
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 21 August 2024
Sec. Endovascular and Interventional Neurology
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1339144
 Junqiang Feng1†
Junqiang Feng1† Yudi Tang2†
Yudi Tang2† Wei You2
Wei You2 Yuhua Jiang2
Yuhua Jiang2 Zhengkun Xu3
Zhengkun Xu3 Yan Zhao4
Yan Zhao4 Xinke Liu2
Xinke Liu2 Jian Lv2
Jian Lv2 Peng Liu2
Peng Liu2 Haining Wei5
Haining Wei5 Mahmud Mossa-Basha6
Mahmud Mossa-Basha6 Youxiang Li2*
Youxiang Li2* Yang Wang1*
Yang Wang1* Chengcheng Zhu6
Chengcheng Zhu6Background and objective: The rupture risk of intracranial aneurysms (IAs) is related to their arterial origin, but whether the different segments of the artery have different risks and act as independent risk factors is still unknown. Our study aimed to investigate the rupture risk of IAs in different arterial segments in a large Chinese cohort.
Methods: Imaging and clinical data of consecutive patients with IAs diagnosed by Computed Tomography angiography (CTA) from January 2013 to December 2022 were collected. Two neuroradiologists independently identified ruptured and unruptured IAs based on imaging and medical records. The internal carotid artery (ICA), middle cerebral artery (MCA), anterior cerebral artery (ACA), vertebral artery (VA), and posterior cerebral artery (PCA) were segmented according to the Bouthillier and Fischer segmentation methods. Stenoses of the proximal parent vessel were evaluated and documented. The Institutional Review Board (IRB) at Beijing Tiantan Hospital approved this retrospective study.
Results: A total of 3,837 aneurysms {median size 3.5 mm [interquartile range (IQR) 2.6–5.1 mm]; 532 ruptured} were included in this study from 2,968 patients [mean age: 57 years (IQR 50–64); male patients: 1,153]. Ruptured aneurysms were most commonly located in the posterior inferior cerebellar artery (PICA) (52.9%), anterior communicating artery (ACoA) (33.8%), other locations (33.3%), ACA (22.4%), and basilar artery (BA) (21.4%). The locations with the highest likelihood of rupture were the C7 ICA (21.3%), M2 MCA (24.0%), distal MCA (25.0%), and A2 ACA (28.1%). IAs originating from the C7 (p < 0.001), dM1 (p = 0.022), and dA1 (p = 0.021) segments were independent risk factors for rupture. IAs without stenosis of the proximal parent vessel were associated with a higher risk of rupture (p = 0.023).
Conclusion: There are unique associations between the origins of aneurysms from various arterial segments. Aneurysms originating from the anterior communicating artery (ACoA), BA, PICA, A2, dA, C7, and M2 indicate a higher risk of rupture. Aneurysms originating from C4, C5, and C6 indicate a lower risk of rupture. C7 IAs, ACoA IAs, and PICA IAs seem to be independent risk factors.
Intracranial aneurysms (IAs) are a common vascular pathology found in roughly 2–3% of the general population (1, 2). In most cases, IAs are asymptomatic and the rupture rate is very low (≤ 1% per year) (3). IA rupture can, however, lead to subarachnoid hemorrhage (SAH), which is associated with high mortality and morbidity (case fatality ≈ 40%) (3). Although many factors have shown associations with IA rupture in cohort studies (4–6), their predictive ability remains limited with moderate accuracy (7). A more accurate assessment of IA rupture risk factors is needed.
The origin of an aneurysm has been associated with aneurysm rupture (5). Aneurysms originating from the anterior communicating artery (ACoA) and posterior circulating artery (PCA) are usually considered to have a high risk of rupture (8). IAs originating from different segments of the same artery may have different rupture risks (9), but it has been rarely studied. Our study aimed to investigate the rupture risk of IAs in different artery segments in a large Chinese cohort.
The Institutional Review Board (IRB) at Beijing Tiantan Hospital approved this retrospective study with a waiver of informed consent. Imaging data and clinical baseline data of 2,968 patients with saccular IAs detected on head CTA at Beijing Tiantan Hospital from January 2013 to December 2022 were included in this study. Patients with incomplete medical records, cerebral vascular malformations, moyamoya disease, or traumatic or inflammatory IAs were excluded. In addition, patients with a history of subarachnoid hemorrhage were not included because of incomplete medical data. Patients' demographics including age, gender, blood lipids, blood glucose, blood pressure, and smoking history were recorded.
All data were anonymized before processing. Two neuroradiologists with 12 and 13 years of experience independently identified ruptured and unruptured aneurysms based on existing CTA images according to previously developed criteria, which included the identification of the rupture site in patients with SAH and multiple aneurysms (10). In case of disagreement on rupture status, a third neuroradiologist with 21 years of experience adjudicated according to the imaging, medical record, and his experience. Finally, all data were divided into a ruptured and an unruptured group. The proportion of the ruptured aneurysms was defined as the ratio of ruptured aneurysms on a certain artery (or a certain segment) to all aneurysms on this artery (or this segment).
