
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 25 July 2024
Sec. Stroke
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1364875
This article is part of the Research Topic Signaling Pathways and Therapeutic Targets in Stroke View all 6 articles
 Jingfang Long1,2†
Jingfang Long1,2† Jiahao Chen1†
Jiahao Chen1† Guiqian Huang1,3†
Guiqian Huang1,3† Zhen Chen4†
Zhen Chen4† Heyu Zhang1
Heyu Zhang1 Ye Zhang1
Ye Zhang1 Qi Duan1
Qi Duan1 Beilan Wu1
Beilan Wu1 Jincai He1*
Jincai He1*Introduction: Hemorrhagic transformation (HT) is a serious complication that can occur spontaneously after an acute ischemic stroke (AIS) or after a thrombolytic/mechanical thrombectomy. Our study aims to explore the potential correlations between fibrinogen levels and the occurrence of spontaneous HT (sHT) and HT after mechanical thrombectomy (tHT).
Methods: A total of 423 consecutive AIS patients diagnosed HT who did not undergone thrombolysis and 423 age- and sex-matched patients without HT (non-HT) were enrolled. Fibrinogen levels were measured within 24 h of admission after stroke. The cohorts were trisected according to fibrinogen levels. The HT were further categorized into hemorrhagic infarction (HI) or parenchymal hematoma (PH) based on their imaging characteristics.
Results: In sHT cohort, fibrinogen levels were higher in HT patients than non-HT patients (p < 0.001 versus p = 0.002). High fibrinogen levels were associated with the severity of HT. HT patients without atrial fibrillation (AF) had higher levels of fibrinogen compared to non-HT (median 3.805 vs. 3.160, p < 0.001). This relationship did not differ among AF patients. In tHT cohort, fibrinogen levels were lower in HT patients than non-HT patients (p = 0.002). Lower fibrinogen levels were associated with the severity of HT (p = 0.004). The highest trisection of fibrinogen both in two cohorts were associated with HT [sHT cohort: OR = 2.515 (1.339–4.725), p = 0.016; that cohort: OR = 0.238 (0.108–0.523), p = 0.003].
Conclusion: Our study suggests that lower fibrinogen level in sHT without AF and higher fibrinogen level in tHT are associated with more severe HT.
Hemorrhagic transformation (HT) is known as a natural progression of acute ischemic stroke (AIS) caused by the restoration of blood flow in the ischemic area (1). This condition can often lead to a deterioration of neurological function. HT includes spontaneous hemorrhage (spontaneous hemorrhagic transformation) and post-interventional hemorrhage (including thrombolysis, thrombectomy, and anticoagulation, etc.) after AIS (2). Existing findings suggest that the Asian population is significantly more prone to HT than the western population (3, 4). A study from China in 2016 reported that the incidence of HT after endovascular therapy was as high as 49.5%, with symptomatic intracranial hemorrhage (sICH) at 16.0% (5). Existing studies on HT have mainly focused on thrombolytic therapy, with fewer studies on spontaneous HT (sHT) and HT after mechanical thrombectomy (tHT).
The European Cooperative Acute Stroke Study (ECASS) classifies hemorrhagic stroke based on radiological findings, as hemorrhagic infarction (HI) and parenchymal hematoma (PH) (6). PH has been associated with a higher risk of clinical complications, as well as a longer hospital stay and a severe clinical prognosis when compared to HI, often results in death at discharge (7, 8).
The AIS is often associated with atherosclerosis, and plaque instability can lead to the formation of blood clots, resulting in abnormal cerebral blood flow (9). During the treatment window period for stroke, reperfusion therapy is an effective means of improving stroke prognosis, but there is a risk of severe hemorrhage (10). Studies have found that blood biomarkers (blood glucose, magnesium), inflammation levels, blood–brain barrier disruption, fibrinolysis/antifibrinolytic disorders, oxidative stress, etc., are associated with hemorrhagic transformation after AIS (11, 12). Several previous studies indicate that HT is connected with abnormalities in the coagulation and fibrinolysis systems (13). Fibrinogen is a clotting protein involved in primary and secondary hemostasis that plays a crucial role in platelet aggregation and building the fibrin network (13). Additionally, fibrinogen is an acute-phase protein that undergoes significant upregulation during systemic inflammation (14). Studies have reported a close relationship between fibrinogen levels and stroke prognosis. It has been reported that a good prognosis in patients with spontaneous brain hemorrhage during non-surgical treatment is associated with low levels of fibrinogen (15, 16). Hemorrhagic transformation is one of the serious complications of intravenous thrombolysis with tissue plasminogen activator (t-PA), and the vast majority of currently published studies have focused on reducing or preventing hemorrhagic transformation after thrombolysis (17, 18), and our team previously reported higher fibrinogen in HT patients who did not receive thrombolysis (19), However, there is currently limited research comparing fibrinogen with different types of HT (spontaneous hemorrhagic transformation and hemorrhagic transformation after mechanical thrombectomy).
The aim of our study is to investigate whether serum fibrinogen, an early serum biomarker, can aid in identifying stroke patients who might be at risk of HT and are appropriate for early thrombectomy, thereby enhancing clinical outcomes and guiding appropriate treatment.
This retrospective cohort study was conducted at the Stroke Center of the First Affiliated Hospital of Wenzhou Medical University, China, and included consecutive patients aged 18 years or older confirmed the diagnosis of HT following AIS between January 2012 and February 2023.
This study was approved by the Institutional Review Board and Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. Informed consent was waived since all data were anonymized and this was a retrospective study.
Upon admission, the diagnosis of first-ever AIS was confirmed using computed tomography (CT) or magnetic resonance imaging (MRI). The exclusion criteria were: (1) patients with hemorrhagic stroke or transient ischemic attacks (TIA); (2) patients with severe liver or kidney dysfunction; (3) patients failed to receive a repeat CT/MRI scan; (4) patients received intravenous thrombolytic therapy; (5) patients with incomplete medical data.
Finally, this study included 423 consecutive patients who were diagnosed with HT after AIS, comprising of 262 patients with spontaneously occurring HT (sHT) and 161 patients with HT following thrombectomy (tHT). For each cohort, an equal number of age- and sex-matched AIS patients without HT (Non-HT) were randomly selected as controls from the Stroke Center of our institution between January 2017 and February 2023. All patients met the inclusion criteria.
Demographic characteristics, including age and sex, were collected and data including the history of atrial fibrillation (AF), diabetes mellitus, hypertension, coronary heart disease (CHD), current cigarette smoking, and current drinking status were obtained to assess stroke risk.
Laboratory tests were conducted within 24 h of hospital admission under fasting conditions. Laboratory inspection, including erythrocyte count, leukocyte count, platelet (PLT) count, hemoglobin count, fasting blood glucose, prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT) and fibrinogen. Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria were used to classify the ischemic stroke subtypes (20). In addition, the use of anticoagulant and antiplatelet therapies for AIS during hospitalization before the onset of HT was recorded. The severity of stroke was evaluated within 24 h of admission by qualified neurologists using the National Institutes of Health Stroke Scale (NIHSS) score.
To maintain adequate statistical power within each category, all patients were divided into trisection based on the distribution of their baseline serum fibrinogen levels (21). This allowed for the examination of potential improvements in performance across the trisection.
Within 24 h of stroke onset, all patients underwent a brain CT scan or MRI, which included diffusion-weighted imaging (DWI) and T2-weighted gradient-echo. A subsequent CT/MRI was performed 7 ± 2 days after stroke onset or whenever the patient’s clinical condition worsened, in order to diagnose HT. Two neurologists, who were blinded to the clinical and laboratory measurements, independently evaluated the CT/MRI scans and diagnosed HT. HT was categorized radiologically according to the recommendations of the ECASS (22, 23): HI type 1 (small petechiae along the periphery of the infarct), HI type 2 (more confluent petechiae around the infarcted area without a space-occupying effect), PH type 1 (hematoma <30% of the infarcted area with a mild space-occupying effect), and PH type 2 (hematoma >30% of the infarcted area with a significant space-occupying effect).
