- 1Psychiatry Neuroimaging Laboratory, Department of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Somerville, MA, United States
- 2cBRAIN, Department of Child and Adolescent Psychiatry, Psychosomatics, and Psychotherapy, Ludwig-Maximilians-University, Munich, Germany
- 3Department of Psychiatry and Psychotherapy, Jena University Hospital, Jena, Germany
- 4Department of Clinical Psychology, Friedrich Schiller University Jena, Jena, Germany
- 5German Center for Mental Health (DZPG), Halle-Jena-Magdeburg, Germany
- 6Center for Intervention and Research on Adaptive and Maladaptive Brain Circuits underlying Mental Health (C-I-R-C), Halle-Jena-Magdeburg, Germany
- 7Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States
- 8Department of Neurology, Ludwig-Maximilians-University, Munich, Germany
- 9Department of Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
- 10Department of Software Engineering and IT, École de technologie supérieure, Montreal, QC, Canada
- 11Translational Research Center for TBI and Stress Disorders (TRACTS) and Geriatric Research, Education and Clinical Center (GRECC), VA Boston Healthcare System, Boston, MA, United States
- 12Neuroimaging Research for Veterans (NeRVe) Center, VA Boston Healthcare System, Boston, MA, United States
- 13Massachusetts General Hospital Department of Radiology, Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, United States
- 14Department of Radiology and Neurology, Uniformed Services University, Bethesda, MD, United States
- 15Department of Rehabilitation and Human Performance, Icahn School of Medicine at Mount Sinai, New York, NY, United States
- 16Department of Psychiatry, Harvard Medical School, Boston, MA, United States
- 17Graduate School of Systemic Neuroscience, Ludwig-Maximilians-University, Munich, Germany
Background: Intimate partner violence (IPV) perpetration is highly prevalent among veterans. Suggested risk factors of IPV perpetration include combat exposure, post-traumatic stress disorder (PTSD), depression, alcohol use, and mild traumatic brain injury (mTBI). While the underlying brain pathophysiological characteristics associated with IPV perpetration remain largely unknown, previous studies have linked aggression and violence to alterations of the limbic system. Here, we investigate whether IPV perpetration is associated with limbic microstructural abnormalities in military veterans. Further, we test the effect of potential risk factors (i.e., PTSD, depression, substance use disorder, mTBI, and war zone-related stress) on the prevalence of IPV perpetration.
Methods: Structural and diffusion-weighted magnetic resonance imaging (dMRI) data were acquired from 49 male veterans of the Iraq and Afghanistan wars (Operation Enduring Freedom/Operation Iraqi Freedom; OEF/OIF) of the Translational Research Center for TBI and Stress Disorders (TRACTS) study. IPV perpetration was assessed using the psychological aggression and physical assault sub-scales of the Revised Conflict Tactics Scales (CTS2). Odds ratios were calculated to assess the likelihood of IPV perpetration in veterans with either of the following diagnoses: PTSD, depression, substance use disorder, or mTBI. Fractional anisotropy tissue (FA) measures were calculated for limbic gray matter structures (amygdala-hippocampus complex, cingulate, parahippocampal gyrus, entorhinal cortex). Partial correlations were calculated between IPV perpetration, neuropsychiatric symptoms, and FA.
Results: Veterans with a diagnosis of PTSD, depression, substance use disorder, or mTBI had higher odds of perpetrating IPV. Greater war zone-related stress, and symptom severity of PTSD, depression, and mTBI were significantly associated with IPV perpetration. CTS2 (psychological aggression), a measure of IPV perpetration, was associated with higher FA in the right amygdala-hippocampus complex (r = 0.400, p = 0.005).
Conclusion: Veterans with psychiatric disorders and/or mTBI exhibit higher odds of engaging in IPV perpetration. Further, the more severe the symptoms of PTSD, depression, or TBI, and the greater the war zone-related stress, the greater the frequency of IPV perpetration. Moreover, we report a significant association between psychological aggression against an intimate partner and microstructural alterations in the right amygdala-hippocampus complex. These findings suggest the possibility of a structural brain correlate underlying IPV perpetration that requires further research.
Introduction
Intimate partner violence (IPV) perpetration is highly prevalent among military veterans, with ~66 to 91% of veterans engaging in psychological aggression, and ~ 27% reporting physical assaults against their relationship partners (1). Psychological aggression refers to verbal and non-verbal behavior used to dominate, belittle, criticize, isolate, and instill fear in an intimate partner. Physical assault involves the intentional affliction of harm to an intimate partner through physical violence, including shaking, pushing, blows to the head or other parts of the body, and strangulation or other forms of impeded breathing (2). There are several factors that may contribute to the high prevalence of IPV among veterans. War zone-related trauma exposure and stress is tightly bound to the development of psychiatric disorders (3, 4), which, in turn, have been associated with IPV perpetration (5–7). In addition, psychiatric conditions commonly seen in veterans (e.g., posttraumatic stress disorder (PTSD), and depression) are linked to emotion regulation deficits (8–10). Such deficits may further contribute to the risk of perpetrating IPV. Comorbid substance abuse, which is commonly observed in veterans, may further amplify this link to IPV perpetration (11–14).
Moreover, there is a large overlap between psychiatric diagnoses and mTBI among veterans (15–18). Post-concussive symptoms generally subside after a couple of days or weeks following acute head injury (19). However, approximately 15 to 30% of those experiencing mTBI develop long-term impairments (19–21) that may persist even years after the sustained injury. These symptoms may include anger and aggressiveness (19–21) and may also increase the risk of IPV perpetration. While an association between persistent post-concussive symptoms and IPV perpetration has been suggested in a recent study (22), evidence is still limited and requires further investigation. Most importantly, rather than endorsing a direct causal link between mTBI or post-concussive symptoms and IPV perpetration, the individual contribution of post-concussive sub-components (i.e., physical and affective symptoms) needs to be taken into account. Additionally, despite the urgent need for preventive and treatment efforts in this population, even less is known about the underlying patho-mechanism of IPV perpetration among veterans who evince comorbidity of mTBI and psychiatric disorders. Neuroimaging research serves as a tool to unravel brain alterations associated with IPV perpetration, thereby opening the possibility of identifying brain correlates that may serve as treatment targets beyond routine interventions.
IPV perpetration and neuroimaging
War zone-related stress (23), post-deployment psychiatric disorders (24, 25), and mTBI (26) are associated with alterations in brain structure. While brain research in the population of IPV perpetrators is relatively scarce, magnetic resonance imaging (MRI) studies report altered brain function and structure associated with aggressiveness. More specifically, altered functional activity of the brain’s limbic system [a brain circuit primarily responsible for emotion processing (27)] in otherwise healthy individuals with high aggressiveness, and aggressive individuals with borderline and antisocial personality disorder has been reported (28–30). Moreover, decreased volume of the limbic system, particularly the amygdala, has been shown in individuals with aggressive behavior (29, 31, 32). In accordance with these findings, there is initial evidence that IPV perpetration is associated with greater functional activation in limbic areas (33) and smaller amygdala volume (34).
While IPV perpetration may be associated with altered limbic macrostructure, to date, a potential relationship between IPV perpetration and brain microstructural alterations has not yet been investigated. Alterations in brain microstructure may provide additional information about underlying abnormal neuronal processes (35, 36), such as tissue composition [e.g., glial changes (37–39), alterations in dendritic arborization (40–42)] or atrophic processes (43).
Diffusion-weighted MRI (dMRI) provides information on even subtle brain microstructural alterations by quantifying the motion of water molecules in tissue (44). Associations between altered limbic gray matter microstructure and war zone-related stress (23), psychiatric disorders (24, 25), and mTBI (26) have previously been reported, while findings on IPV perpetration are lacking.
Given the detrimental outcome of IPV perpetration and the immense overall economic burden of increased healthcare costs and criminal justice persecution (45), improved knowledge and understanding about the associated risk factors and underlying patho-mechanisms of IPV perpetration is needed to establish options for treatment and prevention.
In this study, comprised of a sample of US Iraq/Afghanistan veterans with available dMRI, neuropsychiatric, and IPV assessments, we focus on two core objectives. The first objective is to investigate the association between neuropsychiatric conditions and IPV perpetration. More specifically, we test the likelihood of perpetrating IPV (i.e., psychological aggression and physical assault) in the presence of neuropsychiatric disorders (i.e., PTSD, mood disorder, substance use disorder, or mTBI). Moreover, we test whether greater war zone stress, and neuropsychiatric symptom severity (i.e., PTSD, depressive, and post-concussive symptoms, and alcohol use) are associated with higher IPV perpetration frequency. In the second objective of the study, we employ dMRI to investigate whether IPV perpetration is associated with alterations in limbic gray matter microstructure that may improve our understanding of the neurobiological patho-mechanisms underlying IPV perpetration.
Methods
Participants
Veterans of the Iraq and Afghanistan wars (Operation Enduring Freedom/Operation Iraqi Freedom; OEF/OIF) were recruited as part of the Translational Research Center for TBI and Stress Disorders (TRACTS) study (46). Out of the first 384 consecutively recruited veterans, a small subset of 49 male veterans had assessments available on IPV perpetration, and structural and dMRI data. All 49 veterans consented to sharing their data with investigators outside of TRACTS and provided written informed consent. Study protocols were approved by the Institutional Review Board of the VA Boston Healthcare System.
Diagnostic and clinical assessment
Assessment of intimate partner violence
Perpetration of violence against a relationship partner in the past year was assessed using the psychological aggression and physical assault scales of the Revised Conflict Tactics Scales (CTS2) (47), a 78-item questionnaire referring to IPV. The frequency of psychological aggression (8 items: e.g., “I called my partner fat or ugly,” “I shouted or yelled at my partner”) and physical assault (12 items: e.g., “I beat my partner up,” “I choked my partner,” “I threw something at my partner that could hurt”) were assessed on a 6-point scale (0 = never to 6 = more than 20 times). A summed score was computed from all of the items from each sub-scale (CTS2 psychological aggression and CTS2 physical assault).
Assessment of war zone-related stress
War zone-related stress was assessed with the combat experiences and post-battle experiences sub-scales of the Deployment Risk & Resilience Inventory (DRRI) (48). The DRRI sub-scales (DRRI-Combat and DRRI-Other) consist of 16 questions concerning combat or war zone-related events (e.g., DRRI-Combat: “I personally witnessed someone from my unit or an ally being seriously wounded or killed,” DRRI-Other: “I saw civilians after they had been severely wounded or disfigured”). The DRRI-Combat uses a 5-point scale (0 = never to 4 = daily or almost daily), while the DRRI-Other scale uses a binary response format (0 = no and 1 = yes). Summed scores were computed from all items of each sub-scale.
Assessment of PTSD
Current diagnosis of PTSD and PTSD symptom severity were assessed using the 30-item Clinician-Administered PTSD Scale for DSM-IV (CAPS-IV) (49) which captures reexperiencing, avoidance and numbing, and hyperarousal symptoms of the traumatic event. Sub-scores for each sub-scale and a total PTSD symptom severity score were calculated by summing frequency and intensity scores rated from 0 = absent to 4 = extreme/incapacitating.
Assessment of depression
Mood disorder was diagnosed with the non-patient research version of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/NP) (50). Depression severity was assessed with the Depression, Anxiety and Stress Scale 21-items (DASS-21) (51). The depression sub-scale comprises seven items (e.g., “I felt down-hearted and blue,” “I felt I wasn’t worth much as a person”) that are scored from 0 = did not apply to me at all to 3 = applied to me very much or most of the time. Scores of the seven items were summed into a total score.
Assessment of substance use and drinking history
Substance use disorder was diagnosed with the non-patient research version of the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/NP) (50). Lifetime burden of alcohol consumption was estimated using a retrospective, interview-based procedure measuring total lifetime exposure to alcohol by assessing the number of standard drinks consumed (lifetime drinking history total).