Arteries that originate aneurysm were documented, which included the internal carotid artery (ICA), anterior cerebral artery (ACA), anterior communicating artery (ACoA), anterior choroidal artery (AchoA), ophthalmic artery (OA), middle cerebral artery (MCA), posterior cerebral artery (PCA), posterior communicating artery (PcoA), basilar artery (BA), vertebral artery (VA), posterior inferior cerebellar artery (PICA), superior cerebellar artery (SCA), and anterior inferior cerebellar artery (AICA).
The ICA was divided into seven segments according to the Bouthillier segmentation method (11) (cervical segment C1, petrous segment C2, lacerum segment C3, cavernous segment C4, clinoid segment C5, ophthalmic segment C6, and communicating segment C7). The MCA was divided into five segments according to the Fischer segmentation method (horizontal segment M1, insular segment M2, opercula segment M3, and cortical segments M4 and M5). The ACA was divided into five segments according to the Fischer segmentation method (12) (suprachiasmatic segment A1, subcallosal segment A2, genu segment A3, the frontal lobe segment of the pericallosal artery A4, and the parietal lobe segment of the pericallosal artery A5). The VA was divided into four segments (pre-foraminal segment V1, foraminal segment V2, external spinal segment V3, and intradural segment V4).
The aneurysms were less distributed in some locations; thus, C1–C3 were merged into pC, M3–M5 were merged into dM, A3–A5 were merged into dA, and the SCA, AICA, and OA were merged into others. Considering that there were many aneurysms at the bifurcation of C7 and PcoA, we merged them into PcoA-C7 for further analysis.
In the analysis of the independent risk factors of the MCA, ACA, and PCA, M2–M5 were merged into dM1, A2–A5 were merged into dA1, and P2–M4 were merged into dP1.
The cerebral arteries were reconstructed from bone-subtracted CTA images using a 3D slicer (Version 4.10.1). The two neuroradiologists independently determined the origin of the aneurysms, whether it was a bifurcation aneurysm and whether it had a daughter sac, as well as the degree of stenosis of the proximal parent artery (no, mild <50%, moderate 50–70%, severe 70%, occluded) using reconstructed three-dimensional CTA datasets. In case of any interrater discrepancies, the senior neuroradiologist adjudicated. The aneurysm size was measured as the maximum height from the midpoint of the neck to the dome of the aneurysm.
Normally distributed data and non-normally distributed data were expressed as mean ± standard deviation and median (interquartile range), respectively. Categorical data were expressed as percentiles. The proportion of each factor was statistically compared between the ruptured group and the unruptured group to evaluate its correlation with rupture. A t-test was used for the parameters of the normally distributed data, and a Mann–Whitney U-test was used for the parameters of the non-normally distributed data. Pearson's chi-square test, logistic regression analysis, and propensity score matching (PSM)(Match Tolerance = 0.001) were used to assess the correlation between the risk factors and the ruptured aneurysms. Pearson's chi-square test with continuity correction and the Fisher's exact test were used to assess the correlation between the aneurysm location and rupture risk. t-tests were also used to assess the correlation between the aneurysm origin and risk factors. PSM was performed between the ruptured and unruptured groups with a 1:1 ratio. Differences with a p-value of <0.05 were considered significant. Data were analyzed with SPSS 23.0 software (SPSS Inc, Chicago, IL).
A total of 3,837 aneurysms (ruptured 532) from 2,968 patients [age 57 (50–64)] were included. More detailed characteristics are shown in Tables 1, 2. The aneurysm size and location are shown in Table 3.

Table 2. Characteristics of the aneurysms and logistic regression results between the two IA groups.