The normality of the data distribution was tested using the Kolmogorov–Smirnov test. For continuous variables with normal distributions, the mean ± standard deviation was used, and for those with non-normal distributions, the median with interquartile range was used. Categorical variables were expressed as relative frequency and percentage. Student’s t-test or the Mann–Whitney U test was used to compare continuous variables, as appropriate. The chi-square test or Fisher’s exact test was used for categorical variables. One-way analysis of variance (ANOVA) or Kruskal–Wallis test was used to perform statistical comparisons of fibrinogen stratification for continuous variables, while Pearson’s chi-square test or Fisher’s exact test was used for categorical variables. To determine whether the fibrinogen stratification was an independent predictor of HT after AIS, a multivariate-adjusted conditional binary logistic regression was performed after adjusting for conventional confounding factors and significant variables (p < 0.1) identified in univariate conditional logistic regression analysis. A two-tailed p < 0.05 was taken to indicate statistical significance.
This study enrolled 846 stroke patients divided in two cohorts, including 322 with MT treatment and 524 without MT treatment (Figure 1).
Supplementary Table S1 shows the baseline characteristics and laboratory findings of AIS patients. The median age of the sHT cohort is 67(interquartile range, 59–74), with 373 males (71.2%), and the NIHSS score at discharge is 5 (1–10). The median age of the tHT cohort is even older, at 70 (interquartile range, 61–77, p < 0.001), with 222 males (68.9%), and a NIHSS score of 10 (4–19) at discharge. The tHT cohort has a higher history of AF, with 127 cases (39.4%), and a higher history of hyperlipidemia, with 187 cases (58.1%). The fibrinogen levels in the tHT cohort were lower than those in the sHT cohort (p = 0.002). The differences in the baseline characteristics of the AIS patients with and without HT in the two cohorts are shown in Table 1, patient with HT have higher mRS and NIHSS scores on both admission and discharge, higher RBC, WBC, CRP level than patients without HT. In sHT cohort, the level of fibrinogen of HT patients is higher than non-HT patients (3.76 versus 3.21 g/L, p < 0.001), while in tHT cohort, fibrinogen level in patients with HT is lower than non-HT patients (2.91 versus 3.10 g/L, p = 0.002). HT patients are more likely to receive anticoagulants and antiplatelets treatment in sHT cohort.
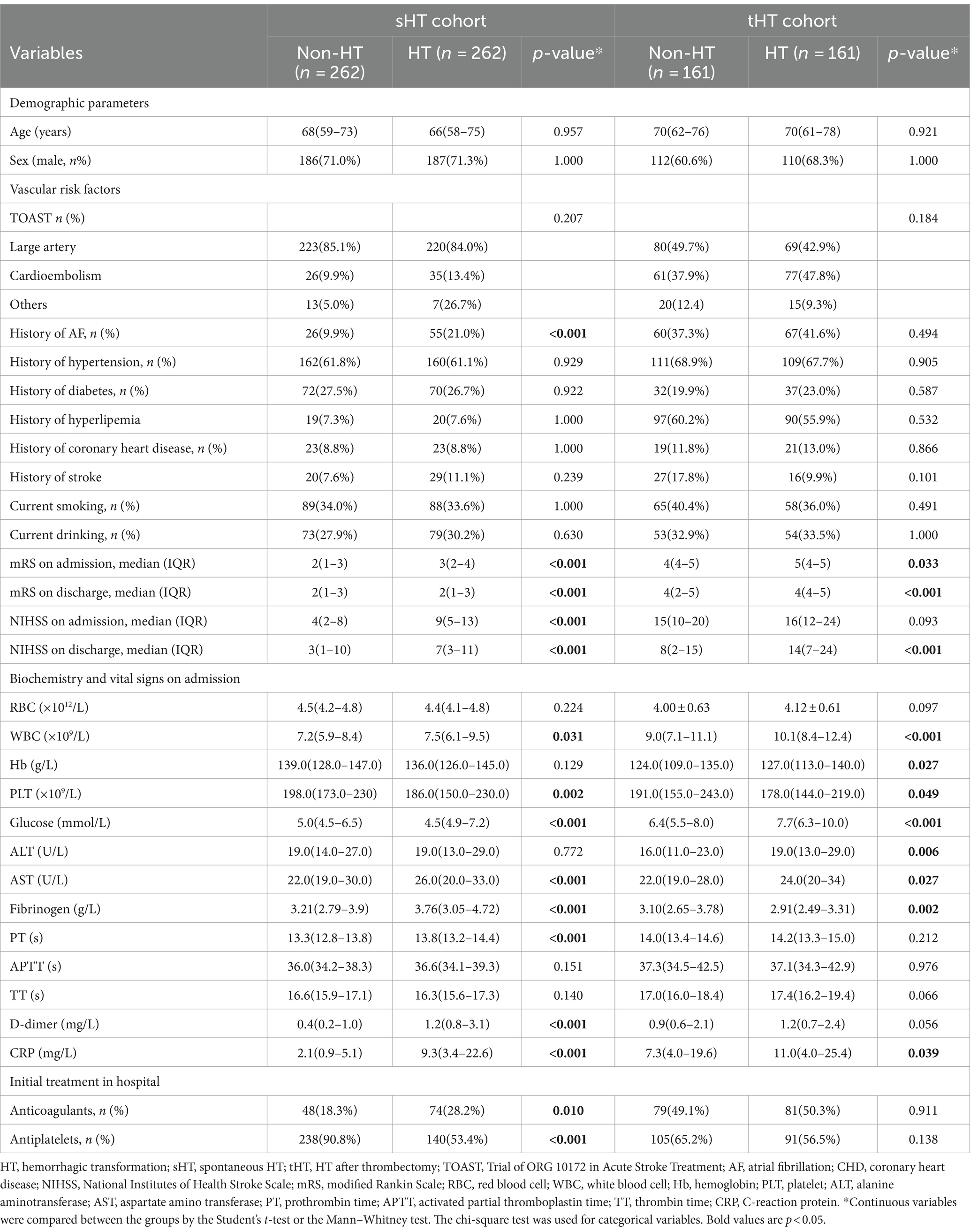
Table 1. Differences in the baseline characteristics of the AIS patients with and without HT in the two cohorts.
We divided two cohorts into three equal parts based on the distribution of fibrinogen (Supplementary Tables S2, S3): for sHT level range (0.95–1.00 μmol/L), T1 was 0.95–3.09 μmol/L, T2 was 3.10–3.95 μmol/L, and T3 was 3.96–10.00 μmol/L; for tHT level range (1.01–9.46 μmol/L), T1 was 1.01–2.71 μmol/L, T2 was 2.72–3.34 μmol/L, and T3 was 3.35–9.46 μmol/L. The demographic characteristics, vascular risk factors, laboratory test results, TOAST classification, and initial hospital treatment for the two cohorts of cases were presented using tables based on the fibrinogen trisection. The clotting time (TT) gradually decreased as fibrinogen gradually increased in both groups. A higher incidence of HT was associated with higher fibrinogen levels (p < 0.001) in the sHT cohort. In contrast, a higher incidence of HT was related to lower fibrinogen levels (p = 0.004) in the tHT cohort. The sHT cohort with high fibrinogen levels showed a higher prevalence of hyperlipidemia, platelets, white blood cell (WBC), C-reaction protein (CRP), and a lower usage rate of antiplatelet drugs compared to the subjects with low fibrinogen levels. Patients with higher fibrinogen level in the tHT cohort had a higher prevalence of hyperlipidemia, platelet count, globulin, CRP, in addition, lower PT when compared to those with lower fibrinogen levels.
