
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 25 March 2024
Sec. Neurological Biomarkers
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1351925
This article is part of the Research Topic Multi omics Approach to Identify the Neurological Biomarkers and Understand its Significance Associated with Immune Profiling and Bioenergetics View all 3 articles
Background and Aim: The prognostic potential of cardiac troponin (cTn) in acute stroke patients has been a subject of ongoing debate. Our objective was to provide a comprehensive evidence for predicting mortality in acute stroke patients by using the elevated troponin levels.
Methods: We conducted an extensive literature search, including PubMed, EMbase, and Trip Databases, covering studies published up to September 30, 2023. We computed risk ratios (RR) with 95% confidence intervals (CIs), performed sensitivity analysis, and conducted trial sequential analysis (TSA).
Results: In total, 53 studies were analyzed, with 37 focusing on acute ischemic stroke (AIS), 11 on subarachnoid hemorrhage (SAH), and 7 on Intracerebral hemorrhage (ICH). Elevated cTn levels were significantly showed a higher predictive risk for In-hospital mortality in both AIS (RR=3.80, 95% CI; 2.82 to 5.12) as well as SAH (RR=2.23, 95% CI; 1.64 to 3.02). However, no significant predictive risk between elevated cTn levels and in-hospital mortality for ICH patients (RR=1.13, 95% CI: 0.46 to 2.79). A similar pattern was observed for elevated cTn levels, indicating an increased risk of last follow-up mortality for AIS (RR=2.41, 95% CI: 1.98 to 2.93) and SAH (RR=3.08, 95% CI: 2.25 to 4.21).
Conclusion: Elevated troponin levels can serve as a promising predictive marker for both in-hospital and last follow-up mortality in AIS and SAH patients but not in ICH patients. Further prospective studies are needed to validate our findings along with exploring the preventive management of mortality in acute stroke settings.
Acute stroke represents a critical medical condition with substantial implications for patient outcomes and healthcare systems (1). In this context, the timely and accurate identification of prognostic markers capable of predicting stroke-associated mortality emerges as a paramount imperative (2). Such markers not only facilitate risk assessment but also guide precise and targeted clinical interventions, ultimately influencing patient outcomes and optimizing healthcare resource allocation (3). Cardiac Troponin (cTn), widely recognized as a cardinal biomarker in cardiology, has recently attracted attention for its potential role as an indicator of mortality risk in patients afflicted by acute stroke (4).
In the short term, the immediate post-stroke period, in-hospital mortality rates for acute stroke patients can exhibit alarming elevations, with figures occasionally reaching a staggering 50% (5). The major driving force behind such acute fatalities often lies in cardiac-related complications. These complications manifest through the release of cardiac troponin-T (cTnT), cardiac troponin-I (cTnI), High-sensitive cardiac Troponin I (hs-cTnI) and High-sensitive cardiac Troponin T (hs-cTnT) proteins into the circulatory system. Importantly, during the acute phase of ischemic stroke (IS), a marked elevation in serum levels of cTnT or cTnI is frequently observed, establishing a robust link between cardiac injury and the stroke itself (6). The precise and timely prognosis of this cardiac involvement not only impacts therapeutic strategies but also plays a pivotal role in the monitoring and management of patient outcomes, fundamentally shaping the quality of healthcare delivery (7).
However, despite the potential of cardiac troponin as a prognostic marker, the scientific literature presents contrasting findings regarding its association with the risk of all-cause mortality in patients afflicted with acute stroke (8). Moreover, the precision of risk estimates demonstrates considerable variability across individual research studies. Troponin elevations have been reported in a substantial proportion of stroke patients, with prevalence rates ranging from 27% to 34% (9). These elevations have been consistently linked to heightened mortality in various stroke subtypes, encompassing IS, Intracerebral hemorrhage (ICH), and subarachnoid hemorrhage (SAH). Nevertheless, previous meta-analyses (6, 10–17) have failed to definitively establish troponin elevation as an independent and unequivocal prognostic factor in the context of acute stroke patients. As a result, the debate concerning the predictive utility of elevated cardiac troponin levels in the stroke population continues to be a subject of scientific scrutiny. Therefore, we aimed to conduct a systematic review and meta-analysis, aptly named “Elevated Troponin Levels as a Mortality Predictor in Acute Stroke,” in order to explore the utility of cTn in predicting mortality following acute stroke.
The Cochrane Handbook (version 5.1.0) was used to conduct a systematic review and meta-analysis (18). The preferred reporting items for systematic reviews and meta-analyses (PRISMA) were followed throughout the systematic review’s creation (19). Electronic searches were conducted in databases including PubMed, EMbase, and Trip Databases up to 30th September 2023. Additionally, the reference list of retrieved studies and previous meta-analyses, was manually search for collecting more relevant studies often missed while performing the electronic search. The search aimed to identify studies the prediction of cTn level with the mortality rate in patient with acute stroke. The search utilized a set of specific keywords, including (“Troponin” OR “Cardiac Troponin” OR “cTnT” OR “cTnI” OR “Troponin I” OR “Troponin T” OR “High Sensitive Troponin” OR “High Sensitive Troponin I” OR “hTnI” OR “High Sensitive Troponin T” OR “hTnT”) AND (“Stroke” OR “Brain Stroke” OR “Acute Stroke” OR “Ischemic Stroke” OR “Cerebral Infarction” OR “Intracerebral Hemorrhage” OR “Hemorrhagic Stroke” OR “Subarachnoid Hemorrhage” OR “Aneurysmal Subarachnoid Hemorrhage”) AND (“Mortality” OR “Mortality Rate” OR “Death” OR “Death Rate” OR “Prognosis” OR “Outcome”).
Inclusion Criteria:
1. Published observational studies, including prospective cohort and retrospective cohort studies that investigate the predictive utility of Cardiac Troponin (cTn) as a biomarker for mortality in patients with acute stroke.
2. Studies that report data related to either in-hospital mortality and/or last follow-up mortality as endpoints.
3. Studies that provide data on cTn assay results at least for a single time point during the course of the study.
4. Studies involving human participants.
Exclusion Criteria:
1. Case reports, case series, review articles, gray literature, and editorials.
2. Studies not reporting relevant outcomes.
3. Unavailability of full-texts.
Two independent authors (“A” and “MS”) conducted a meticulous evaluation of the identified articles to assess their eligibility for inclusion in our study. Upon the initial screening, the authors proceeded to a comprehensive examination of the full-text articles to validate their eligibility and to systematically extract relevant data. To facilitate this process, a standardized data extraction form was utilized, ensuring uniformity and thoroughness in data collection process. The following data was extracted from studies: First Author’s Name, Published year, Country, Study design, Study period, Sample size of elevated cTn and normal cTn groups, source, cTn cut-off value, cTn assessment time point, cTn type, cTn estimation method, In-hospital mortality, Last follow-up mortality outcome measured. The values of cTn levels were reported with different units in the included studies and were converted to similar units for analysis purpose using online unit conversion tools.1,2 Any disagreement were resolved through discussion with the corresponding author.
The quality of the included studies was assessed using the Newcastle-Ottawa Scale (NOS) for observational cohort studies (20). The NOS evaluates study quality across three domains: selection of participants, comparability of groups, and ascertainment of outcomes. Studies were assigned scores ranging from 0 to 9, with higher scores indicating higher quality. Any discrepancies in quality scores were resolved through consensus with the corresponding author.
To assess publication bias, we employed a funnel plot analysis (21). Egger’s regression test was used to ascertain the asymmetry of funnel plots (22).
A fixed/random-effects model was used to calculate the pooled risk ratio (RR) with 95% confidence interval (CI). Heterogeneity was calculated with the I2 statistic. The heterogeneity was considered as significant in case of I2 more than 50% for which random effects model was applied, on the other hand, if I2 was less than 50%, then fixed-effect model was applied. Sensitivity analysis was performed by sequentially omitting a single study in each turn, to validate the pooled observed effect. In order to ascertain the sufficiency of our sample size and the statistical power of our meta-analysis, we conducted Trial Sequential Analysis (TSA). Tests were considered statistically significant at a p-value less than 0.05. All statistical analyses were carried out using STATA, version 12.0 (Stata Statistical Software, Release 12; StataCorp LP, College Station, TX).
