
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 27 March 2024
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1355437
Voltage gated calcium channels (VGCCs) play a critical role in neural transmission. Antibodies that target these ion channels can disrupt cellular signal transmission resulting in various clinical presentations. VGCC antibodies are most commonly associated with paraneoplastic syndromes such as Lambert-Eatons myasthenic syndrome. Here, we report a 47-year-old female with Stage IV appendiceal adenocarcinoma status post appendectomy and right hemicolectomy, who presented with progressive memory impairment, aphasia, ataxia, weakness, and headache. Neurologic exam was notable for right-sided parietal drift, decreased right arm swing, and ataxia of the bilateral upper extremities, more prominent on the right side. MRI of the brain with and without contrast was unremarkable. Cerebrospinal fluid (CSF) was notable for an elevated myelin basic protein (4.9 ng/mL, normal reference 0.0–3.7 ng/mL) with normal cell count, flow cytometry, and cytology. An extensive serum autoimmune neurology antibody evaluation revealed elevated VGCC autoantibodies (observed value: 96.1 pmol/L, normal range 0.0–30.0 pmol/L). A diagnosis of paraneoplastic voltage gated calcium channel antibodies secondary to appendiceal adenocarcinoma was made. The patient was treated with five exchanges with plasmapheresis over 10 days with significant clinical improvement in her symptoms. Upon literature review, this would be the first reported case of VGCC antibodies associated with appendiceal adenocarcinoma.
Voltage gated calcium channels play a critical role in cellular signaling and neural transmission. When activated, these ion channels trigger calcium influx into the cell, which triggers neurotransmitter release (1). This sequence can have a variety of effects on the post-synaptic cell, including muscle contraction in skeletal, cardiac, and smooth muscle cells. VGCCs also play a role in hormone secretion, gene expression, enzyme activation, and many other physiologic processes (1). Lambert-Eaton myasthenia syndrome (LEMS) is an autoimmune neuromuscular junction disorder where antibodies are produced against VGCCs, which can manifest as a paraneoplastic syndrome most commonly associated with small cell lung cancer (SCLC). We present a unique case of VGCC antibodies associated with appendiceal adenocarcinoma.
A 47-year-old female with a history of migraines initially presented with progressive right lower quadrant (RLQ) pain radiating to the periumbilical region that partially improved with antacids. CT scan showed perforated appendicitis with a large RLQ abscess (Figure 1). Patient was treated conservatively with antibiotics and IR guided drainage of the abscess. The patient then underwent a laparoscopic appendectomy with biopsy. The histopathological specimen revealed a positive KRAS mutation and well-differentiated grade I invasive appendiceal adenocarcinoma measuring 3.5 cm with invasion of the visceral peritoneum. Lab results were significant for a carcinoembryonic antigen (CEA) titer level of 9.0 ng/mL (normal reference 0.0–3.0 ng/mL). Further investigation with PET imaging showed residual adenocarcinoma in the terminal ileum, and the patient subsequently underwent a laparoscopic right hemicolectomy. Pathological analysis of 24 lymph nodes were negative for metastatic disease but invasion to the visceral peritoneum and abdominal wall was noted. The staging of the tumor was pT4a, pN0, pM1a (clinically Stage IV). Follow-up PET scan was negative for any local recurrence or distant metastases.
