- 1Department of Psychiatry, Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 2Department of Imaging and Interventional Radiology, Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
Background: Post-stroke behavioral disinhibition (PSBD) is common in stroke survivors and often presents as impulsive, tactless or vulgar behavior. However, it often remains undiagnosed and thus untreated, even though it can lead to a longer length of stay in a rehabilitation facility. The proposed study will aim to evaluate the clinical, neuropsychological and magnetic resonance imaging (MRI) correlates of PSBD in a cohort of stroke survivors and describe its 12-month course.
Methods: This prospective cohort study will recruit 237 patients and will be conducted at the Neurology Unit of the Prince of Wales Hospital. The project duration will be 24 months. The patients will be examined by multiple MRI methods, including diffusion-weighted imaging, within 1 week after stroke onset. The patients and their caregivers will receive a detailed assessment at a research clinic at 3, 9 and 15 months after stroke onset (T1, T2 and T3, respectively). The disinhibition subscale of the Frontal Systems Behavior Scale (FrSBe) will be completed by each subject and caregiver, and scores ≥65 will be considered to indicate PSBD.
A stepwise logistic regression will be performed to assess the importance of lesions in the regions of interest (ROIs), together with other significant variables identified in the univariate analyses. For patients with PSBD at T1, the FrSBe disinhibition scores will be compared between the groups of patients with and without ROI infarcts, using covariance analysis. The demographic, clinical and MRI variables of remitters and non-remitters will be examined again at T2 and T3 by logistic regression.
Discussion: This project will be the first MRI study on PSBD in stroke survivors. The results will shed light on the associations of lesions in the orbitofrontal cortex, anterior temporal lobe and subcortical brain structures with the risk of PSBD. The obtained data will advance our understanding of the pathogenesis and clinical course of PSBD in stroke, as well as other neurological conditions. The findings are thus likely to be applicable to the large population of patients with neurological disorders at risk of PSBD and are expected to stimulate further research in this field.
Background
For the purpose of this proposed study, behavioral disinhibition (BD) is defined as the inability to inhibit inappropriate behavior (1). Disinhibition interferes with the ability to inhibit automatic behavior, urges and emotions. It also impedes goal-directed behavior such as resisting temptation, delaying gratification and controlling impulses. Examples of BD include inappropriate comments, jokes, flamboyancy, lack of shame, impulsive behavior, disregard for conventions, poor risk assessment, undue familiarity, sexual acting out and vulgarity.
BD is a common phenomenon in cases of cerebral diseases such as frontal tumor (2), frontotemporal dementia (2–4), progressive supranuclear palsy (5, 6), amyotrophic lateral sclerosis (7, 8), multiple sclerosis (9), traumatic brain injury (1, 10, 11) and stroke (12, 13). For instance, the prevalence of BD in patients with frontotemporal dementia varies from 42 to 83% (3, 4, 14). BD is also common in patients with head injury, with a prevalence ranging from 19 to 32% (1, 10, 15). BD is associated with poor quality of life (7) and suicidality (16) in patients and with burden (7, 17, 18) and stress (19, 20) in their caregivers.
Post-stroke BD (PSBD) is common in stroke survivors and often presents as impulsive, tactless or vulgar behavior (21). Various studies have reported that 5 to 76% of stroke patients had PSBD at 4 days to 4 years post-stroke (12, 21–27). The frequency of PSBD, detected using the Neuropsychiatric Inventory (28), has been reported to range from 5 to 29% (12, 21, 24–26). Two local studies have found the frequency of PSBD to be 5 to 17% (25, 27). The clinical correlates of PSBD are unknown, while the correlates of BD in other neurological disorders have been suggested to include male sex (29, 30), severity of disease (20, 29), disability (10) and depressive and anxiety symptoms (9, 31).
The course of PSBD is uncertain. In a study of only 10 stroke survivors with PSBD, the remission rate at a 1-year follow-up was 90% (26). In contrast, another cross-sectional study of 274 stroke survivors reported the prevalence of PSBD as 22, 34 and 31% at 2.5 years, 2.5–5.5 years and beyond 5.5 years post-stroke, respectively, suggesting possible chronicity of PSBD (12). There is a lack of large-scale longitudinal studies on the course of PSBD. Similarly, the predictors of persistence of PSBD are unknown. Our previous research revealed the non-remission rate of post-stroke depression, another neuropsychiatric condition, at 1 year to be 66%, and the clinical correlates of persistence of post-stroke depression were found to be severity of depression, severity of stroke and cognitive functioning at baseline (32).
PSBD often remains undiagnosed and thus untreated, even though it can lead to a longer length of stay in a rehabilitation facility (26). Single case reports have suggested that transcranial direct current stimulation is useful in alleviating PSBD (13, 33). However, no high-quality trials on pharmacological and psychosocial treatments for PSBD have been conducted to date. Selective serotonin reuptake inhibitors (34, 35), bupropion (35), trazodone (36), aripiprazole (35), dextroamphetamine (35) and donepezil (37) may be useful pharmacological treatments for reducing BD in cases of neurological diseases, whereas cognitive and behavioral interventions may be useful non-pharmacological treatments (35, 38).
Starkstein and Robinson (39) suggested that most patients with BD have orbitofrontal cortex (OFC) and/or basotemporal dysfunction. Based on both contextual cues and object–reward associative memory, the OFC may promote or inhibit behavior that is programmed in the dorsal cortex (40). The basotemporal cortex and the OFC share prominent anatomical connections that could underlie the association between frontal lobe-related volitional and psychomotor behavior and limbic system-related emotional drive. Thus, dysfunction of this system may result in motor disinhibition, instinctive disinhibition and emotional disinhibition. Tekin and Cummings (41) proposed that BD occurs due to dysfunction of the orbitofrontal subcortical circuit (OFSC). The principal components of this brain circuit are the medial OFC, frontal subcortical white matter, caudate and thalamus. The relationships between BD and dysfunctions in the main components of this circuit are discussed in the following paragraphs.
BD is common in patients with frontal lobe pathologies (42) such as frontal tumors (2), frontotemporal dementia (4, 43) and frontal injuries (1). Frontal lobe stroke is associated with reduced emotional intelligence (44). BD is a common sequela of frontal lobe tumors and is related to lesions in the OFC in patients with traumatic brain injuries (1, 45). In patients with mild cognitive impairment, dementia or frontotemporal dementia, BD is positively correlated with atrophy (31, 46, 47) and hypometabolism in the OFC (48). Inhibitory dysfunction in patients with Parkinson’s disease is also related to OFC atrophy (49). Our team previously demonstrated the association of frontal infarcts with another post-stroke neuropsychiatric condition, namely anxiety (50).