Figure 1 illustrates the PRISMA flow diagram detailing the inclusion and exclusion criteria for studies in our systematic review and meta-analysis. Initially, 1,234 studies were identified across three databases and from other sources. After removing duplicates, 237 articles remained, and following further exclusions, 62 full-text articles were assessed for eligibility. Finally, 53 studies (23–73) were included that examined the role of elevated troponin for predicting mortality in acute stroke. Among these studies, 37 studies focused on AIS patients (23–59), 11 on SAH patients (28, 53, 60–68), and 7 on ICH patients (28, 53, 69–73). The AIS studies involved 3,606 patients with elevated troponin and 14,099 patients with normal troponin, the SAH studies involved 450 patients with elevated troponin and 980 patients with normal troponin, and the ICH studies involved 1,041 patients with elevated troponin and 1,259 patients with normal troponin. Geographically, 24 AIS studies were conducted in Caucasian regions (23–38, 41, 42, 44, 49, 50, 53, 55, 56), and 13 in Asian regions (39, 40, 43, 45–48, 51, 52, 54, 57–59). For SAH, 10 studies were in Caucasian regions (28, 53, 60–66, 68), with only 1 in an Asian region (67). In ICH, 4 studies were in Caucasian regions (28, 53, 69, 72), and 3 in Asian regions (70, 71, 73). Two studies (28, 53) were common for AIS, SAH, and ICH subjects. The publication years ranged from 2000 to 2023, and the sample sizes varied from 43 to 1,718, with detailed baseline characteristics provided in Table 1.
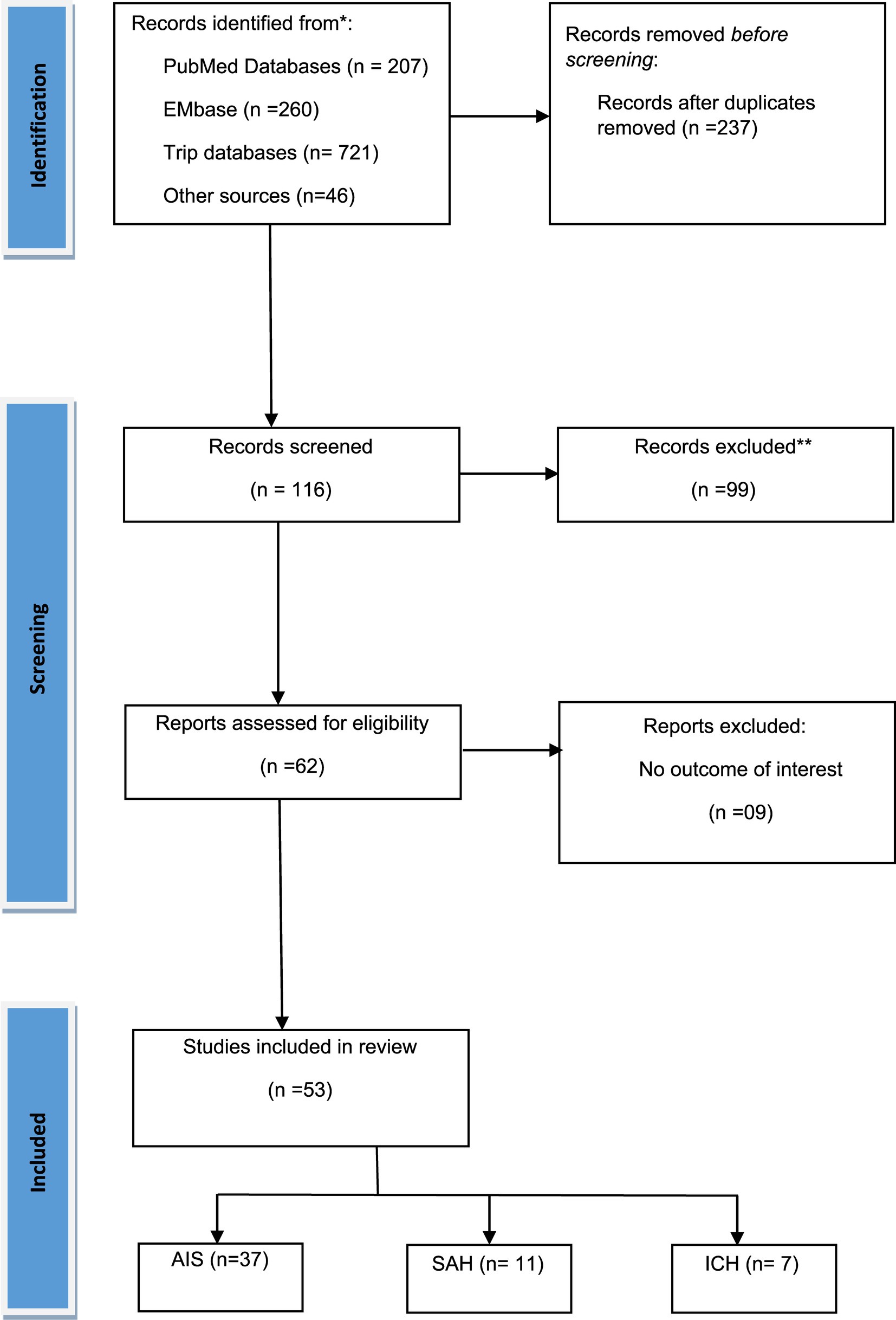
Figure 1. PRISMA flow diagram for the selection of studies and specific reasons for exclusion from the present meta-analysis.
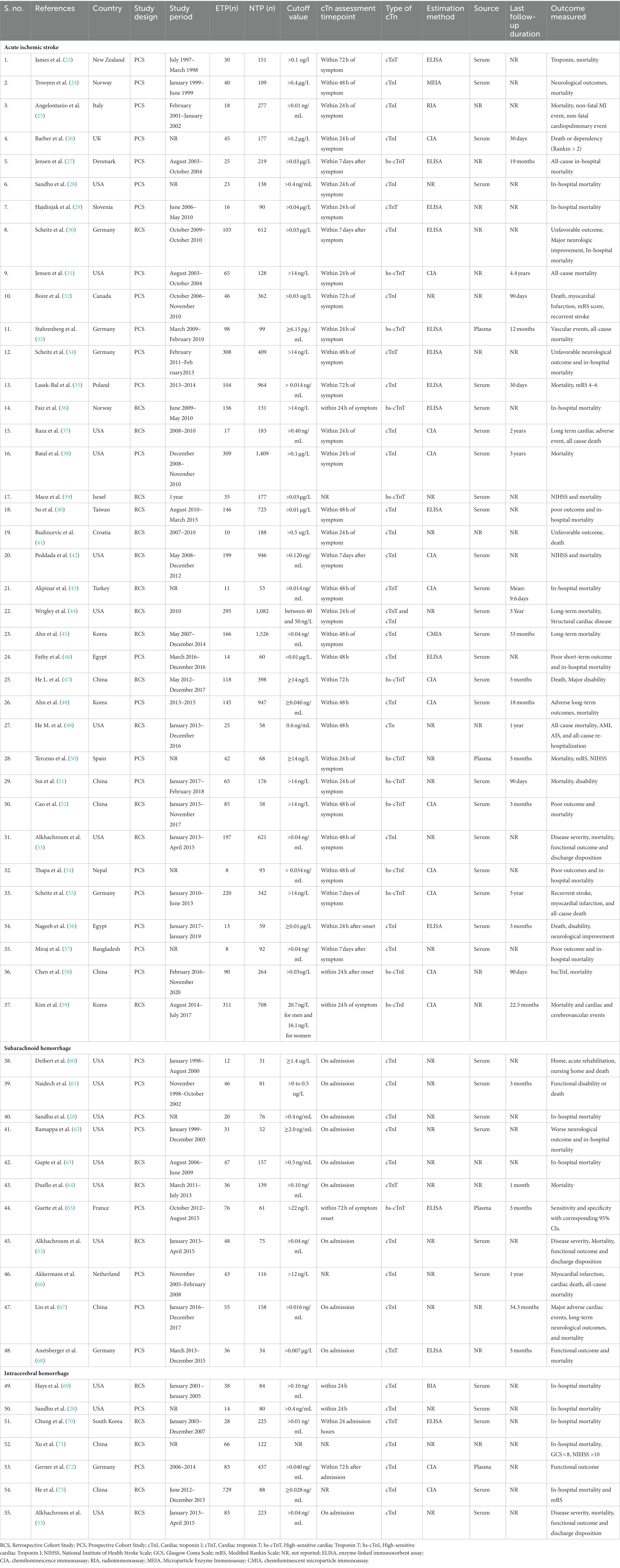
Table 1. Baseline characteristics for the included studies investigating for elevated Troponin level in predicting mortality for acute stroke patients.