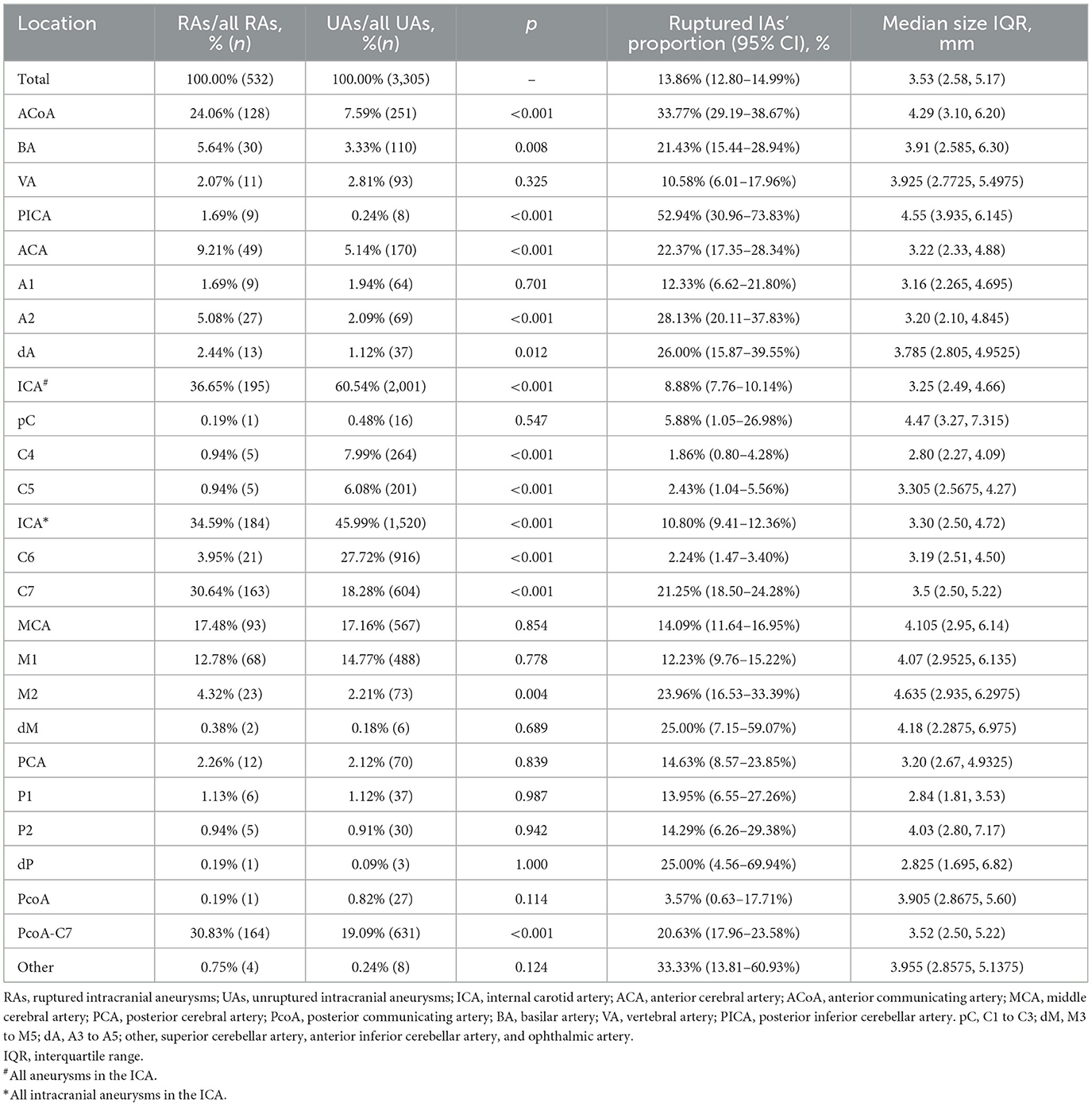
Table 3. The distribution of aneurysms of the two groups, the ruptured IAs' proportions, and the size of aneurysms of each location.
As shown in Figure 1, the ICA is the most common location of IAs, with the most frequent segment being C6. Figure 2 shows the distribution of the aneurysms of each artery segment. Table 3 shows the distribution of the ruptured and unruptured IAs.
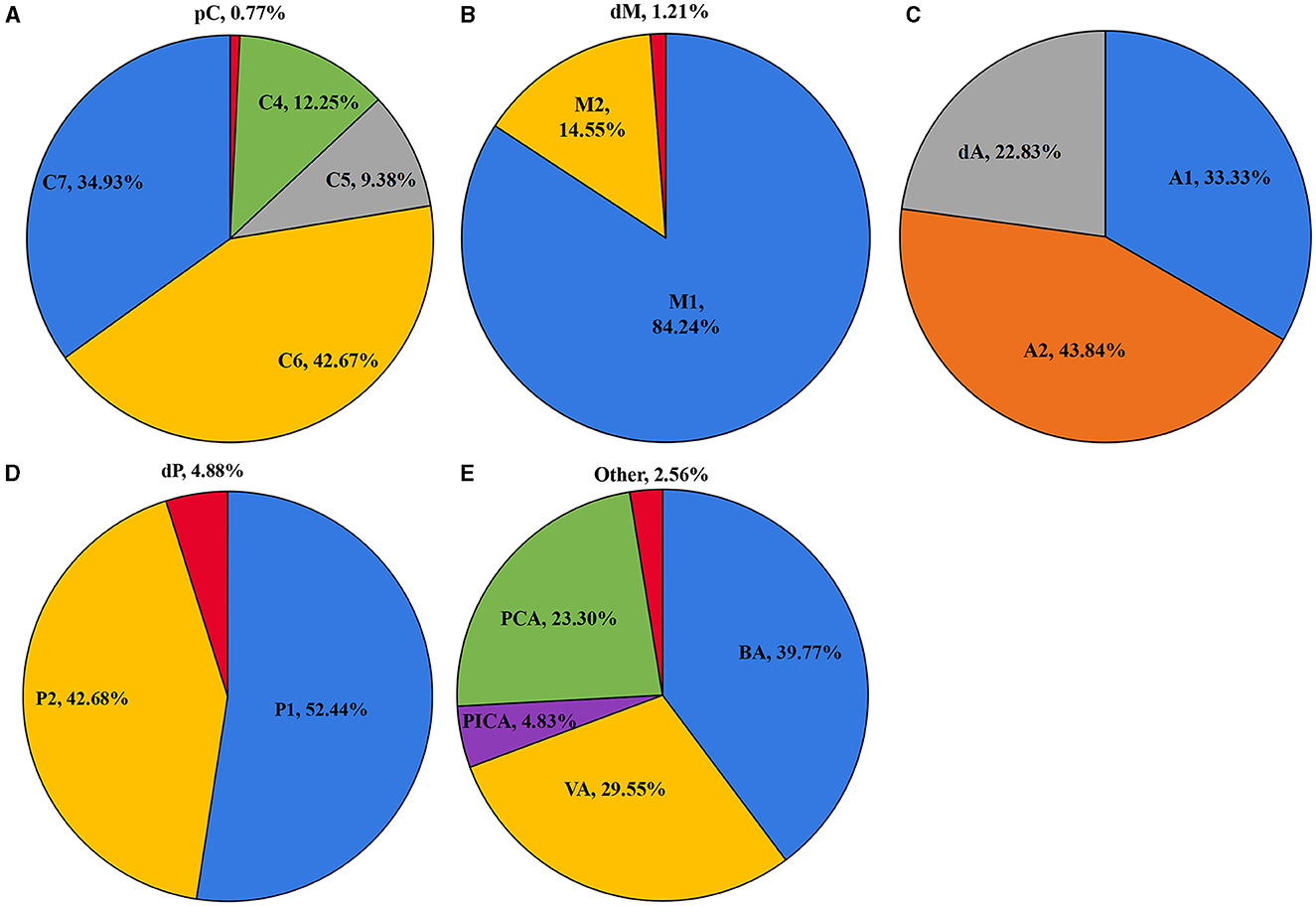