Assessment of mild traumatic brain injury and post-concussive symptoms
History of mTBI was assessed using the Boston Assessment of TBI-Lifetime (BAT-L) (52). The BAT-L classifies a TBI as mild if loss of consciousness equals 30 min or less, and posttraumatic amnesia or an altered mental status does not exceed 24 h. Mild TBI is further classified into grade 1–3, where a higher grade refers to greater mTBI severity. A total mTBI frequency and severity score was computed from the number and severity of all mTBIs prior to, during, and post-military deployment.
Post-concussive symptoms during the last two weeks were assessed using the Neurobehavioral Symptom Inventory (NSI), a 22-item self-report questionnaire (53). Severity of vestibular, somatosensory, cognitive, and affective persistent post-concussive neurobehavioral symptoms (e.g., “feeling dizzy,” “headaches,” “difficulty making decisions,” “feeling anxious”) were rated on a 5-point scale (0 = none to 4 = very severe). Sub-scores for each sub-scale, as well as a summed total score, were calculated.
Magnetic resonance imaging
Image acquisition
MRI data were acquired on a 3-Tesla Siemens TIM Trio scanner (Siemens Healthcare, Erlangen, Germany) at the VA Medical Center in Boston, MA, United States. T1-weighted structural MPRAGE scans (256 slices, T1 = 1,000 ms, TR = 2,530 ms, TW = 3.32 ms, voxel size = 1 mm3, flip angle = 7°, FOV = 256 × 256 mm2) and dMRI scans using a single-shot echo-planar sequence with a twice-refocused spin-echo pulse (64 axial slices with no inter-slice gap, 60 gradient directions with a b-value of 700 s/mm2 and 10 additional scans with b = 0 gradients, TR = 10.000 ms, TE = 103 ms, voxel size = 2 mm3, FOV = 256 mm2) were obtained.
Image processing
Pre-processing of the structural T1-weighted and dMRI data was performed using our in-house image processing pipeline. First, the images were axis-aligned, centered, and motion-corrected. DMRI data was corrected for eddy current effects using the FMRIB Software Library (version 5.1) (54, 55). Image quality of T1-weighted and dMRI data was checked for artifacts using the 3D Slicer program (version 4.5) (56). T1-weighted and diffusion masks covering the entire brain were automatically created and manually corrected in 3D Slicer where necessary. Automated segmentation of brain regions from T1-weighted data was performed using FreeSurfer (version 5.1.0) (57).
Using in-house software (58), free-water (FW) imaging was also implemented to obtain voxel-wise free-water corrected fractional anisotropy (FA) measures for each participant. By separating the MRI signal into two compartments (58), FW imaging is able to eliminate partial volume with extracellular FW [e.g., caused by cerebrospinal fluid (CSF) contamination, edema, or atrophy] in each voxel. Given the correction for FW, FA serves as a more accurate marker for tissue than the conventional FA measure (59). FreeSurfer parcellation label maps were non-linearly registered from the individual T1-weighted space to the respective diffusion MRI space to obtain diffusion metrics for selected limbic regions (amygdala-hippocampus complex, cingulate, entorhinal, and parahippocampal cortex). Amygdala and hippocampus were combined into one region of interest to ensure higher parcellation accuracy (60). Average diffusion measures (FA) were calculated for limbic gray matter structures.
Statistical analysis
Descriptive statistics for demographic and clinical variables were performed using IBM SPSS Statistics 27 (61). A false discovery rate (FDR)-corrected (62) p-value of 0.05 was chosen to indicate statistical significance.
IPV perpetration and neuropsychiatric conditions
Percentages were calculated to display the proportion of veterans who engaged in IPV perpetration (i.e., psychological aggression; physical assault). Odds ratios were calculated to portray the odds of IPV perpetration in veterans with PTSD, mood disorder, substance use disorder, or mTBI. To assess the associations between neuropsychiatric symptom severity and IPV perpetration frequency, we calculated partial correlations between frequency of IPV perpetration (CTS2 psychological aggression and CTS2 physical assault) and (1) war zone-related stress (combat and post-battle experiences), (2) psychiatric symptoms (PTSD symptoms, depressive symptoms, lifetime drinking), and (3) mTBI (mTBI frequency and severity, post-concussive symptoms). Age was included as a covariate.
Associations between limbic microstructure and IPV perpetration
To assess whether IPV perpetration was linked to brain structural alterations, we calculated partial correlations between frequency of IPV perpetration (CTS2 psychological aggression and CTS2 physical assault) and limbic microstructure (left/right amygdala-hippocampus complex, cingulate, parahippocampal gyrus, entorhinal cortex FA). Age was included as a covariate. In an additional step, clinical risk factors that showed significant correlations with IPV perpetration were included as covariates.
CTS2 physical assault perpetration deviated from normality according to the Kolmogorov–Smirnov test (D = 0.405(49), p < 0.001). We, thus, performed non-parametric partial correlations between frequency of CTS2 physical assault and war zone-related stress, psychiatric and mTBI symptoms and limbic microstructure.
Results
IPV perpetration and neuropsychiatric conditions
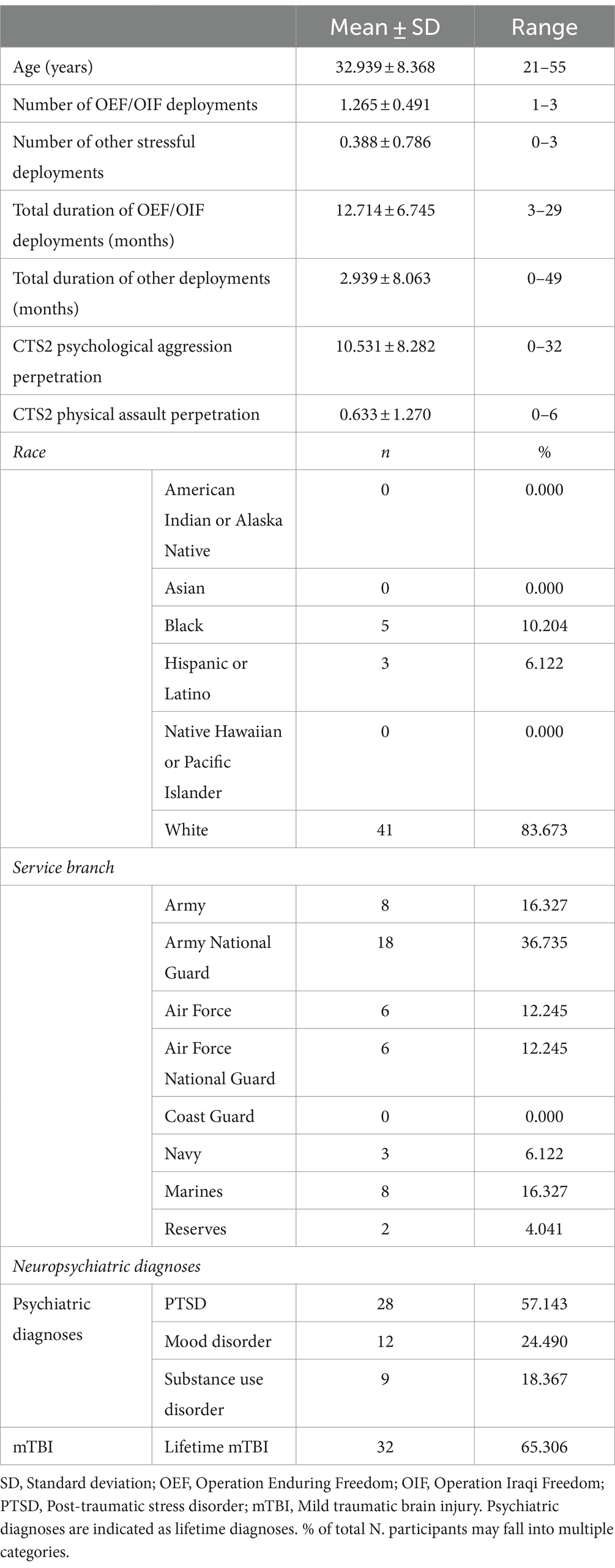
Sample characteristics are displayed in Table 1. In the total sample (N = 49), the following neuropsychiatric conditions were present: PTSD (n = 28, 57.143%), mood (n = 12, 24.490%), substance disorder (n = 9, 18.367%), and mTBI (n = 32, 65.306%). Notably, there was substantial overlap of the neuropsychiatric diagnoses (Figure 1), meaning that the majority of individuals had more than one diagnosis.
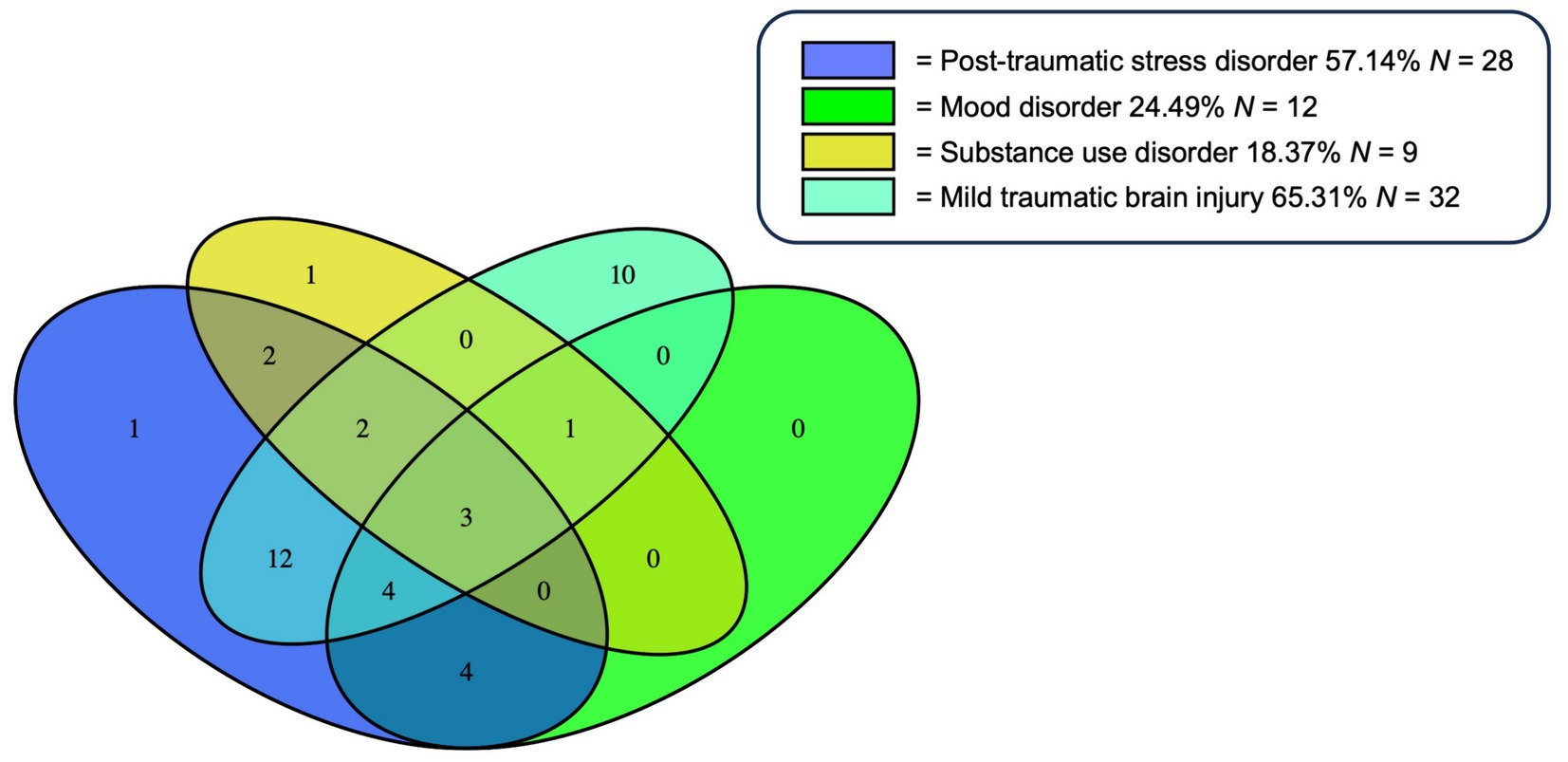
Figure 1. Overlap of Neuropsychiatric Diagnoses. % of total N (=49). Of the total sample (N = 49), 40 participants had at least one but often times multiple neuropsychiatric diagnoses. This Venn diagram illustrates the overlap of the neuropsychiatric diagnoses: Posttraumatic stress disorder (PTSD), mild traumatic brain injury (mTBI), substance use, and mood disorder. Percentages given for each diagnosis in the legend represent the proportion in relation to the total sample (N = 49). Of note, the sum of all percentages does not equal 100% due to comorbidity of multiple diagnoses in some participants.