Regarding predictive mortality outcomes, 15 studies with AIS patients (23–25, 28–30, 34, 36, 39–43, 54, 57), 6 with SAH (28, 53, 60, 62, 63, 67), and 7 with ICH (28, 53, 69–73) reported data for In-hospital mortality. For last follow-up mortality, 22 AIS studies (26, 27, 31–35, 37, 38, 44–53, 56, 58, 59) and 6 SAH studies (61, 64–68) provided relevant data. Only a single study (67) reported data both In-hospital mortality and last follow-up mortality for SAH subjects. The majority of studies included in our review received high ratings on the NOS Scale, indicating strong methodological quality (Table 2). Each study’s total score, ranging from 6 to 9, reflects its overall quality, supporting the reliability and validity of the evidence presented in this review.
Elevated cTn levels were significantly showed a higher predictive risk for In-hospital mortality in both AIS (RR = 3.80, 95% CI; 2.82 to 5.12; Figure 2A) as well as SAH (RR = 2.23, 95% CI; 1.64 to 3.02; Figure 2B). However, no significant predictive risk between elevated cTn levels and in-hospital mortality for ICH patients (RR = 1.13, 95% CI: 0.46 to 2.79; Figure 2C).
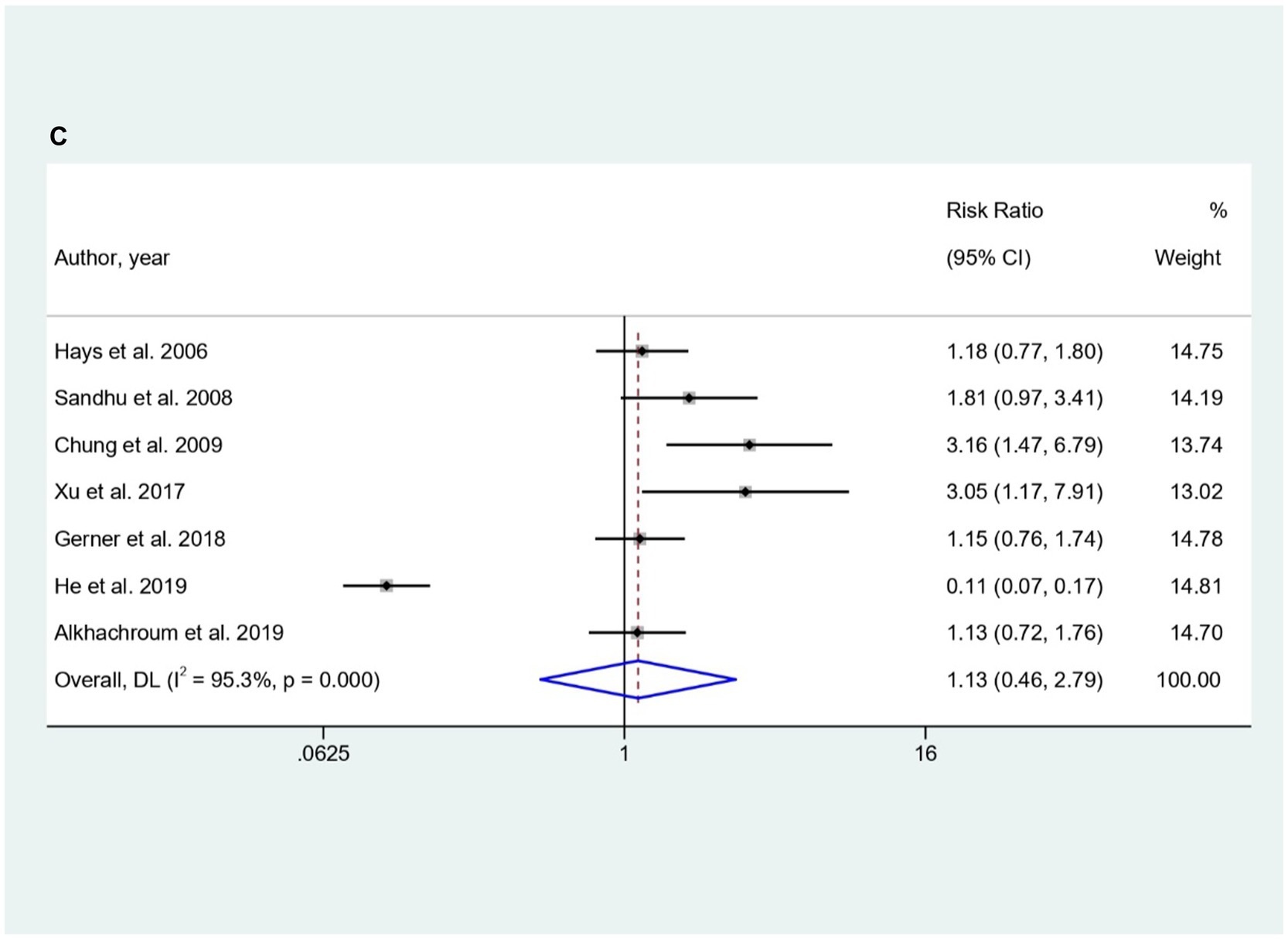
Figure 2. (A–C) Forest plot for the prediction of In-hospital mortality in (A) AIS, (B) SAH, and (C) ICH with respect to elevated cTn Levels.
Further subgroup analysis based on type of cTn, study design, Troponin cut-off value, and estimation method of troponin showed diverse patterns. Specifically for AIS subgroup analysis based on type of troponin, cTnI had a higher prediction risk (RR: 3.93; 95% CI: 2.34 to 6.60) than cTnT (RR: 3.63; 95% CI: 2.24 to 5.88) and hs-cTnT (RR: 3.34; 95% CI: 1.32 to 8.47) while hscTnI had (RR: 6.40; 95% CI: 1.21 to 33.88; Supplementary Table S1A). Based on study design, prospective cohort studies (PCS) had a higher risk (RR: 4.00; 95% CI: 2.43 to 6.57) compared to retrospective cohort studies (RCS) [RR: 3.66; 95% CI: 2.45 to 5.47]. Troponin cut-off values also showed variation, with 0.01 to 0.05 μg/L at RR: 4.55 (95% CI: 3.29 to 6.29) and 0.1 to 0.5 μg/L at RR: 2.86 (95% CI: 1.70 to 4.83). Assessment time-points within 24, 48, and 72 h had different risks.
The choice of troponin estimation methods significantly influenced risk, with Enzyme-Linked Immunosorbent Assay (ELISA) resulting in RR: 3.56 (95% CI: 2.07 to 6.12), Radioimmunoassay (RIA) at RR: 4.57 (95% CI: 2.23 to 9.34), and chemiluminescence immunoassay (CIA) at RR: 2.92 (95% CI: 2.16 to 3.96). Prediction risks varied at assessment time-points within 24, 48, and 72 h, but stability was observed for studies examining troponin levels within 24 h after Acute Ischemic Stroke (AIS). For SAH patients, subgroup analysis for showed that cTnI had a higher predictive risk for In-hospital mortality (RR = 2.23; 95% CI: 1.64 to 3.02). Both PCS (RR = 2.31; 95% CI: 1.49 to 3.58) and RCS (RR = 2.15; 95% CI: 1.40 to 3.31) also showed higher predictive role of elevated troponin for the In-hospital mortality in SAH patients (Supplementary Table S1B). For ICH, subgroup analysis revealed non-significant predictive risk between elevated cTn levels and in-hospital mortality for ICH patients (Supplementary Table S1C). Only significant association for the assessment time point of troponin levels within 24 h and within 48 h after ICH (RR = 1.76; 95% CI: 1.01 to 3.06) and (RR = 3.05; 95% CI: 1.17 to 7.91) respectively were observed (Supplementary Table S1C).
