front Neurosci. (2023) 17:1158737. doi: 10.3389/fnins.2023.1158737
12. Yu, S, and Zheng, X. Observation of the therapeutic effect of transcranial magnetic stimulation in the treatment of postherpetic neuralgia (in Chinese). J Third Mil Med Univ. (2010) 32:567–75. doi: 10.16016/j.1000-5404.2010.06.033
13. Yin, Z, Shen, Y, Dai, W, and Qian, K. Efficacy of high-frequency repetitive transcranial magnetic stimulation in the treatment of postherpetic neuralgia (in Chinese). Jiangsu Med J. (2017) 43:1331–4. doi: 10.19460/j.cnki.0253-3685.2017.018.014
14. Pei, Q, Wu, B, Tang, Y, Yang, X, Song, L, Wang, N, et al. Repetitive transcranial magnetic stimulation at different frequencies for postherpetic neuralgia: a double-blind, sham-controlled. Randomized Trial Pain Physician. (2019) 22:E303–13. doi: 10.36076/ppj/2019.22.E303
15. Pu, Y. Observation of the therapeutic effect of repetitive transcranial magnetic stimulation in the treatment of postherpetic neuralgia (in Chinese) [master], (2016)
16. Chen, X, Liao, P, Shi, Q, Zhou, L, Luo, Q, Yun, D, et al. The effect of qi-boosting and blood-activating analgesic decoction combined with high-frequency repetitive transcranial magnetic stimulation on pain, sleep quality, and short- and long-term therapeutic outcomes in elderly patients with postherpetic neuralgia (in Chinese). Chin Gen Practice. (2021) 24:2174. doi: 10.12114/j.issn.1007-9572.2021.00.490
17. Ma, SM, Ni, JX, Li, XY, Yang, LQ, Guo, YN, and Tang, YZ. High-frequency repetitive transcranial magnetic stimulation reduces pain in postherpetic neuralgia. Pain Med. (2015) 16:2162–70. doi: 10.1111/pme.12832
18. de Wit, R, van Dam, F, Abu-Saad, HH, Loonstra, S, Zandbelt, L, van Buuren, A, et al. Empirical comparison of commonly used measures to evaluate pain treatment in cancer patients with chronic pain. J Clin Oncol. (1999) 17:1280. doi: 10.1200/jco.1999.17.4.1280
19. Feine, JS, Lavigne, GJ, Dao, TTT, Morin, C, and Lund, JP. Memories of chronic pain and perceptions of relief. Pain. (1998) 77:137–41. doi: 10.1016/s0304-3959(98)00089-x
20. Melzack, R . The short-form McGill pain questionnaire. Pain. (1987) 30:191–7. doi: 10.1016/0304-3959(87)91074-8
21. Bouhassira, D, and Attal, N. All in one: is it possible to assess all dimensions of any pain with a simple questionnaire? Pain. (2009) 144:7–8. doi: 10.1016/j.pain.2009.04.001
22. Lefaucheur, JP, Drouot, X, Menard-Lefaucheur, I, Zerah, F, Bendib, B, Cesaro, P, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry. (2004) 75:612–6. doi: 10.1136/jnnp.2003.022236
23. Passard, A, Attal, N, Benadhira, R, Brasseur, L, Saba, G, Sichere, P, et al. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain. (2007) 130:2661–70. doi: 10.1093/brain/awm189
24. Onesti, E, Gabriele, M, Cambieri, C, Ceccanti, M, Raccah, R, di Stefano, G, et al. H-coil repetitive transcranial magnetic stimulation for pain relief in patients with diabetic neuropathy. Eur J Pain. (2013) 17:1347–56. doi: 10.1002/j.1532-2149.2013.00320.x
25. Dall’Agnol, L, Medeiros, LF, Torres, IL, Deitos, A, Brietzke, A, Laste, G, et al. Repetitive transcranial magnetic stimulation increases the corticospinal inhibition and the brain-derived neurotrophic factor in chronic myofascial pain syndrome: an explanatory double-blinded, randomized, sham-controlled trial. J Pain. (2014) 15:845–55. doi: 10.1016/j.jpain.2014.05.001
26. Nahmias, F, Debes, C, de Andrade, DC, Mhalla, A, and Bouhassira, D. Diffuse analgesic effects of unilateral repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers. Pain. (2009) 147:224–32. doi: 10.1016/j.pain.2009.09.016