The frequency of psychological aggression and physical assault in the context of neuropsychiatric diagnoses is shown in Figure 2.
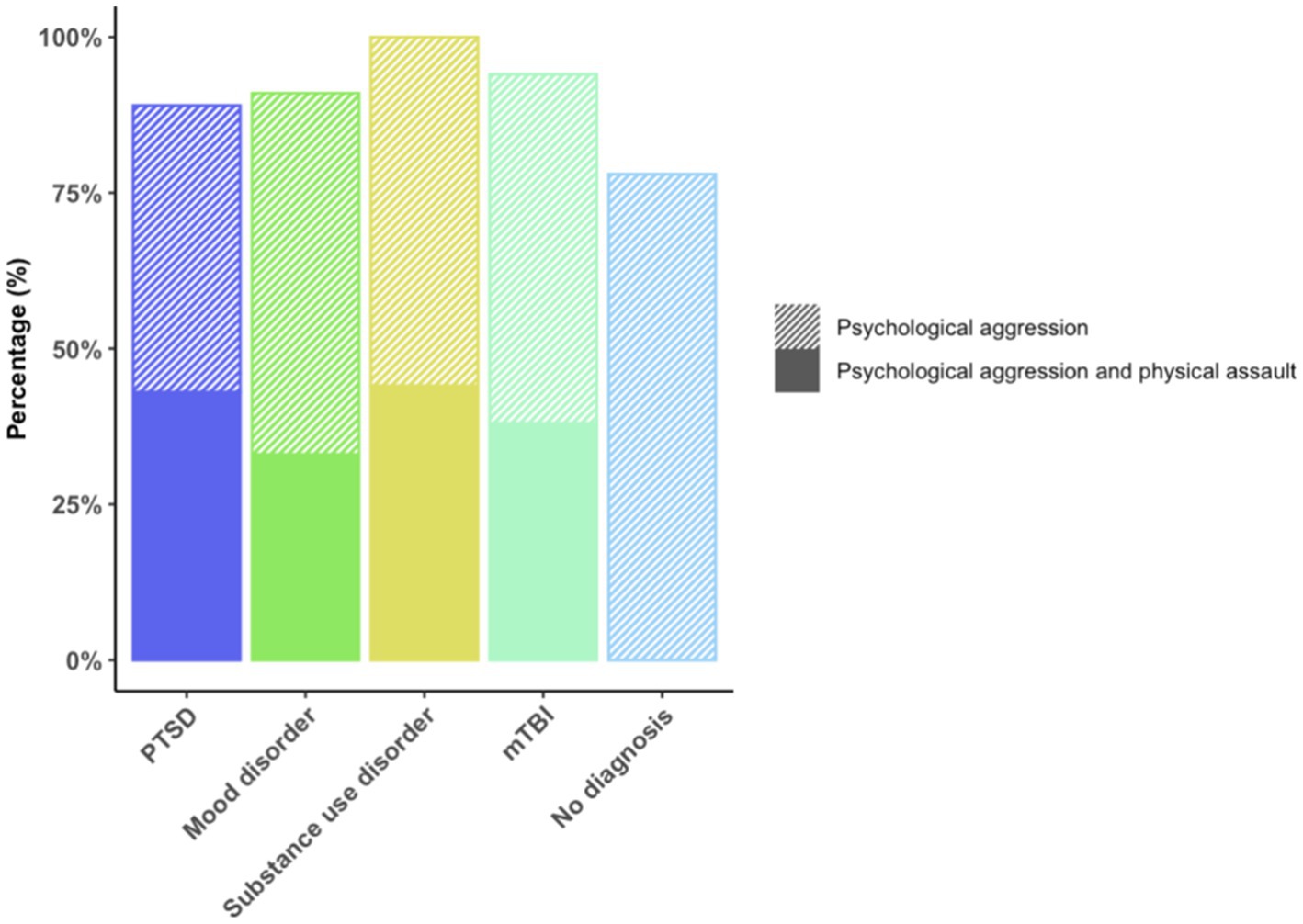
Figure 2. Frequency of psychological aggression and physical assault in the context of neuropsychiatric diagnoses. % of psychological aggression or combined psychological aggression and physical assault among veterans with the respective neuropsychiatric diagnosis.
Forty-three veterans (87.755%) engaged in psychological aggression against their intimate partners, and 14 (28.571%) exerted physical assault at least once or twice during the past year. Frequency of IPV perpetration (none to severe) is displayed in Figure 3. Veterans with PTSD, mood disorder, substance use disorder, and mTBI had higher odds of engaging in IPV perpetration compared to those with none of these afflictions (Figure 4).
Figure 3. Frequency of IPV Perpetration. % of total (N =49). This figure displays the frequency of IPV perpetration (i.e., psychological aggression and physical assault).
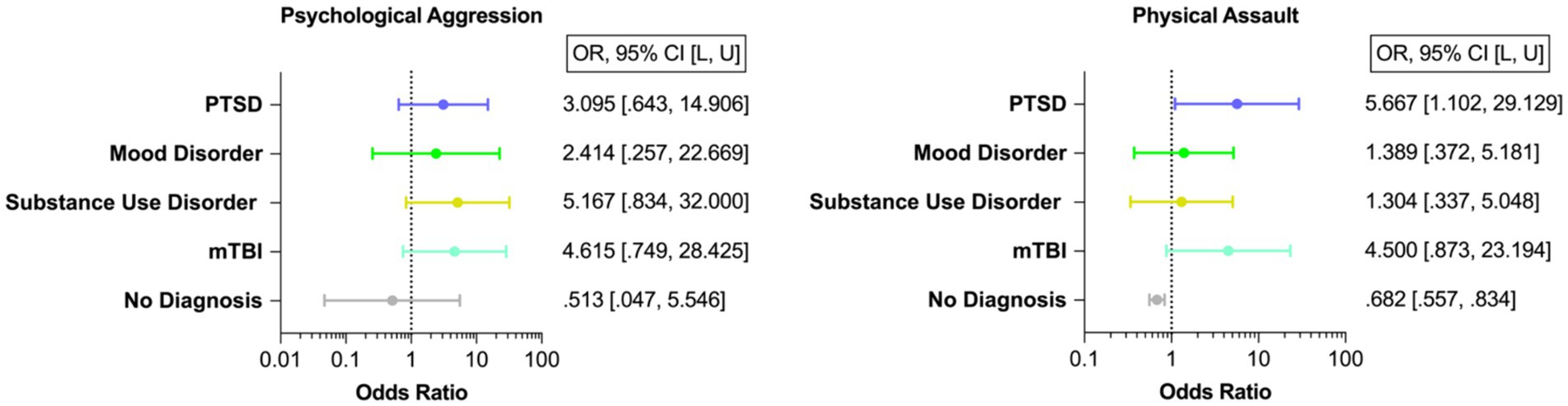
Figure 4. Odds of IPV Perpetration. PTSD, Post-traumatic stress disorder; mTBI, Mild traumatic brain injury. This figure displays the odds of IPV perpetration (i.e., psychological aggression and physical assault) in veterans with PTSD, mood disorder, substance use disorder, and mTBI. Please note that there is a large overlap of neuropsychiatric diagnosis and mTBI among veterans. Of the total sample (N = 49), 12 participants were diagnosed with one single neuropsychiatric diagnosis (24.49%). Most individuals were diagnosed with more than one disorder (n = 28, 70% of the individuals with a neuropsychiatric diagnosis; please refer to Figure 1).
This figure displays the frequency of IPV perpetration (i.e., psychological aggression and physical assault).
This figure displays the odds of IPV perpetration (i.e., psychological aggression and physical assault) in veterans with PTSD, mood disorder, substance use disorder, and mTBI. Please note that there is a large overlap of neuropsychiatric diagnosis and mTBI among veterans (15–18). Of the total sample (N = 49), 12 participants were diagnosed with one single neuropsychiatric diagnosis (24.49%). Most individuals were diagnosed with more than one disorder (n = 28, 70% of the individuals with a neuropsychiatric diagnosis; please refer to Figure 1).
Partial correlations showed significant associations between higher scores on CTS2 psychological aggression and greater war zone-related stress, PTSD, depressive, and post-concussive symptoms (Table 2). Moreover, CTS2 psychological aggression was significantly associated with all PTSD sub-scales (reexperiencing; avoidance and numbing; and hyperarousal symptoms) and with all post-concussive sub-scales (vestibular; somatosensory; cognitive; and affective symptoms). In contrast, higher scores on the CTS2 physical assault measures were significantly associated with greater post-concussive symptoms, and with PTSD, and depressive symptoms, although only correlations between avoidance and numbing symptoms of PTSD, and vestibular, somatosensory, and cognitive sub-symptoms of post-concussive symptoms were significant (Table 2). Associations between CTS2 psychological aggression and physical assault with lifetime drinking failed to reach significance.
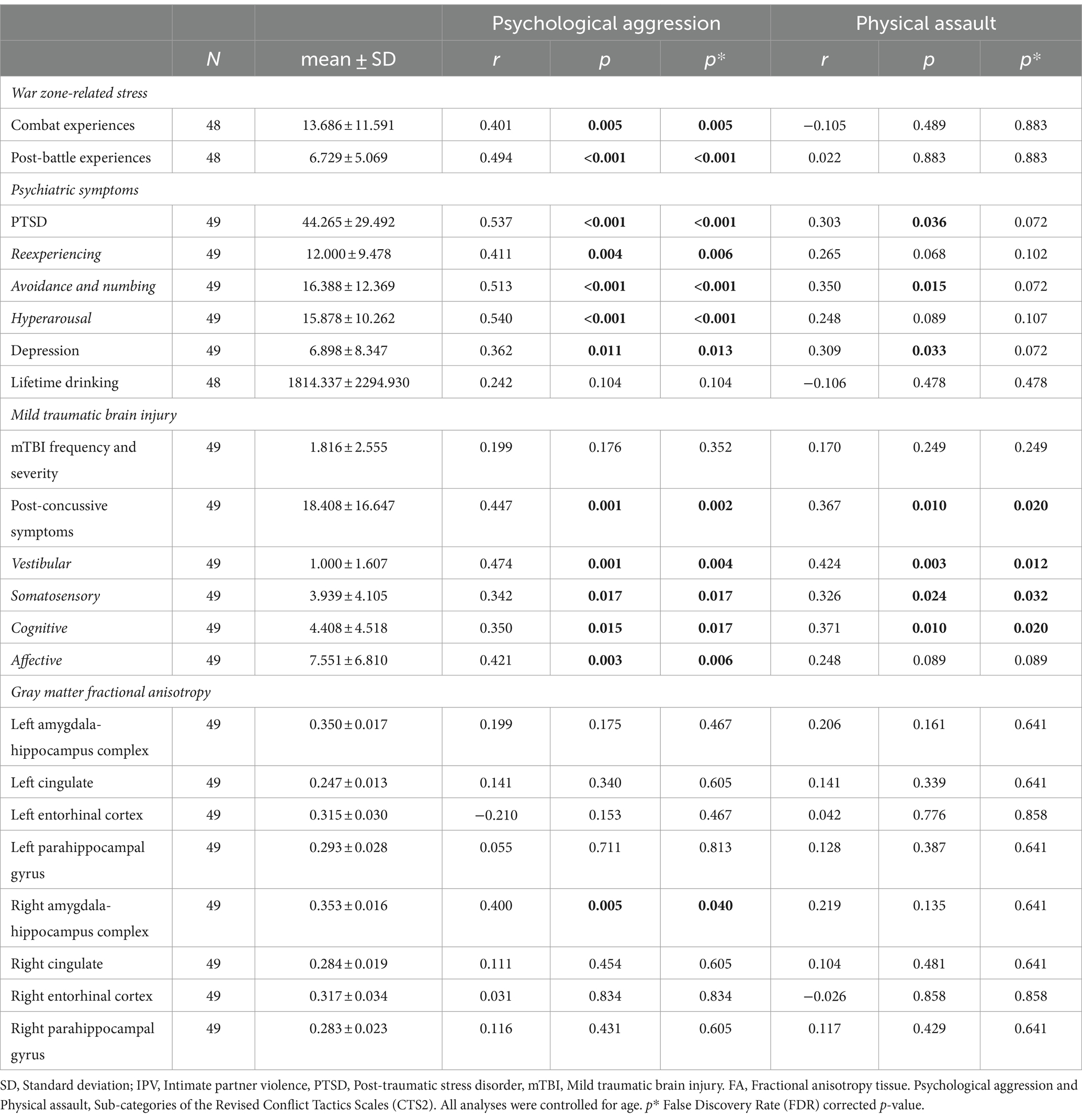
Table 2. Associations between IPV perpetration, war zone-related stress, Psychiatric symptoms, mTBI, and limbic microstructure.