Elevated cTn levels were also significantly showed a higher predictive risk for last follow-up mortality in both AIS (RR = 2.41, 95% CI: 1.98 to 2.93; Figure 3A) and SAH (RR = 3.08, 95% CI: 2.25 to 4.21; Figure 3B). Subgroup analysis showed hs-cTnT stood out with a significantly higher prediction risk (RR: 6.02; 95% CI: 2.60 to 13.93) in AIS, compared to cTnT and cTnI (Supplementary Table S2A). Both prospective and retrospective study designs, indicated an elevated predictive role of elevated troponin for last follow-up mortality in AIS and SAH subgroup analysis (Supplementary Tables S2A,B). Predictive risk remained stable for studies examining troponin levels within 24 h after AIS and SAH. Troponin estimation methods varied for AIS studies, with most utilizing CIA method, while for SAH, the predominant method was ELISA. Analysis on last follow-up mortality in ICH group was not feasible as only a single study provided data for it.
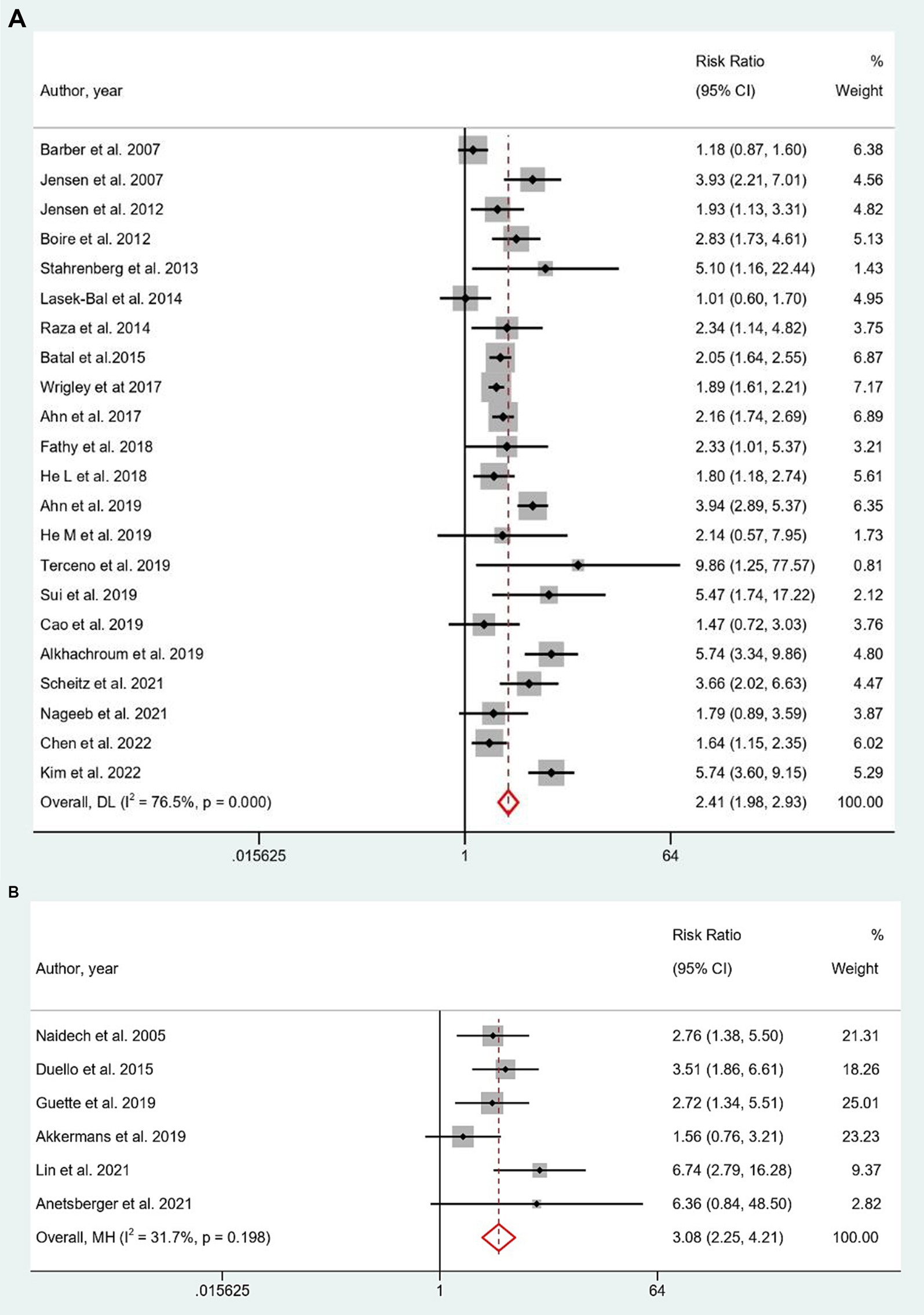
Figure 3. (A,B) Forest plot for the prediction of last follow-up mortality in (A) AIS and (B) SAH with respect to elevated cTn Levels.
Funnel plot analysis did not reveal any apparent signs of asymmetry. The distribution of studies around the estimated effect appeared to be even, suggesting the absence of significant publication bias that could impact the results. Funnel plots for the in-hospital mortality in patients with AIS (p = 0.45), SAH (p = 0.10), and ICH (p = 0.19), and last follow-up mortality for AIS (p = 0.13), and SAH (p = 0.42), are represented in Supplementary Figures S1A–C, S2A,B.
Sensitivity analysis indicated that the pooled risk estimates for in-hospital mortality in patients with AIS, SAH, and ICH was not significantly affected by the removal of any individual study (Supplementary Figures S3A–C). Similarly, the pooled risk estimates for last follow-up mortality in patients with AIS and SAH was not significantly affected by the removal of any individual study (Supplementary Figures S4A,B). Our findings suggest that the results of the meta-analysis are robust and are not driven by any single study which provides additional confidence in the validity of the findings.
Our Trial sequential analysis (TSA) showed a very promising strength with 77% power with total sample size of 4,939 AIS patients and 55% power for the studies with 3,062 HS subjects. TSA plot are represented in Figures 4A,B. The high power of the TSA suggests that the meta-analysis is unlikely to be influenced by chance or random fluctuations in the data. Overall, the TSA results provide strong evidence that the meta-analysis is well-powered and that the findings are reliable and generalizable. This reinforces the importance of considering troponin elevation as a crucial factor in risk stratification and treatment decisions for patients with acute stroke.