Figure 2. The distribution of the aneurysms of the ICA (A), MCA (B), ACA (C), PCA (D), and posterior circulation (E).
We analyzed the ruptured aneurysm proportions of different cerebral arteries and different arterial segments (Table 3). The ranking of the ruptured aneurysm proportions is shown in Figure 3.
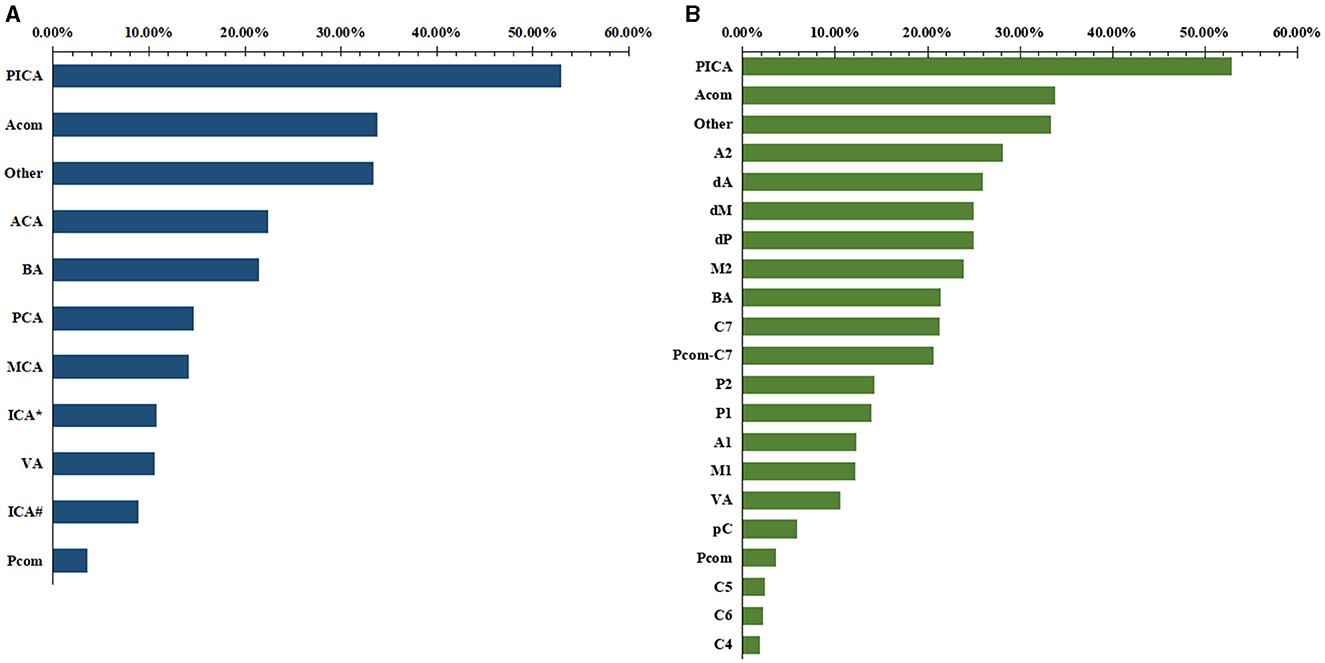
Figure 3. The ranking of the proportions of ruptured aneurysms of different locations. (A) Cerebral artery. (B) arterial segment.