Associations between IPV perpetration and limbic microstructure
Higher scores on CTS2 psychological aggression were significantly associated with higher FA in the right amygdala-hippocampus complex (r = 0.400, p = 0.005, Table 2, Figure 5). This association remained significant when additionally controlling for war zone-related stress, PTSD, depressive, and post-concussive symptoms (r = 0.389, p = 0.011). There was no significant association between CTS2 physical assault and limbic microstructure.
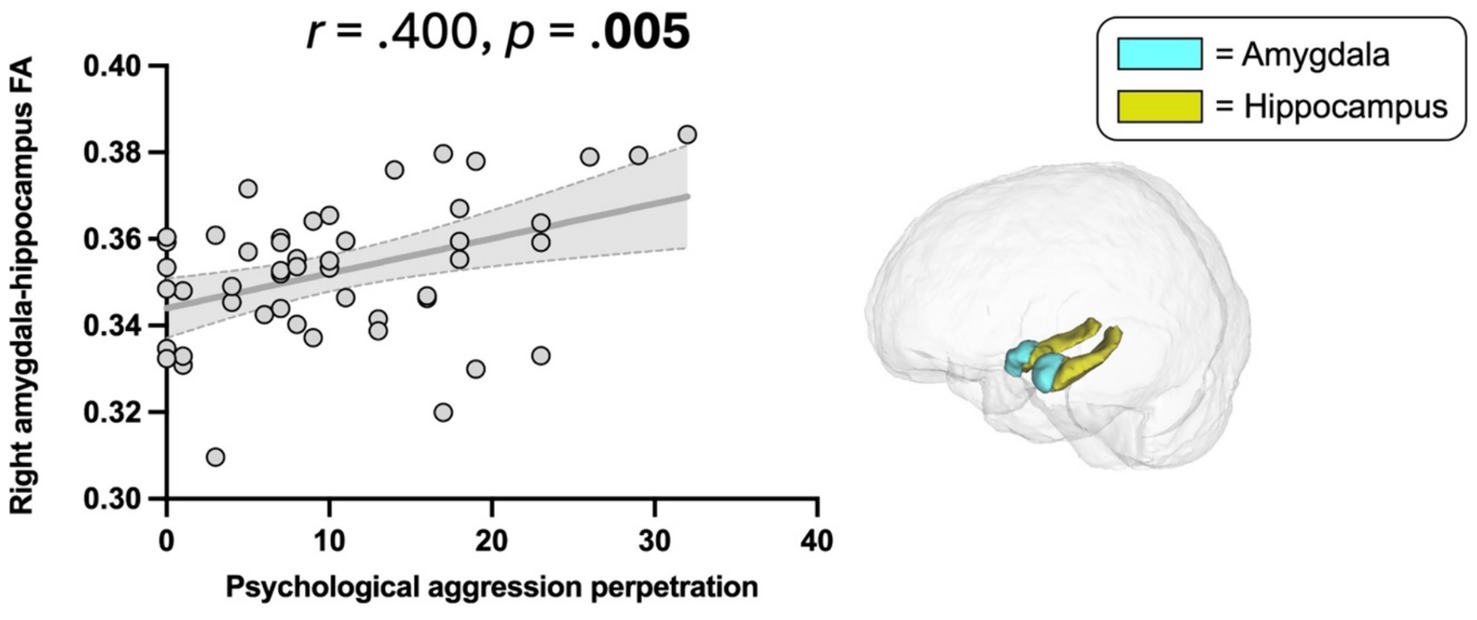
Figure 5. Association between Psychological aggression perpetration and amygdala-hippocampus complex FA. Psychological aggression, revised conflict tactics scales (CTS2) (47); FA, Fractional anisotropy. Scatter plot illustrating the significant correlation between psychological aggression perpetration and higher FA in the right amygdala-hippocampus complex (p = 0.005).
Discussion
In the present study, OEF/OIF veterans diagnosed with PTSD, mood disorder, substance use disorder, or mTBI had increased odds of perpetrating IPV (i.e., psychological aggression and physical assault). Moreover, greater war zone-related stress, PTSD, depressive, and post-concussive symptoms were significantly associated with increased IPV perpetration frequency, while lifetime drinking failed to reach significance. The diffusion imaging analysis of limbic microstructure showed a significant association between greater IPV psychological aggression and higher FA in the right amygdala-hippocampus complex, suggesting the possibility of a structural brain correlate underlying IPV perpetration that requires further investigation.
IPV perpetration and neuropsychiatric conditions
Consistent with previous findings (1), we found that approximately 90% of veterans in this study perpetrated psychological aggression and approximately 30% physically assaulted their relationship partner at least once or twice during the past year. We also observed higher odds of IPV perpetration in veterans with psychiatric disorders (i.e., PTSD, mood, substance use disorder) and mTBI. Most remarkably, veterans with PTSD had six times higher odds of physically assaulting their relationship partners than those without PTSD. While several psychiatric disorders have been associated with an increased likelihood of IPV perpetration (14, 63), a particularly strong relationship between PTSD and IPV perpetration has repeatedly been observed (64). However, it is important to note the preliminary nature of the current study due to the limited sample size.
Anger and aggressiveness are common features of PTSD (65), and the hyperarousal symptoms of PTSD have been especially associated with an increased likelihood of perpetrating IPV (66–69). Individuals with PTSD experience a hyper aroused “survival mode” state (70), constantly scanning their environment for potentially threatening triggers to be prepared for fight or flight (71). It has been suggested that there is a hypersensitivity to even mildly threatening affective provocations that the perpetrator may be unable to control and deal with appropriately (72).
Interestingly, while reexperiencing, avoidance and numbing and hyperarousal symptoms of PTSD were all associated with psychological aggression perpetration, it was only avoidance and numbing symptoms that were significantly associated with physical assault perpetration. Thus, while associations between reexperiencing, hyperarousal, and physical assault may have failed to reach significance due to the limited overall sample size and limited variance in the physical assault measure, there, nonetheless, appears to be a particularly strong connection between avoidance and numbing and emotion regulation deficits.
Individuals with PTSD who perpetrate IPV often exhibit information processing and emotion regulation deficits, therefore misinterpreting their partners intentions. This may lead to excessive rage that translates into violence (8, 73, 74). Further, avoidance of unpleasant memories or thoughts is a maladaptive emotion regulation strategy that disrupts the process of integrating traumatic memories (75) and may, therefore, reinforce a volatile internal environment that may contribute to impulsive or aggressive behavior as a means of releasing or managing overwhelming emotions.
Deficiencies in emotion processing and regulation have similarly been observed in individuals suffering from a mood disorder (9, 76), which may explain the link between depression and IPV perpetration (14, 77, 78). The depressed perpetrator interprets their intimate partner’s behavior as negative or intentional and is, therefore, more inclined to engage in psychological and physical violence (78).
In addition, our findings confirm previous reports of greater odds of IPV perpetration in veterans with substance use disorder (79, 80). Intoxication impairs rational cognitive functioning and diminishes behavioral inhibition, lowering an individual’s threshold to engage in violence (81). Moreover, substance abuse reinforces PTSD and depressive symptoms, further exacerbating the risk of IPV perpetration (82). Previous studies have shown that alcohol use may moderate the relationship between other risk factors (i.e., PTSD, depression) and IPV perpetration (83–85). While we report higher odds of IPV perpetration in veterans with a clinician-diagnosed substance use disorder, alcohol use frequency, in and of itself, in the absence of a diagnosis of substance abuse, was not significantly associated with IPV perpetration. Thus, despite the well-established link between alcohol use and IPV perpetration (11–14, 86), our study did not reveal a significant association between lifetime drinking history and IPV perpetration. This discrepancy may be attributed to the nuanced nature of the relationship between alcohol and IPV perpetration, where diagnosable alcohol use disorder could be a more critical factor than mere frequency of alcohol consumption. Notably, in our study only n = 9 veterans qualified for a diagnosable substance use disorder. It is, thus, possible that the limited variance in the data and the small sample size of those with a diagnosable substance use disorder may have impacted our ability to detect an effect. Moreover, of the n = 9 veterans with substance use disorder, n = 8 were also diagnosed with PTSD, mTBI, or a mood disorder.
Our study’s unique contribution lies in shedding light on the complex interplay of neuropsychiatric symptoms and IPV perpetration among veterans. It is crucial to recognize that while alcohol use is a relevant factor, its relationship with neuropsychiatric conditions, such as PTSD, mTBI and depression, may have a more direct impact on the risk of IPV perpetration. Future research, employing larger samples and multivariate statistical modeling, can probe these intricate relationships, offering a more nuanced understanding of the factors influencing IPV within the veteran population.
IPV perpetration and post-concussive symptoms
Remarkably, there is also a high overlap between psychiatric and persistent post-concussive symptoms, and both have been shown to be associated with IPV perpetration. Particularly interesting, we observed significant associations between post-concussive symptoms and IPV perpetration, while there was no significant relationship between the number of sustained mTBIs and IPV perpetration. A recent veteran study revealed similar findings, reporting that only persistent post-concussive symptoms but not TBI diagnosis itself predicted IPV perpetration at a one-year follow-up (22). Indeed, there are some indications that it may not be those who experience mTBI, but rather a minority of individuals who develop persistent post-concussive symptoms, who are at risk for engaging in IPV (22, 87).
Post-concussive symptoms greatly overlap with psychiatric symptoms and especially comorbidity with PTSD is common in the veteran population (10, 17). However, the analysis of the sub-components of persistent post-concussive neurobehavioral symptoms in our study revealed that not only the cognitive and affective post-concussive symptoms but also vestibular and somatosensory symptoms were associated with IPV perpetration.
Vestibular symptoms may encompass dizziness, vertigo, and general problems with balance and spatial orientation, while somatosensory symptoms include headaches, sensitivity to light and noise or vision problems. It has previously been shown that physical health conditions lead to higher odds of perpetrating IPV (88), as physical health conditions are often associated with discomfort or even pain that has been shown to increase aggressive behavior (89). It has been argued that increased stress due to physical health complaints depletes stress regulation resources, thus enabling aggressive behaviors. Moreover, Individuals with physical health issues may experience a sense of diminished control over their own bodies which they may compensate by attempting to exert control over their partners (88). It is also conceivable that physical health complaints lead to significant emotional distress that further reinforces a relationship with IPV perpetration. Moreover, affective symptoms including anxiety, depression, and irritability as well as cognitive complaints such as poor concentration, forgetfulness and difficulty making decisions are common long-term symptoms following mTBI. Cognitive and affective complaints may lead to poor frustration tolerance. Indeed, post-concussive symptoms have been found to significantly interfere with emotion regulation and psychosocial functioning (10), potentially explaining the link with IPV perpetration (90). Interestingly, it has even been suggested that deficits in emotion regulation can discern between those who do or do not perpetrate violence (91). Thus, addressing emotion regulation deficits emerges as a critical factor in facilitating the recovery of both psychiatric and post-concussive symptoms (92). In doing so, interventions have the potential to mitigate the impact of these symptoms and to proactively prevent acts of violence.