According to the percentage analysis of fibrinogen level grouping in the subtypes of HT, in the sHT cohort (Figure 2A), non-HT patients with low Fibrinogen T1 had the highest proportion (44.1%), while the PH-1 and PH-2 had the highest proportion of T3, which was higher than that of HI-1 and HI-2. In the tHT cohort (Figure 2B), non-HT patients with high Fibrinogen T3 had the highest proportion (41.1%), and PH-2 had the highest proportion of T1 (51.2%). Fibrinogen level was directly proportional to the severity of HT in sHT (Figure 3A). However, in tHT cohort, fibrinogen level was inversely proportional to the severity of HT (Figure 3B). Taking HT as the dependent variable and fibrinogen lowest trisection (T1) as the reference, the adjusted multivariate logistic regression analysis results are shown in Table 2. According to the univariate analysis (Supplementary Table S4), the unadjusted OR value of fibrinogen T3 in sHT was 3.120 (95%CI: 2.170–4.489, p < 0.001), and the OR value of T3 in tHT was 0.419 (95%CI: 0.263–0.666, p = 0.002). AF was independently correlated in sHT with an OR value of 2.408 (95%CI: 1.580–3.670, p = 0.001), but not statistically significant in tHT. Glucose, WBC, PLT, discharge NIHSS, admission and discharge mRS, CRP were significantly correlated with HT in both cohorts. After adjusting for confounding factors such as age, sex, history (hypertension, hyperlipidemia, diabetes, etc.), and laboratory tests (such as WBC, PLT, APTT, glucose, etc.), as well as antiplatelet and anticoagulant drugs after admission, T3 in both cohorts remained significantly and independently associated with HT risk, T3: model1: OR = 1.676 (95%CI: 1.121–2.508, p < 0.001), model2: OR = 2.839 (95%CI: 1.887–4.271, p < 0.001), model3: OR = 2.515 (95%CI: 1.339–4.725, p = 0.002). tHT: model1: OR = 0.417 (95%CI: 0.263–0.665, p = 0.002), model2: OR: 0.424 (95%CI: 0.243–0.740, p = 0.011), model3: OR = 0.238 (95%CI: 0.108–0.523, p = 0.003). Additionally, after adjusting for confounding factors, fibrinogen T2 in sHT was also significantly and independently associated with HT risk, model2: OR = 1.676 (95%CI: 1.121–2.508, p = 0.035), model3: OR = 2.182 (95%CI, 1.207–3.945, p = 0.030).
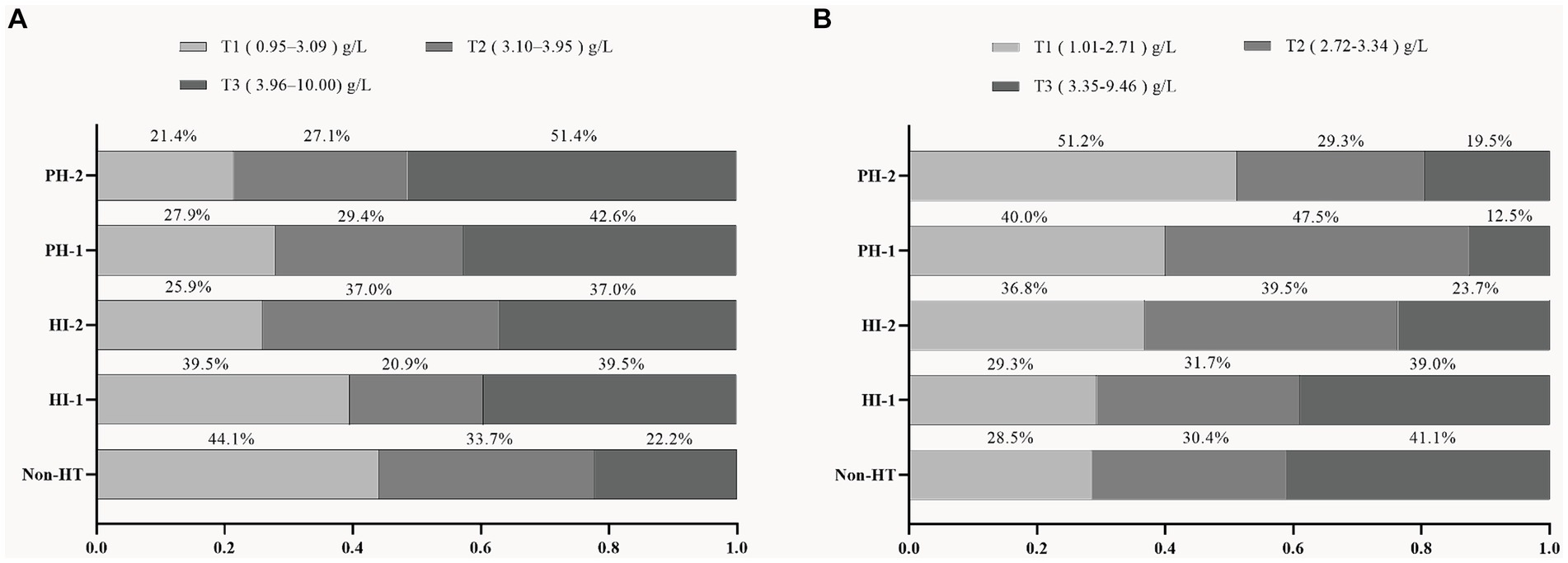
Figure 2. Proportion of patients in each fibrinogen trisection among AIS patients with different HT subtypes in the two cohorts. (A) sHT cohort, (B) tHT cohort. HT, hemorrhagic transformation; HI, hemorrhagic infarction; PH, parenchymal hematoma.
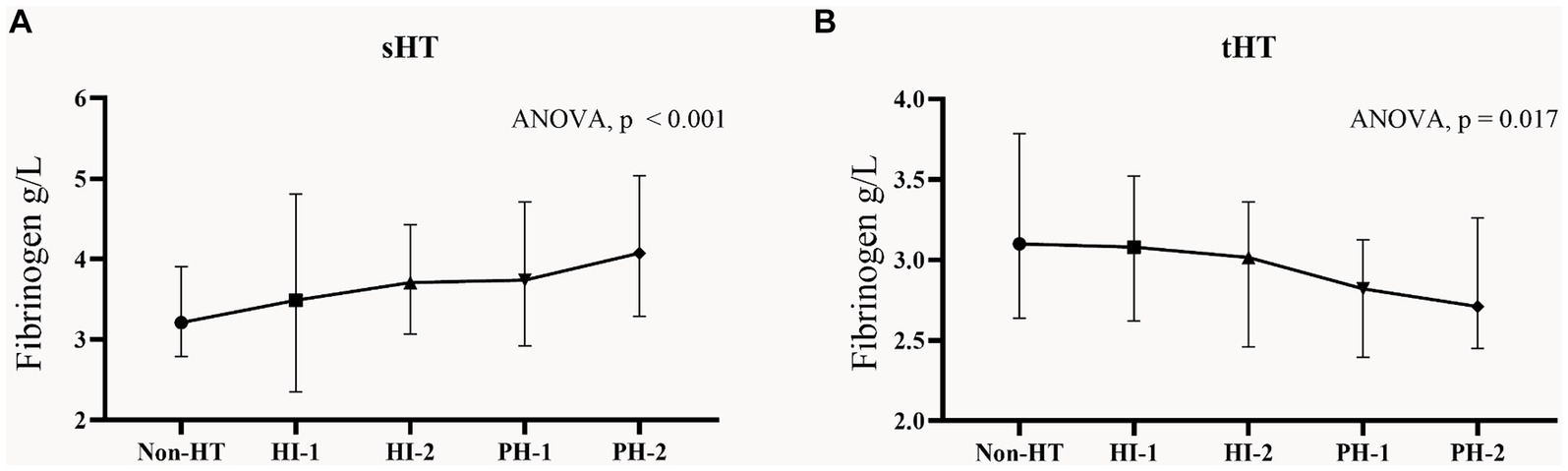
Figure 3. Fibrinogen concentrations in subgroups of HT in the two cohorts. Each data point and error bar correspond to the median and interquartile range of fibrinogen levels in the subgroups of HT. (A) sHT cohort, (B) tHT cohort. HT, hemorrhagic transformation; HI, hemorrhagic infarction; PH, parenchymal hematoma.