The ruptured aneurysm proportion of C7 (21.25%) was significantly higher than that of C4 (5.88%, p < 0.001), C5 (1.86%, p < 0.001), and C6 (2.24%, p < 0.001). The ruptured aneurysm proportion of M2 (23.96%) was significantly higher than that of M1 (12.23%, p = 0.002). The ruptured aneurysm proportion of A2 (28.13%) was significantly higher than that of A1 (12.33%, p = 0.013).
Factors such as bifurcation, presence of a daughter sac, size, age, presence of multiple aneurysms, gender, parent artery stenosis, artery of origin, hyperlipidemia, diabetes mellitus, hypertension, and smoking history were included in the analysis.
A comparative analysis between the ruptured and unruptured IAs was conducted. As shown in Table 2, gender (p = 0.066), age (p = 0.974), diabetes (p = 0.601), hypertension (p = 0.359), and smoking history (p = 0.312) were not significantly associated with rupture. Non-hyperlipidemia (p < 0.001), bifurcation (p < 0.001), larger size (p = 0.001), single aneurysm (p < 0.001), presence of daughter aneurysms (p < 0.001) and non-stenotic proximal parent artery (p = 0.011) were found to be risk factors associated with rupture through a logistic regression analysis (Table 2).
The segments of the origins of IAs can be a risk factor according to the logistic regression analysis. For ICA IAs, C7 was an independent risk factor for IA rupture compared with C6 (p < 0.001, OR: 7.569; 95% CI: 4.540–12.618). For MCA IAs, dM1 was correlated with a higher rupture risk than M1 IAs (p = 0.044, OR: 1.961; 95% CI: 1.020–3.770). For ACA IAs, dA1 IAs were correlated with a higher rupture risk than A1 (p = 0.014, OR: 3.182; 95% CI: 1.264–8.013). For PCA IAs, different segment positions were not considered independent risk factors (p = 0.855). Other independent risk factors of the ICA, MCA, ACA, and PCA are shown in Supplementary Tables 1–4.
Non-hyperlipidemia, bifurcation, diameter, single aneurysm, presence of daughter aneurysms, and non-stenotic proximal parent artery were the risk factors included in the PSM. A total of 776 aneurysms were selected from all aneurysms. The segments of the origins of IAs were, after further analysis, found to be a risk factor for rupture. The proportions of C4 (p = 0.002), C5 (p = 0.015), C6 (p < 0.001), and M1 (p < 0.001) in the unruptured group were significantly higher than those in the ruptured group. The proportions of C7 (p < 0.001), the ACoA (p = 0.002), and the PICA (p = 0.011) in the ruptured group were significantly higher than those in the unruptured group. Although the ACA (p = 0.028) had a higher proportion in the ruptured group, A1 (p = 0.203), A2 (p = 0.109), and dA (p = 0.387) had no significant difference in the two groups. More details are provided in Table 4.
The origins of IAs and their imaging characteristics are well-known factors associated with rupture. However, to date, studies have focused only on the artery of origin and not the specific arterial segment. In addition, the contribution of preaneurysmal arterial stenosis has rarely been described. Our large-scale study showed that a segment-specific evaluation of aneurysm origin may play an important role in aneurysm instability, with C7, M2, dM, A2, and dA IAs having a higher rupture risk. These results provide new information about the assessment of IA stability, which was not reported previously.
Greving et al. (6) analyzed six prospective cohort studies and found that the ICA and MCA were the most common locations of IAs but had a low rupture risk compared to the ACoA, ACA, BA, and PCA. However, our results showed that several specific segments of the ICA and MCA (C7, M2, and dM) had a very high rupture risk, only second to the ACoA.
