- 1Department of Neurology, Faculty of Medicine and Health Sciences, University Putra Malaysia, Serdang, Selangor, Malaysia
- 2Department of Otorhinolaryngology, Faculty of Medicine and Health Sciences, University Putra Malaysia, Serdang, Selangor, Malaysia
- 3Department of Emergency, Faculty of Medicine and Health Sciences, University Putra Malaysia, Serdang, Selangor, Malaysia
- 4Department of Pathology, Faculty of Medicine and Health Sciences, University Putra Malaysia, Serdang, Selangor, Malaysia
Accurate and timely diagnosis of posterior circulation stroke in patients with acute dizziness is a challenge that can lead to misdiagnosis and significant harm. The present review sought to identify and describe published research on the clinical application of vHIT in posterior circulation stroke. vHIT, a portable device, has gained prominence in evaluating peripheral vestibular disorders and offers potential applications in diagnosing neurological disorders, particularly posterior circulation stroke. Several studies have shown that vHIT can differentiate between stroke and vestibular neuritis based on VOR gain values, with high sensitivity and specificity. The manuscript also discusses vHIT’s performance in differentiating between types of posterior circulation stroke, such as PICA, AICA, and SCA strokes. While vHIT has demonstrated promise, the review emphasizes the need for further research to validate its use as a tool to rule out stroke in acute dizziness patients in the emergency department. In conclusion, the manuscript underscores the potential of vHIT as a valuable addition to the diagnostic arsenal for acute dizziness, particularly in the context of posterior circulation stroke. It calls for further research and wider adoption of vHIT in clinical settings to improve patient care and reduce unnecessary costs associated with misdiagnoses.
Introduction
Acute vestibular syndrome (AVS) is characterized by the abrupt onset of vertigo or dizziness, nausea or vomiting, intolerance to head motion, and an unsteady feeling. Vascular vertigo or dizziness should be considered in patients who show signs of AVS. According to the latest guideline published by the Committee for the Classification of Vestibular Disorders of the Barany Society, the following conditions can result in vascular vertigo/dizziness: stroke, transient ischemic attack (TIA), isolated labyrinthine infarction/hemorrhage, and vertebral artery compression syndrome (VACS) (1). The difficulties of distinguishing peripheral vestibular disorders such as vestibular neuritis (VN) vs. posterior circulation stroke (PCS) have always been a challenge to the frontliners, leading to frequent misdiagnosis and potentially serious harm to patients.
The video head impulse test (vHIT) provides a portable and objective method for distinguishing between these conditions. It utilizes head-mounted goggles equipped with a high-speed camera and sensors to evaluate head velocity (2). Worn by the patient, these goggles record the movement of their eyes while their head undergoes rapid rotations in various directions. This allows the clinician to assess the gain, latency, and symmetry of the vestibulo-ocular reflex (VOR), a crucial reflex enabling us to maintain a fixed gaze on a target while our head is in motion. In contrast to subjectively observing corrective saccades resulting from the VOR, vHIT offers an objective measurement of both overt and covert corrective saccades. Physiologically, vHIT works by measuring the VOR gain of each semi-circular canal by calculating the duration ratio between head impulse and gaze deviation (3). A normal VOR gain value is >−0.81 for horizontal canals and >−0.71 for vertical canals. Any value below the cut-off threshold is considered abnormal (4).