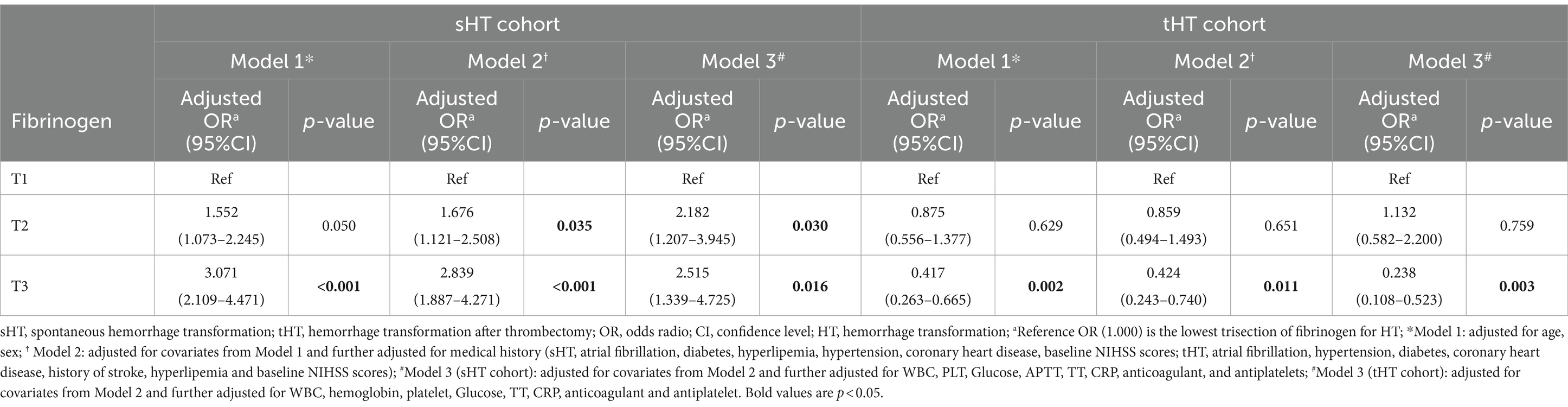
Table 2. Multivariate logistic regression analysis of the association between fibrinogen level and HT in the two cohorts.
We divided the two cohorts into two subgroups based on whether or not they had AF history (Supplementary Table S5). In the sHT cohort, HT patients without AF had higher levels of fibrinogen compared to non-HT (median 3.805 vs. 3.160, p < 0.001), while no difference was found between the two groups among patients with AF. The incidence of HT was higher in the cohort with a history of AF compared to the cohort without AF. In the tHT cohort, among patients without AF, those non-HT had higher levels of non-HT fibrinogen compared to those with HT (3.100 versus 2.935, p = 0.034), while in the AF group, non-HT fibrinogen remained higher than HT fibrinogen (3.040 versus 2.855, p = 0.024). Adjusted multivariate logistic regression analysis showed that in the AF-absence subgroup of sHT (Table 3), T2 and T3 were significantly and independently associated with HT risk after adjusting for confounding factors. T2: model1: OR = 1.662 (95%CI: 1.105–2.501, p = 0.041), model2: OR = 1.687 (95%CI: 1.089–2.611, p = 0.049). T3: model1: OR = 3.975 (95%CI: 2.614–6.044, p < 0.001), model2: OR = 3.479 (95%CI: 2.216–5.463, p < 0.001), model3: OR = 3.458 (95%CI: 2.010–5.947, p < 0.001). However, in the AF subgroup, after adjustment, any of the trisection of fibrinogen showed no significant correlation with HT risk. In tHT shown in Table 4, regardless of the presence of AF, the OR value of T3 after adjustment was significantly correlated with HT risk. In the AF-absence subgroup: T3: model1: OR = 0.446 (95%CI: 0.244–0.812, p = 0.027), model2: OR = 0.381 (95%CI: 0.184–0.786, p = 0.029), model3: OR = 0.195 (95%CI: 0.066–0.578, p = 0.013). In the AF-presence subgroup: model1: OR = 0.327 (95%CI: 0.122–0.877, p = 0.027), model3: OR = 0.161 (95%CI: 0.036–0.714, p = 0.044).
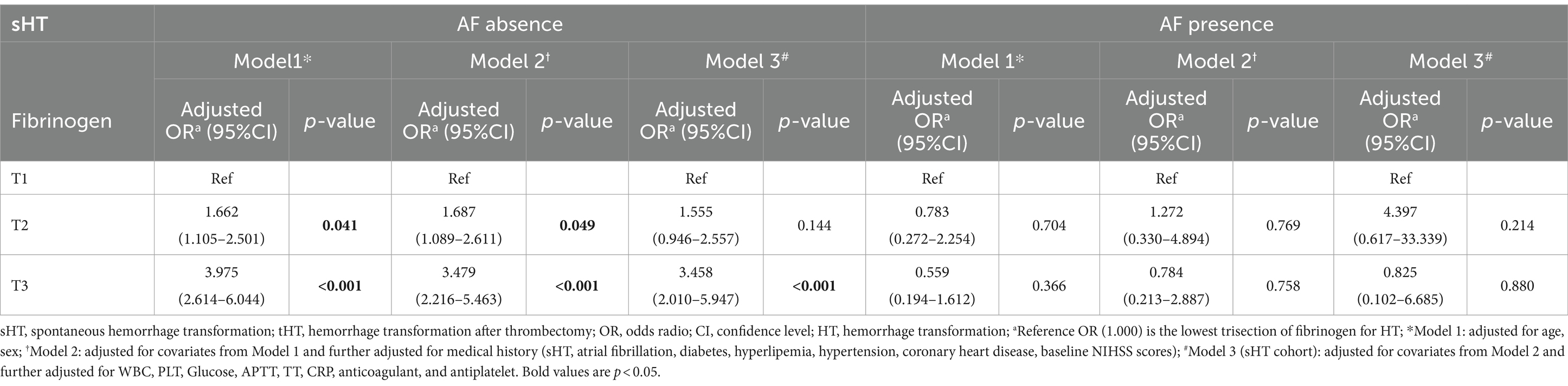
Table 3. Multivariate logistic regression analysis of the association between fibrinogen and HT with and without AF in sHT.
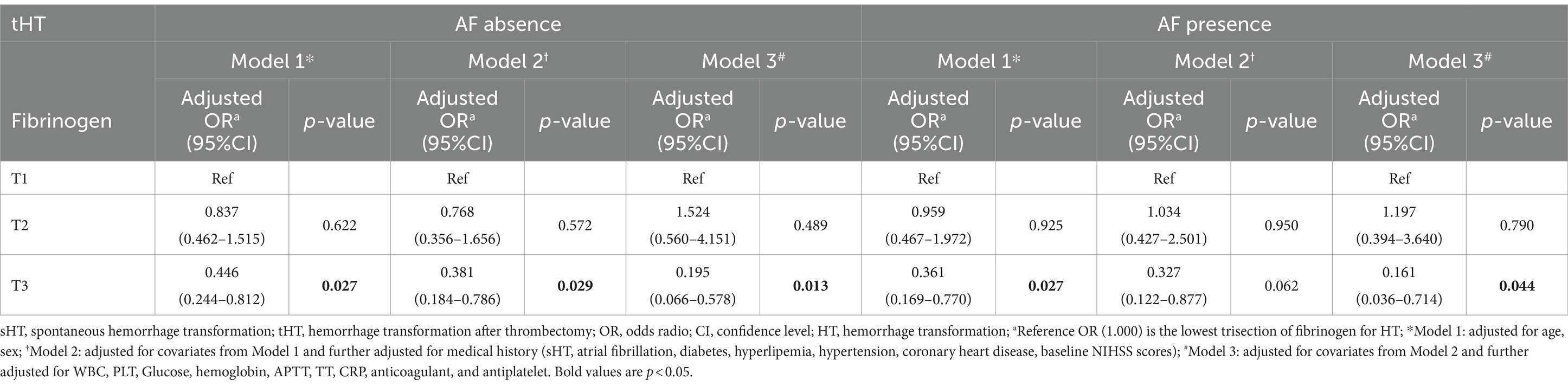
Table 4. Multivariate logistic regression analysis of the association between fibrinogen and HT with and without AF in tHT.