In addition to the frontal cortex, temporal lobe structures have been implicated in BD. Socially appropriate behavior requires knowledge of adequate social actions within a given sequential context. Such social knowledge or concepts or emotions are represented in the anterior temporal lobe (ATL) (51, 52). There is strong evidence that the ATL is involved in inappropriate social behavior (53, 54). For example, BD symptoms were found to be present in 65% of patients with temporal lobe atrophy (55). BD in patients with temporal-variant frontotemporal dementia has been found to be related to temporal atrophy (56, 57). Zahn et al. (51) reported that patients with frontotemporal lobar degeneration and corticobasal syndrome accompanied by ATL degeneration had significantly more impairment in social concepts and showed more BD symptoms than those without ATL degeneration. In a lesion–symptom mapping study of patients with traumatic brain injuries, damage to the right temporal lobe, including the pole, was associated with greater BD symptoms (1).
BD has been linked to thalamic and caudate lesions and is thought to arise due to the interruption of the prefrontal–subcortical network (58). PSBD has been linked to paramedian thalamic infarction (59–61). BD is also a known feature of basal ganglia disorders (61–65). Mendez et al. (66) attributed BD to ventromedial caudate lesions. Case reports have linked BD to caudate infarction (64, 67). BD is present in 9 to 20% of patients with Parkinson’s disease (19, 63, 68) and 11% of patients with caudate lesions (62). Reduced gray matter density and altered metabolic connectivity in the striatum have also been associated with BD in patients with frontotemporal dementia (62, 69). Our team found that caudate infarcts are linked to a post-stroke neuropsychiatric condition, namely fatigue (70).
Very few structural brain imaging studies have been published on PSBD (12, 22, 58, 59, 71). Single case reports and case series have linked PSBD to paramedian thalamic (58, 59), caudate (55), supratentorial (22) and subtentorial (71) infarcts. Van Almenkerk et al. (12) reported no association between the prevalence of PSBD and the laterality of stroke. An increase in BD symptoms was noted in 79 patients with subtentorial infarcts compared with 10 patients with parietal/occipital infarcts (71). The limitations of these studies include mixed cohorts of acute and chronic stroke survivors (12) and a lack of detailed radiological examination. Furthermore, the classification of infarct locations was rather crude, namely subtentorial versus parietal/occipital or supratentorial (22, 71), left versus right (12, 22), cortical versus subcortical and anterior versus posterior (22). This proposed project will be the first magnetic resonance imaging (MRI) study on PSBD in stroke survivors. The results of our investigation of the associations between lesions in the OFC, ATL and subcortical structures and the risk of PSBD can advance our understanding of the pathogenesis and clinical course of PSBD in stroke as well as other neurological conditions.
Aims and hypotheses to be tested
The main objective of the proposed study will be to evaluate the clinical and MRI correlates and the 12-month course of PSBD in a cohort of stroke survivors. The regions of interest (ROIs) will be the OFC, ATL, thalamus, and caudate. In addition to individual brain regions, the presence of infarcts affecting structures of the OFSC will be evaluated. The occipital and parietal lobes will be included as control regions.
Hypotheses
Four hypotheses will be tested: (i) patients with PSBD have more infarcts in the ROIs, but not in the control regions, than those without PSBD; (ii) there is a significant positive correlation between the number of infarcts in the ROIs and the severity of PSBD; (iii) 66% (32) of patients with PSBD at baseline continue to have PSBD 12 months after the first assessment; and (iv) the severity of PSBD, severity of stroke and level of cognitive functioning at baseline predict the persistence of PSBD (32).
Methods
Recruitment of subjects
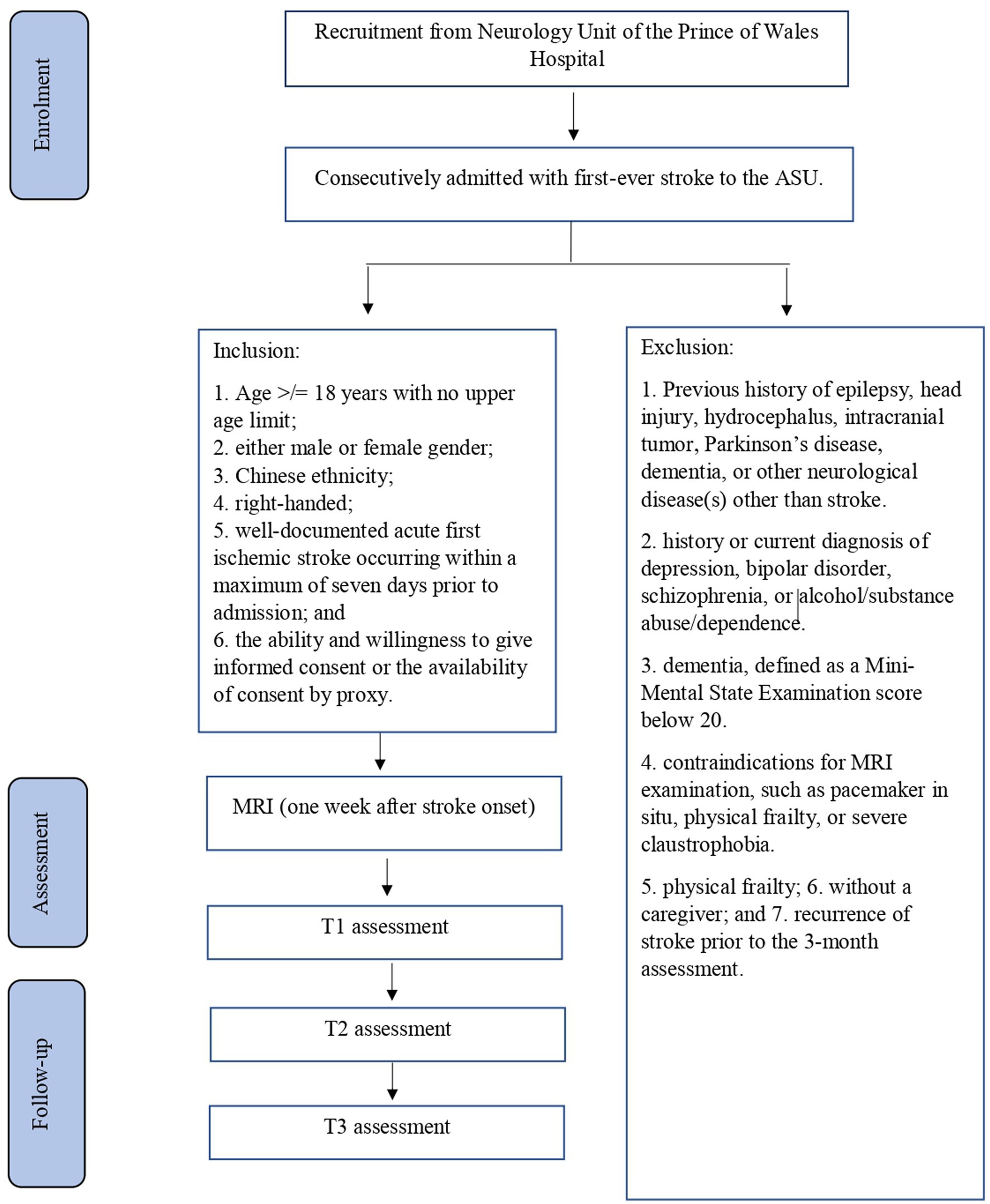
The planned study will be a prospective cohort study of stroke survivors. Details of recruitment are shown in Figure 1. Subjects will be recruited from among patients with first-ever stroke who are consecutively admitted to the Acute Stroke Unit (ASU) of the Prince of Wales Hospital (PWH). The PWH is a general hospital serving a population of 800,000 in Hong Kong. The ASU treats approximately 93% of all acute stroke patients admitted to the PWH, with the majority of the remaining 7% admitted to the neurosurgery unit. All of the acute stroke patients (n = 500) consecutively admitted to the ASU over a 12-month period will be invited to participate in the study. A research assistant (RA) will visit the ASU daily to identify eligible patients and obtain their written consent for inclusion in the study. It is estimated that approximately 80% of these 500 patients (n = 500 × 80% = 400) will have ischemic stroke and that MRI examination will be contraindicated in 10% of them, leaving 360 potential subjects (400 × 90%). According to our previous findings (72), the mortality rate at 3 months post-stroke is around 12%; thus, 316 [360 × (100% − 12%)] potential subjects will be approached. Of these survivors, 25% will not meet the inclusion criteria (72). Hence, the number of possible subjects will be around 237 [316 × (100% − 25%)] (72). Assuming a dropout rate of 20%, 190 [237 × (100–20%)] patients are expected to complete the 12-month follow-up assessment.