The reason behind C7 IAs having a high rupture risk may be due to a complex hemodynamic environment caused by higher blood flow velocities and smaller lumen areas (13). The high rupture risk of C7 IAs may be masked by the low risk of C6 aneurysms. Detmer et al. (14) showed a high risk of rupture of carotid bifurcation aneurysms, which was consistent with our results. A Japanese cohort study (15) had findings similar to ours, showing that C7-Pcom origin IAs were at a higher risk of rupture, whereas other ICA IAs were at a lower risk of rupture.
Although the size of the M2 and M1 IAs were similar in the current study, the smaller diameter (16) and thinner wall (17, 18) of M2 arterial segments likely contributed to increased M2 IA instability. Our study also found that the IAs at dA1 had a much higher risk than the A1 IAs. Previous studies of ACA origin IAs (19, 20) had shown that A1, A2, or distal ACA (A3 or beyond) aneurysms had thin walls, complex hemodynamics, and were more likely to rupture at smaller sizes than aneurysms at other locations. Lehecka et al. (21) speculated that A2 IAs had a higher rupture risk because of the perforator origin, broad base configuration, thin walls, and abnormal hemodynamics. In addition, dA1 IAs had a smaller parent artery diameter compared to A1 IAs (22), which may be associated with increased IA instability (19, 23, 24).
Similar to prior studies, we found a high rupture risk of the posterior circulation origin IAs (1, 6); however, we also found that only the BA and PICA showed a high risk of rupture compared to the mean risk of all IAs. Similar to other studies (25, 26), BA IAs were the most common posterior circulation aneurysms, accounting for 39.8% in our study. The high prevalence of BA IAs may have contributed to an overestimation of the overall posterior circulation IA rupture risk. In addition, considering the small data on posterior circulation aneurysms, further verification may be necessary.
Similar to previous studies, the risk factors for IA rupture included bifurcation origin, presence of a daughter sac, large diameter, increased age, and no stenosis of the parent artery (1, 6, 9, 14, 25, 27, 28). The aneurysms with a non-stenotic parent artery usually exhibited lower wall shear stress (29), and this phenomenon may have led to a higher rupture risk (30) because of inflammation (31). The current study did not find female sex to be a risk factor for rupture. Age and menopause have a significant impact on aneurysms in female patients, which may explain the discrepancies in the results of different studies regarding sex as a risk factor for rupture.
After adjusting for the risks attributed to aneurysm rupture, C7 IAs, ACoA IAs, and PICA IAs showed a high risk of rupture and C4 IAs, C5 IAs, C6 IAs, and M1 IAs showed a low risk of rupture. This finding indicates that the origin of an aneurysm is also a risk factor. However, after PSM, the number of aneurysms included in the study was significantly reduced, necessitating further investigation of the results.
There were a number of limitations of this study. First, this was a single-center retrospective study including a Chinese cohort. Most of the patients included were local residents, especially those with ruptured aneurysms. In addition, many unruptured aneurysms are found through physical examination or in relation to other reasons. These aspects led to population bias in our data, which may not translate to other populations. Second, most aneurysms were treated soon after discovery, with patients not undergoing a long-term follow-up, which may have resulted in bias toward those aneurysms deemed to not require initial treatment. Third, every aneurysm was represented in this study as a single data point. All risk factors were summaries of existing cases, and there was a lack of follow-up observation and strict control of variables. The study could not completely exclude the interference of confounding factors. Aneurysms were so numerous and rare in some segments that they had to be merged into others and could not be assessed for risk. In addition, patients with prior subarachnoid hemorrhage were not included in the study because many patients were diagnosed at other hospitals and did not have complete images and medical records of ruptured aneurysms. This study could not determine the effect of previous subarachnoid hemorrhage on aneurysm rupture, potentially contributing to bias. Therefore, further investigation in a large prospective longitudinal cohort is necessary.
Beyond the already established risk associated with IA rupture at the artery of origin, this study showed that further risk can be attributed to specific segments of origin, which might improve predictions of aneurysm instability. Aneurysms originating from the ACoA, BA, and PICA and A2, dA, C7, and M2 indicated a higher rupture risk. Aneurysms originating from C4, C5, and C6 indicated a lower rupture risk. C7 IAs, ACoA IAs, and PICA IAs appeared to be independent risk factors. A multicenter prospective cohort study can better refine the findings of this study.
The corresponding author will provide data supporting the conclusions of this article upon reasonable request.