The study of two cohorts revealed that fibrinogen levels after admission were significantly correlated with the development of HT. The sHT cohort demonstrated a positive correlation between higher fibrinogen levels and more severe HT. Conversely, lower fibrinogen levels correlated positively with more severe HT in the tHT cohort. Specifically, the presence of AF was shown to have a significant impact on the development of HT in the sHT cohort.
Fibrinogen, a soluble 340 kDa glycoprotein, is synthesized by hepatocytes in the liver. Under normal physiological conditions, the plasma fibrinogen concentration typically ranges between 2 and 4 g/L (24). Our study is the first to compare the relationship between fibrinogen and HT in two different cohorts. We identified a positive correlation between fibrinogen levels and HT severity in sHT patients. Conversely, we observed a negative correlation between fibrinogen levels and HT severity in tHT patients, indicating distinct pathogenesis between the two cohorts.
Previous clinical studies have found that high fibrinogen is associated with the development of HT in stroke patients who have not been thrombolytic (25, 26). Delayed time to fibrinogen-to-fibrin conversion, as indicated by prolonged TT, is independently and negatively associated with spontaneous HT (27). Fibrinogen is a crucial component of the coagulation system and is linked with vasculitis disorders (28). In the presence of an intact blood–brain barrier, the central nervous system cannot detect fibrinogen. Following an acute ischemic stroke (AIS), there is a breakdown in blood–brain barrier (BBB) formation and increased permeability during reperfusion, resulting in the infiltration of fibrinogen into the central nervous system. After AIS, vascular endothelial cells are damaged, resulting in damage to the blood–brain barrier (BBB) and increased permeability during reperfusion, resulting in fibrinogen infiltration and deposition in the central nervous system (29, 30). The increased permeability during reperfusion leads to the infiltration of fibrinogen into the central nervous system. This subsequently results in the conversion of fibrinogen into fibrin, which has the potential to bind to the CD11b/CD18 integrin receptor and induce microglia activation (30). Fibrinogen can also promote the entry of inflammatory monocytes/macrophages, monocytes synthesize thromboinflammatory proteins via de novo synthesis (31). This mechanism has been studied in the context of multiple sclerosis (32). Moreover, fibrinogen can activate matrix metalloproteinase-9 (MMP-9) expression, which is recognized as a pro-inflammatory factor in the context of neurovascular unit injury and blood–brain barrier breakdown (33). Higher fibrinogen delays the constriction of thrombotic clots, worsens vascular occlusion, and leads to increased levels of inflammation (34). At the same time, elevated levels of inflammation biomarkers such as IL-1, IL-6, CRP, TNF-α, and blood clots contribute to the activation of inflammatory pathways (35–38). In our study, CRP levels were higher in the sHT cohort with high fibrinogen levels (T3) (11.4, 95% CI 4.2–27.5). Therefore, ischemia/reperfusion injury to tissues can contribute to blood–brain barrier dysfunction, ultimately leading to hemorrhagic transformation (39, 40).
In the sHT cohort, we found that patients who developed HT had higher levels of hyperfibrinogen, which was consistent with previous findings (41). However, it has been reported in the literature of some rt-PA treatments that low fibrinogen <1.5 g/L is associated with a high incidence of HT (42), which may be due to the significant disruption of the fibrinolytic system caused by rt-PA, and the reduction of fibrinogen in both non-HT and HT patients compared with baseline after thrombolytic therapy. Ye et al. (27) found no significant difference in fibrinogen and HT correlation, but in their study, a higher proportion of patients with a history of atrial fibrillation were higher among non-HT patients, which is consistent with our findings. Studies have shown a higher incidence of AF in the HT group than in the non-HT group, and cardioembolic strokes can be used to predict early HT (23, 43), which is consistent with our study. Our study excluded confounding factors, such as anticoagulant and antiplatelet therapy, which demonstrated that fibrinogen and HT are still independently associated. Previous reports have shown the high safety profile of anticoagulant or antiplatelet therapy for MT (44–46). Cardioembolic stroke is linked to fibrin-rich white thrombi, while non-cardioembolic cases (atherosclerotic thrombi and cryptogenic thrombi) are linked to erythrocyte-rich thrombi. Cardioembolism shows a higher ratio of fibrin to platelets than non-cardioembolism (47–49). Erythrocyte-rich thrombi can induce oxidative stress (50) through increased hemoglobin, resulting in dysregulation of heme/iron metabolism. Previous studies have found that AF is associated with more severe baseline hypoperfusion (51), leading to more severe HT (52, 53). Moreover, we found that hypertriglyceridemia was more common in patients without AF than in those with AF. Swarowska et al. (54) also concluded that persistent fibrinogen elevation in the AF group after hospitalization was associated with high triglycerides, while high triglycerides were related to systemic inflammation. But the relationship between increased fibrinogen and increased triglycerides after AIS still requires further investigation. Above findings suggest that we found no significant difference in fibrinogen levels in patients in the AF group in the non-HT and HT group. Therefore, we speculate that the HT in the subgroups of AF may be influenced by AF itself, and the predictive ability of fibrinogen in this group is influenced by AF, so that the correlation between fibrinogen and HT only suitable in sHT patients without AF. As a result, when treating stroke patients with AF, medical professionals should closely monitor their fibrinogen levels to guide clinical treatment and health management. Also, it’s worth noting that in the sHT group, 28.2% of patients received anticoagulant therapy for the first time after stroke, which was associated with 21% of patients with a history of atrial fibrillation. We found that anticoagulation therapy was a risk factor for HT (OR = 1.753, 95CI: 1.240–2.478, p = 0.008), while antiplatelet therapy was a protective factor for HT (OR = 0.116, 95CI: 0.077–0.175, p < 0.001), and we hypothesized that patients with antiplatelet therapy had a lower proportion of cardioembolism (26), while thrombin was a key factor in the conversion of soluble fibrinogen to insoluble fibrin, which would be elevated when anticoagulation was taken.
In patients with tHT, studies have found a decrease in fibrinogen levels after thrombectomy, and lower fibrinogen levels predict a higher risk of HT (13, 55). The mechanism of fibrinogen production may be attributed to surgical procedures that lead to the accumulation of fibrinogen in blood vessels, forming a thrombus. Additionally, physical instruments used to stretch and compress blood vessels may cause vascular endothelium damage (56) or vessel occlusion (57), initiating a series of reactions, such as platelet activation, coagulation factor release, and vasoconstriction (28, 58). These reactions increase the risk of fibrinogen buildup on the blood vessel walls, forming a stable thrombus that is subsequently depleted (59). Hemodilution during surgery may cause a decrease in fibrinogen (60). Besides, anticoagulant treatment may be applied during thrombectomy to prevent thrombosis, which can also impact fibrinogen synthesis and release. Studies on fibrinogen in patients undergoing thrombolysis have shown that fibrinogen depletion after AIS might lead to potential hemorrhagic infarcts converting into PH or an increase in the volume of PH, ultimately increasing the incidence of HT (61, 62). During cardiac surgery, postoperative fibrinogen-fibrin products are elevated (63) due to intraoperative extracorporeal circulation hemodilution and clotting factor depletion (64), postoperative fibrinogen levels are an independent risk factor for excessive hemorrhage, and decreased fibrinogen levels increase the risk of postoperative hemorrhage (59, 65, 66). Suitable levels of fibrinogen are necessary to facilitate effective thrombosis and prevent HT consequences.