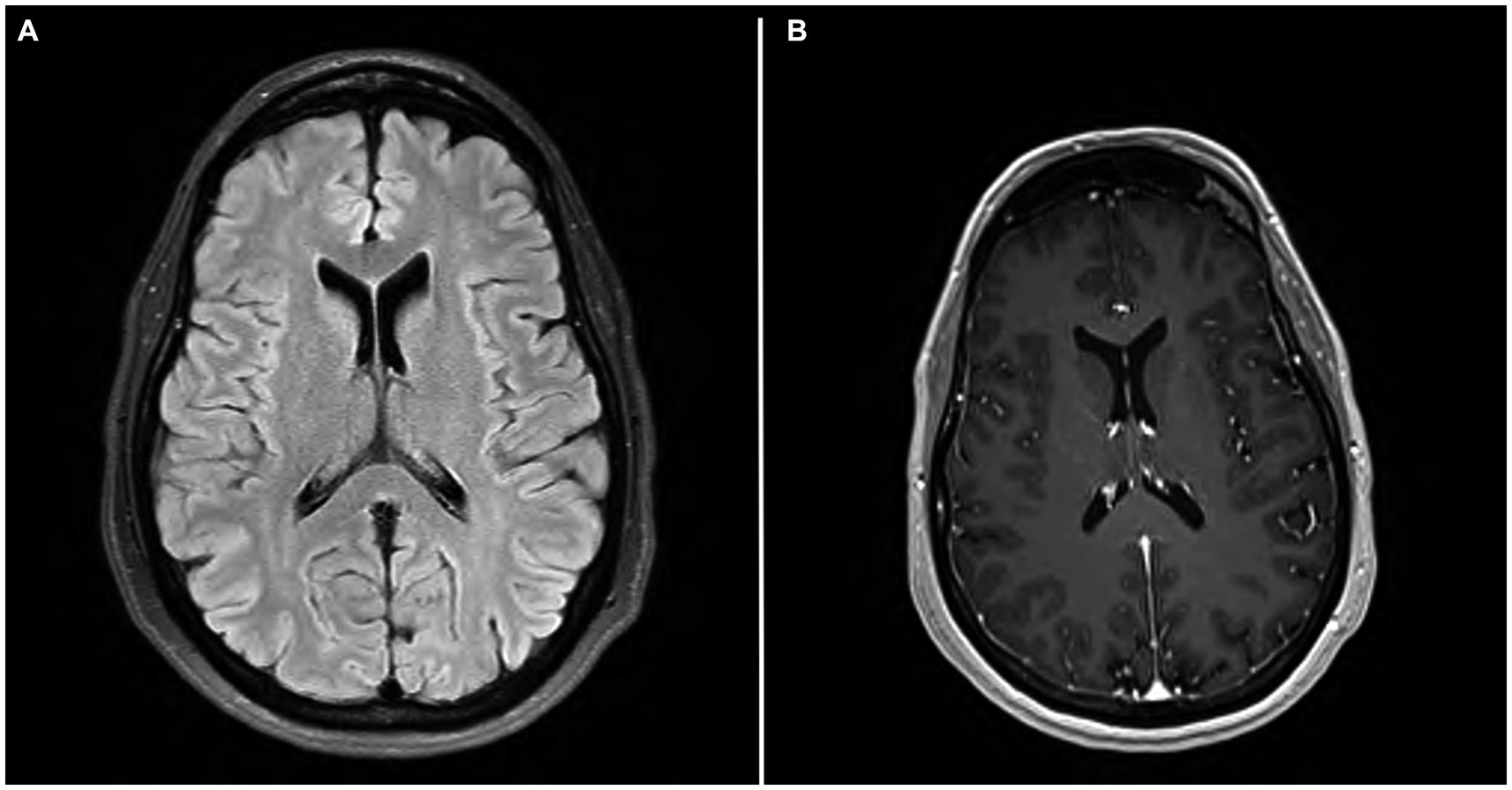
Figure 1. (A) MRI brain axial T2 FLAIR. (A) showing scattered minimal T2 and FLAIR hyperintensities in the periventricular and subcortical white matter. (B) MRI brain axial T1 with contrast showing no evidence of enhancement.
Two months after surgical intervention, the patient presented with fatigue, progressive memory impairment, concentration difficulties, issues with balance, weakness, and bilateral frontal headaches. Both short term and long-term memory was impaired, along with working memory, procedural memory and declarative memory. The patient was no longer able to perform routine tasks at work and instrumental activities of daily living such as grocery shopping were no longer possible. She had episodes of aphasia, which she described as difficulty getting words out that would last a few hours and spontaneously resolve. She had two falls recently, which she attributed to worsening imbalance, clumsiness, and weakness. She also reported daily tension-like headaches without pertinent associations.