Associations between limbic microstructure and IPV perpetration
We showed a significant association between psychological aggression perpetration and higher FA of the right amygdala-hippocampus complex. Higher gray matter FA may reflect greater tissue density, and an enhanced tissue organization through strengthened axonal and dendritic connections (35, 36, 40–42). To date, findings on gray matter diffusion in psychiatric samples are limited. We previously showed an association between higher FA of the amygdala-hippocampus complex and greater war zone-related stress (23) and PTSD symptoms (24), suggesting that stress-related experiences alter limbic microstructural integrity. Similarly, increased diffusion in several regions (including the amygdala and hippocampus) was shown in individuals with persistent post-concussive symptoms following mTBI compared to controls (26). In accordance with these findings, our study revealed an association between higher amygdala-hippocampus FA and greater psychological aggression perpetration frequency. We speculate that our findings may constitute a brain structural reflection of the previously observed greater amygdala and hippocampus activity that coincides with IPV perpetration (33, 72).
Hyperresponsivity of the amygdala and related limbic structures is not only a core feature of violence perpetration (33, 72, 93, 94), but also of PTSD symptomatology (95–100) and the associated emotion regulation deficits (101). Similarly, individuals with post-concussive symptoms exhibit functional and structural alterations of the amygdala that have been linked to emotion dysregulation (102, 103). The amygdala and hippocampus are vulnerable to extensive stress hormone exposure (104, 105) and the adverse effects of head impacts (106–108), contributing to the emergence and maintenance of both post-concussive symptoms and PTSD symptoms (109–111). Moreover, since the amygdala is immediately activated in threatening situations (112) and the hippocampus is crucial for memory retrieval and consolidation (113), it has been suggested that even in response to only mildly threatening situations, the IPV perpetrator may remember previous encounters of threat and social conflict that elicit inappropriate hyperarousal and consequent psychological abuse or physical assault (72). Indeed, it has been suggested that IPV perpetrators have lower perspective-taking abilities in the face of greater personal distress (114), show deficits in information processing, and misinterpret their partners intentions (73). In turn, constant hyperarousal states prohibit the adequate judgment of social cues, and increase the likelihood of misinterpreting them for malicious intentions which may further translate into violence perpetration (70, 73).
Notably, our findings persisted even when accounting for PTSD, depressive, and post-concussive symptoms, suggesting that amygdala microstructure is associated with psychological aggression above and beyond other clinical risk factors for IPV perpetration. A potential explanation may be the link between emotion dysregulation and IPV perpetration (115). Indeed, it has previously been shown that emotion dysregulation fully accounts for the association between PTSD and IPV perpetration (74).
Interestingly, we demonstrated an association between psychological aggression perpetration and enhanced amygdala-hippocampus microstructure only in the right hemisphere. The right amygdala has been suggested to be particularly involved in affective information retrieval (116) and unconscious information processing (117). Unconscious threat perception elicits inappropriate hyper-aroused reactions that may translate into violence (72). The right hemisphere, and particularly the right amygdala may, thus, contribute to violent outbursts through emotion dysregulation and information processing deficits. To date, one study linked right amygdala volume to IPV perpetration (34). Others, however, have associated violence perpetration with the left amygdala, showing an increased connectivity between limbic and paralimbic structures in the left hemisphere of violent offenders (94). Future research needs to address whether IPV perpetration is associated with lateralization of limbic structural alterations.
While we report a significant association between higher FA in the amygdala-hippocampus complex, we did not detect significant associations between the cingulate, parahippocampal gyrus, entorhinal cortex, and IPV perpetration. The amygdala-hippocampus complex has well-established roles in emotional processing and memory, both relevant to IPV perpetration. It is possible that the surrounding limbic structures – while part of a larger operating network responsible for emotional processing – are not the primary loci for the specific behavior under consideration. Moreover, the multifaceted nature of IPV, with psychological and physical components explored in this study, could mean that different brain regions contribute to distinct aspects of the behavior. The limited overall sample size in our sample poses challenges, as the variability might not have been sufficient to establish a robust relationship with structural alterations in these particular brain regions. It is possible that our sample of 49 veterans was not sufficiently powered to detect a significant relationship between IPV perpetration and microstructural alterations. Moreover, while a large proportion of veterans in our sample engaged in moderate or even severe psychological aggression perpetration (~90% N = 43), only a minority third reported physical assaults (~30% N = 14). Consequently, there may not have been enough variance in the physical assault measure to capture a relationship with brain alterations. Further research with more robust samples is warranted to deepen our understanding of the nuanced neural underpinnings of IPV perpetration.
Limitations and future directions
We acknowledge several study limitations. First, our findings are based on a relatively small sample of male military veterans. Consequently, the results of the current study represent preliminary results that require validation in larger and more diverse samples for robustness and generalizability. A replication of our study using a larger sample size and including female perpetrators is required. It is worth noting that according to the Centers for Disease Control and Prevention (CDC), approximately 1 in 2 women and 2 in 5 men have reported experiencing contact sexual violence, physical violence, and/or stalking victimization by an intimate partner at some point in their lifetime (118). While studies have shown that men are more likely to perpetrate physical violence against intimate partners (119, 120), it is important to acknowledge that women can also be engage in IPV, and their perpetration may manifest in different forms. Moreover, underreporting of IPV is common due to various factors such as stigma, fear, and cultural norms. Additionally, research on IPV perpetration by females is still evolving, and further studies are needed to better understand the prevalence and dynamics of female-perpetrated IPV. Second, our findings result from a cross-sectional study design, meaning that we cannot infer causality between the studied variables. While we assume that deployment-related mental health issues fuel IPV perpetration, it is similarly possible that IPV perpetration reinforces the emergence and maintenance of neuropsychiatric symptoms. Third, we captured psychological aggression and physical assault perpetration, but not sexual abuse, which may require special preventive and treatment efforts. In general, therapeutic options for IPV perpetration may particularly focus on PTSD alleviation, as successful PTSD treatment reduces anger and aggressiveness among veterans (121, 122). In turn, specific anger and aggression management programs for veterans may lower PTSD and related symptoms and improve anger expression. For example, STEP-Home is a transdiagnostic civilian reintegration 12-week workshop for 9/11 veterans, specifically designed to improve emotional regulation and impulse control, thereby decreasing anger and aggressiveness (123). In addition, novel treatment options, such as neurofeedback, may assist with regulating amygdala activity and, thus, control overreactive emotional states that lead to violence (124–126). Fourth, early life stress and childhood trauma history was not directly assessed in this study, despite their previously reported association with brain structure and function (127). Future studies should consider incorporating direct assessment measures such as the Childhood Trauma Questionnaire (CTQ) (128). Last, the analysis of gray matter diffusivity comes with resolution constraints, limiting the characterization of microstructural features. The complexity of gray matter organization poses challenges in accurately discerning diffusion orientations and partial volume effects arising from the proximity of gray matter to cerebrospinal fluid and white matter, impacting measurement precision. Despite our attempts to limit these issues using free-water modeling, the FA measure remains unspecific and is only an approximation of underlying microstructural characteristics.
Conclusion
Veterans with psychiatric disorders and/or mTBI exhibit higher odds of engaging in IPV perpetration. Further, the more severe the symptoms of PTSD, depression or mTBI, and the greater the experienced war zone-related stress, the greater the frequency of IPV perpetration. Emotion regulation and information processing deficits may underlie the link between neuropsychiatric symptoms and IPV perpetration. Moreover, we report a significant association between psychological aggression against an intimate partner and microstructural alterations in the right amygdala-hippocampus complex. The findings suggest the possibility of a structural brain correlate underlying IPV perpetration which may constitute a brain structural reflection of previously reported limbic hyperresponsivity during aggressive states.
Author’s note
SH’s contribution to this body of work represents his own expertise and opinions and should not be viewed as the opinion of the Uniformed Services University, Defense Health Agency, Department of Defense, or the Federal government.
Data availability statement
The data are not publicly available because the dataset contains information that could compromise the privacy of research participants. The data that support the findings of this study are available upon reasonable request.
Ethics statement
The studies involving humans were approved by Institutional Review Board VA Boston Healthcare System. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PR: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. CH: Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. JS-H: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing. EK: Data curation, Methodology, Writing – original draft, Writing – review & editing, Validation. VS: Data curation, Methodology, Writing – original draft, Writing – review & editing. LB: Visualization, Writing – original draft, Writing – review & editing. LP: Visualization, Writing – original draft, Writing – review & editing. YR: Methodology, Software, Writing – original draft, Writing – review & editing. SB: Methodology, Software, Writing – original draft, Writing – review & editing. OP: Methodology, Software, Writing – original draft, Writing – review & editing. DS: Writing – original draft, Writing – review & editing. SH: Writing – original draft, Writing – review & editing. CE: Conceptualization, Validation, Writing – original draft, Writing – review & editing. CF: Resources, Validation, Writing – original draft, Writing – review & editing, Conceptualization, Investigation. WM: Conceptualization, Investigation, Resources, Validation, Writing – original draft, Writing – review & editing. MS: Conceptualization, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. IK: Conceptualization, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The current research received support from the Translational Research Center for TBI and Stress Disorders (TRACTS) through a VA Rehabilitation Research and Development National Network Research Center for Traumatic Brain Injury Grant (B3001-C), which was originally awarded to the regretfully deceased Regina E. McGlinchey. Further support was received through a VA Merit Award (I01RX000928-01A2) to MS, National Institute of Neurological Disorders and Stroke (NINDS) Awards (R01NS100952) (R01NS115957) to IK, and a National Institutes of Health Neuroimage Analysis Center grant (NIH P41EB015902) to OP. The authors further acknowledge support from the Harvard Medical School Livingston Fellowship Award (JS-H), the BBRF Young investigator grant (JS-H, funded by Mary and John Osterhaus and the Brain and Behavior Research Foundation), the German Society for Clinical Neurophysiology and Functional Imaging (DGKN) Fellowship (EK), and the Fulbright commission (PR).
Acknowledgments
The authors express special gratitude to the veterans participating in this study and the whole TRACTS team for data collection and management.