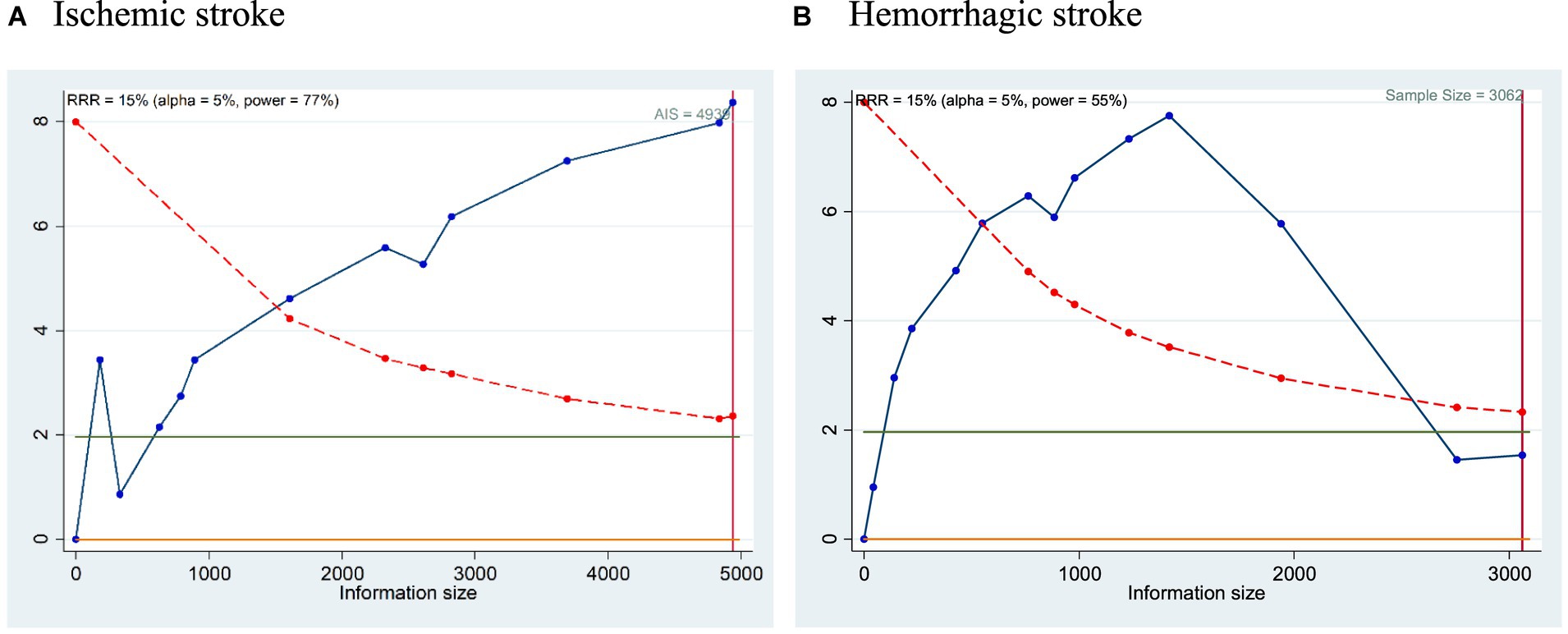
Figure 4. (A,B) TSA plot for the prediction of mortality in (A) Ischemic stroke and (B) Hemorrhagic stroke with respect to elevated cTn Levels.
Our findings highlights the significance of routinely assessing admission cTn in stroke patients, emphasizing its association with higher mortality rates. Existing literature has consistently reported the association between elevated troponin levels and all-cause mortality across various stroke types (10, 12). Studies have shown that baseline troponin levels predict poor outcomes in both in-hospital and last follow-up scenarios, irrespective of acute coronary syndrome (69, 71, 72). Furthermore, higher blood troponin levels during the acute phase of acute spontaneous ICH are linked with unfavorable outcomes (69, 71, 72). Similar findings are observed in patients with spontaneous subarachnoid hemorrhage, where an increased risk of cerebral ischemia and death is demonstrated (13, 14, 60).
The current meta-analysis supports and reinforces these findings, indicating that elevated troponin levels serve as predictors for both in-hospital and follow-up mortalities for AIS with additional finding of other subtypes of stroke including SAH and ICH. Elevated cTn levels were significantly showed a higher predictive risk for In-hospital mortality and last follow-up mortality in both AIS as well as SAH. However, no significant predictive risk between elevated cTn levels and in-hospital mortality for ICH patients. However, it’s crucial to note that this observation may be influenced by the limited inclusion of only seven studies in the overall analysis.
Previous findings have reported that elevation of cardiac troponin has been reported to occur in ICH patients along with only 1.2% of them died of cardiac causes (69). Although previous studies have been tried to address hypothesis that elevated cardiac troponin might serve as prognostic markers for prediction of adverse clinical events, the results were largely inconsistent and inconclusive with regard to ICH. The previous study demonstrated that elevated troponin levels were associated with higher mortality following ICH (69). Subsequently, the utility of elevated troponin levels for prediction of mortality was confirmed in surgical ICH patients (74), but was not found to be consistently associated with in-hospital mortality in Chinese ICH patients (71). Small sample sizes (less than 240 stroke patients) and ethnic variability probably contribute to the negative results and discrepancies. Similarly in our meta-analysis, only seven ICH studies were included and we observed non-significant association with the elevated level of Troponin.
Approximately 18%–20% (75–77) of ischemic stroke patients present with elevated high-sensitive Troponin T (hsTnT) levels on admission, which can be attributed to various factors including renal failure, chronic heart failure, myocardial infarction (MI), and stress-related cardiomyopathies such as neurogenic stunned myocardium (NSM). NSM, characterized by contraction band necrosis at the cellular level, manifests with elevated hsTnT levels and electrocardiographic abnormalities (78–81). While some patients can be differentiated using ECG and echocardiography, overlap in diagnostic criteria complicates diagnosis for others. Despite common assumptions in stroke centers, attributing elevated hsTnT levels solely to brain or heart origin may not always hold true, as MI patients have an increased risk of stroke (82), even with marginally elevated hsTnT levels, raising questions about predictive value in differentiating NSM from MI (83).
As per the 2023 Guideline for the Management of Patients with SAH by AHA/ASA (84), certain medical parameters, such as BMI, hypertension, hyperglycemia, troponin levels, hyperthermia, peak white blood cell count, C-reactive protein, and high neutrophil counts, have been linked to clinical outcomes in SAH. However, it is emphasized that additional investigation is necessary to determine their prognostic value and influence on treatment outcomes. Furthermore, the guideline underscores the active exploration of novel biomarkers, encompassing imaging, serum, and cerebrospinal fluid (CSF), in the field of SAH. Ongoing research incorporating advanced proteomic, genomic, and other biological marker methods, in conjunction with existing clinical, radiographic, and physiological monitoring data, is deemed essential. These efforts aim to shed light on the potential use of biomarkers for prognosis and interventions, ultimately contributing to improved outcomes in patients with SAH.
The COVID-19 pandemic has placed unprecedented strain on stroke services globally, disrupting healthcare delivery and raising concerns about its impact on stroke outcomes (85). Despite these challenges, our meta-analysis did not identify any explicit instances linking the pandemic with influential impacts on stroke management or patient outcomes across included studies. This highlights the need for further research to understand the nuanced interplay between COVID-19 and stroke care delivery, while also emphasizing the importance of developing adaptive strategies and fostering interdisciplinary collaborations to mitigate the pandemic’s adverse effects on stroke services and ensure optimal patient care.
Clinical decision-making regarding troponin elevations in stroke patients is a multifaceted challenge that requires careful consideration of various factors. While elevated troponin levels in stroke patients can indicate myocardial injury, termed “troponitis,” it’s essential to recognize that other contributing factors such as stress, sepsis, or renal dysfunction may also lead to troponin elevation, particularly in the acute phase of a stroke (86). Therefore, clinicians must conduct a thorough evaluation to differentiate between myocardial infarction and other causes of troponin elevation (87). This evaluation typically involves a comprehensive clinical assessment, including medical history, physical examination, electrocardiography, and imaging studies such as echocardiography or cardiac MRI (88).
Additionally, clinicians may utilize risk stratification tools or algorithms specific to stroke patients with troponin elevations to guide further diagnostic and therapeutic interventions. Collaboration between neurologists, cardiologists, and other specialists is crucial in developing a cohesive management plan tailored to the individual patient’s needs, balancing the risks and benefits of interventions such as antiplatelet therapy, anticoagulation, or coronary angiography (89). Ultimately, the decision-making process should prioritize patient safety, optimizing outcomes, and improving overall care in stroke units. Ongoing research and consensus guidelines from professional societies play a vital role in informing evidence-based practices and advancing our understanding of troponin elevations in stroke patients.
While the study has strengths, it acknowledges limitations, including: (1) using a single baseline cTn assessment time point and non-standardized measurement procedures, potentially introducing misclassification; (2) lacking information on important confounders like infarction locations, stroke severity, time from symptom onset, and type of recanalization therapies, which could bias the relationship between elevated cTn and mortality; (3) absence of data on functional outcomes like the modified Rankin Scale; and (4) variations in cut-off values, assay methods, and follow-up periods across individual studies contributed to high heterogeneity from pooled analysis by troponin assays, complicating result interpretation.