Materials and methods
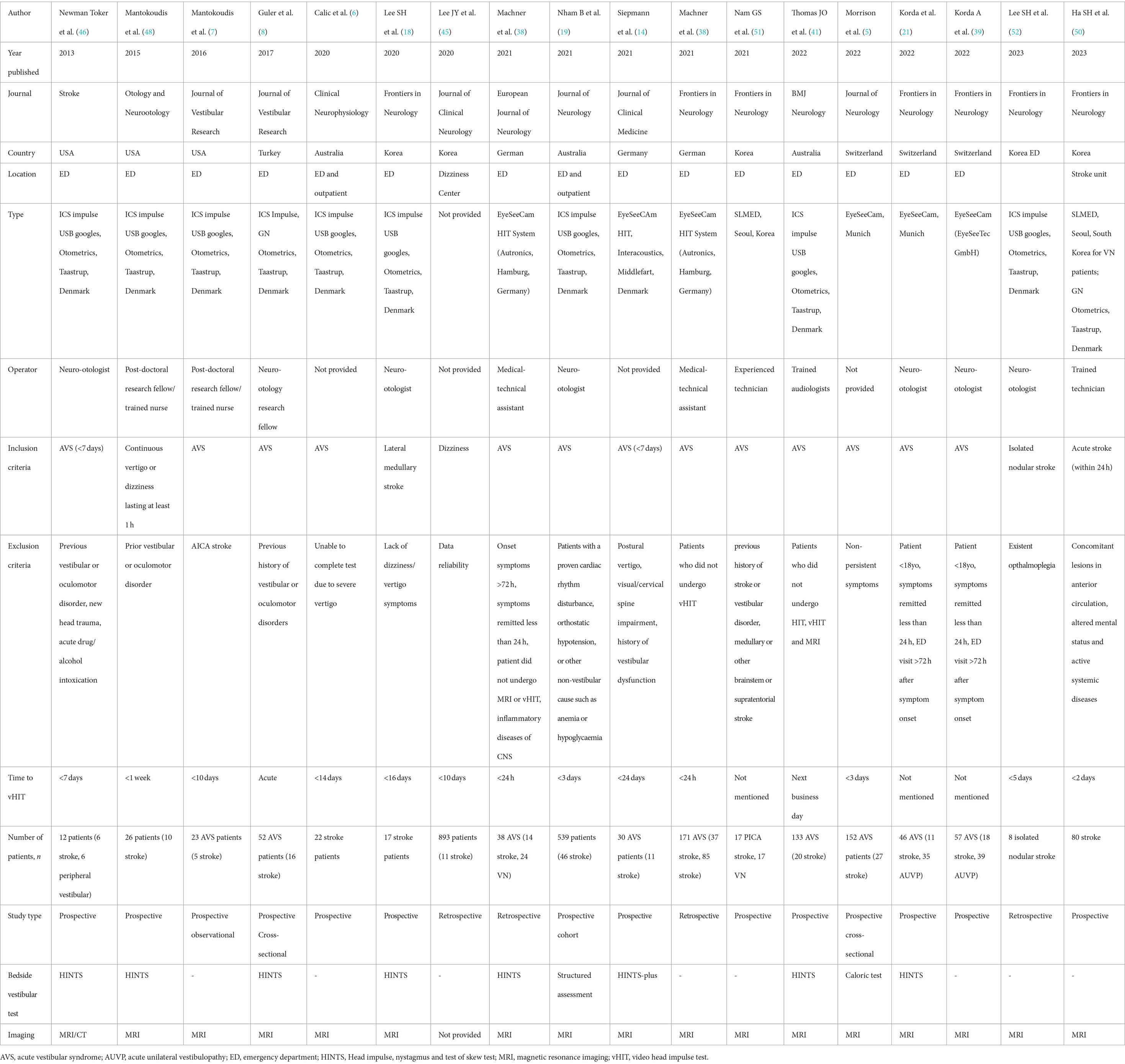
An electronic search was performed by searching the following databases: PubMed, Scopus and Google Scholar. Articles are searched using the following search terms: video head impulse test, video oculography, acute vestibular syndrome, posterior circulation stroke, acute vertigo, and acute dizziness. We only consider publications in the English language before 31 October 2023. Congress-related published abstracts were excluded. Reference lists of related publications were also examined for further sources not identified in online searches. This search strategy yielded 18 published works.
Thus, a total of 18 clinical studies were selected and their clinical data were summarized in Table 1.
Due to the shortage of research on video head impulse tests in stroke, there was a lack of consistency in the outcome measures used and in the theoretical and methodological approaches employed. Furthermore, the gain measurements for the primary VOG device, primarily obtained using the EyeSeeCam and the Otometrics device, exhibit variations due the difference in measurement methods. Consequently, direct comparisons of the calculations are not feasible due to these differences.
Results
All studies included in this review were published between 2013 and 2023. Regarding study design, there were 14 prospective and 4 retrospective studies. The sample size ranged from 12 to 893 participants. As expected, there was a smaller number of stroke patients compared to peripheral causes. Most studies use MRI scans as neuroimaging of choice, while 1 study uses both CT and MRI. 1 study did not provide this information.