Mental status exam was significant for poor concentration, inability to perform serial 7’s, one out of 3 memory recall after 5 min, and decreased processing speed. Neurologic exam was significant for parietal drift of the right arm, decreased right arm swing, and ataxia of the bilateral upper extremities, which was more pronounced on the right side. Additionally, the patient exhibited a broad-based gait. Bilateral biceps, triceps, brachioradialis, patellar, and Achilles reflexes were 2+ and symmetric. Romberg test was negative. No sensory deficits or other focal neurologic deficits were noted. MRI brain with and without IV contrast was unremarkable for evidence of brain metastasis; findings of scattered minimal T2 and FLAIR hyperintensities in the periventricular and subcortical white matter, presumed to be secondary to sequelae of headaches, were noted (Figure 2). The CSF analysis demonstrated normal glucose, cell count, and total protein. CSF findings (Table 1) were notable for rare B cells, presence of one oligoclonal band, and elevated myelin basic protein (4.9 ng/mL, normal reference 0.0–3.7 ng/mL). CSF flow cytometry and cytology studies were unremarkable. In view of recent diagnosis of appendiceal adenocarcinoma, paraneoplastic syndromes of the nervous system were considered. An autoimmune neurology antibody (Table 2) evaluation revealed elevated antibodies against VGCC (observed value: 96.1 pmol/L, normal range 0.0–30.0 pmol/L). VGCC antibodies secondary to paraneoplastic appendiceal adenocarcinoma was made. The antibody analysis was negative for Anti-HU, Anti-RI, Anti-YO, AMPA, NMDA, and GABA.
Patient was treated with plasma exchange (PLEX). A total of five exchanges (3 to 5 L of plasma each) over the next 10 days was performed. Within days of completing PLEX treatment, patient had significant improvement in her ataxia, weakness, and memory/concentration difficulties. Repeat neurologic exam showed full strength of all extremities, no signs of ataxia in the bilateral upper extremities, and normal gait. Patient was discharged home after completion of PLEX treatment. Patient was followed closely for 1 year with no recurrence of symptoms and repeat VGCC antibody testing was unremarkable (see Figures 1, 2).
VGCCs are located on the presynaptic terminal of the neural junction of many different types of cells, including skeletal muscle, cardiac muscle, smooth muscle, endocrine cells, and numerous others. The activation of VGCCs by action potentials allows calcium influx into the cell, which triggers neurotransmitter release and subsequent depolarization of the post synaptic membrane. This depolarization is responsible for many physiologic processes, including muscle contraction. VGCCs are divided into five types L, P/Q, N, R, T based on tissue and pharmacological properties (2) (see Tables 1, 2).
Antibodies against VGCCs are most commonly associated with LEMS but have also been linked to paraneoplastic cerebellar degeneration (PCD) and malignant hyperthermia. SCLC is the most common underlying malignancy noted in patients with VGCC antibodies. The rarely reported case suggests an association with non-SCLC, prostate cancer, and thymoma in adults (3–5). VGCC antibodies have also been reported in neuroblastoma and lymphoproliferative malignancy in children. Upon literature review, there has never been a reported case of VGCC antibodies associated with appendiceal adenocarcinoma. This would be the first noted case thus far.
The prevalence of VGCC antibodies in asymptomatic patients without known cancer is less than 2% (6, 7). Neoplasms of the appendix are extremely rare with an estimated rate of 0.12–2.6 cases per million people (8). There have been few isolated case reports of other paraneoplastic syndromes such as Myasthenia gravis and amphiphysin-IgG autoimmunity associated with appendiceal adenocarcinoma (8, 9). Most studies regarding LEMS as a paraneoplastic syndrome have been conducted in patients with SCLC. It has been shown that functional VGCCs are expressed on the surface membranes of cancer cells in SCLC which provides a probable mechanism for antibody development (10).