Sample size estimation
Two hundred and thirty-seven patients will be recruited. If no significant correlations between BD and lesion site are found in a sample of this size, it is unlikely that any clinically meaningful effects of lesions would be found with a larger sample. As there are no published data on the location of infarcts in patients with PSBD, we calculated the sample size using the figures reported for another neuropsychiatric disorder in stroke. In a report on anxiety in patients with stroke, 21.4% of those with anxiety had frontal infarcts versus only 8.6% of patients without anxiety, and the odds ratio was 2.9 (50). Using these figures as the estimate, a sample size of 237 will have 94.7% power in identifying frontal infarcts as a predictor of PSBD in stroke, using a multivariate logistic regression analysis (73). Give that 190 patients are expected to complete the 12-month assessment, this will provide at least 88% power in identifying the severity of PSBD, severity of stroke and cognitive functioning at baseline as predictors of the persistence of PSBD, using a two-sample t-test (32).
Eligibility criteria
Inclusion and exclusion criteria
The following inclusion criteria will be applied: (i) Age ≥ 18 years with no upper age limit; (ii) either male or female gender; (iii) well-documented acute first ischemic stroke that has occurred within a maximum of 7 days prior to admission; and (iv) the ability and willingness to give informed consent or the availability of consent by proxy, obtained from patients’ next of kin.
The following exclusion criteria will be applied: (i) A history of epilepsy, head injury, hydrocephalus, intracranial tumor, Parkinson’s disease, dementia or neurological disease (s) other than stroke; (ii) history or current diagnosis of depression, bipolar disorder, schizophrenia or alcohol/substance abuse/dependence; (iii) dementia, defined as a Mini-Mental State Examination score below 20; (iv) contraindications for MRI examination, such as a pacemaker in situ, physical frailty or severe claustrophobia; and (v) recurrence of stroke prior to the 3-month assessment.
Data collection
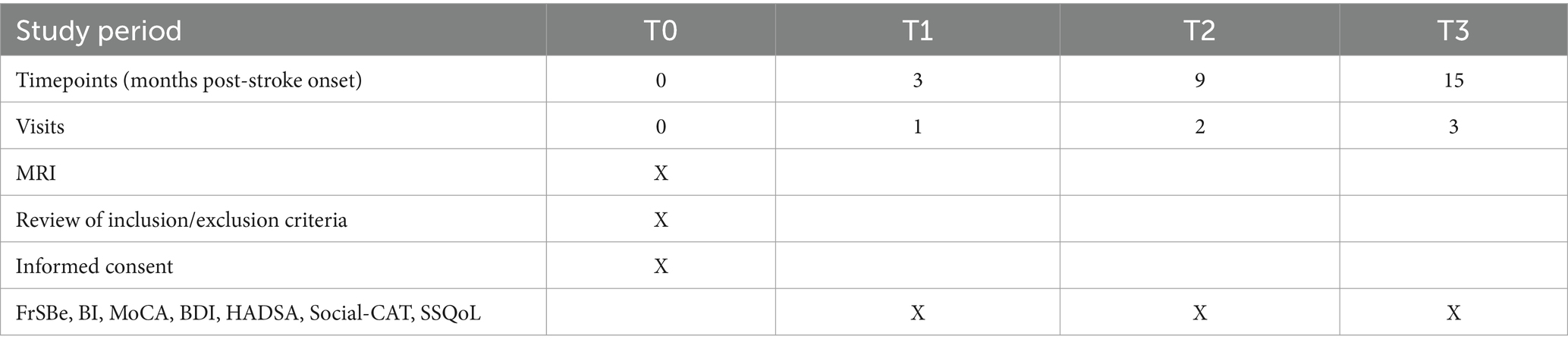
Details of the data collection schedule are shown in Appendix Table 1. Written or proxy consent will be obtained from all of the patients. The number of patients excluded and reasons for exclusion will be recorded. The following demographic, psychosocial and medical data will be collected from all subjects: age, sex, education and date of stroke onset. Subjects’ clinical data and information on neurological impairments, including aphasia and dysarthria measured using the National Institute of Health Stroke Scale (74) (NIHSS), will be extracted from the Stroke Registry, which is maintained by a full-time, trained research nurse.