Elevated troponin levels can serve as a promising predictive marker for both in-hospital and last follow-up mortality in AIS and SAH patients but not in ICH patients. Further prospective studies are needed to validate our findings along with exploring the preventive management of mortality in acute stroke settings.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
AG: Writing – review & editing, Writing – original draft, Methodology, Data curation. MS: Investigation, Writing – review & editing, Methodology, Data curation, Project administration. PK: Writing – review & editing, Writing – original draft, Supervision, Software, Formal analysis, Conceptualization.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1351925/full#supplementary-material
1. Tadi, P, and Lui, F. Acute Stroke In: StatPearls. Treasure Island (FL): StatPearls Publishing (2023)
2. Roman-Filip, C, Catană, M-G, and Mihăilă, R-G. Prognostic markers for ischemic stroke—are they truly reliable? Med Pharm Rep. (2023) 96:65–70. doi: 10.15386/mpr-2365
3. Saceleanu, VM, Toader, C, Ples, H, Covache-Busuioc, RA, Costin, HP, Bratu, BG, et al. Integrative approaches in acute ischemic stroke: from symptom recognition to future innovations. Biomedicines. (2023) 11:2617. doi: 10.3390/biomedicines11102617
4. Wettersten, N, and Maisel, A. Role of cardiac troponin levels in acute heart failure. Card Fail Rev. (2015) 1:102–6. doi: 10.15420/cfr.2015.1.2.102
5. Grefkes, C, and Fink, GR. Recovery from stroke: current concepts and future perspectives. Neurol Res Pract. (2020) 2:17. doi: 10.1186/s42466-020-00060-6
6. Fan, Y, Jiang, M, Gong, D, Man, C, and Chen, Y. Cardiac troponin for predicting all-cause mortality in patients with acute ischemic stroke: a meta-analysis. Biosci Rep. (2018) 38:BSR20171178. doi: 10.1042/BSR20171178
7. Bohr, A, and Memarzadeh, K. The rise of artificial intelligence in healthcare applications. Artif Intell Healthc. (2020) 25–60:2. doi: 10.1016/B978-0-12-818438-7.00002-2
8. Abdi, S, Oveis-Gharan, S, Sinaei, F, and Ghorbani, A. Elevated troponin T after acute ischemic stroke: association with severity and location of infarction. Iran J Neurol. (2015) 14:35–40.
9. Etgen, T, Baum, H, Sander, K, and Sander, D. Cardiac troponins and N-terminal pro-brain natriuretic peptide in acute ischemic stroke do not relate to clinical prognosis. Stroke. (2005) 36:270–5. doi: 10.1161/01.STR.0000151364.19066.a1
10. Alhazzani, A, Kumar, A, Algahtany, M, and Rawat, D. Role of troponin as a biomarker for predicting outcome after ischemic stroke. Brain Circ. (2021) 7:77–84. doi: 10.4103/bc.bc_51_20
11. Broersen, LHA, Stengl, H, Nolte, CH, Westermann, D, Endres, M, Siegerink, B, et al. Association between high-sensitivity cardiac troponin and risk of stroke in 96 702 individuals: a Meta-analysis. Stroke. (2020) 51:1085–93. doi: 10.1161/STROKEAHA.119.028323
12. Dous, GV, Grigos, AC, and Grodman, R. Elevated troponin in patients with acute stroke – is it a true heart attack? Egypt Heart J. (2017) 69:165–70. doi: 10.1016/j.ehj.2017.01.005
13. Memar Montazerin, S, Chi, G, Marandi, R, Najafi, H, Shojaei, F, Lee, JJ, et al. Evaluation of cardiac troponin and adverse outcomes after aneurysmal subarachnoid hemorrhage: a systematic review and Meta-analysis. Neurocrit Care. (2022) 36:650–61. doi: 10.1007/s12028-021-01368-0
14. Zhang, L, Wang, Z, and Qi, S. Cardiac troponin elevation and outcome after subarachnoid hemorrhage: a systematic review and Meta-analysis. J Stroke Cerebrovasc Dis. (2015) 24:2375–84. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.030
15. Zhang, Y, Ouyang, M, Qiu, J, Cao, X, Xu, B, and Sui, Y. Prognostic value of serum cardiac troponin in acute ischemic stroke: an updated systematic review and Meta-analysis. J Stroke Cerebrovasc Dis. (2022) 31:106444. doi: 10.1016/j.jstrokecerebrovasdis.2022.106444
16. Lippi, G, Cervellin, G, and Sanchis-Gomar, F. Predicting mortality with cardiac troponins: recent insights from meta-analyses. Diagnosi. (2021) 8:37–49. doi: 10.1515/dx-2019-0061
17. Pitliya, A, AlEdani, EM, Bhangu, JK, Javed, K, Manshahia, PK, Nahar, S, et al. The impact of elevated troponin levels on clinical outcomes in patients with acute ischemic stroke: a systematic review. Ann Indian Acad Neurol. (2023) 26:641–54. doi: 10.4103/aian.aian_567_23
18. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
19. Moher, D, Shamseer, L, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
20. Stang, A . Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
21. Begg, CB, and Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
22. Egger, M, Smith, GD, Schneider, M, and Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
23. James, P . Relation between troponin T concentration and mortality in patients presenting with an acute stroke: observational study. BMJ. (2000) 320:1502–4. doi: 10.1136/bmj.320.7248.1502
24. Troøyen, M, and Indredavik, B. Myocardial damage in acute stroke assessed with troponin I. Tidsskr Nor Laegeforen. (2001) 121:421–5.
25. Di Angelantonio, E . Prognostic significance of admission levels of troponin I in patients with acute ischaemic stroke. J Neurol Neurosurg Psychiatry. (2005) 76:76–81. doi: 10.1136/jnnp.2004.041491
26. Barber, M, Morton, JJ, Macfarlane, PW, Barlow, N, Roditi, G, and Stott, DJ. Elevated troponin levels are associated with Sympathoadrenal activation in acute Ischaemic stroke. Cerebrovasc Dis. (2007) 23:260–6. doi: 10.1159/000098325
27. Jensen, JK, Kristensen, SR, Bak, S, Atar, D, Høilund-Carlsen, PF, and Mickley, H. Frequency and significance of troponin T elevation in acute ischemic stroke. Am J Cardiol. (2007) 99:108–12. doi: 10.1016/j.amjcard.2006.07.071
28. Sandhu, R, Aronow, WS, Rajdev, A, Sukhija, R, Amin, H, D'aquila, K, et al. Relation of cardiac troponin I levels with in-hospital mortality in patients with ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. Am J Cardiol. (2008) 102:632–4. doi: 10.1016/j.amjcard.2008.04.036
29. Hajdinjak, E, Klemen, P, and Grmec, Š. Prognostic value of a single prehospital measurement of N-terminal pro-brain natriuretic peptide and troponin T after acute Ischaemic stroke. J Int Med Res. (2012) 40:768–76. doi: 10.1177/147323001204000243
30. Scheitz, JF, Endres, M, Mochmann, H-C, Audebert, HJ, and Nolte, CH. Frequency, determinants and outcome of elevated troponin in acute ischemic stroke patients. Int J Cardiol. (2012) 157:239–42. doi: 10.1016/j.ijcard.2012.01.055
31. Jensen, JK, Ueland, T, Aukrust, P, Antonsen, L, Kristensen, SR, Januzzi, JL, et al. Highly sensitive troponin T in patients with acute ischemic stroke. Eur Neurol. (2012) 68:287–93. doi: 10.1159/000341340
32. Beaulieu-Boire, I, Leblanc, N, Berger, L, and Boulanger, J-M. Troponin elevation predicts atrial fibrillation in patients with stroke or transient ischemic attack. J Stroke Cerebrovasc Dis. (2013) 22:978–83. doi: 10.1016/j.jstrokecerebrovasdis.2012.01.008