As for the country of origin, 3 studies were conducted in the US, 5 in Korea, 3 in Australia, 3 in Switzerland, 3 in Germany, and 1 in Turkey. Various types of vHIT machines were used in the study, most used is the ICS impulse USB googles, Otometrics, Taastrup, Denmark while 2 studies used the EyeSeeCam, Munich and another used the EyeSeeCAm HIT (Interacoustics, Middlefart, Denmark). Another study from Korea did not provide the name of the machine used, but another 2 studies in this country used SLMED, South Korea. In terms of the operator for the machine, most were performed by neuro-otologists.
vHIT was conducted shortly after the onset of AVS symptoms in the majority of studies. AVS is defined as acute onset of continuous vertigo or dizziness lasting at least 1 h. In addition to AVS, our review encompassed investigations on vHIT involving isolated nodular stroke and lateral medullary stroke. Many studies opted to exclude individuals with prior vestibular or oculomotor disorders, recent head trauma, and acute drug/alcohol intoxication, as these conditions can elicit nystagmus. Furthermore, some studies ruled out participants with cervical impairment, given the infeasibility of performing vHIT on individuals with spine or neck injuries. Additionally, certain studies integrated bedside clinical assessments alongside vHIT or other tests evaluating vestibular function. vHIT was performed acutely after the onset of dizziness in most studies Apart from AVS, we also included studies of vHIT on isolated nodular stroke and lateral medullary stroke.
Comparison of VHIT to bedside testing
HINTS constitutes a battery of bedside clinical tests, including head impulse test (HIT), assessment of nystagmus, and evaluation of skew deviation (22–24). HIT is a straightforward bedside clinical examination in which the clinician passively rotates a patient’s head abruptly while observing the VOR (3).
Despite its usefulness as a bedside tool in patients presenting with acute dizziness in the emergency department, the major drawback to HINT is its subjectivity, making it highly operator-dependent (16, 25, 26). Detecting overt corrective saccades requires experience, and an inexperienced operator may easily overlook these findings (7, 8, 27).
Hotson and Baloh (28) have shown that the presence of direction-changing horizontal nystagmus in any gaze direction consistently implies a central localization. Conversely, unidirectional horizontal nystagmus can happen in lesions that are peripheral or central (29). A significant decrease in the horizontal head impulse VOR gains can occur from unilateral or bilateral (positive HITs) lesions of the vestibular nucleus, flocculus, or nucleus prepositus hypoglossi (30–32). In such cases, it is imperative to assess the integrity of the horizontal VOR using vHIT to distinguish between central and peripheral localization. If the vHIT results are normal, it is evident that the lesion causing the unidirectional nystagmus spares the vestibular periphery, pointing toward a central lesion as the likely cause.
When skew deviation is detected during cover testing, a central lesion is likely present. A substantial skew deviation, as seen in AVS, is more frequently linked to acute stroke than minor skew deviations found by cover testing, which may occasionally appear in vestibular neuritis. Such a pronounced skew deviation should be regarded as a red flag, prompting the need for additional investigations (3, 33, 34). The combination of these three oculomotor findings has proven to be more sensitive (96.5%) and specific (84.4%) than MRI brain imaging for detecting posterior circulation stroke (13, 35).
HINTS examination has also been combined with other bedside examinations, such as the Dix-Hallpike test, STANDING, ABCD2 score and HINTS Plus to enhance stroke detection (11, 14, 36).
HINTS exam has demonstrated high sensitivity in detecting PCS (7, 8, 18, 37). However, vHIT is shown to be more specific in detecting PCS (16, 38). This is particularly useful in settings without neuro-otological experts who can perform the HINT bedside examination reliably. vHIT demonstrated an overall accuracy of 94.2% in detecting central pathology, boasting 100% sensitivity and 88.9% specificity. In contrast, experts evaluating bedside HINTS achieved slightly lower accuracy at 88.3%, comprising 90.9% sensitivity and 85.7% specificity (39).
Common neurological bedside examinations like NIHSS (National Institutes of Health Stroke Scale) scoring are also insensitive to detecting posterior circulation stroke, making it challenging for ED physicians to confidently rule out acute stroke in patients with acute dizziness (40).