Assessment of PSBD
Three months after the onset of the index stroke (T1), the patients and their caregivers will receive the following assessments at a research clinic. The timing of the assessment is consistent with other studies of PSBD (24, 27). A psychiatrist blind to the subjects’ radiological data will conduct a clinical interview at the research clinic. PSBD will be assessed using the disinhibition subscale of the validated Frontal Systems Behavior Scale (FrSBe). The FrSBe is a 46-item questionnaire that assesses three frontal behavioral domains: apathy, disinhibition and executive dysfunction. The disinhibition subscale contains 15 items that assess problems with inhibitory control of actions and emotions, including impulsivity, hyperactivity, social inappropriateness, emotional lability, explosiveness and irritability. All items are rated on a 5-point Likert scale: 1 (almost never), 2 (seldom), 3 (sometimes), 4 (frequently), 5 (almost always). Raw scores are converted to normative T-scores (sex-, age-and education-matched) for each behavior, and an overall “frontal dysfunction” score is also calculated. Higher scores indicate greater dysfunction. Scores ≥65 are considered clinically significant, 60–64 are considered borderline and < 60 are considered normal (75, 76). The FrSBe has a clear three-factor structure, and the corresponding subscales have previously shown good validity and reliability. The disinhibition subscale has high internal consistency, indicated by a Cronbach’s alpha coefficient of 0.89 (77). The FrSBe has been used for the assessment of frontal system dysfunction in stroke survivors (44, 78, 79).
A trained RA, blind to the subjects’ radiological data, will measure the level of physical functioning, depressive and anxiety symptoms, cognitive functioning, social functioning, quality of life and anosognosia using the Barthel Index (80) (BI), the Beck Depression Inventory (81) (BDI), the anxiety subscale of the Hospital Anxiety Depression Scale (82) (HADSA), the Montreal Cognitive Assessment (MoCA) (83), the Computerized Adaptive Test of Social Functioning (Social-CAT) (84), the Stroke-Specific Quality of Life Scale (SSQoL) (85) and the Self-Awareness of Deficits Interview (SADI) (86), respectively. Proxy ratings by the caregivers will be obtained for subjects with marked aphasia.
Follow-up assessments of PSBD will be conducted for all subjects at 9 months (T2) and 15 months (T3) post-stroke. All of the instruments (FrSBe, BI, MoCA, BDI, HADSA, Social-CAT and SSQoL) will be repeated during the follow-up assessments (Appendix Table 1).
MRI examination and analysis
Patients will be examined by MRI within 1 week after stroke onset. All scans will be performed using a 3 T scanner (Philips Achieva 3.0 T, X Series, Quasar Dual MRI System) with standardized sequences, including diffusion-weighted imaging (DWI), 3D T1-weighted, T2-weighted, fluid-attenuated inversion recovery (FLAIR) and susceptibility-weighted imaging (SWI). An experienced neuroradiologist blind to the subjects’ PSBD status will assess the MRI images. Acute infarct will be defined as a hyperintense lesion on DWI with corresponding hypointensity on the apparent diffusion coefficient map. White matter hyperintensities (WMH) will be defined as hyperintensities ≥5 mm that are ill-defined on FLAIR images but are isointense with normal brain parenchyma on T1-weighted images. Lesions equivalent to the signal characteristics of cerebrospinal fluid on T1-weighted images and measuring more than 3 mm in diameter, as well as wedge-shaped cortico-subcortical lesions, will be regarded as old/lacunar infarcts. Microbleeds will be defined as dot-like hypointensities on SWI. The total number of microbleeds will be determined. The number of microbleeds in the basal ganglia and thalamus will also be noted separately. All raw data will be transferred to the PALS system (Carestream Solutions).
An ordinal scale devised and validated by Staals et al. (87) will be used to estimate the total small vessel disease (SVD) burden. Briefly, the presence of each of the four MRI markers for SVD (WMHs, lacunae, cerebral microbleeds and perivascular spaces) will be summed to form a total SVD score ranging from 0 to 4. WMHs will be assessed using the Fazekas scale, with scores ranging from 0 to 3. Extensive WMH will be indicated by deep WMHs that score 2 or 3 or by periventricular WMHs that score 3. One point will be given for any extensive WMH, cerebral microbleed or lacuna. One point will be awarded to perivascular spaces when more than 10 are located on one side of a single slice in the basal ganglia.
MRI pre-processing
This will include non-uniformity correction (88), spatial standardization and brain extraction (excluding the skull). To ensure that the brain structure volumes are comparable among subjects, the MRI data of each subject will be transformed from its original space to a common stereotactic space using multi-scale affine registration (89). Brain regions will be automatically segmented from the head MRI data using the brain extraction tool (90).
Brain segmentation
Brain tissue will be classified into gray matter, white matter and cerebrospinal fluid (91). Whole-brain segmentation will be achieved using an atlas-based approach (92), which automatically adjusts the existing atlas intensity model to newly inputted data. The ROIs and other brain regions will be segmented and their volumes quantified using the Tamarac brain atlas (93) and demon registration (94).
Infarct segmentation and quantification
Infarcts will be delineated semi-automatically as high-intensity regions on diffusion-weighted images and WMHs as high-density regions on FLAIR images (and isointense on T1-weighted images) using ITK-SNAP software. The segmented infarct and WMH regions will be combined with the ROI and other brain-region masks generated in the previous step. The infarct and WMH pixels that fall within the ROIs and other brain regions will then be calculated.
Statistical analysis
All of the variables will be tested for normality using Kolmogorov–Smirnov tests with a significance threshold of p < 0.05. Demographic, clinical and MRI variables (age; gender; NIHSS, BI, HADSA, Social-CAT, SSQoL, BDI and MoCA scores; ROI and OFSC infracts; microbleeds; WMH volumes; and total SVD scores) will be compared between patients with and without PSBD at T1 using the χ2 test, Student’s t-test or the Mann–Whitney U test, as appropriate. Stepwise logistic regression will be performed to assess the importance of lesions in the ROIs, together with other significant variables identified in the above univariate analyses. For patients with PSBD at T1, the FrSBe disinhibition scores for the groups with and without ROI infarcts will be compared using covariance analysis. The demographic, clinical and MRI variables of remitters and non-remitters at T2 and T3 will be examined again using logistic regression. We will also test a series of generalized estimating equation models to evaluate the association between the clinical and brain MRI characteristics and risk of PSBD across all follow-up assessments (T1, T2 and T3). First, we will run a univariate model to fit a logistic regression. Next, we will examine the association between the demographic variables and concurrent medical diseases and the risk of PSBD. The second model will comprise baseline FrSBe disinhibition, NIHSS and MoCA scores added to the previous model. The brain MRI characteristics will be entered in the final model. The level of significance will be set at 0.05.In addition to the above pre-planned analysis, an exploratory voxel-based analysis will be performed.
Discussion
We will try to achieve a homogeneous patient population by narrowing the criteria of age and duration of PSBD. Patients with other causes of PSBD, such as psychiatric or neurological disorders, will be excluded. This project will be the first longitudinal study to examine the role of the OFC, ATL, caudate and thalamus in a large sample of consecutively admitted stroke survivors with PSBD. The results will shed light on the association between the above brain regions and PSBD. They are thus likely to be applicable to the large population of patients with neurological disorders at risk of BD and should also stimulate further research in this field.