33. Stahrenberg, R, Niehaus, CF, Edelmann, F, Mende, M, Wohlfahrt, J, Wasser, K, et al. High-sensitivity troponin assay improves prediction of cardiovascular risk in patients with cerebral ischaemia. J Neurol Neurosurg Psychiatry. (2013) 84:479–87. doi: 10.1136/jnnp-2012-303360
34. Scheitz, JF, Mochmann, HC, Erdur, H, Tütüncü, S, Haeusler, KG, Grittner, U, et al. Prognostic relevance of cardiac troponin T levels and their dynamic changes measured with a high-sensitivity assay in acute ischaemic stroke: analyses from the TRELAS cohort. Int J Cardiol. (2014) 177:886–93. doi: 10.1016/j.ijcard.2014.10.036
35. Lasek-Bal, A, Kowalewska-Twardela, T, Gąsior, Z, Warsz-Wianecka, A, Haberka, M, Puz, P, et al. The significance of troponin elevation for the clinical course and outcome of first-ever Ischaemic stroke. Cerebrovasc Dis. (2014) 38:212–8. doi: 10.1159/000365839
36. Faiz, KW, Thommessen, B, Einvik, G, Brekke, PH, Omland, T, and Rønning, OM. Determinants of high sensitivity cardiac troponin T elevation in acute ischemic stroke. BMC Neurol. (2014) 14:96. doi: 10.1186/1471-2377-14-96
37. Raza, F, Alkhouli, M, Sandhu, P, Bhatt, R, and Bove, AA. Elevated cardiac troponin in acute stroke without acute coronary syndrome predicts long-term adverse cardiovascular outcomes. Stroke Res Treat. (2014) 2014:1–6. doi: 10.1155/2014/621650
38. Batal, O, Jentzer, J, Balaney, B, Kolia, N, Hickey, G, Dardari, Z, et al. The prognostic significance of troponin I elevation in acute ischemic stroke. J Crit Care. (2016) 31:41–7. doi: 10.1016/j.jcrc.2015.09.018
39. Maoz, A, Rosenberg, S, and Leker, RR. Increased high-sensitivity troponin-T levels are associated with mortality after ischemic stroke. J Mol Neurosci. (2015) 57:160–5. doi: 10.1007/s12031-015-0593-7
40. Su, Y-C, Huang, K-F, Yang, F-Y, and Lin, S-K. Elevation of troponin I in acute ischemic stroke. PeerJ. (2016) 4:e1866. doi: 10.7717/peerj.1866
41. Budincevic, H, Sremec, J, Crnac, P, Ostojic, V, Galic, E, and Bielen, I. Impact of troponin I on outcome of ischemic stroke patients. Rom J Intern Med. (2017) 55:19–22. doi: 10.1515/rjim-2016-0044
42. Peddada, K, Cruz-Flores, S, Goldstein, LB, Feen, E, Kennedy, KF, Heuring, T, et al. Ischemic stroke with troponin elevation: patient characteristics, resource utilization, and in-hospital outcomes. Cerebrovasc Dis. (2016) 42:213–23. doi: 10.1159/000445526
43. Akpinar, O . Prognostic value of troponin t in patients with acute ischemic stroke in EMERGENCY department. Acta Medica Mediterr. (2017) 757–761:111. doi: 10.19193/0393-6384_2017_5_111
44. Wrigley, P, Khoury, J, Eckerle, B, Alwell, K, Moomaw, CJ, Woo, D, et al. Prevalence of positive troponin and echocardiogram findings and association with mortality in acute ischemic stroke. Stroke. (2017) 48:1226–32. doi: 10.1161/STROKEAHA.116.014561
45. Ahn, S-H, Lee, JS, Kim, YH, Kim, BJ, Kim, YJ, Kang, DW, et al. Prognostic significance of troponin elevation for long-term mortality after ischemic stroke. J Stroke. (2017) 19:312–22. doi: 10.5853/jos.2016.01942
46. Fathy, HA, Ashour, WMR, Elserafy, TS, and Amer, MM. The prognostic value of elevated cardiac troponin-I in short-term outcome of acute ischemic stroke. Int J Clin Exp Neurol. (2018) 6:1–7.
47. He, L, Wang, J, and Dong, W. The clinical prognostic significance of hs-cTnT elevation in patients with acute ischemic stroke. BMC Neurol. (2018) 18:118. doi: 10.1186/s12883-018-1121-5
48. Ahn, S-H, Kim, YH, Lee, JS, Han, JH, Kim, SY, Kang, DW, et al. Troponin I levels and long-term outcomes in acute ischemic stroke patients. J Am Coll Cardiol. (2019) 73:525–6. doi: 10.1016/j.jacc.2018.11.022
49. He, M, Panchangam, S, Cruz, B, and Mukherjee, D. Underutilization of cardiac therapies in patients with acute ischemic stroke and elevated troponin. Cardiovasc Hematol Agents Med Chem. (2019) 17:144–51. doi: 10.2174/1871525717666191019115338
50. Terceño, M, Silva, Y, Bashir, S, Vera-Monge, V, Buxó, M, and Serena, J. Troponin T predicts Cardioembolic Aetiology and clinical outcome in undetermined Ischaemic stroke in Hyperacute phase. J Stroke Cerebrovasc Dis. (2020) 29:104528. doi: 10.1016/j.jstrokecerebrovasdis.2019.104528
51. Sui, Y, Liu, T, Luo, J, Xu, B, Zheng, L, Zhao, W, et al. Elevation of high-sensitivity cardiac troponin T at admission is associated with increased 3-month mortality in acute ischemic stroke patients treated with thrombolysis. Clin Cardiol. (2019) 42:881–8. doi: 10.1002/clc.23237
52. Cao, Y-Z, Zhao, LB, Liu, S, Liu, QH, Jiang, L, Zhou, CG, et al. Prognostic value of elevated high-sensitivity cardiac troponin T levels in patients with acute ischemic stroke treated with endovascular thrombectomy. J Clin Neurosci. (2019) 64:145–9. doi: 10.1016/j.jocn.2019.03.030
53. Alkhachroum, AM, Miller, B, Chami, T, Tatsuoka, C, and Sila, C. A troponin study on patients with ischemic stroke, intracerebral hemorrhage and subarachnoid hemorrhage: type II myocardial infarction is significantly associated with stroke severity, discharge disposition and mortality. J Clin Neurosci. (2019) 64:83–8. doi: 10.1016/j.jocn.2019.04.005
54. Thapa, P, Agrawal, JP, and Baniya, R. A study on elevation of troponin I in ischemic stroke as an independent prognostic marker of outcomes. Nepal J Neurosci. (2020) 17:26–34. doi: 10.3126/njn.v17i2.30224
55. Scheitz, JF, Stengl, H, Nolte, CH, Landmesser, U, and Endres, M. Neurological update: use of cardiac troponin in patients with stroke. J Neurol. (2021) 268:2284–92. doi: 10.1007/s00415-020-10349-w
56. Nageeb, RS, Omran, AA, and Mohamed, WS. Troponin-I elevation predicts outcome after thrombolysis in ischemic stroke patients. Egypt J Neurol Psychiatry Neurosurg. (2021) 57:4. doi: 10.1186/s41983-020-00256-2
57. Miraj, AA, Mohammad, QD, Rahman, S, and Miah, S. Elevation of troponin I in ischemic stroke of outcomes in patients with acute stroke. Acute Stroke Br J Res. (2022) 9:120. doi: 10.21767/2394-3718.9.12.120
58. Chen, F, Bai, X, Wang, X, Zhang, L, Wang, F, Huang, L, et al. Impact of high-sensitivity troponin elevation and dynamic changes on 90-day mortality in patients with acute ischemic stroke after mechanical thrombectomy: results from an observational cohort. J NeuroInterventional Surg. (2022) 15:1142–7. doi: 10.1136/jnis-2022-019682
59. Kim, BS, Park, JJ, Chang, H, Kim, SH, Kwon, CH, Chung, SM, et al. Association of High-Sensitivity Troponin I with cardiac and cerebrovascular events in patient after ischemic stroke. Cerebrovasc Dis. (2023) 52:153–9. doi: 10.1159/000525920
60. Deibert, E, Barzilai, B, Braverman, AC, Edwards, DF, Aiyagari, V, Dacey, R, et al. Clinical significance of elevated troponin I levels in patients with nontraumatic subarachnoid hemorrhage. J Neurosurg. (2003) 98:741–6. doi: 10.3171/jns.2003.98.4.0741