The studies involving humans were approved by the Institutional Review Board at Beijing Tiantan Hospital, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
JF: Conceptualization, Investigation, Methodology, Writing – original draft. YT: Formal analysis, Visualization, Writing – original draft. WY: Data curation, Investigation, Writing – original draft. YJ: Investigation, Writing – original draft. ZX: Data curation, Formal analysis, Writing – original draft. YZ: Data curation, Investigation, Writing – original draft. XL: Investigation, Writing – original draft. JL: Data curation, Investigation, Writing – original draft. PL: Data curation, Writing – original draft. HW: Data curation, Writing – original draft. MM-B: Writing – original draft. YL: Writing – review & editing, Funding acquisition. YW: Writing – review & editing, Data curation, Methodology, Project administration, Supervision, Writing – original draft. CZ: Methodology, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The views and opinions expressed in this manuscript are those of the authors and do not necessarily reflect the official policy or position of the institution or funder. The authors are solely responsible for ensuring that the research presented in this manuscript is original and that all sources have been appropriately cited.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1339144/full#supplementary-material
1. Rinkel G, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. (1998) 29:251–6. doi: 10.1161/01.STR.29.1.251
2. Detmer F, Lückehe D, Mut F, Slawski M, Hirsch S, Bijlenga P, et al. Comparison of statistical learning approaches for cerebral aneurysm rupture assessment. Int J Comput Assist Radiol Surg. (2020) 15:141–50. doi: 10.1007/s11548-019-02065-2
3. Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke. (2013) 44:2414–21. doi: 10.1161/STROKEAHA.113.001838
4. You S, Kong D, Kim J, Jeon P, Kim K, Roh H, et al. Characteristic features of unruptured intracranial aneurysms: predictive risk factors for aneurysm rupture. J Neurol Neurosurg Psychiatry. (2010) 81:479–84. doi: 10.1136/jnnp.2008.169573
5. Mocco J, Brown R, Torner J, Capuano A, Fargen K, Raghavan M, et al. Aneurysm morphology and prediction of rupture: an international study of unruptured intracranial aneurysms analysis. Neurosurgery. (2018) 82:491–6. doi: 10.1093/neuros/nyx226
6. Greving J, Wermer M, Brown R, Morita A, Juvela S, Yonekura M, et al. Development of the PHASES score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. (2014) 13:59–66. doi: 10.1016/S1474-4422(13)70263-1
7. Kleinloog R, de Mul N, Verweij B, Post J, Rinkel G, Ruigrok Y. Risk factors for intracranial aneurysm rupture: a systematic review. Neurosurgery. (2018) 82:431–40. doi: 10.1093/neuros/nyx238
8. Vlak M, Rinkel G, Greebe P, van der Bom J, Algra A. Trigger factors for rupture of intracranial aneurysms in relation to patient and aneurysm characteristics. J Neurol. (2012) 259:1298–302. doi: 10.1007/s00415-011-6341-1
9. AlMatter M, Bhogal P, Aguilar Pérez M, Schob S, Hellstern V, Bäzner H, et al. The size of ruptured intracranial aneurysms: a 10-year series from a single center. Clin Neuroradiol. (2019) 29:125–33. doi: 10.1007/s00062-017-0632-6
10. Fung C, Mavrakis E, Filis A, Fischer I, Suresh M, Tortora A, et al. Anatomical evaluation of intracranial aneurysm rupture risk in patients with multiple aneurysms. Neurosurg Rev. (2019) 42:539–47. doi: 10.1007/s10143-018-0998-1
11. Bouthillier A, van Loveren H, Keller J. Segments of the internal carotid artery: a new classification. Neurosurgery. (1996) 38:425–32; discussion 432–3. doi: 10.1227/00006123-199603000-00001