Despite the high specificity and sensitivity of vHIT in detecting acute stroke, the importance of clinical assessment should not be undermined. The integration of subjective and objective evaluations of VOR gain, as seen in the combination of HINT and vHIT, has demonstrated an enhancement in distinguishing between PCS and VN (13, 41). Re-examining patients at the conclusion of the 24-h period is imperative, it should be noted, for the purpose of differentiating between vertigo and other conditions, such as migraine, Meniere’s disease, and TIA (1).
vHIT vs. another non-invasive vestibular function test
Scleral search coil
Before the development of vHIT, the gold standard for measuring VOR gain was the scleral search coil (SSC). This technique involves a lightweight copper coil implanted into a circular-shaped silicon, which is later attached to the sclera (42). However, due to its invasive nature, it is impractical for clinical usage (43). Chen et al. examined the differences in HIT gains and compensatory saccades in PCS and VN using dual-search coils. In VN, there were asymmetric gain reductions and uneven compensatory saccades. In contrast, AICA strokes resulted in bilaterally reduced HITs, along with relatively small corrective saccades. Finally, PICA strokes demonstrated a directional bias, with HIT gains increased toward the opposite side of the lesion, accompanied by the smallest saccades overall (44). The emergence of vHIT as a quick, reliable, and validated tool for measuring VOR gain has led to its comparison with the SSC in several clinical studies (1, 10, 17).
Caloric test
vHIT has also advanced to replace the caloric test in assessing semi-circular canal function in vestibular patients (2, 5, 9, 12). However, caloric testing may be more relevant in conditions like Meniere’s disease and vestibular migraine (5).
In this test, patients lie supine at 30 degrees, and warm and cold-water solutions are irrigated into the affected ear within 25 to 30 s, with resulting nystagmus observed (37, 45). Compared to vHIT, bithermal caloric testing has lower sensitivity and specificity in detecting stroke in patients with acute dizziness. An abnormal caloric test and normal vHITs are associated with peripheral causes of dizziness (6). In cases of normal vHIT, it is advisable to proceed with caloric testing to rule out peripheral vertigo.
Vestibular-evoked myogenic potentials
Another vestibular function test is the Vestibular-Evoked Myogenic Potentials (VemPs), which consist of two major components: cVEMPs and oVEMPs. Asymmetry ratios (AR) are then used to detect unilateral otolith dysfunction. Although vEMPs have high specificity (90.9%) for detecting vestibular neuritis, especially in the absence or asymmetry of oVEMPs, they are less useful in detecting posterior circulation stroke. cVEMPs also show similar abnormal AR in both VN and PCS (19).
Subjective visual horizontal
Subjective Visual Horizontal (SVH) is performed by asking the patient to sit upright in a dark room, looking at an illuminated line presented at various angles from a 1.5 m distance. Contraversive SVH deviation, indicating lesions rostral to the pons, is a precise yet insensitive indicator of PCS. SVH is more likely to produce abnormal results in either VN or PCS, making it a poor discriminatory test to distinguish between the two (19, 37).
Several studies have incorporated the use of multiple tools to improve the detection of stroke in acute vestibular syndrome (9, 46). Structured history-taking and physical examination, including HINTS/HINTS PLUS, serve as the backbone in most of these studies. This is then followed by additional vestibular testing, such as vHIT, VEMP, and bithermal caloric testing. When combined, these tools have shown promising results in enhancing sensitivity and specificity.
vHIT vs. neuroimaging
Regrettably, neuroimaging studies run the risk of overlooking PCS. The commonly employed investigation modality for stroke in emergency departments, brain computed tomography (CT), exhibits notably low sensitivity in identifying this condition. Retrospective studies suggest that CT may have as low as 42% sensitivity for ischemic stroke in cases of dizziness. However, a lack of awareness regarding CT’s limitations in assessing dizziness may contribute to its overuse and the potential for misdiagnosis. Additionally, even early MRI Brain with diffusion-weighted imaging may fail to detect up to 20% of acute PCS (47). The current gold standard for detecting PCS is an MRI of the brain performed more than 48 h after the onset of dizziness. In accordance with published studies, the majority employed MRI brain as a confirmatory test for PCS, with the exception of a 2013 study by Newman Toker et al., which utilized either CT or MRI brain. Although MRI is more commonly available nowadays, carrying out this procedure for every patient experiencing dizziness in a busy and overcrowded emergency department is not feasible and would consume a significant amount of time.