Conflict of interest
IK is a professor at Ludwig-Maximilians-University Munich (paid position). She serves as European Editor at Journal of Neurotrauma (unpaid position) and as Vice President of the European Neurotrauma Organization (unpaid position). She receives research grant funding from the National Institutes of Health, the European Research Council, the German Ministry for Research and Education. She receives funding for a research study on sport-related concussion from Abbott Inc. The Ludwig-Maximilians-University hospital received donations for her research from the Schatt Foundation and from Mary Ann Liebert Inc. She receives royalties for book chapters published by Thieme Publishers. Her spouse is employee at Siemens and she thus holds stock options at Siemens and Siemens Healthineers. IK’s in-kind contributions: PhD students working under her supervision receive scholarships from the Villigst Foundation, the Konrad Adenauer Foundation, the Studienstiftung des deutschen Volkes, the China Scholarship Council collaboration with Ludwig-Maximilians-University Munich, the Harvard Munich Club, and Fulbright. MS is Professor of Psychiatry and Radiology at Harvard Medical School and is employed by Brigham and Women’s Hospital. She has grant funding from the National Institute of Mental Health and formerly from VA Merit Awards and from the National Institute of Neurological Diseases and Stroke. She serves on the editorial board of several professional journals (unpaid), on NIH study sections (Honoria minimum), and she receives royalties for book chapters and books published by Thieme Publishers, Elsevier, and Cambridge University Press (minimal). She receives funding also for legal cases as a consultant and an expert witness related to mild traumatic brain injury and the use of diffusion tensor imaging in the courtroom. MS’s in-kind contributions include PhD, MD, MD-PhD trainees and junior faculty working under her mentorship, many of whom have received K awards and grant support for their research. EK received speaker honoraria and financial compensation for travel expenses from Medtronic, UCB, Livanova, and Eisai and has participated in clinical trials for Medtronic, UCB and Precisis, all unrelated to the submitted work. Her research is supported by the Medical Clinical Scientist Program (MCSP). SH is a retired United States Army Medical Corps Officer. He is a consultant for the National Football League Players Association, Major League Soccer Players Association, SCS Consulting LLC. He is an advisory board member for the Project Enlist (Veterans Advisory Board of the Concussion Legacy Foundation), NanoDX, Prevent Biometrics, Owl Therapeutics Inc., University of Michigan Concussion Center, and Collaborative Neuropathology Network Characterizing Outcomes of TBI (CONNECT-TBI). He is a co-PI for the Long-term Impact of Military-relevant, Brain Injury Consortium – Chronic Effects of Neurotrauma Consortium (LIMBIC-CENC).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kwan, J, Sparrow, K, Facer-Irwin, E, Thandi, G, Fear, NT, and MacManus, D. Prevalence of intimate partner violence perpetration among military populations: a systematic review and meta-analysis. Aggress Violent Behav. (2020) 53:101419. doi: 10.1016/j.avb.2020.101419
2. Breiding, MJ, Basile, KC, Smith, SG, Black, MC, and Mahendra, RR. Intimate partner violence surveillance: Uniform definitions and recommended data elements, version 2.0. Atlanta (GA): National Center for Injury Prevention and Control, Centers for Disease Control and Prevention (2015).
3. Orsillo, SM, Weathers, FW, Litz, BT, Steinberg, HR, Huska, JA, and Keane, TM. Current and lifetime psychiatric disorders among veterans with war zone-related posttraumatic stress disorder. J Nerv Ment Dis. (1996) 184:307–13. doi: 10.1097/00005053-199605000-00007
4. Prigerson, HG, Maciejewski, PK, and Rosenheck, RA. Population attributable fractions of psychiatric disorders and behavioral outcomes associated with combat exposure among US men. Am J Public Health. (2002) 92:59–63. doi: 10.2105/AJPH.92.1.59
5. Taft, CT, Stalans, LJ, Pless, AP, Koenen, KC, King, LA, and King, DW. Risk factors for partner violence among a national sample of combat veterans. J Consult Clin Psychol. (2005) 73:151–9. doi: 10.1037/0022-006X.73.1.151
6. Maguen, S, Lucenko, BA, Reger, MA, Gahm, GA, Litz, BT, Seal, KH, et al. The impact of reported direct and indirect killing on mental health symptoms in Iraq war veterans. J Trauma Stress. (2010) 23:86–90. doi: 10.1002/jts.20434
7. Van Winkle, EP, and Safer, MA. Killing versus witnessing in combat trauma and reports of PTSD symptoms and domestic violence. J Trauma Stress. (2011) 24:107–10. doi: 10.1002/jts.20614
8. Neilson, EC, Gulati, NK, Stappenbeck, CA, George, WH, and Davis, KC. Emotion regulation and intimate partner violence perpetration in undergraduate samples: a review of the literature. Trauma Violence Abus. (2021) 24:576–96. doi: 10.1177/15248380211036063
9. Murphy, CM . Social information processing and the perpetration of intimate partner violence: it is (and isn’t) what you think. Psychol Viol. (2013) 3:212–7. doi: 10.1037/a0033344
10. Verfaellie, M, Lafleche, G, Spiro, A, Tun, C, and Bousquet, K. Chronic Postconcussion symptoms and functional outcomes in OEF/OIF veterans with self-report of blast exposure. J Int Neuropsychol Soc. (2013) 19:1–10. doi: 10.1017/S1355617712000902
11. Rosen, LN, Parmley, AM, Knudson, KH, and Fancher, P. Intimate partner violence among married male U.S. Army soldiers: ethnicity as a factor in self-reported perpetration and victimization. Violence Vict. (2002) 17:607–22. doi: 10.1891/vivi.17.5.607.33716
12. Brewster, AL, Milner, JS, Mollerstrom, WW, Saha, BT, and Harris, N. Evaluation of spouse abuse treatment: description and evaluation of the air force family advocacy programs for spouse physical abuse. Mil Med. (2002) 167:464–9. doi: 10.1093/milmed/167.6.464
13. Slep, AMS, Foran, HM, Heyman, RE, and Snarr, JD. USAF family advocacy research program. Identifying unique and shared risk factors for physical intimate partner violence and clinically-significant physical intimate partner violence. Aggress Behav. (2015) 41:227–41. doi: 10.1002/ab.21565
14. Zamorski, MA, and Wiens-Kinkaid, ME. Cross-sectional prevalence survey of intimate partner violence perpetration and victimization in Canadian military personnel. BMC Public Health. (2013) 13:1–18. doi: 10.1186/1471-2458-13-1019
15. Combs, HL, Berry, DTR, Pape, T, Babcock-Parziale, J, Smith, B, Schleenbaker, R, et al. The effects of mild traumatic brain injury, post-traumatic stress disorder, and combined mild traumatic brain injury/post-traumatic stress disorder on returning veterans. J Neurotrauma. (2015) 32:956–66. doi: 10.1089/neu.2014.3585
16. Vasterling, JJ, Aslan, M, Lee, LO, Proctor, SP, Ko, J, Jacob, S, et al. Longitudinal associations among posttraumatic stress disorder symptoms, traumatic brain injury, and neurocognitive functioning in Army soldiers deployed to the Iraq war. J Int Neuropsychol Soc. (2018) 24:311–23. doi: 10.1017/S1355617717001059
17. Lagarde, E, Salmi, L-R, Holm, LW, Contrand, B, Masson, F, Ribéreau-Gayon, R, et al. Association of symptoms following mild traumatic brain injury with posttraumatic stress disorder vs. postconcussion syndrome. JAMA Psychiatry. (2014) 71:1032–40. doi: 10.1001/jamapsychiatry.2014.666
18. Seal, KH, Bertenthal, D, Samuelson, K, Maguen, S, Kumar, S, and Vasterling, JJ. Association between mild traumatic brain injury and mental health problems and self-reported cognitive dysfunction in Iraq and Afghanistan veterans. J Rehabil Res Dev. (2016) 53:185–98. doi: 10.1682/JRRD.2014.12.0301
19. Gonschorek, AS, Schwenkreis, P, and Guthke, T. Psychische Störungen nach leichtem Schädel-Hirn-Trauma. Nervenarzt. (2016) 87:567–79. doi: 10.1007/s00115-016-0119-8
20. Selassie, AW, Zaloshnja, E, Langlois, JA, Miller, T, Jones, P, and Steiner, C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil. (2008) 23:123–31. doi: 10.1097/01.HTR.0000314531.30401.39
21. Cassidy, JD, Cancelliere, C, Carroll, LJ, Côté, P, Hincapié, CA, Holm, LW, et al. Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the international collaboration on mild traumatic brain injury prognosis. Arch Phys Med Rehabil. (2014) 95:S132–51. doi: 10.1016/j.apmr.2013.08.299
22. Portnoy, GA, Relyea, MR, Presseau, C, Orazietti, S, Martino, S, Brandt, CA, et al. Longitudinal analysis of persistent Postconcussion symptoms, probable TBI, and intimate partner violence perpetration among veterans. J Head Trauma Rehabil. (2022) 37:34–42. doi: 10.1097/HTR.0000000000000759
23. Kaufmann, E, Rojczyk, P, Sydnor, VJ, Guenette, JP, Tripodis, Y, Kaufmann, D, et al. Association of war Zone-Related Stress with Alterations in limbic Gray matter microstructure. JAMA Netw Open. (2022) 5:1–17. doi: 10.1001/jamanetworkopen.2022.31891
24. Sydnor, VJ, Bouix, S, Pasternak, O, Hartl, E, Levin-Gleba, L, Reid, B, et al. Mild traumatic brain injury impacts associations between limbic system microstructure and post-traumatic stress disorder symptomatology. NeuroImage Clin. (2020) 26:102190. doi: 10.1016/j.nicl.2020.102190
25. Berman, Z, Assaf, Y, Tarrasch, R, and Joel, D. Macro-and microstructural gray matter alterations in sexually assaulted women. J Affect Disord. (2020) 262:196–04. doi: 10.1016/j.jad.2019.10.024
26. Bouix, S, Pasternak, O, Rathi, Y, Pelavin, PE, Zafonte, R, and Shenton, ME. Increased Gray matter diffusion anisotropy in patients with persistent post-concussive symptoms following mild traumatic brain injury. PLoS One. (2013) 8:e66205. doi: 10.1371/journal.pone.0066205
27. RajMohan, V, and Mohandas, E. The limbic system. Indian J Psychiatry. (2007) 49:132–9. doi: 10.4103/0019-5545.33264
28. Coccaro, EF, McCloskey, MS, Fitzgerald, DA, and Phan, KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. (2007) 62:168–78. doi: 10.1016/j.biopsych.2006.08.024
29. Rosell, DR, and Siever, LJ. The neurobiology of aggression and violence. CNS Spectr. (2015) 20:254–79. doi: 10.1017/S109285291500019X
30. Siever, LJ . Neurobiology of aggression and violence. Am J Psychiatry. (2008) 165:429–42. doi: 10.1176/appi.ajp.2008.07111774
31. Yang, Y, Raine, A, Narr, KL, Colletti, P, and Toga, AW. Localization of deformations within the amygdala in individuals with psychopathy. Arch Gen Psychiatry. (2009) 66:986–94. doi: 10.1001/archgenpsychiatry.2009.110
32. Pardini, DA, Raine, A, Erickson, K, and Loeber, R. Lower amygdala volume in men is associated with childhood aggression, early psychopathic traits, and future violence. Biol Psychiatry. (2014) 75:73–80. doi: 10.1016/j.biopsych.2013.04.003
33. Bueso-Izquierdo, N, Verdejo-Román, J, Contreras-Rodríguez, O, Carmona-Perera, M, Pérez-García, M, and Hidalgo-Ruzzante, N. Are batterers different from other criminals? An fMRI study. Soc Cogn Affect Neurosci. (2016) 11:852–62. doi: 10.1093/scan/nsw020
34. Zhang, L, Kerich, M, Schwandt, ML, Rawlings, RR, McKellar, JD, Momenan, R, et al. Smaller right amygdala in Caucasian alcohol-dependent male patients with a history of intimate partner violence: a volumetric imaging study. Addict Biol. (2013) 18:537–47. doi: 10.1111/j.1369-1600.2011.00381.x
35. Sagi, Y, Tavor, I, Hofstetter, S, Tzur-Moryosef, S, Blumenfeld-Katzir, T, and Assaf, Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. (2012) 73:1195–03. doi: 10.1016/j.neuron.2012.01.025
36. Blumenfeld-Katzir, T, Pasternak, O, Dagan, M, and Assaf, Y. Diffusion MRI of structural brain plasticity induced by a learning and memory task. PLoS One. (2011) 6:e20678. doi: 10.1371/journal.pone.0020678
37. Sizonenko, SV, Camm, EJ, Garbow, JR, Maier, SE, Inder, TE, Williams, CE, et al. Developmental changes and injury induced disruption of the radial organization of the cortex in the immature rat brain revealed by in vivo diffusion tensor MRI. Cereb Cortex. (2007) 17:2609–17. doi: 10.1093/cercor/bhl168
38. Budde, MD, Janes, L, Gold, E, Turtzo, LC, and Frank, JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. (2011) 134:2248–60. doi: 10.1093/brain/awr161
39. Seehaus, A, Roebroeck, A, Bastiani, M, Fonseca, L, Bratzke, H, Lori, N, et al. Histological validation of high-resolution DTI in human post mortem tissue. Front Neuroanat. (2015) 9:1–12. doi: 10.3389/fnana.2015.00098
40. Bock, AS, Olavarria, JF, Leigland, LA, Taber, EN, Jespersen, SN, and Kroenke, CD. Diffusion tensor imaging detects early cerebral cortex abnormalities in neuronal architecture induced by bilateral neonatal enucleation: an experimental model in the ferret. Front Syst Neurosci. (2010) 4:4. doi: 10.3389/fnsys.2010.00149
41. Dean, JM, McClendon, E, Hansen, K, Azimi-Zonooz, A, Chen, K, Riddle, A, et al. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci Transl Med. (2013) 5:168ra7. doi: 10.1126/scitranslmed.3004669
42. Leigland, LA, Budde, MD, Cornea, A, and Kroenke, CD. Diffusion MRI of the developing cerebral cortical gray matter can be used to detect abnormalities in tissue microstructure associated with fetal ethanol exposure. NeuroImage. (2013) 83:1081–7. doi: 10.1016/j.neuroimage.2013.07.068
43. Laitinen, T, Sierra López, A, Bolkvadze, T, Pitkänen, A, and Gröhn, O. Diffusion tensor imaging detects chronic microstructural changes in white and grey matter after traumatic brain injury in rat. Front Neurosci. (2015) 9:9(MAR). doi: 10.3389/fnins.2015.00128
44. Koerte, IK, and Muehlmann, M. Diffusion tensor imaging In: MRI in psychiatry. Berlin, Heidelberg: Springer (2014)
45. Peterson, C, Kearns, MC, McIntosh, WL, Estefan, LF, Nicolaidis, C, McCollister, KE, et al. Lifetime economic burden of intimate partner violence among U.S. adults. Am J Prev Med. (2018) 55:433–44. doi: 10.1016/j.amepre.2018.04.049
46. McGlinchey, RE, Milberg, WP, Fonda, JR, and Fortier, CB. A methodology for assessing deployment trauma and its consequences in OEF/OIF/OND veterans: the TRACTS longitudinal prospective cohort study. Int J Methods Psychiatr Res. (2017) 26:1–15. doi: 10.1002/mpr.1556
47. Straus, MA, Hamby, SL, Boney-Mccoy, S, and Sugarman, DB. The revised conflict tactics scales (CTS2). J Fam Issues. (1996) 17:283–16. doi: 10.1177/019251396017003001
48. King, LA, King, DW, Vogt, DS, Knight, J, and Samper, RE. Deployment risk and resilience inventory: a collection of measures for studying deployment-related experiences of military personnel and veterans. Mil Psychol. (2006) 18:89–120. doi: 10.1207/s15327876mp1802_1
49. Weathers, FW, Bovin, MJ, Lee, DJ, Sloan, DM, Schnurr, PP, Kaloupek, DG, et al. The clinician-administered PTSD scale for DSM–5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess. (2018) 30:383–95. doi: 10.1037/pas0000486
50. First, MB, Gibbon, M, Spitzer, RL, and Williams, JBW. Structured clinical interview for DSM-IV Axis I disorders (SCID-I). Washington, DC: American Psychiatric Press (1997).