12. Fischer E. Die lageabweichungen der vorderen hirnarterie im gefassbild. Zbl Neurochir. (1938) 3:300–12.
13. van Tuijl R, Ruigrok Y, Velthuis B, van der Schaaf I, Rinkel G, Zwanenburg J. Velocity pulsatility and arterial distensibility along the internal carotid artery. J Am Heart Assoc. (2020) 9:e016883. doi: 10.1161/JAHA.120.016883
14. Detmer F, Chung B, Jimenez C, Hamzei-Sichani F, Kallmes D, Putman C, et al. Associations of hemodynamics, morphology, and patient characteristics with aneurysm rupture stratified by aneurysm location. Neuroradiology. (2019) 61:275–84. doi: 10.1007/s00234-018-2135-9
15. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. (2012) 366:2474–82. doi: 10.1056/NEJMoa1113260
16. Rai A, Hogg J, Cline B, Hobbs G. Cerebrovascular geometry in the anterior circulation: an analysis of diameter, length and the vessel taper. J Neurointerv Surg. (2013) 5:371–5. doi: 10.1136/neurintsurg-2012-010314
17. Hademenos G, Massoud T, Valentino D, Duckwiler G, Viñuela F. A nonlinear mathematical model for the development and rupture of intracranial saccular aneurysms. Neurol Res. (1994) 16:376–84. doi: 10.1080/01616412.1994.11740257
18. Dhar S, Tremmel M, Mocco J, Kim M, Yamamoto J, Siddiqui A, et al. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery. (2008) 63:185–96; discussion 196–7. doi: 10.1227/01.NEU.0000316847.64140.81
19. Cebral J, Raschi M. Suggested connections between risk factors of intracranial aneurysms: a review. Ann Biomed Eng. (2013) 41:1366–83. doi: 10.1007/s10439-012-0723-0
20. Dashti R, Hernesniemi J, Lehto H, Niemelä M, Lehecka M, Rinne J, et al. Microneurosurgical management of proximal anterior cerebral artery aneurysms. Surg Neurol. (2007) 68:366–77. doi: 10.1016/j.surneu.2007.07.084
21. Lehecka M, Dashti R, Hernesniemi J, Niemelä M, Koivisto T, Ronkainen A, et al. Microneurosurgical management of aneurysms at the A2 segment of anterior cerebral artery (proximal pericallosal artery) and its frontobasal branches. Surg Neurol. (2008) 70:232–46; discussion 246. doi: 10.1016/j.surneu.2008.03.008
22. Zurada A, Gielecki J, Tubbs R, Loukas M, Cohen-Gadol A, Chlebiej M, et al. Three-dimensional morphometry of the A2 segment of the anterior cerebral artery with neurosurgical relevance. Clin Anat. (2010) 23:759–69. doi: 10.1002/ca.21036
23. Ma D, Tremmel M, Paluch R, Levy E, Meng H, Mocco J. Size ratio for clinical assessment of intracranial aneurysm rupture risk. Neurol Res. (2010) 32:482–6. doi: 10.1179/016164109X12581096796558
24. Matsukawa H, Kamiyama H, Kinoshita Y, Saito N, Hatano Y, Miyazaki T, et al. Morphological parameters as factors of 12-month neurological worsening in surgical treatment of patients with unruptured saccular intracranial aneurysms: importance of size ratio. J Neurosurg. (2018) 131:852–8. doi: 10.3171/2018.4.JNS173221
25. Wang C, Shi W, Zhang G, Lu H, Ma J. Flow diverter treatment of posterior circulation aneurysms. A meta-analysis. Neuroradiology. (2016) 58:391–400. doi: 10.1007/s00234-016-1649-2
26. Li M, Jiang Z, Yu H, Hong T. Size ratio: a morphological factor predictive of the rupture of cerebral aneurysm? Can J. (2013) 40:366–71. doi: 10.1017/S0317167100014323
27. Fukuda S, Shimogonya Y, Yonemoto N. Differences in cerebral aneurysm rupture rate according to arterial anatomies depend on the hemodynamic environment. Am J Neuroradiol. (2019) 40:834–9. doi: 10.3174/ajnr.A6030
28. Juvela S. Treatment scoring of unruptured intracranial aneurysms. Stroke. (2019) 50:2344–50. doi: 10.1161/STROKEAHA.119.025599
29. Antonov A, Kono K, Greim-Kuczewski K, Hippelheuser J, Lauric A, Malek A. Proximal stenosis is associated with rupture status in middle cerebral artery aneurysms. World Neurosurg. (2018) 109:e835–44. doi: 10.1016/j.wneu.2017.10.108
30. Axier A, Rexiati N, Wang Z, Cheng X, Su R, Aikeremu R, et al. Effect of hemodynamic changes on the risk of intracranial aneurysm rupture: a systematic review and meta-analysis. Am J Transl Res. (2022) 14:4638–47.
31. Yudi T, Haining W, Zihao Z, Mingzhu F, Junqiang F, Zhixin L, et al. Transition of intracranial aneurysmal wall enhancement from high to low wall shear stress mediation with size increase: a hemodynamic study based on 7T magnetic resonance imaging. Heliyon. (2024) 10:e30006. doi: 10.1016/j.heliyon.2024.e30006
Keywords: aneurysm, aneurysm rupture risk, aneurysm rupture, risk assessment, CT angiography
Citation: Feng J, Tang Y, You W, Jiang Y, Xu Z, Zhao Y, Liu X, Lv J, Liu P, Wei H, Mossa-Basha M, Li Y, Wang Y and Zhu C (2024) Risk analysis of intracranial aneurysm rupture based on the arterial segment of origin. Front. Neurol. 15:1339144. doi: 10.3389/fneur.2024.1339144
Received: 15 November 2023; Accepted: 31 July 2024;
Published: 21 August 2024.
Edited by:
Markus Holtmannspötter, Klinikum Nürnberg, GermanyReviewed by:
Hans Gram Værum Novrup, Aalborg University Hospital, DenmarkCopyright © 2024 Feng, Tang, You, Jiang, Xu, Zhao, Liu, Lv, Liu, Wei, Mossa-Basha, Li, Wang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youxiang Li, bGl5b3V4aWFuZ0BtYWlsLmNjbXUuZWR1LmNu; Yang Wang, d2FuZ3lhbmc3ODM5QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.