Patients generally tolerated vHIT examination well (7, 48). In the future, vHIT could be a valuable diagnostic tool for patients suspected of stroke who cannot undergo an MRI due to reasons such as claustrophobia or the presence of incompatible MRI devices on-site. It is also noteworthy that vHIT is considerably more cost-effective than an MRI machine, making it potentially useful in district hospitals or settings with limited resources.
Findings in posterior circulation stroke
The utilization of vHIT for detecting PCS in cases of AVS has been established through various clinical studies (21, 47, 48). HIT gain and catch-up saccades characteristics can distinguish between PCS and VN (6, 19, 37, 46, 47). Mean VOR gain assessment achieves a 91% accuracy in differentiating PICA strokes from VN. PCS patients exhibited low or normal VOR gain, increased catch-up saccade amplitude, and saccade frequency compared to healthy age-matched controls (46). There is also significant refixation-saccade prevalence difference between PCS and VN. Additionally, in normal controls, the first and cumulative saccade amplitudes, initial saccade peak velocities, and duration were smaller, while in PCS, they were higher, and in VN, they were the highest. Utilizing artificial intelligence (AI) for the analysis of video Head Impulse Test (vHIT) data indicates a promising direction for future exploration (24).
PICA stroke
The territory most affected in PCS is the PICA (4, 15, 41, 49, 50). In a study by Mantokoudis et al., involving 26 patients with AVS and utilizing ICS impulse, a vHIT device, it was found that among the 7 patients diagnosed with PICA stroke, the mean VOR HIT gains fell within the normal range, with no discernible difference between ipsilesional and contralesional infarcts (21). Similar qualitative findings were observed, with over 99% of PICA strokes displaying normal clinical horizontal head impulse test results (18, 20, 51).
Another study by Guler et al. concluded that VOR gains in PICA-SCA stroke (pure cerebellar stroke with no brainstem involvement) were normal, showing no asymmetry. In AICA-PICA stroke, low VOR gain was observed on both ipsilesional and contralesional sides compared to healthy controls (8). Meanwhile, Calic et al. combined VOR gain and individual saccade parameters to enhance PCS detection, revealing that PCS is associated with normal or reduced VOR gain. Yet, the combined measures did not improve the differentiation between PCS and VN (19).
PICA stroke was found to be associated with preserved VOR gain and smaller corrective saccade amplitude in the ipsilesional horizontal canals (51, 52). While VOR gain proves useful in distinguishing between PCS and VN, there is no significant difference in VOR when comparing lesions in the midbrain, medulla, or pons of PCS (15). A small single-center study also concluded that preserved aVOR gain was found in patients with isolated heminodular stroke, which involves the nodulus with or without associated cerebellar structures supported by the medial posterior inferior cerebellar artery (mPICA) (52). The combination of normal VOR gain and absence of VOR asymmetry allowed investigators to correctly identify PCS in patients with AVS.
Lateral medullary stroke
The lateral medullary region of the brainstem is primarily supplied by PICA. A complete infarction in this area can lead to Wallenberg syndrome, characterized by acute vertigo, ipsilateral Horner’s syndrome, ataxia, and facial hypesthesia, along with contralateral hemisensory deficits. Examinations may also reveal associated nystagmus and skew deviation (53).
In a study conducted in a South Korean university hospital involving 17 patients with unilateral lateral medullary syndrome, the majority of patients (88%) exhibited normal aVOR gain. Only two patients demonstrated unilaterally reduced aVOR gains, both of which were mild and restricted to specific semicircular canals. The investigators attributed these findings to minimal or no involvement of the vestibular nuclei, as evidenced by MRI brain scans (51).
AICA stroke
More pronounced changes in VOR gain were observed in cases involving AICA strokes. Both vHIT studies demonstrated bilaterally reduced VOR gains without asymmetry in this specific subtype of PCS (8, 49). Guler et al. concluded that VOR gain is more likely to be impacted in AICA-PICA stroke (brainstem infarct) since the AICA supplies the vestibulo-oculomotor system and connecting oligosynaptic pathways (8). Similarly, in VN, VOR gains are significantly reduced bilaterally, potentially causing confusion with AICA strokes (5). False positive HIT examinations are more likely to be encountered in AICA infarction compared to PICA (19, 32, 54, 55).