27. Mhalla, A, Baudic, S, de Andrade, DC, Gautron, M, Perrot, S, Teixeira, MJ, et al. Long-term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. Pain. (2011) 152:1478–85. doi: 10.1016/j.pain.2011.01.034
28. Attal, N, Poindessous-Jazat, F, de Chauvigny, E, Quesada, C, Mhalla, A, Ayache, SS, et al. Repetitive transcranial magnetic stimulation for neuropathic pain: a randomized multicentre sham-controlled trial. Brain. (2021) 144:3328–39. doi: 10.1093/brain/awab208
29. Goto, T, Saitoh, Y, Hashimoto, N, Hirata, M, Kishima, H, Oshino, S, et al. Diffusion tensor fiber tracking in patients with central post-stroke pain; correlation with efficacy of repetitive transcranial magnetic stimulation. Pain. (2008) 140:509–18. doi: 10.1016/j.pain.2008.10.009
30. Tamura, Y, Okabe, S, Ohnishi, T, Saito, DN, Arai, N, Mochio, S, et al. Effects of 1-Hz repetitive transcranial magnetic stimulation on acute pain induced by capsaicin. Pain. (2004) 107:107–15. doi: 10.1016/j.pain.2003.10.011
31. Ahmed, MA, Mohamed, SA, and Sayed, D. Long-term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta-endorphin in patients with phantom pain. Neurol Res. (2011) 33:953–8. doi: 10.1179/1743132811y.0000000045
32. Moisset, X, de Andrade, DC, and Bouhassira, D. From pulses to pain relief: an update on the mechanisms of rTMS-induced analgesic effects. Eur J Pain. (2016) 20:689–700. doi: 10.1002/ejp.811
33. Xu, J, and Guo, T. Inhibitory effects of repetitive transcranial magnetic stimulation on astrocytes in the spinal cord of rats with neuropathic pain (in Chinese). Chin J Phys Med Rehabil. (2016) 38:659–63. doi: 10.3760/cma.j.issn.0254-1424.2016.09.004
34. Jiang, X, Yan, W, Wan, R, Lin, Y, Zhu, X, Song, G, et al. Effects of repetitive transcranial magnetic stimulation on neuropathic pain: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2022) 132:130–41. doi: 10.1016/j.neubiorev.2021.11.037
35. Jin, Y, Xing, G, Li, G, Wang, A, Feng, S, Tang, Q, et al. High frequency repetitive transcranial magnetic stimulation therapy for chronic neuropathic pain: a meta-analysis. Pain Physician. (2015) 18:E1029–46. doi: 10.36076/ppj.2015/18/E1029
36. Hu, X, Jiang, X, and Zhang, T. Progress in the application of repetitive transcranial magnetic stimulation therapy in post-stroke aphasia (in Chinese). Chin J Rehabil Theory Practice. (2015) 21:138–41. doi: 10.3969/j.issn.1006-9771.2015.02.004
37. Meissner, K, Fässler, M, Rücker, G, Kleijnen, J, Hróbjartsson, A, Schneider, A, et al. Differential effectiveness of placebo treatments: a systematic review of migraine prophylaxis. JAMA Intern Med. (2013) 173:1941–51. doi: 10.1001/jamainternmed.2013.10391
38. Majumder, P, Balan, S, Gupta, V, Wadhwa, R, and Perera, TD. The safety and efficacy of repetitive transcranial magnetic stimulation in the treatment of major depression among children and adolescents: a systematic review. Cureus. (2021) 13:e14564. doi: 10.7759/cureus.14564
Keywords: repetitive transcranial magnetic stimulation, postherpetic neuralgia, meta-analysis, efficacy, systematic review
Citation: Dai Q, Xu A, Wang K, Yang Y, Shao Y and Sun Y (2024) The efficacy of repetitive transcranial magnetic stimulation in postherpetic neuralgia: a meta-analysis of randomized controlled trials. Front. Neurol. 15:1365445. doi: 10.3389/fneur.2024.1365445
Received: 06 January 2024; Accepted: 30 May 2024;
Published: 11 June 2024.
Edited by:
Tim P. Jürgens, University Hospital Rostock, GermanyReviewed by:
Eleonora Vecchio, University of Bari Aldo Moro, ItalyCopyright © 2024 Dai, Xu, Wang, Yang, Shao and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongxin Sun, c3VueW9uZ3hpbkBjbXUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.