The highlight of the study is that for the first time, we found that fibrinogen levels in patients with atrial fibrillation were positively correlated with the development of HT in the sHT group, but showed an inverse relationship in the tHT cohort, which has not been demonstrated in other studies. These findings suggest that fibrinogen levels on admission may help clinicians identify patients at increased risk of HT, and that for patients not receiving reperfusion therapy, timely detection and intervention of high fibrinogen levels can reduce the risk of HT development, while for patients undergoing MT surgery, low fibrinogen levels should be avoided.
Our study has some limitations. Firstly, it was a retrospective, single-center study, we could not establish the exact causality, and fibrinogen levels were only measured only once after admission, and future studies need to investigate the fibrinogen dynamics during hospitalization, fibrinogen may be measured at different concentrations at different times of detection (67), and Lip et al. (68) found that fibrinogen peaked 1 week after stroke, but Tamam et al. (69) found that h-CRP and fibrinogen reached their highest values on day 3 after stroke. Secondly, baseline imaging data, such as infarct area and site, were not included, even though they potentially affect the occurrence and development of hemorrhagic transformation. Thirdly, patients who underwent thrombolysis-alone and bridging treatments were excluded, and the comparison of fibrinogen levels with HT in these two cohorts needs further exploration. In addition, we did not include inflammatory factors such as IL-1 and IL-6, and analyzed the relationship between fibrinogen levels and them. Although we adjusted for confounders, we had to admit that gender, age, diabetes, hypertension, lipids, and initial stroke severity were all associated with HT (5, 33). In MT surgery, the occurrence and severity of HT are also related to factors such as surgical time (70), the method of clot retrieval (71), thrombectomy passes and others. Thrombosis in fibrin-rich tissues has been found to be associated with a higher number of recanalization operations and a longer recanalization time required during surgery (48, 72).
In conclusion, for sHT patients with non-AF, high fibrinogen levels are associated with the incidence of HT, while in tHT patients, there is a correlation between low fibrinogen and the occurrence of HT.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study involving humans was approved by The Institutional Review Board and Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. Informed consent was waived since all data were anonymized and this was a retrospective study.
JL: Data curation, Writing – original draft. JC: Data curation, Software, Writing – review & editing. GH: Conceptualization, Writing – review & editing. ZC: Data curation, Writing – review & editing. HZ: Data curation, Writing – review & editing. YZ: Data curation, Writing – review & editing. QD: Data curation, Writing – review & editing. BW: Data curation, Writing – review & editing. JH: Investigation, Project administration, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by grants from the Projects of National Natural Science Foundation of China (No. 81873799) and the Wenzhou Municipal Sci-Tech Bureau Program (Y20210166).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1364875/full#supplementary-material
1. Hong, JM, Kim, DS, and Kim, M. Hemorrhagic transformation after ischemic stroke: mechanisms and management. Front Neurol. (2021) 12:703258. doi: 10.3389/fneur.2021.703258
2. Alvarez-Sabin, J, Maisterra, O, Santamarina, E, and Kase, CS. Factors influencing haemorrhagic transformation in ischaemic stroke. Lancet Neurol. (2013) 12:689–705. doi: 10.1016/S1474-4422(13)70055-3
3. Krishnamurthi, RV, Feigin, VL, Forouzanfar, MH, Mensah, GA, Connor, M, Bennett, DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the global burden of disease study 2010. Lancet Glob Health. (2013) 1:e259–81. doi: 10.1016/S2214-109X(13)70089-5
4. Shen, AY, Yao, JF, Brar, SS, Jorgensen, MB, and Chen, W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. (2007) 50:309–15. doi: 10.1016/j.jacc.2007.01.098
5. Dong, S, Yu, C, Wu, Q, Xia, H, Xu, J, Gong, K, et al. Predictors of symptomatic intracranial Hemorrhage after endovascular thrombectomy in acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis. (2022) 52:363–375. doi: 10.1159/000527193
6. Hacke, W, Kaste, M, Fieschi, C, Toni, D, Lesaffre, E, Von Kummer, R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European cooperative acute stroke study (ECASS). JAMA. (1995) 274:1017–25. doi: 10.1001/jama.1995.03530130023023
7. D'amelio, M, Terruso, V, Famoso, G, Di Benedetto, N, Realmuto, S, Valentino, F, et al. Early and late mortality of spontaneous hemorrhagic transformation of ischemic stroke. J Stroke Cerebrovasc Dis. (2014) 23:649–54. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.005
8. Rao, NM, Levine, SR, Gornbein, JA, and Saver, JL. Defining clinically relevant cerebral hemorrhage after thrombolytic therapy for stroke: analysis of the National Institute of Neurological Disorders and Stroke tissue-type plasminogen activator trials. Stroke. (2014) 45:2728–33. doi: 10.1161/STROKEAHA.114.005135
10. Powers, WJ, Rabinstein, AA, Ackerson, T, Adeoye, OM, Bambakidis, NC, Becker, K, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
11. Sun, A, Cao, Y, Jia, Z, Zhao, L, Shi, H, and Liu, S. Analysis of influencing factors of hemorrhagic transformation in patients with large vessel occlusion stroke after mechanical thrombectomy. Am J Transl Res. (2023) 15:6304–13.
12. Thomas, SE, Plumber, N, Venkatapathappa, P, and Gorantla, V. A review of risk factors and predictors for Hemorrhagic transformation in patients with acute ischemic stroke. Int J Vasc Med. (2021) 2021:4244267. doi: 10.1155/2021/4244267
13. Lin, C, Pan, H, Qiao, Y, Huang, P, Su, J, and Liu, J. Fibrinogen level combined with platelet count for predicting Hemorrhagic transformation in acute ischemic stroke patients treated with mechanical thrombectomy. Front Neurol. (2021) 12:716020. doi: 10.3389/fneur.2021.716020
14. Fenger-Eriksen, C, Ingerslev, J, and Sorensen, B. Fibrinogen concentrate – a potential universal hemostatic agent. Expert Opin Biol Ther. (2009) 9:1325–33. doi: 10.1517/14712590903193051
15. Castellanos, M, Leira, R, Tejada, J, Gil-Peralta, A, Davalos, A, Castillo, J, et al. Predictors of good outcome in medium to large spontaneous supratentorial intracerebral haemorrhages. J Neurol Neurosurg Psychiatry. (2005) 76:691–5. doi: 10.1136/jnnp.2004.044347
16. Peycheva, M, Deneva, T, and Zahariev, Z. The role of fibrinogen in acute ischaemic stroke. Neurol Neurochir Pol. (2021) 55:74–80. doi: 10.5603/PJNNS.a2020.0094
17. Gallego-Fabrega, C, Temprano-Sagrera, G, Cárcel-Márquez, J, Muiño, E, Cullell, N, Lledós, M, et al. A multitrait genetic study of hemostatic factors and hemorrhagic transformation after stroke treatment. J Thromb Haemost. (2024) 22:936–50. doi: 10.1016/j.jtha.2023.11.027
18. Huang, P, and Yi, XY. Predictive role of admission serum glucose, baseline NIHSS score, and fibrinogen on hemorrhagic transformation after intravenous thrombolysis with alteplase in acute ischemic stroke. Eur Rev Med Pharmacol Sci. (2023) 27:9710–20. doi: 10.26355/eurrev_202310_34141
19. Huang, GQ, Zeng, YY, Cheng, QQ, Cheng, HR, Ruan, YT, Yuan, CX, et al. Low triiodothyronine syndrome is associated with hemorrhagic transformation in patients with acute ischaemic stroke. Aging. (2019) 11:6385–97. doi: 10.18632/aging.102195
20. Adams, HP, Bendixen, BH, Kappelle, LJ, Biller, J, Love, BB, Gordon, DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
21. Di Napoli, M, Papa, F, and Bocola, V. Prognostic influence of increased C-reactive protein and fibrinogen levels in ischemic stroke. Stroke. (2001) 32:133–8. doi: 10.1161/01.STR.32.1.133
22. Hacke, W, Kaste, M, Fieschi, C, Von Kummer, R, Davalos, A, Meier, D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian acute stroke study Investigators. Lancet. (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
23. Paciaroni, M, Agnelli, G, Corea, F, Ageno, W, Alberti, A, Lanari, A, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke. (2008) 39:2249–56. doi: 10.1161/STROKEAHA.107.510321
24. Tennent, GA, Brennan, SO, Stangou, AJ, O'Grady, J, Hawkins, PN, and Pepys, MB. Human plasma fibrinogen is synthesized in the liver. Blood. (2007) 109:1971–4. doi: 10.1182/blood-2006-08-040956
25. Kochetov, AG, Karpova, OV, Arkhipkin, AA, Novozhenova, IV, Shamalov, NA, Ramazanov, GR, et al. The prognostic significance of fibrinogen concentration in patients with ischemic stroke without thrombolytic treatment. Zh Nevrol Psikhiatr Im S S Korsakova. (2010) 110:46–51.