Ethics statement
The studies involving humans were approved by Joint Chinese University of Hong Kong–New Territories East Cluster Clinical Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WKT: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. EH: Conceptualization, Methodology, Writing – review & editing. TWHL: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Knutson, KM, Dal Monte, O, Schintu, S, Wassermann, EM, Raymont, V, Grafman, J, et al. Areas of brain damage underlying increased reports of behavioral disinhibition. J Neuropsychiatry Clin Neurosci. (2015) 27:193–8. doi: 10.1176/appi.neuropsych.14060126
2. Gregg, N, Arber, A, Ashkan, K, Brazil, L, Bhangoo, R, Beaney, R, et al. Neurobehavioral changes in patients following brain tumour: patients and relatives perspective. Support Care Cancer. (2014) 22:2965–72. doi: 10.1007/s00520-014-2291-3
3. Srikanth, S, Nagaraja, AV, and Ratnavalli, E. Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer’s disease, vascular dementia and frontotemporal dementia. J Neurol Sci. (2005) 236:43–8. doi: 10.1016/j.jns.2005.04.014
4. Shea, YF, Ha, J, and Chu, L. Comparisons of clinical symptoms in biomarker-confirmed Alzheimer’s disease, dementia with Lewy bodies, and frontotemporal dementia patients in a local memory clinic. Psychogeriatrics. (2014) 15:235–41. doi: 10.1111/psyg.12103
5. Litvan, I, Mega, MS, Cummings, JL, and Fairbanks, L. Neuropsychiatric aspects of progressive supranuclear palsy. Neurology. (1996) 47:1184–9. doi: 10.1212/WNL.47.5.1184
6. Wiener, J, Moran, MT, and Haut, MW. Completed suicide in a case of clinically diagnosed progressive supranuclear palsy. Neurodegenerative Dis Manag. (2015) 5:289–92. doi: 10.2217/nmt.15.24
7. Chiò, A, Vignola, A, Mastro, E, Giudici, AD, Iazzolino, B, Calvo, A, et al. Neurobehavioral symptoms in ALS are negatively related to caregivers’ burden and quality of life. Eur J Neurol. (2010) 17:1298–303. doi: 10.1111/j.1468-1331.2010.03016.x
8. Pender, N, Pinto-Grau, M, and Hardiman, O. Cognitive and behavioral impairment in amyotrophic lateral sclerosis. Curr Opin Neurol. (2020) 33:649–54. doi: 10.1097/WCO.0000000000000862
9. Chiaravalloti, ND, and DeLuca, J. Assessing the behavioral consequences of multiple sclerosis: an application of the frontal systems behavior scale (FrSBe). Cogn Behav Neurol. (2003) 16:54–67. doi: 10.1097/00146965-200303000-00007
10. Ciurli, P, Formisano, R, Bivona, U, Cantagallo, A, and Angelelli, P. Neuropsychiatric disorders in persons with severe traumatic brain injury. J Head Trauma Rehabil. (2011) 26:116–26. doi: 10.1097/HTR.0b013e3181dedd0e
11. Osborne-Crowley, K, and McDonald, S. A review of social disinhibition after traumatic brain injury. J Neuropsychol. (2018) 12:176–99. doi: 10.1111/jnp.12113
12. van Almenkerk, S, Depla, MFIA, Smalbrugge, M, Eefsting, JA, and Hertogh, CMPM. Institutionalized stroke patients: status of functioning of an under researched population. J Am Med Dir Assoc. (2012) 13:634–9. doi: 10.1016/j.jamda.2012.05.008
13. Campanella, W, Pedrini, R, Vestito, L, Marinelli, L, Trompetto, C, and Mori, L. Transcranial direct current stimulation in the treatment of subacute post-stroke thalamic aphasia. European J Case Reports in Internal Med. (2020) 7:001794. doi: 10.12890/2020_001794
14. Schwertner, E, Pereira, JB, Xu, H, Secnik, J, Winblad, B, Eriksdotter, M, et al. Behavioral and Psychological Symptoms of Dementia in Different Dementia Disorders: A Large-Scale Study of 10,000 Individuals. J Alzheimers Dis. (2022) 87:1307–18. doi: 10.3233/JAD-215198
15. Filipčíková, M, Wearne, T, Li, R, and McDonald, S. The prevalence, predictors, associated symptoms, and outcomes of social disinhibition following moderate-to-severe TBI: a scoping review of quantitative evidence. J Clin Exp Neuropsychol. (2021) 43:716–36. doi: 10.1080/13803395.2021.2000589
16. Juengst, SB, Kumar, RG, Arenth, PM, and Wagner, AK. Exploratory associations with tumor necrosis factor-α, disinhibition and suicidal endorsement after traumatic brain injury. Brain Behav Immun. (2014) 41:134–43. doi: 10.1016/j.bbi.2014.05.020
17. Soileau, MJ, Persad, C, Taylor, J, Patil, PG, and Chou, KL. Caregiver burden in patients with Parkinson disease undergoing deep brain stimulation: an exploratory analysis. J Parkinsons Dis. (2014) 4:517–21. doi: 10.3233/JPD-140380
18. Jabbarinejad, R, Cohen-Zimerman, S, Wagner, AK, and Grafman, J. Determinants of caregiver burden in male patients with epilepsy following penetrating traumatic brain injury. Epilepsy Behav. (2021) 116:107768. doi: 10.1016/j.yebeh.2021.107768
19. McKinlay, A, Grace, RC, Dalrymple-Alford, JC, Anderson, TJ, Fink, J, and Roger, D. Neuropsychiatric problems in Parkinson’s disease: comparisons between self and caregiver report. Aging Ment Health. (2008) 12:647–53. doi: 10.1080/13607860802343225