The management of paraneoplastic VGCC antibodies revolves around treatment of the underlying cancer. Stabilizing symptoms requires adequate tumor management with or without adjuvant therapy (11). In patients with malignancy-induced LEMS characterized by moderate–severe weakness that hinders daily function, the cornerstone of management is adequate immunomodulatory treatment along with treatment of the primary malignancy. Intravenous immunoglobulin therapy has been utilized in various cases of myasthenia gravis and has also been shown to have benefit in cases involving LEMS by reducing the number of VGCC antibodies in addition to clinical improvement of symptoms (12, 13). These studies suggest that long-term follow up with IVIG showed improvement in PEFR and electrophysiological measurements, and limb strength. These improvements were maintained at a 2 year follow-up period (14). The usual course of IVIG is a total dosage of 2 g/kg given over 2–5 days with maintenance therapy requiring repeat infusions at 4–12 week intervals showing satisfactory results. However, the peak effects of IVIG were seen to occur at 2–4 weeks, which is followed by a gradual return of muscle weakness (12, 15, 16).
Plasma exchange is another therapy for antibody-mediated disorders and has been utilized in several cases of LEMS (17–20). Typical protocol for plasmapheresis dictates 5 exchanges of approximately 3–5 L of plasma over the course of 1–2 weeks, similar to what was done for our patient. However, data suggests that the beneficial effects of plasmapheresis, which lead to clinical and electrophysiologic improvements, maybe short-lived. Trials involving combination of plasmapheresis with immunosuppressive therapy such as prednisone and azathioprine showed greater improvement than either regimen alone (20). Immunosuppressive therapy alone with agents such as prednisone, azathioprine, mycophenolate mofetil, cyclosporine, and even rituximab in refractory cases has been documented. However, the side effects associated with these agents require periodic surveillance and regular laboratory monitoring (21–23). Our patient did not have recurrence of symptoms during her 1 year follow up and repeat levels of VGCC antibodies were within the reference range. As with many paraneoplastic syndromes, the prognosis of these patients depends on the treatment and/or progression of the underlying neoplasm.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
GP: Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing. AB: Writing – original draft, Writing – review & editing. AP: Writing – original draft, Writing – review & editing. VB: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Flavell, SW, and Greenberg, ME. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci. (2008) 31:563–90. doi: 10.1146/annurev.neuro.31.060407.125631
2. Catterall, WA . Voltage-gated calcium channels. Cold Spring Harb Perspect Biol. (2011) 3:a003947. doi: 10.1101/cshperspect.a003947
3. Bekircan-Kurt, CE, Derle Çiftçi, E, Kurne, AT, and Anlar, B. Voltage gated calcium channel antibody-related neurological diseases. World J Clin Cases. (2015) 3:293–00. doi: 10.12998/wjcc.v3.i3.293
4. Wirtz, PW, Smallegange, TM, Wintzen, AR, and Verschuuren, JJ. Differences in clinical features between the Lambert-Eaton myasthenic syndrome with and without cancer: an analysis of 227 published cases. Clin Neurol Neurosurg. (2002) 104:359–63. doi: 10.1016/S0303-8467(02)00054-9
5. Benatar, M, Blaes, F, Johnston, I, Wilson, K, Vincent, A, Beeson, D, et al. Presynaptic neuronal antigens expressed by a small cell lung carcinoma cell line. J Neuroimmunol. (2001) 113:153–62. doi: 10.1016/s0165-5728(00)00431-8
6. Zalewski, NL, Lennon, VA, Lachance, DH, Klein, CJ, Pittock, SJ, and Mckeon, A. P/Q- and N-type calcium-channel antibodies: Oncological, neurological, and serological accompaniments. Muscle Nerve. (2016) 54:220–7. doi: 10.1002/mus.25027
7. Connor, SJ, Hanna, GB, and Frizelle, FA. Appendiceal tumors: retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum. (1998) 41:75–80. doi: 10.1007/BF02236899
8. Jayarangaiah, A, and Theetha, KP. Lambert Eaton Myasthenic Syndrome. StatPearls. Treasure Island (FL): StatPearls Publishing (2023).