SCA stroke
Similar to PICA stroke, VOR gain findings in SCA stroke can be normal or slightly diminished without asymmetry (7, 44).
Limitations of vHIT
Despite the numerous benefits it offers, the vHIT has certain limitations. Factors like artifacts and technical issues, such as goggle slippage, can potentially affect vHIT results, emphasizing the need for a proficient operator to improve accuracy. Additionally, the vHIT machine relies on the operator’s skills and experience, making it operator-dependent and influencing the test’s overall quality. Moreover, vHIT might not be suitable for certain patients, particularly those with a prior history of neck or spine injuries. Financial considerations, encompassing both purchase and maintenance costs, pose additional constraints on vHIT. Furthermore, there is a notable learning curve essential for attaining proficiency in employing vHIT across diverse settings like the emergency department, wards, and outpatient clinic.
Conclusion and perspective/general conclusions and suggestions for future research
The purpose of this review was to identify published studies that have used vHIT in detecting posterior circulation stroke. There has been growing interest among neurologists and researchers on the topic of vHIT in recent years. However, these studies involved a small number of patients, and most were conducted in a single-center setting. This may be attributed to the high cost of performing MRI and the lack of experienced personnel to conduct and interpret the vHIT machine.
While the vHIT does have some limitations, its many advantages make it a critical tool in the diagnosis and treatment of acute dizziness. In a busy emergency department, vHIT may be useful in triaging patients with acute dizziness to speed up the diagnosis of acute stroke. In addition, many acutely dizzy patients with peripheral vestibular causes for their symptoms are over-tested, misdiagnosed, and undertreated. The expenses associated with unwarranted imaging and hospital admissions for these patients can be substantial. Thus, accurate and efficient diagnosis for these patients will likely save lives and reduce costs through prompt and appropriate treatments.
Patients with vHIT findings that are suggestive of peripheral vestibulopathy may be triaged to the green zone or seen in outpatient settings. This will lessen the workload of emergency department staff and will in turn improve the hyperacute care of stroke patients. Similarly, patients who presented to district hospitals can be effectively triaged into those that need to be sent to tertiary hospitals to receive higher level stroke care or those that can be treated conservatively.
In summary, vHIT demonstrated encouraging indications as a diagnostic tool for identifying posterior circulation stroke in acute situations. Further research is essential to validate the efficacy of vHIT as a diagnostic test for ruling out stroke in patients with acute dizziness in the emergency department.
Author contributions
NJ: Writing – original draft. MM: Data curation, Supervision, Writing – review & editing. AM: Resources, Writing – review & editing. HM: Supervision, Writing – review & editing. AH: Writing – original draft. WW: Conceptualization, Methodology, Supervision, Writing – review & editing. HB: Writing – review & editing. LI: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was conducted under the research grant support FRGS/1/2020/SKK08/UPM/02/3.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kim, JS, Newman-Toker, DE, Kerber, KA, Jahn, K, Bertholon, P, Waterston, J, et al. Vascular vertigo and dizziness: Diagnostic criteria. J Vestib Res. (2022) 32:205–22. doi: 10.3233/VES-210169
2. MacDougall, HG, Weber, KP, McGarvie, LA, Halmagyi, GM, and Curthoys, I. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. (2009) 73:1134–41. doi: 10.1212/WNL.0b013e3181bacf85