20. Mukherjee, A, Biswas, A, Roy, A, Biswas, S, Gangopadhyay, G, and Das, SK. Behavioral and psychological symptoms of dementia: correlates and impact on caregiver distress. Dementia and Geriatric Cogn Disords Extra. (2017) 7:354–65. doi: 10.1159/000481568
21. Angelelli, P, Paolucci, S, Bivona, U, Piccardi, L, Ciurli, P, Cantagallo, A, et al. Development of neuropsychiatric symptoms in poststroke patients: a cross-sectional study. Acta Psychiatr Scand. (2004) 110:55–63. doi: 10.1111/j.1600-0447.2004.00297.x
22. Aybek, S, Carota, A, Ghika-Schmid, F, Berney, A, Melle, GV, Guex, P, et al. Emotional behavior in acute stroke. Cogn Behav Neurol. (2005) 18:37–44. doi: 10.1097/01.wnn.0000152226.13001.8a
23. Ghika-Schmid, F, van Melle, G, Guex, P, and Bogousslavsky, J. Subjective experience and behavior in acute stroke: the Lausanne emotion in acute stroke study. Neurology. (1999) 52:22–8. doi: 10.1212/WNL.52.1.22
24. Greenop, KR, Almeida, OP, Hankey, GJ, van Bockxmeer, F, and Lautenschlager, NT. Premorbid personality traits are associated with post-stroke behavioral and psychological symptoms: a three-month follow-up study in Perth. Western Australia Int Psychogeriatrics. (2009) 21:1063–71. doi: 10.1017/S1041610209990457
25. Mok, VCT, Wong, A, Wong, K, Chu, WCW, Xiong, Y, Chan, AYY, et al. Executive dysfunction and left frontal white matter hyperintensities are correlated with neuropsychiatric symptoms in stroke patients with confluent white matter hyperintensities. Dement Geriatr Cogn Disord. (2010) 30:254–60. doi: 10.1159/000318744
26. Buijck, BI, Zuidema, SU, Eijk, M, Geurts, AC, and Koopmans, RT. Neuropsychiatric symptoms in geriatric patients admitted to skilled nursing facilities in nursing homes for rehabilitation after stroke: a longitudinal multicenter study. Int J Geriatr Psychiatry. (2012) 27:734–41. doi: 10.1002/gps.2781
27. Wong, A, Cheng, S-T, Lo, ES, Kwan, PW, Law, LS, Chan, AY, et al. Validity and reliability of the neuropsychiatric inventory questionnaire version in patients with stroke or transient ischemic attack having cognitive impairment. J Geriatr Psychiatry Neurol. (2014) 27:247–52. doi: 10.1177/0891988714532017
28. Cummings, JL, Mega, M, Gray, K, Rosenberg-Thompson, S, Carusi, DA, and Gornbein, J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. (1994) 44:2308–14. doi: 10.1212/WNL.44.12.2308
29. Treiber, KA, Lyketsos, CG, Corcoran, C, Steinberg, M, Norton, M, Green, RC, et al. Vascular factors and risk for neuropsychiatric symptoms in Alzheimer’s disease: the Cache County study. Int Psychogeriatr. (2008) 20:538–53. doi: 10.1017/S1041610208006704
30. Eikelboom, WS, Pan, M, Ossenkoppele, R, Coesmans, M, Gatchel, JR, Ismail, Z, et al. Sex differences in neuropsychiatric symptoms in Alzheimer’s disease dementia: a meta-analysis. Alzheimers Res Ther. (2021) 17:e055542. doi: 10.1002/alz.055542
31. Godefroy, V, Tanguy, D, Bouzigues, A, Sezer, I, Ferrand-Verdejo, J, Azuar, C, et al. Frontotemporal dementia subtypes based on behavioral inhibition deficits. Alzheimer’s & Dementia: Diagnosis, Assess Dis Monitor. (2021) 13:e12178. doi: 10.1002/dad2.12178
32. Tang, WK, Chen, Y, Liang, H, Chu, WC, Mok, VC, Ungvari, GS, et al. Cerebral microbleeds as a predictor of 1-year outcome of poststroke depression. Stroke. (2014) 45:77–81. doi: 10.1161/STROKEAHA.113.002686
33. Ahn, HC, and Kim, KT. Case report: improved behavioral and psychiatric symptoms with repetitive transcranial magnetic stimulation at the bilateral DLPFC combined with cognitive and behavioral therapy in a patient with unilateral thalamic hemorrhage. Front Neurol. (2022) 13:13. doi: 10.3389/fneur.2022.880161
34. Stewart, JT, and Shin, KJ. Paroxetine treatment of sexual disinhibition in dementia. Am J Psychiatry. (1997) 154:1474–11474. doi: 10.1176/ajp.154.10.1474a
35. Magrath, GN, Zapata-Restrepo, LM, and Miller, BL. Advances in treatment of frontotemporal dementia. J Neuropsychiatry Clin Neurosci. (2022) 34:316–27. doi: 10.1176/appi.neuropsych.21060166
36. Trieu, C, Gossink, F, Stek, ML, Scheltens, P, Pijnenburg, YAL, and Dols, A. Effectiveness of pharmacological interventions for symptoms of behavioral variant frontotemporal dementia: a systematic review. Cogn Behav Neurol. (2020) 33:1–15. doi: 10.1097/WNN.0000000000000217
37. Cummings, J, Lai, T, Hemrungrojn, S, Mohandas, E, Yun Kim, S, Nair, G, et al. Role of donepezil in the management of neuropsychiatric symptoms in Alzheimer’s disease and dementia with Lewy bodies. CNS Neurosci Ther. (2016) 22:159–66. doi: 10.1111/cns.12484
38. Wick, JY, and Zanni, GR. Disinhibition: clinical challenges in the long-term care facility. Consult Pharm. (2005) 20:1006–18. doi: 10.4140/TCP.n.2005.1006
39. Starkstein, SE, and Robinson, RG. Mechanism of disinhibition after brain lesions. J Nerv Ment Dis. (1997) 185:108–14. doi: 10.1097/00005053-199702000-00007
40. Goldar, JC. Anatomia de la Mente: Ensayo sobre Los Fundamentos Neurobiológicos de la psiquiatría. Buenos Aires, Argentina: Editorial Salerno (1993).