26. Ruan, Y, Yuan, C, Liu, Y, Zeng, Y, Cheng, H, Cheng, Q, et al. High fibrinogen-to-albumin ratio is associated with hemorrhagic transformation in acute ischemic stroke patients. Brain Behav. (2020) 11:e01855. doi: 10.1002/brb3.1855
27. Ye, C, Wang, Y, Song, Q, Liu, J, Wei, C, and Liu, M. Association between coagulation function and spontaneous Hemorrhagic transformation in acute ischemic stroke. Curr Neurovasc Res. (2020) 17:344–53. doi: 10.2174/1567202617666200514114258
28. Davalos, D, and Akassoglou, K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. (2012) 34:43–62. doi: 10.1007/s00281-011-0290-8
29. Di Biase, L, Bonura, A, Pecoraro, PM, Carbone, SP, and Di Lazzaro, V. Unlocking the potential of stroke blood biomarkers: early diagnosis, ischemic vs. haemorrhagic differentiation and haemorrhagic transformation risk: a comprehensive review. Int J Mol Sci. (2023) 24:24. doi: 10.3390/ijms241411545
30. Petersen, MA, Ryu, JK, and Akassoglou, K. Fibrinogen in neurological diseases: mechanisms, imaging and therapeutics. Nat Rev Neurosci. (2018) 19:283–301. doi: 10.1038/nrn.2018.13
31. Campbell, RA, Vieira-De-Abreu, A, Rowley, JW, Franks, ZG, Manne, BK, Rondina, MT, et al. Clots are potent triggers of inflammatory cell gene expression: indications for timely fibrinolysis. Arterioscler Thromb Vasc Biol. (2017) 37:1819–27. doi: 10.1161/ATVBAHA.117.309794
32. Davalos, D, Ryu, JK, Merlini, M, Baeten, KM, Le Moan, N, Petersen, MA, et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. (2012) 3:1227. doi: 10.1038/ncomms2230
33. Jickling, GC, Liu, D, Stamova, B, Ander, BP, Zhan, X, Lu, A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. J Cereb Blood Flow Metab. (2014) 34:185–99. doi: 10.1038/jcbfm.2013.203
34. Tutwiler, V, Peshkova, AD, Andrianova, IA, Khasanova, DR, Weisel, JW, and Litvinov, RI. Contraction of blood clots is impaired in acute ischemic stroke. Arterioscler Thromb Vasc Biol. (2017) 37:271–9. doi: 10.1161/ATVBAHA.116.308622
35. Mackman, N . The many faces of tissue factor. J Thromb Haemost. (2009) 7:136–9. doi: 10.1111/j.1538-7836.2009.03368.x
36. Perez, RL, and Roman, J. Fibrin enhances the expression of Il-1 beta by human peripheral blood mononuclear cells. Implications in pulmonary inflammation. J Immunol. (1995) 154:1879–87.
37. Sower, LE, Froelich, CJ, Carney, DH, Fenton, JW, and Klimpel, GR. Thrombin induces IL-6 production in fibroblasts and epithelial cells. Evidence for the involvement of the seven-transmembrane domain (STD) receptor for alpha-thrombin. J Immunol. (1995) 155:895–901. doi: 10.4049/jimmunol.155.2.895
38. Welsh, P, Lowe, GD, Chalmers, J, Campbell, DJ, Rumley, A, Neal, BC, et al. Associations of proinflammatory cytokines with the risk of recurrent stroke. Stroke. (2008) 39:2226–30. doi: 10.1161/STROKEAHA.107.504498
39. Ninomia, T, Wang, L, Kumar, SR, Kim, A, and Zlokovic, BV. Brain injury and cerebrovascular fibrin deposition correlate with reduced antithrombotic brain capillary functions in a hypertensive stroke model. J Cereb Blood Flow Metab. (2000) 20:998–1009. doi: 10.1097/00004647-200006000-00012
40. Wang, X, and Lo, EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. (2003) 28:229–44. doi: 10.1385/MN:28:3:229
41. Cheng, HR, Chen, YB, Zeng, YY, Ruan, YT, Yuan, CX, Cheng, QQ, et al. Hemostasis functions are associated with hemorrhagic transformation in non-atrial fibrillation patients: a case-control study. BMC Neurol. (2021) 21:36. doi: 10.1186/s12883-021-02065-3
42. Wang, R, Zeng, J, Wang, F, Zhuang, X, Chen, X, and Miao, J. Risk factors of hemorrhagic transformation after intravenous thrombolysis with rt-pa in acute cerebral infarction. QJM. (2019) 112:323–6. doi: 10.1093/qjmed/hcy292
43. Chen, X, Wang, Y, Fu, M, Lei, H, Cheng, Q, and Zhang, X. Plasma immunoproteasome predicts early Hemorrhagic transformation in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. (2017) 26:49–56. doi: 10.1016/j.jstrokecerebrovasdis.2016.08.027
44. Černík, D, Šaňák, D, Divišová, P, Köcher, M, Cihlář, F, Zapletalová, J, et al. Mechanical thrombectomy in patients with acute ischemic stroke on anticoagulation therapy. Cardiovasc Intervent Radiol. (2018) 41:706–11. doi: 10.1007/s00270-018-1902-7
45. Kingma, JG . Effect of platelet GPIIb/IIIa receptor blockade with MK383 on infarct size and myocardial blood flow in a canine Reocclusion model. J Cardiovasc Pharmacol Ther. (2019) 24:182–92. doi: 10.1177/1074248418808389
46. Wong, JWP, Churilov, L, Dowling, R, Mitchell, P, Bush, S, Kanesan, L, et al. Safety of endovascular thrombectomy for acute ischaemic stroke in anticoagulated patients ineligible for intravenous thrombolysis. Cerebrovasc Dis. (2018) 46:193–9. doi: 10.1159/000493801
47. Alkarithi, G, Duval, C, Shi, Y, Macrae, FL, and Ariens, RAS. Thrombus structural composition in cardiovascular disease. Arterioscler Thromb Vasc Biol. (2021) 41:2370–83. doi: 10.1161/ATVBAHA.120.315754
48. Maekawa, K, Shibata, M, Nakajima, H, Mizutani, A, Kitano, Y, Seguchi, M, et al. Erythrocyte-rich thrombus is associated with reduced number of Maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra. (2018) 8:39–49. doi: 10.1159/000486042
49. Sporns, PB, Hanning, U, Schwindt, W, Velasco, A, Minnerup, J, Zoubi, T, et al. Ischemic stroke: what does the histological composition tell us about the origin of the thrombus? Stroke. (2017) 48:2206–10. doi: 10.1161/STROKEAHA.117.016590
50. Cubedo, J, Blasco, A, Padro, T, Ramaiola, I, Juan-Babot, O, Goicolea, J, et al. Molecular signature of coronary stent thrombosis: oxidative stress and innate immunity cells. Thromb Haemost. (2017) 117:1816–27. doi: 10.1160/TH17-03-069
51. Tu, HT, Campbell, BC, Christensen, S, Desmond, PM, De Silva, DA, Parsons, MW, et al. Worse stroke outcome in atrial fibrillation is explained by more severe hypoperfusion, infarct growth, and hemorrhagic transformation. Int J Stroke. (2015) 10:534–40. doi: 10.1111/ijs.12007
52. Campbell, BC, Christensen, S, Butcher, KS, Gordon, I, Parsons, MW, Desmond, PM, et al. Regional very low cerebral blood volume predicts hemorrhagic transformation better than diffusion-weighted imaging volume and thresholded apparent diffusion coefficient in acute ischemic stroke. Stroke. (2010) 41:82–8. doi: 10.1161/STROKEAHA.109.562116
53. Kim, JH, Bang, OY, Liebeskind, DS, Ovbiagele, B, Kim, GM, Chung, CS, et al. Impact of baseline tissue status (diffusion-weighted imaging lesion) versus perfusion status (severity of hypoperfusion) on hemorrhagic transformation. Stroke. (2010) 41:e135–42. doi: 10.1161/STROKEAHA.109.563122
54. Swarowska, M, Janowska, A, Polczak, A, Klimkowicz-Mrowiec, A, Pera, J, Slowik, A, et al. The sustained increase of plasma fibrinogen during ischemic stroke predicts worse outcome independently of baseline fibrinogen level. Inflammation. (2014) 37:1142–7. doi: 10.1007/s10753-014-9838-9
55. Yang, X, Jia, X, Ren, H, and Zhang, H. The short-and long-term efficacies of endovascular interventions for the treatment of acute ischemic stroke patients. Am J Transl Res. (2021) 13:5436–43.