51. Osman, A, Wong, JL, Bagge, CL, Freedenthal, S, Gutierrez, PM, and Lozano, G. The depression anxiety stress Scales-21 (DASS-21): further examination of dimensions, scale reliability, and correlates. J Clin Psychol. (2012) 68:1322–38. doi: 10.1002/jclp.21908
52. Fortier, CB, Amick, MM, Grande, L, McGlynn, S, Kenna, A, Morra, L, et al. The Boston assessment of traumatic brain injury-lifetime (BAT-L) semistructured interview: evidence of research utility and validity. J Head Trauma Rehabil. (2014) 29:89–98. doi: 10.1097/HTR.0b013e3182865859
53. Schumm, JA, Gore, WL, Chard, KM, and Meyer, EC. Examination of the World Health Organization disability assessment system as a measure of disability severity among veterans receiving cognitive processing therapy. J Trauma Stress. (2017) 30:704–9. doi: 10.1002/jts.22243
54. Greve, DN, and Fischl, B. Accurate and robust brain image alignment using boundary-based registration. NeuroImage. (2009) 48:63–72. doi: 10.1016/j.neuroimage.2009.06.060
55. Jenkinson, M, Bannister, P, Brady, M, and Smith, S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. (2002) 17:825–41. doi: 10.1006/nimg.2002.1132
56. Fedorov, A, Beichel, R, Kalpathy-Cramer, J, Finet, J, Fillion-Robin, JC, Pujol, S, et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging. (2012) 30:1323–41. doi: 10.1016/j.mri.2012.05.001
57. Destrieux, C, Fischl, B, Dale, A, and Halgren, E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. (2010) 53:1–15. doi: 10.1016/J.NEUROIMAGE.2010.06.010
58. Pasternak, O, Sochen, N, Gur, Y, Intrator, N, and Assaf, Y. Free water elimination and mapping from diffusion MRI. Magn Reson Med. (2009) 62:717–30. doi: 10.1002/MRM.22055
59. Metzler-Baddeley, C, O’Sullivan, MJ, Bells, S, Pasternak, O, and Jones, DK. How and how not to correct for CSF-contamination in diffusion MRI. NeuroImage. (2012) 59:1394–03. doi: 10.1016/J.NEUROIMAGE.2011.08.043
60. Guenette, JP, Stern, RA, Tripodis, Y, Chua, AS, Schultz, V, Sydnor, VJ, et al. Automated versus manual segmentation of brain region volumes in former football players. NeuroImage Clin. (2018) 18:888–96. doi: 10.1016/j.nicl.2018.03.026
62. Hochberg, Y, and Benjamini, Y. More powerful procedures for multiple significance testing. Stat Med. (1990) 9:811–8. doi: 10.1002/sim.4780090710
63. Thomas, JL, Wilk, JE, Riviere, LA, McGurk, D, Castro, CA, and Hoge, CW. Prevalence of mental health problems and functional impairment among active component and national guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. (2010) 67:614–23. doi: 10.1001/archgenpsychiatry.2010.54
64. Trevillion, K, Williamson, E, Thandi, G, Borschmann, R, Oram, S, and Howard, LM. A systematic review of mental disorders and perpetration of domestic violence among military populations. Soc Psychiatry Psychiatr Epidemiol. (2015) 50:1329–46. doi: 10.1007/s00127-015-1084-4
65. Jakupcak, M, Conybeare, D, Phelps, L, Hunt, S, Holmes, HA, Felker, B, et al. Anger, hostility, and aggression among Iraq and Afghanistan war veterans reporting PTSD and subthreshold PTSD. J Trauma Stress. (2007) 20:945–54. doi: 10.1002/jts.20258
66. Marshall, AD, Panuzio, J, and Taft, CT. Intimate partner violence among military veterans and active duty servicemen. Clin Psychol Rev. (2005) 25:862–76. doi: 10.1016/j.cpr.2005.05.009
67. Bell, KM, and Orcutt, HK. Posttraumatic stress disorder and male-perpetrated intimate partner violence. J Am Med Assoc. (2009) 302:562–4. doi: 10.1001/jama.2009.1126
68. Birkley, EL, Eckhardt, CI, and Dykstra, RE. Posttraumatic stress disorder symptoms, intimate partner violence, and relationship functioning: a Meta-analytic review. J Trauma Stress. (2016) 29:397–05. doi: 10.1002/jts.22129
69. Taft, CT, Kaloupek, DG, Schumm, JA, Marshall, AD, Panuzio, J, King, DW, et al. Posttraumatic stress disorder symptoms, physiological reactivity, alcohol problems, and aggression among military veterans. J Abnorm Psychol. (2007) 116:498–07. doi: 10.1037/0021-843X.116.3.498
70. Chemtob, CM, Novaco, RW, Hamada, RS, Gross, DM, and Smith, G. Anger regulation deficits in combat-related posttraumatic stress disorder. J Trauma Stress. (1997) 10:17–36. doi: 10.1023/A:1024852228908
71. Sherin, J, and Nemeroff, C. Post-traumatic stress disorder: the neurobiological impact of psychological trauma. Dialogues Clin Neurosci. (2011) 13:263–78. doi: 10.31887/DCNS.2011.13.2/jsherin
72. Lee, TMC, Chan, SC, and Raine, A. Hyperresponsivity to threat stimuli in domestic violence offenders: a functional magnetic resonance imaging study. J Clin Psychiatry. (2009) 70:36–45. doi: 10.4088/JCP.08m04143
73. Taft, CT, Schumm, JA, Marshall, AD, Panuzio, J, and Holtzworth-Munroe, A. Family-of-origin maltreatment, posttraumatic stress disorder symptoms, social information processing deficits, and relationship abuse perpetration. J Abnorm Psychol. (2008) 117:637–46. doi: 10.1037/0021-843X.117.3.637
74. Miles, SR, Menefee, DS, Wanner, J, Teten Tharp, A, and Kent, TA. The relationship between emotion dysregulation and impulsive aggression in veterans with posttraumatic stress disorder symptoms. J Interpers Violence. (2016) 31:1795–16. doi: 10.1177/0886260515570746
75. Miethe, S, Wigger, J, Wartemann, A, Fuchs, FO, and Trautmann, S. Posttraumatic stress symptoms and its association with rumination, thought suppression and experiential avoidance: a systematic review and Meta-analysis. J Psychopathol Behav Assess. (2023) 45:480–95. doi: 10.1007/s10862-023-10022-2
76. Smith, HL, Summers, BJ, Dillon, KH, Macatee, RJ, and Cougle, JR. Hostile interpretation bias in depression. J Affect Disord. (2016) 203:9–13. doi: 10.1016/j.jad.2016.05.070
77. Oram, S, Trevillion, K, Khalifeh, H, Feder, G, and Howard, LM. Systematic review and meta-analysis of psychiatric disorder and the perpetration of partner violence. Epidemiol Psychiatr Sci. (2014) 23:361–76. doi: 10.1017/S2045796013000450
78. Barros-Gomes, P, Kimmes, J, Smith, E, Cafferky, B, Stith, S, Durtschi, J, et al. The role of depression in the relationship between psychological and physical intimate partner violence. J Interpers Violence. (2019) 34:3936–60. doi: 10.1177/0886260516673628
79. Yu, R, Nevado-Holgado, AJ, Molero, Y, D’Onofrio, BM, Larsson, H, Howard, LM, et al. Mental disorders and intimate partner violence perpetrated by men towards women: a Swedish population-based longitudinal study. PLoS Med. (2019) 16:e1002995–19. doi: 10.1371/journal.pmed.1002995
80. Van Dorn, R, Volavka, J, and Johnson, N. Mental disorder and violence: is there a relationship beyond substance use? Soc Psychiatry Psychiatr Epidemiol. (2012) 47:487–03. doi: 10.1007/s00127-011-0356-x
81. Fals-Stewart, W, Leonard, KE, and Birchler, GR. The occurrence of male-to-female intimate partner violence on days of men’s drinking: the moderating effects of antisocial personality disorder. J Consult Clin Psychol. (2005) 73:239–48. doi: 10.1037/0022-006X.73.2.239
82. Cauley, M (2012) Posttraumatic stress disorder and co-occurring substance use disorders-advances in assessment and treatment.Pdf.