41. Tekin, S, and Cummings, JL. Frontal–subcortical neuronal circuits and clinical neuropsychiatry. J Psychosom Res. (2002) 53:647–54. doi: 10.1016/S0022-3999(02)00428-2
42. Chow, TW. Personality in frontal lobe disorders. Curr Psychiatry Rep. (2000) 2:446–51. doi: 10.1007/s11920-000-0031-5
43. Sheelakumari, R, Bineesh, C, Varghese, T, Kesavadas, C, Verghese, J, and Mathuranath, PS. Neuroanatomical correlates of apathy and disinhibition in behavioral variant frontotemporal dementia. Brain Imaging Behav. (2019) 14:2004–11. doi: 10.1007/s11682-019-00150-3
44. Hoffmann, M, Cases, LB, Hoffmann, B, and Chen, R. The impact of stroke on emotional intelligence. BMC Neurol. (2010) 10:103. doi: 10.1186/1471-2377-10-103
45. Jang, SH, and Kwon, HG. Severe disinhibition due to injuries of neural tracts related to emotion circuit in a patient with traumatic brain injury. Medicine. (2017) 96:e9493. doi: 10.1097/MD.0000000000009493
46. Heflin, LH, Laluz, V, Jang, J, Ketelle, R, Miller, BL, and Kramer, JH. Let’s inhibit our excitement: the relationships between Stroop, behavioral disinhibition, and the frontal lobes. Neuropsychology. (2011) 25:655–65. doi: 10.1037/a0023863
47. Hornberger, M, Geng, J, and Hodges, JR. Convergent grey and white matter evidence of orbitofrontal cortex changes related to disinhibition in behavioral variant frontotemporal dementia. Brain. (2011) 134:2502–12. doi: 10.1093/brain/awr173
48. Peters, F, Perani, D, Herholz, K, Holthoff, V, Beuthien-Baumann, B, Sorbi, S, et al. Orbitofrontal dysfunction related to both apathy and disinhibition in frontotemporal dementia. Dement Geriatr Cogn Disord. (2006) 21:373–9. doi: 10.1159/000091898
49. O’Callaghan, C, Naismith, SL, Hodges, JR, Lewis, SJG, and Hornberger, M. Fronto-striatal atrophy correlates of inhibitory dysfunction in Parkinson’s disease versus behavioral variant frontotemporal dementia. Cortex. (2013) 49:1833–43. doi: 10.1016/j.cortex.2012.12.003
50. Tang, WK, Chen, Y, Lu, J, Liang, H, Chu, WC, Tong Mok, VC, et al. Frontal infarcts and anxiety in stroke. Stroke. (2012) 43:1426–8. doi: 10.1161/STROKEAHA.111.640482
51. Zahn, R, Moll, J, Iyengar, V, Huey, ED, Tierney, M, Krueger, F, et al. Social conceptual impairments in frontotemporal lobar degeneration with right anterior temporal hypometabolism. Brain. (2009) 132:604–16. doi: 10.1093/brain/awn343
52. Nakatani, H, Nonaka, Y, Muto, S, Asano, M, Fujimura, T, Nakai, T, et al. Trait respect is linked to reduced gray matter volume in the anterior temporal lobe. Front Hum Neurosci. (2020) 14:344. doi: 10.3389/fnhum.2020.00344
53. Wong, C, and Gallate, J. The function of the anterior temporal lobe: a review of the empirical evidence. Brain Res. (2012) 1449:94–116. doi: 10.1016/j.brainres.2012.02.017
54. Zahn, R, Green, S, Beaumont, H, Burns, A, Moll, J, Caine, D, et al. Frontotemporal lobar degeneration and social behavior: dissociation between the knowledge of its consequences and its conceptual meaning. Cortex. (2017) 93:107–18. doi: 10.1016/j.cortex.2017.05.009
55. Chan, D, Anderson, V, Pijnenburg, Y, Whitwell, J, Barnes, J, Scahill, R, et al. The clinical profile of right temporal lobe atrophy. Brain. (2009) 132:1287–98. doi: 10.1093/brain/awp037
56. Thompson, SA, Patterson, K, and Hodges, JR. Left/right asymmetry of atrophy in semantic dementia: behavioral-cognitive implications. Neurology. (2003) 61:1196–203. doi: 10.1212/01.WNL.0000091868.28557.B8
57. García-Alberca, JM, Florido, M, Cáceres, M, Sánchez-Toro, A, Lara, JP, and García-Casares, N. Medial temporal lobe atrophy is independently associated with behavioral and psychological symptoms in Alzheimer’s disease. Psychogeriatrics. (2018) 19:46–54. doi: 10.1111/psyg.12363
58. Rusconi, ML, Carelli, L, Stampatori, C, and Mattioli, F. Cognitive and behavioral deficits following bilateral thalamic stroke: a longitudinal study. Neurocase. (2013) 20:501–9. doi: 10.1080/13554794.2013.826682
59. Carrera, E, and Bogousslavsky, J. The thalamus and behavior: effects of anatomically distinct strokes. Neurology. (2006) 66:1817–23. doi: 10.1212/01.wnl.0000219679.95223.4c
60. Baird, A, and Robinson, GA. Novel cognitive insights from the first year after bi-thalamic infarct. Neurocase. (2018) 24:76–81. doi: 10.1080/13554794.2018.1444779
61. Bhatia, KP, and Marsden, CD. The behavioral and motor consequences of focal lesions of the basal ganglia in man. Brain. (1994) 117:859–76. doi: 10.1093/brain/117.4.859
62. Zamboni, G, Huey, ED, Krueger, F, Nichelli, PF, and Grafman, J. Apathy and disinhibition in frontotemporal dementia: insights into their neural correlates. Neurology. (2008) 71:736–42. doi: 10.1212/01.wnl.0000324920.96835.95
63. Lee, W-J, Tsai, C-F, Gauthier, S, Wang, S-J, and Fuh, J-L. The association between cognitive impairment and neuropsychiatric symptoms in patients with Parkinson’s disease dementia. Int Psychogeriatr. (2012) 24:1980–7. doi: 10.1017/S1041610212001317
64. Meguro, K, Meguro, M, and Akanuma, K. Recurrent delusional ideas due to left caudate head infarction, without dementia. Psychogeriatrics. (2012) 12:58–61. doi: 10.1111/j.1479-8301.2011.00385.x
65. Fabbrini, G, Fabbrini, A, and Suppa, A. Progressive supranuclear palsy, multiple system atrophy and corticobasal degeneration. Psychopharmacol Neurolog Dis. (2019) 165:155–77. doi: 10.1016/B978-0-444-64012-3.00009-5
66. Mendez, MF, Adams, NL, and Lewandowski, KS. Neurobehavioral changes associated with caudate lesions. Neurology. (1989) 39:349–54. doi: 10.1212/WNL.39.3.349
67. Balcioglu, YH, Dogan, M, Incı, I, and Solmaz, M. Sexual behavioral disinhibition associated with nucleus lentiformis lesion: a forensic neuroscience perspective through a case. J Forensic Sci. (2020) 65:1779–83. doi: 10.1111/1556-4029.14477
68. Yao, M, Zhang, H, Xu, Y, Zhang, S, Gao, Y, Shu, M, et al. Neuropsychiatric symptoms and cognitive impairment in Chinese patients with Parkinson’s disease in Han and hui ethnicity. Current Med Sci. (2019) 39:122–6. doi: 10.1007/s11596-019-2009-3
69. Liu, L, Chu, M, Nie, B, Jiang, D, Xie, K, Cui, Y, et al. Altered metabolic connectivity within the limbic cortico-striato-thalamo-cortical circuit in presymptomatic and symptomatic behavioral variant frontotemporal dementia. Alzheimers Res Ther. (2023) 15:3. doi: 10.1186/s13195-022-01157-7
70. Tang, WK, Liang, HJ, Chen, YK, Chu, WCW, Abrigo, J, Mok, VCT, et al. Poststroke fatigue is associated with caudate infarcts. J Neurol Sci. (2013) 324:131–5. doi: 10.1016/j.jns.2012.10.022
71. Hoffmann, M, and Cases, LB. Etiology of frontal network syndromes in isolated subtentorial stroke. Behav Neurol. (2008) 20:101–5. doi: 10.1155/2008/635187
72. Tang, WK, Chan, SS, Chiu, HF, Ungvari, GS, Wong, KS, Kwok, TC, et al. Poststroke depression in Chinese patients: frequency, psychosocial, clinical, and radiological determinants. J Geriatr Psychiatry Neurol. (2005) 18:45–51. doi: 10.1177/0891988704271764
73. Cohen, J. Statistical power analysis for the behavioral sciences. Hillsdale, New Jersey: Lawrence Erlbaum Associates (1988).