56. Nogueira, RG, Levy, EI, Gounis, M, and Siddiqui, AH. The Trevo device: preclinical data of a novel stroke thrombectomy device in two different animal models of arterial thrombo-occlusive disease. J Neurointerv Surg. (2012) 4:295–300. doi: 10.1136/neurintsurg-2011-010053
57. Singh, P, Doostkam, S, Reinhard, M, Ivanovas, V, and Taschner, CA. Immunohistochemical analysis of thrombi retrieved during treatment of acute ischemic stroke: does stent-retriever cause intimal damage? Stroke. (2013) 44:1720–2. doi: 10.1161/STROKEAHA.113.000964
58. Krishnan, R, Mays, W, and Elijovich, L. Complications of mechanical thrombectomy in acute ischemic stroke. Neurology. (2021) 97:S115–25. doi: 10.1212/WNL.0000000000012803
59. Pikija, S, Trkulja, V, Mutzenbach, JS, Mccoy, MR, Ganger, P, and Sellner, J. Fibrinogen consumption is related to intracranial clot burden in acute ischemic stroke: a retrospective hyperdense artery study. J Transl Med. (2016) 14:250. doi: 10.1186/s12967-016-1006-6
60. Ng, KF, Lam, CC, and Chan, LC. In vivo effect of haemodilution with saline on coagulation: a randomized controlled trial. Br J Anaesth. (2002) 88:475–80. doi: 10.1093/bja/88.4.475
61. Matosevic, B, Knoflach, M, Werner, P, Pechlaner, R, Zangerle, A, Ruecker, M, et al. Fibrinogen degradation coagulopathy and bleeding complications after stroke thrombolysis. Neurology. (2013) 80:1216–24. doi: 10.1212/WNL.0b013e3182897015
62. Yan, S, Zhang, X, Zhang, R, Xu, J, and Lou, M. Early fibrinogen depletion and symptomatic intracranial Hemorrhage after reperfusion therapy. Stroke. (2019) 50:2716–21. doi: 10.1161/STROKEAHA.119.025711
63. Hall, TS, Sines, JC, and Spotnitz, AJ. Hemorrhage related reexploration following open heart surgery: the impact of pre-operative and post-operative coagulation testing. Cardiovasc Surg. (2002) 10:146–53. doi: 10.1177/096721090201000210
64. Kindo, M, Hoang Minh, T, Gerelli, S, Perrier, S, Meyer, N, Schaeffer, M, et al. Plasma fibrinogen level on admission to the intensive care unit is a powerful predictor of postoperative bleeding after cardiac surgery with cardiopulmonary bypass. Thromb Res. (2014) 134:360–8. doi: 10.1016/j.thromres.2014.05.008
65. Vandelli, L, Marietta, M, Gambini, M, Cavazzuti, M, Trenti, T, Cenci, MA, et al. Fibrinogen decrease after intravenous thrombolysis in ischemic stroke patients is a risk factor for intracerebral hemorrhage. J Stroke Cerebrovasc Dis. (2015) 24:394–400. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.005
66. Walden, K, Jeppsson, A, Nasic, S, Backlund, E, and Karlsson, M. Low preoperative fibrinogen plasma concentration is associated with excessive bleeding after cardiac operations. Ann Thorac Surg. (2014) 97:1199–206. doi: 10.1016/j.athoracsur.2013.11.064
67. Marquardt, L, Ruf, A, Mansmann, U, Winter, R, Buggle, F, Kallenberg, K, et al. Inflammatory response after acute ischemic stroke. J Neurol Sci. (2005) 236:65–71. doi: 10.1016/j.jns.2005.05.006
68. Lip, GY, Blann, AD, Farooqi, IS, Zarifis, J, Sagar, G, and Beevers, DG. Sequential alterations in haemorheology, endothelial dysfunction, platelet activation and thrombogenesis in relation to prognosis following acute stroke: the West Birmingham stroke project. Blood Coagul Fibrinolysis. (2002) 13:339–47. doi: 10.1097/00001721-200206000-00010
69. Tamam, Y, Iltumur, K, and Apak, I. Assessment of acute phase proteins in acute ischemic stroke. Tohoku J Exp Med. (2005) 206:91–8. doi: 10.1620/tjem.206.91
70. Alawieh, A, Pierce, AK, Vargas, J, Turk, AS, Turner, RD, Chaudry, MI, et al. The golden 35 min of stroke intervention with adapt: effect of thrombectomy procedural time in acute ischemic stroke on outcome. J Neurointerv Surg. (2018) 10:213–20. doi: 10.1136/neurintsurg-2017-013040
71. Saver, JL, Chapot, R, Agid, R, Hassan, A, Jadhav, AP, Liebeskind, DS, et al. Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke. (2020) 51:2872–84. doi: 10.1161/STROKEAHA.120.028956
Keywords: hemorrhagic transformation, acute ischemic stroke, fibrinogen, mechanical thrombectomy, stroke
Citation: Long J, Chen J, Huang G, Chen Z, Zhang H, Zhang Y, Duan Q, Wu B and He J (2024) The differences of fibrinogen levels in various types of hemorrhagic transformations. Front. Neurol. 15:1364875. doi: 10.3389/fneur.2024.1364875
Received: 14 February 2024; Accepted: 11 July 2024;
Published: 25 July 2024.
Edited by:
Jee-Yeon Hwang, Creighton University, United StatesReviewed by:
Yagiz Meric Altun, Albert Einstein College of Medicine, United StatesCopyright © 2024 Long, Chen, Huang, Chen, Zhang, Zhang, Duan, Wu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jincai He, aGpjQHdtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.