61. Naidech, AM, Kreiter, KT, Janjua, N, Ostapkovich, ND, Parra, A, Commichau, C, et al. Cardiac troponin elevation, cardiovascular morbidity, and outcome after subarachnoid hemorrhage. Circulation. (2005) 112:2851–6. doi: 10.1161/CIRCULATIONAHA.105.533620
62. Ramappa, P, Thatai, D, Coplin, W, Gellman, S, Carhuapoma, JR, Quah, R, et al. Cardiac troponin-I: a predictor of prognosis in subarachnoid hemorrhage. Neurocrit Care. (2008) 8:398–403. doi: 10.1007/s12028-007-9038-7
63. Gupte, M, John, S, Prabhakaran, S, and Lee, VH. Troponin elevation in subarachnoid hemorrhage does not impact in-hospital mortality. Neurocrit Care. (2013) 18:368–73. doi: 10.1007/s12028-012-9813-y
64. Duello, KM, Nagel, JP, Thomas, CS, Blackshear, JL, and Freeman, WD. Relationship of troponin T and age- and sex-adjusted BNP elevation following subarachnoid hemorrhage with 30-day mortality. Neurocrit Care. (2015) 23:59–65. doi: 10.1007/s12028-014-0105-6
65. Guette, P, Launey, Y, Arnouat, M, Bleichner, JP, Masseret, E, Rousseau, C, et al. Prognostic value of high-sensitivity troponin T in aneurysmal subarachnoid hemorrhage: a prospective observational study. Brain Inj. (2019) 33:1372–8. doi: 10.1080/02699052.2019.1641742
66. Akkermans, A, Peelen, LM, Van Waes, JA, Rinkel, GJ, and Van Klei, WA. Cardiac events within one year after a subarachnoid haemorrhage: the predictive value of troponin elevation after aneurysm occlusion. Eur J Prev Cardiol. (2019) 26:420–8. doi: 10.1177/2047487318776098
67. Lin, F, Chen, Y, He, Q, Zeng, C, Zhang, C, Chen, X, et al. Prognostic value of elevated cardiac troponin I after aneurysmal subarachnoid hemorrhage. Front Neurol. (2021) 12:677961. doi: 10.3389/fneur.2021.677961
68. Anetsberger, A, Jungwirth, B, Blobner, M, Ringel, F, Bernlochner, I, Heim, M, et al. Association of Troponin T levels and functional outcome 3 months after subarachnoid hemorrhage. Sci Rep. (2021) 11:16154. doi: 10.1038/s41598-021-95717-w
69. Hays, A, and Diringer, MN. Elevated troponin levels are CME associated with higher mortality following intracerebral hemorrhage. Neurology. (2006) 66:1330–4. doi: 10.1212/01.wnl.0000210523.22944.9b
70. Chung, P-W, Won, YS, Kwon, YJ, Choi, CS, and Kim, BM. Initial troponin level as a predictor of prognosis in patients with intracerebral hemorrhage. J Korean Neurosurg Soc. (2009) 45:355–9. doi: 10.3340/jkns.2009.45.6.355
71. Xu, M, Lin, J, Wang, D, Liu, M, Hao, Z, and Lei, C. Cardiac troponin and cerebral herniation in acute intracerebral hemorrhage. Brain Behav. (2017) 7:e00697. doi: 10.1002/brb3.697
72. Gerner, ST, Auerbeck, K, Sprügel, MI, Sembill, JA, Madžar, D, Gölitz, P, et al. Peak troponin I levels are associated with functional outcome in intracerebral hemorrhage. Cerebrovasc Dis. (2018) 46:72–81. doi: 10.1159/000492395
73. He, Y, Liu, Q, Wang, J, Wang, DW, Ding, H, and Wang, W. Prognostic value of elevated cardiac troponin I in patients with intracerebral hemorrhage. Clin Cardiol. (2020) 43:338–45. doi: 10.1002/clc.23320
74. Garrett, MC, Komotar, RJ, Starke, RM, Doshi, D, Otten, ML, and Connolly, ES. Elevated troponin levels are predictive of mortality in surgical intracerebral hemorrhage patients. Neurocrit Care. (2010) 12:199–203. doi: 10.1007/s12028-009-9245-5
75. Anders, B, Alonso, A, Artemis, D, Schäfer, A, Ebert, A, Kablau, M, et al. What does elevated high-sensitive troponin I in stroke patients mean: concomitant acute myocardial infarction or a marker for high-risk patients? Cerebrovasc Dis. (2013) 36:211–7. doi: 10.1159/000353875
76. Darki, A, Schneck, MJ, Agrawal, A, Rupani, A, and Barron, JT. Correlation of elevated troponin and echocardiography in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2013) 22:959–61. doi: 10.1016/j.jstrokecerebrovasdis.2011.12.004
77. Kerr, G, Ray, G, Wu, O, Stott, DJ, and Langhorne, P. Elevated troponin after stroke: a systematic review. Cerebrovasc Dis. (2009) 28:220–6. doi: 10.1159/000226773
78. Nguyen, H, and Zaroff, JG. Neurogenic stunned myocardium. Curr Neurol Neurosci Rep. (2009) 9:486–91. doi: 10.1007/s11910-009-0071-0
79. Dombrowski, K, and Laskowitz, D. Cardiovascular manifestations of neurologic disease. Handb Clin Neurol. (2014) 119:3–17. doi: 10.1016/B978-0-7020-4086-3.00001-1
80. Apak, I, Iltumur, K, Tamam, Y, and Kaya, N. Serum cardiac troponin T levels as an indicator of myocardial injury in ischemic and hemorrhagic stroke patients. Tohoku J Exp Med. (2005) 205:93–101. doi: 10.1620/tjem.205.93
81. Mazzeo, AT, Micalizzi, A, Mascia, L, Scicolone, A, and Siracusano, L. Brain-heart crosstalk: the many faces of stress-related cardiomyopathy syndromes in anaesthesia and intensive care. Br J Anaesth. (2014) 112:803–15. doi: 10.1093/bja/aeu046
82. Visser, CA, Kan, G, Lie, KI, and Durrer, D. Left ventricular thrombus following acute myocardial infarction: a prospective serial echocardiographic study of 96 patients. Eur Heart J. (1983) 4:333–7. doi: 10.1093/oxfordjournals.eurheartj.a061470
83. Heldner, MR, Pilgrim, T, Wustmann, K, Hsieh, K, Mattle, HP, Arnold, M, et al. Acute carotid T occlusion in a young patient: cryptogenic origin? Stroke. (2014) 45:e125–7. doi: 10.1161/STROKEAHA.114.005388
84. Hoh, BL, Ko, NU, Amin-Hanjani, S, Chou, SH-Y, Cruz-Flores, S, Dangayach, NS, et al. 2023 guideline for the Management of Patients with Aneurysmal Subarachnoid Hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. (2023) 54:e314–70. doi: 10.1161/STR.0000000000000436
85. Small, C, Mehkri, Y, Panther, E, Felisma, P, and Lucke-Wold, B. Coronavirus Disease-2019 and stroke: pathophysiology and management. Can J Neurol Sci J Can Sci Neurol. (2023) 50:495–502. doi: 10.1017/cjn.2022.267
86. van Beek, DEC, van der Horst, ICC, and Scheeren, TWL. Troponin elevations after cardiac surgery: just “Troponitis”? In: J-L Vincent , editor. Annual update in intensive care and Emergency medicine 2020. Cham: Springer International Publishing (2020). 113–24.
87. Body, R, and Carlton, E. Understanding cardiac troponin part 1: avoiding troponinitis. Emerg Med J EMJ. (2018) 35:120–5. doi: 10.1136/emermed-2017-206812
88. Korff, S, Katus, HA, and Giannitsis, E. Differential diagnosis of elevated troponins. Heart. (2006) 92:987–93. doi: 10.1136/hrt.2005.071282
Keywords: troponin, mortality, acute stroke, ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage
Citation: Gulia A, Srivastava M and Kumar P (2024) Elevated troponin levels as a predictor of mortality in patients with acute stroke: a systematic review and meta-analysis. Front. Neurol. 15:1351925. doi: 10.3389/fneur.2024.1351925
Received: 07 December 2023; Accepted: 29 February 2024;
Published: 25 March 2024.
Edited by:
Faraz Rashid, Henry Ford Health System, United StatesReviewed by:
Brandon Peter Lucke-Wold, University of Florida, United StatesCopyright © 2024 Gulia, Srivastava and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pradeep Kumar, cHJhZGVlcGd1cHRhbmV1cm9AZ21haWwuY29t; cHJhZGVlcGd1cHRhQGFpaW1zLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.