83. Smith Slep, AM, Foran, HM, and Heyman, REUnited States air force family advocacy research program. An ecological model of intimate partner violence perpetration at different levels of severity. J Fam Psychol. (2014) 28:470–82. doi: 10.1037/a0037316
84. Foran, HM, Heyman, RE, Slep, AMS, and Snarr, JD. Hazardous alcohol use and intimate partner violence in the military: understanding protective factors. Psychol Addict Behav. (2012) 26:471–83. doi: 10.1037/a0027688
85. Foran, HM, and O’Leary, KD. Problem drinking, jealousy, and anger control: variables predicting physical aggression against a partner. J Fam Violence. (2008) 23:141–8. doi: 10.1007/s10896-007-9136-5
86. Savarese, VW, Suvak, MK, King, LA, and King, DW. Relationships among alcohol use, hyperarousal, and marital abuse and violence in Vietnam veterans. J Trauma Stress. (2001) 14:717–32. doi: 10.1023/A:1013038021175
87. Morris, DH, Spencer, RJ, Winters, JJ, Walton, MA, Friday, S, and Chermack, ST. Association of persistent postconcussion symptoms with violence perpetration among substance-using veterans. Psychol Violence. (2019) 9:167–76. doi: 10.1037/vio0000175
88. Crane, CA, and Easton, CJ. Physical health conditions and intimate partner violence perpetration among offenders with alcohol use diagnoses. J Interpers Violence. (2017) 32:1678–91. doi: 10.1177/0886260515590124
89. Berkowitz, L . On the formation and regulation of anger and aggression: a cognitive-neoassociationistic analysis. Am Psychol. (1990) 45:494–03. doi: 10.1037/0003-066X.45.4.494
90. Watkins, LE, Schumacher, JA, and Coffey, SF. A preliminary investigation of the relationship between emotion dysregulation and partner violence perpetration among individuals with PTSD and alcohol dependence. J Aggress Maltreatment Trauma. (2016) 25:305–14. doi: 10.1080/10926771.2015.1129657
91. Shorey, RC, Brasfield, H, Febres, J, and Stuart, GL. An examination of the association between difficulties with emotion regulation and dating violence perpetration. J Aggress Maltreat Trauma. (2011) 20:870–85. doi: 10.1080/10926771.2011.629342
92. van der Horn, HJ, Out, ML, de Koning, ME, Mayer, AR, Spikman, JM, Sommer, IE, et al. An integrated perspective linking physiological and psychological consequences of mild traumatic brain injury. J Neurol. (2020) 267:2497–06. doi: 10.1007/s00415-019-09335-8
93. da Cunha-Bang, S, Fisher, PM, Hjordt, LV, Perfalk, E, Persson Skibsted, A, Bock, C, et al. Violent offenders respond to provocations with high amygdala and striatal reactivity. Soc Cogn Affect Neurosci. (2017) 12:802–10. doi: 10.1093/scan/nsx0006
94. Siep, N, Tonnaer, F, van de Ven, V, Arntz, A, Raine, A, and Cima, M. Anger provocation increases limbic and decreases medial prefrontal cortex connectivity with the left amygdala in reactive aggressive violent offenders. Brain Imaging Behav. (2019) 13:1311–23. doi: 10.1007/s11682-018-9945-6
95. Shin, LM, Orr, SP, Carson, MA, Rauch, SL, Macklin, ML, Lasko, NB, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. (2004) 61:168–76. doi: 10.1001/archpsyc.61.2.168
96. Britton, JC, Phan, KL, Taylor, SF, Fig, LM, and Liberzon, I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. (2005) 57:832–40. doi: 10.1016/j.biopsych.2004.12.025
97. Lanius, R, Williamson, PC, Hopper, J, Densmore, M, Boksman, K, Gupta, MA, et al. Recall of emotional states in posttraumatic stress disorder: an fMRI investigation. Biol Psychiatry. (2003) 53:204–10. doi: 10.1016/S0006-3223(02)01466-X
98. Shin, LM, Wright, CI, Cannistraro, PA, Wedig, MM, McMullin, K, Martis, B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. (2005) 62:273–81. doi: 10.1001/archpsyc.62.3.273
99. Bremner, JD, Vermetten, E, Vythilingam, M, Afzal, N, Schmahl, C, Elzinga, B, et al. Neural correlates of the classic color and emotional stroop in women with abuse-related posttraumatic stress disorder. Biol Psychiatry. (2004) 55:612–20. doi: 10.1016/j.biopsych.2003.10.001
100. Shin, LM, Rauch, SL, and Pitman, RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. (2006) 1071:67–79. doi: 10.1196/annals.1364.007
101. Phelps, EA, and LeDoux, JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. (2005) 48:175–87. doi: 10.1016/j.neuron.2005.09.025
102. McCorkle, TA, Barson, JR, and Raghupathi, R. A role for the amygdala in impairments of affective behaviors following mild traumatic brain injury. Front Behav Neurosci. (2021) 15:1–14. doi: 10.3389/fnbeh.2021.601275
103. Han, K, Chapman, SB, and Krawczyk, DC. Altered amygdala connectivity in individuals with chronic traumatic brain injury and comorbid depressive symptoms. Front Neurol. (2015) 6:231. doi: 10.3389/fneur.2015.00231
104. McEwen, BS, Nasca, C, and Gray, JD. Stress effects on neuronal structure: Hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. (2016) 41:3–23. doi: 10.1038/npp.2015.171
105. Lupien, SJ, Juster, R-P, Raymond, C, and Marin, M-F. The effects of chronic stress on the human brain: from neurotoxicity, to vulnerability, to opportunity. Front Neuroendocrinol. (2018) 49:91–05. doi: 10.1016/j.yfrne.2018.02.001
106. Wojtowicz, M, Gardner, AJ, Stanwell, P, Zafonte, R, Dickerson, BC, and Iverson, GL. Cortical thickness and subcortical brain volumes in professional rugby league players. NeuroImage Clin. (2018) 18:377–81. doi: 10.1016/j.nicl.2018.01.005
107. Mu, W, Catenaccio, E, and Lipton, ML. Neuroimaging in blast-related mild traumatic brain injury. J Head Trauma Rehabil. (2017) 32:55–69. doi: 10.1097/HTR.0000000000000213
108. Zagorchev, L, Meyer, C, Stehle, T, Wenzel, F, Young, S, Peters, J, et al. Differences in regional brain volumes two months and one year after mild traumatic brain injury. J Neurotrauma. (2016) 33:29–34. doi: 10.1089/neu.2014.3831
109. McAllister, TW, and Stein, MB. Effects of psychological and biomechanical trauma on brain and behavior. Ann N Y Acad Sci. (2010) 1208:46–57. doi: 10.1111/j.1749-6632.2010.05720.x
110. Dieter, JN, and Engel, SD. Traumatic brain injury and posttraumatic stress disorder: comorbid consequences of war. Neurosci Insights. (2019) 14:1–17. doi: 10.1177/1179069519892933
111. McAllister, TW . Neurobiological consequences of traumatic brain injury. Dialogues Clin Neurosci. (2011) 13:287–00. doi: 10.31887/DCNS.2011.13.2/tmcallister
112. Ressler, KJ . Amygdala activity, fear, and anxiety: modulation by stress. Biol Psychiatry. (2010) 67:1117–9. doi: 10.1016/j.biopsych.2010.04.027
113. Opitz, B . Memory function and the hippocampus. Hippocampus Clin Neurosci. (2014) 34:51–9. doi: 10.1159/000356422
114. Covell, CN, Huss, MT, and Langhinrichsen-Rohling, J. Empathic deficits among male batterers: a multidimensional approach. J Fam Violence. (2007) 22:165–74. doi: 10.1007/s10896-007-9066-2
115. Maloney, MA, Eckhardt, CI, and Oesterle, DW. Emotion regulation and intimate partner violence perpetration: a meta-analysis. Clin Psychol Rev. (2023) 100:102238. doi: 10.1016/j.cpr.2022.102238
116. Markowitsch, HJ . Differential contribution of right and left amygdala to affective information processing. Behav Neurol. (1998) 11:233–44. doi: 10.1155/1999/180434
117. Morris, JS, Ohman, A, and Dolan, RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. (1998) 393:467–70. doi: 10.1038/30976
119. Fleming, PJ, McCleary-Sills, J, Morton, M, Levtov, R, Heilman, B, and Barker, G. Risk factors for Men’s lifetime perpetration of physical violence against intimate partners: results from the international men and gender equality survey (IMAGES) in eight countries. PLoS One. (2015) 10:e0118639. doi: 10.1371/JOURNAL.PONE.0118639
120. Fleming, PJ, Gruskin, S, Rojo, F, and Dworkin, SL. Men’s violence against women and men are inter-related: recommendations for simultaneous intervention. Soc Sci Med. (2015) 146:249–56. doi: 10.1016/J.SOCSCIMED.2015.10.021
121. Miles, SR, Dillon, KH, Jacoby, VM, Hale, WJ, Dondanville, KA, Wachen, JS, et al. Changes in anger and aggression after treatment for PTSD in active duty military. J Clin Psychol. (2020) 76:493–507. doi: 10.1002/jclp.22878
122. Berke, DS, Carney, JR, Rusowicz-Orazem, L, Kline, NK, Grunthal, B, Mintz, J, et al. Parameters of aggressive behavior in a treatment-seeking sample of military personnel: a secondary analysis of three randomized controlled trials of evidence-based PTSD treatments. Behav Ther. (2021) 52:136–48. doi: 10.1016/j.beth.2020.03.007
123. Fortier, CB, Kenna, A, Dams-OʼConnor, K, Fonda, J, Levin, LK, Hursh, C, et al. Feasibility of a skills-based group reintegration workshop for OEF/OIF veterans: STEP-home. J Head Trauma Rehabil. (2018) 33:E17–23. doi: 10.1097/HTR.0000000000000362
124. Zotev, V, Krueger, F, Phillips, R, Alvarez, RP, Simmons, WK, Bellgowan, P, et al. Self-regulation of amygdala activation using real-time FMRI neurofeedback. PLoS One. (2011) 6:e24522. doi: 10.1371/journal.pone.0024522
125. Barreiros, AR, Almeida, I, Baía, BC, and Castelo-Branco, M. Amygdala modulation during emotion regulation training with fMRI-based neurofeedback. Front Hum Neurosci. (2019) 13:89. doi: 10.3389/fnhum.2019.00089
126. Nicholson, AA, Rabellino, D, Densmore, M, Frewen, PA, Paret, C, Kluetsch, R, et al. The neurobiology of emotion regulation in posttraumatic stress disorder: amygdala downregulation via real-time fMRI neurofeedback. Hum Brain Mapp. (2017) 38:541–60. doi: 10.1002/hbm.23402
127. Cooke, EM, Connolly, EJ, Boisvert, DL, and Hayes, BE. A systematic review of the biological correlates and consequences of childhood maltreatment and adverse childhood experiences. Trauma Violence Abuse. (2023) 24:156–73. doi: 10.1177/15248380211021613
Keywords: perpetration of violence, diffusion magnetic resonance imaging, veterans, intimate partner violence, perpetration and victimization, traumatic brain injury, limbic system, psychiatric disorders
Citation: Rojczyk P, Heller C, Seitz-Holland J, Kaufmann E, Sydnor VJ, Berger L, Pankatz L, Rathi Y, Bouix S, Pasternak O, Salat D, Hinds SR, Esopenko C, Fortier CB, Milberg WP, Shenton ME and Koerte IK (2024) Intimate partner violence perpetration among veterans: associations with neuropsychiatric symptoms and limbic microstructure. Front. Neurol. 15:1360424. doi: 10.3389/fneur.2024.1360424
Edited by:
Angela M. Boutte, Aries Biotechnologies, United StatesReviewed by:
Sarah C. Hellewell, Curtin University, AustraliaAlba Scerrati, University of Ferrara, Italy
Copyright © 2024 Rojczyk, Heller, Seitz-Holland, Kaufmann, Sydnor, Berger, Pankatz, Rathi, Bouix, Pasternak, Salat, Hinds, Esopenko, Fortier, Milberg, Shenton and Koerte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Philine Rojczyk, cGhpbGluZS5yb2pjenlrQG1lZC51bmktbXVlbmNoZW4uZGU=
 Philine Rojczyk
Philine Rojczyk Carina Heller
Carina Heller Johanna Seitz-Holland
Johanna Seitz-Holland Elisabeth Kaufmann
Elisabeth Kaufmann Valerie J. Sydnor1
Valerie J. Sydnor1 Yogesh Rathi
Yogesh Rathi Sylvain Bouix
Sylvain Bouix Ofer Pasternak
Ofer Pasternak David Salat
David Salat Sidney R. Hinds
Sidney R. Hinds Carrie Esopenko
Carrie Esopenko Martha E. Shenton
Martha E. Shenton Inga K. Koerte
Inga K. Koerte