74. Brott, T, Adams, HP, Olinger, CP, Marler, JR, Barsan, WG, Biller, J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. (1989) 20:864–70. doi: 10.1161/01.STR.20.7.864
75. Grace, J, and Malloy, PF. Frontal systems behavior scale professional manual. Lutz, Florida: Psychological Assessment Resources (2001).
76. McDowell, LJ, Ringash, J, Xu, W, Chan, B, Lu, L, Waldron, J, et al. A cross sectional study in cognitive and neurobehavioral impairment in long-term nasopharyngeal cancer survivors treated with intensity-modulated radiotherapy. Radiother Oncol. (2019) 131:179–85. doi: 10.1016/j.radonc.2018.09.012
77. Lyvers, M, Carlopio, C, Bothma, V, and Edwards, MS. Mood, mood regulation expectancies and frontal systems functioning in current smokers versus never-smokers in China and Australia. Addict Behav. (2013) 38:2741–50. doi: 10.1016/j.addbeh.2013.07.002
78. Caracuel, A, Verdejo-García, A, Fernández-Serrano, MJ, Moreno-López, L, Santago-Ramajo, S, Salinas-Sánchez, I, et al. Preliminary validation of the Spanish version of the frontal systems behavior scale (FrSBe) using Rasch analysis. Brain Inj. (2012) 26:844–52. doi: 10.3109/02699052.2012.655365
79. Jovanovski, D, Zakzanis, K, Ruttan, L, Campbell, Z, Erb, S, and Nussbaum, D. Ecologically valid assessment of executive dysfunction using a novel virtual reality task in patients with acquired brain injury. Appl Neuropsychol Adult. (2012) 19:207–20. doi: 10.1080/09084282.2011.643956
80. Mahoney, FI, and Barthel, DW. Functional evaluation: the Barthel index. Md State Med J. (1965) 14:61–5.
81. Shek, DT. Reliability and factorial structure of the Chinese version of the Beck depression inventory. J Clin Psychol. (1990) 46:35–43. doi: 10.1002/1097-4679(199001)46:1<35::AID-JCLP2270460106>3.0.CO;2-W
82. Leung, CM, Wing, YK, Kwong, PK, and Shum, ALOK. Validation of the Chinese-Cantonese version of the hospital anxiety and depression scale and comparison with the Hamilton rating scale of depression. Acta Psychiatr Scand. (1999) 100:456–61. doi: 10.1111/j.1600-0447.1999.tb10897.x
83. Yeung, P, Wong, L, Chan, C, Leung, JL, and Yung, C. A validation study of the Hong Kong version of Montreal cognitive assessment (HK-MoCA) in Chinese older adults in Hong Kong. Hong Kong Med J. (2014) 20:504–10. doi: 10.12809/hkmj144219
84. Chiang, H, Chen, P-T, Lee, S-C, Shieh, Y-J, Hsueh, I-P, and Hsieh, C-L. Test–retest reliability and responsiveness of the computerized adaptive test of social functioning in persons with stroke. Arch Phys Med Rehabil. (2023) 104:1432–8. doi: 10.1016/j.apmr.2023.03.017
85. Fleming, JM, Strong, J, and Ashton, R. Self-awareness of deficits in adults with traumatic brain injury: how best to measure? Brain Inj. (1996) 10:1–15. doi: 10.1080/02699050410001720059
86. Staals, J, Makin, SDJ, Doubal, FN, Dennis, MS, and Wardlaw, JM. Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology. (2014) 83:1228–34. doi: 10.1212/WNL.0000000000000837
87. Fong, TCT, Lo, TLT, and Ho, RTH. Psychometric properties of the 12-item stroke-specific quality of life scale among stroke survivors in Hong Kong. Sci Rep. (2023) 13:1510. doi: 10.1038/s41598-023-28636-7
88. Sled, JG, Zijdenbos, AP, and Evans, AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. (1998) 17:87–97. doi: 10.1109/42.668698
89. Collins, DL, Neelin, P, Peters, TM, and Evans, AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. (1994) 18:192–205. doi: 10.1097/00004728-199403000-00005
90. Smith, SM. Fast robust automated brain extraction. Hum Brain Mapp. (2002) 17:143–55. doi: 10.1002/hbm.10062
91. Cocosco, CA, Zijdenbos, AP, and Evans, AC. A fully automatic and robust brain MRI tissue classification method. Med Image Anal. (2003) 7:513–27. doi: 10.1016/S1361-8415(03)00037-9
92. Han, X, and Fischl, B. Atlas renormalization for improved brain MR image segmentation across scanner platforms. IEEE Trans Med Imaging. (2007) 26:479–86. doi: 10.1109/TMI.2007.893282
93. Lancaster, JL, Tordesillas-Gutiérrez, D, Martinez, M, Salinas, F, Evans, A, Zilles, K, et al. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. (2007) 28:1194–205. doi: 10.1002/hbm.20345
94. Thirion, J-P. Image matching as a diffusion process: an analogy with Maxwell’s demons. Med Image Anal. (1998) 2:243–60. doi: 10.1016/S1361-8415(98)80022-4
Appendix
TABLE 1 Data collection schedule.
Keywords: stroke, behavior disinhibition, MRI, prefrontal cortex, anterior temporal lobe, caudate, thalamus
Citation: Tang WK, Hui E and Leung TWH (2024) Behavioral disinhibition in stroke. Front. Neurol. 15:1345756. doi: 10.3389/fneur.2024.1345756
Edited by:
Donna Clark Tippett, Johns Hopkins University, United StatesReviewed by:
Marcelo Mendonça, Champalimaud Foundation, PortugalJaime Daniel Mondragón, San Diego State University, United States
Copyright © 2024 Tang, Hui and Leung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wai Kwong Tang, dGFuZ3drQGN1aGsuZWR1Lmhr
 Wai Kwong Tang
Wai Kwong Tang Edward Hui
Edward Hui Thomas Wai Hong Leung3
Thomas Wai Hong Leung3