9. Lanciano, N, Lawson, V, and Arnold, W. Paraneoplastic myasthenia gravis associated with Appendiceal mucinous neoplasm. Neurology. (2014) 82:P2093. doi: 10.1186/s12883-021-02311-8
10. Mareska, M, and Gutmann, L. Lambert–Eaton myasthenic syndrome. Semin Neurol. (2004) 24:149–53. doi: 10.1055/s-2004-830900
11. Dalmau, J, and Rosenfeld, MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. (2008) 7:327–40. doi: 10.1016/S1474-4422(08)70060-7
12. Bain, PG, Motomura, M, Newsom-Davis, J, Misbah, SA, Chapel, HM, Lee, ML, et al. Effects of intravenous immunoglobulin on muscle weakness and calcium-channel autoantibodies in the Lambert-Eaton myasthenic syndrome. Neurology. (1996) 47:678–83. doi: 10.1212/wnl.47.3.678
13. Keogh, M, Sedehizadeh, S, and Maddison, P. Treatment for Lambert-Eaton myasthenic syndrome. Cochrane Database Syst Rev. (2011) 2011:CD003279. doi: 10.1002/14651858.CD003279.pub3
14. Muchnik, S, Losavio, AS, Vidal, A, Cura, L, and Mazia, C. Long-term follow-up of Lambert-Eaton syndrome treated withintravenousimmunoglobulin. MuscleNerve. (1997) 20:674–8. doi: 10.1002/(sici)1097-4598(199706)20:6<674::aid-mus3>3.0.co;2-5
15. Rich, MM, Teener, JW, and Bird, SJ. Treatment of Lambert-Eaton syndrome with intravenous immunoglobulin. Muscle Nerve. (1997) 20:614–5. doi: 10.1002/(sici)1097-4598(199705)20:5<614::aid-mus13>3.0.co;2-w
16. Bird, SJ . Clinical and electrophysiologic improvement in Lambert-Eaton syndrome with intravenous immunoglobulin therapy. Neurology. (1992) 42:1422–3. doi: 10.1212/wnl.42.7.1422
17. Lang, B, Newsom-Davis, J, Wray, D, Vincent, A, and Murray, N. Autoimmune aetiology for myasthenic (Eaton-Lambert) syndrome. Lancet. (1981) 2:224–6. doi: 10.1016/s0140-6736(81)90474-8
18. Motomura, M, Hamasaki, S, Nakane, S, Fukuda, T, and Nakao, YK. Apheresis treatment in Lambert-Eaton myasthenic syndrome. Ther Apher. (2000) 4:287–90. doi: 10.1046/j.1526-0968.2000.004004287.x
19. Newsom-Davis, J, and Murray, NM. Plasma exchange and immunosuppressive drug treatment in the Lambert-Eaton myasthenic syndrome. Neurology. (1984) 34:480–5. doi: 10.1212/wnl.34.4.480
20. Dau, PC, and Denys, EH. Plasmapheresis and immunosuppressive drug therapy in the Eaton-Lambert syndrome. Ann Neurol. (1982) 11:570–5. doi: 10.1002/ana.410110604
21. Newsom-Davis, J . Therapy in myasthenia gravis and Lambert-Eaton myasthenic syndrome. Semin Neurol. (2003) 23:191–8. doi: 10.1055/s-2003-41135
22. Sanders, DB . Lambert-Eaton myasthenic syndrome: diagnosis and treatment. Ann N Y Acad Sci. (2003) 998:500–8. doi: 10.1196/annals.1254.065
Keywords: VGCC, VGCC – voltage-gated calcium channels, paraneoplastic syndrome, appendiceal adenocarcinoma, carcinoma induced antibodies
Citation: Patel G, Sakiri A, Brown A, Pasha A and Bansal V (2024) Case report: A novel case of paraneoplastic voltage gated calcium channel antibodies secondary to appendiceal adenocarcinoma. Front. Neurol. 15:1355437. doi: 10.3389/fneur.2024.1355437
Received: 13 December 2023; Accepted: 09 February 2024;
Published: 27 March 2024.
Edited by:
Ghazala Hayat, Saint Louis University, United StatesReviewed by:
Ondrej Sobek, Laboratory for CSF and Neuroimmunology, CzechiaCopyright © 2024 Patel, Sakiri, Brown, Pasha and Bansal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ahmet Sakiri, YXNha2lyaTkzQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.