3. Halmagyi, GM, Chen, L, MacDougall, HG, Weber, KP, McGarvie, LA, and Curthoys, IS. The video head impulse test. Front Neurol. (2017) 8:258. doi: 10.3389/fneur.2017.00258
4. Hansson, A, and Salzer, J. Normative video head impulse test data in subjects with and without vascular risk factors. Eur Arch Otorrinolaringol. (2021) 278:2619–24. doi: 10.1007/s00405-020-06332-w
5. Morrison, M, Korda, A, Zamaro, E, Wagner, F, Caversaccio, MD, Sauter, TC, et al. Paradigm shift in acute dizziness: is caloric testing obsolete? J Neurol. (2022) 269:853–60. doi: 10.1007/s00415-021-10667-7
6. Calic, Z, Nham, B, Bradshaw, AP, Young, AS, Bhaskar, S, D'Souza, M, et al. Separating posterior-circulation stroke from vestibular neuritis with quantitative vestibular testing. Clin Neurophysiol. (2020) 131:2047–55. doi: 10.1016/j.clinph.2020.04.173
7. Mantokoudis, G, Saber Tehrani, AS, Wozniak, A, Eibenberger, K, Kattah, JC, Guede, CI, et al. Impact of artifacts on VOR gain measures by video-oculography in the acute vestibular syndrome. J Vestib Res. (2016) 26:375–85. doi: 10.3233/VES-160587
8. Guler, A, Karbek Akarca, F, Eraslan, C, Tarhan, C, Bilgen, C, Kirazli, T, et al. Clinical and video head impulse test in the diagnosis of posterior circulation stroke presenting as acute vestibular syndrome in the emergency department. J Vestib Res. (2017) 27:233–42. doi: 10.3233/VES-170620
9. Rambold, HA. Economic management of vertigo/dizziness disease in a county hospital: video-head-impulse test vs. caloric irrigation. Eur Arch Otorrinolaringol. (2014) 272:2621–8. doi: 10.1007/s00405-014-3205-1
10. Mangabeira Albernaz, PL, Zuma, E, and Maia, FC. The video head impulse test. Acta Otolaryngol. (2014) 134:1245–50. doi: 10.3109/00016489.2014.942439
11. Gerlier, C, Hoarau, M, Fels, A, Vitaux, H, Mousset, C, Farhat, W, et al. Differentiating central from peripheral causes of acute vertigo in an emergency setting with the HINTS, STANDING, and ABCD2 tests: a diagnostic cohort study. Acad Emerg Med. (2021) 28:1368–78. doi: 10.1111/acem.14337
12. Stevens, MN, Garrison, DB, and Kaylie, DM. What is the potential clinical utility of vHIT when assessing adult patients with dizziness? Laryngoscope. (2017) 127:2689–90. doi: 10.1002/lary.26774
13. Rau, CJ, Terling, L, Elkhodair, S, and Kaski, D. Acute vertigo in the emergency department: use of bedside oculomotor examination. Eur J Emerg Med. (2020) 27:381–3. doi: 10.1097/MEJ.0000000000000674
14. Siepmann, T, Gruener, C, Simon, E, Sedghi, A, Kitzler, HH, Pallesen, LP, et al. Video-oculography-assisted head impulse test and caloric testing for detecting stroke in acute vertigo patients via modified HINTS plus. J Clin Med. (2021) 10:4471. doi: 10.3390/jcm10194471
15. Tarnutzer, AA, Berkowitz, AL, Robinson, KA, Hsieh, YH, and Newman-Toker, DE. Does my dizzy patient have a stroke? A systematic review of bedside diagnosis in acute vestibular syndrome. CMAJ. (2011) 183:E571–92. doi: 10.1503/cmaj.100174
16. Machner, B, Erber, K, Choi, JH, Trillenberg, P, Sprenger, A, and Helmchen, C. Usability of the head impulse test in routine clinical practice in the emergency department to differentiate vestibular neuritis from stroke. Eur J Neurol. (2021) 28:1737–44. doi: 10.1111/ene.14707
17. Agrawal, Y, Schubert, MC, Migliaccio, AA, Zee, DS, Schneider, E, Lehnen, N, et al. Evaluation of quantitative head impulse testing using search coils versus video-oculography in older individuals. Otol Neurotol. (2014) 35:283–8. doi: 10.1097/MAO.0b013e3182995227
18. Lee, SH, Kim, JM, Schuknecht, B, and Tarnutzer, AA. Vestibular and ocular motor properties in lateral medullary stroke critically depend on the level of the medullary lesion. Front Neurol. (2020) 11:390. doi: 10.3389/fneur.2020.00390
19. Nham, B, Reid, N, Bein, K, Bradshaw, AP, McGarvie, LA, Argaet, EC, et al. Capturing vertigo in the emergency room: three tools to double the rate of diagnosis. J Neurol. (2021) 269:294–306. doi: 10.1007/s00415-021-10627-1
20. Newman-Toker, DE, Kerber, KA, Hsieh, YH, Pula, JH, Omron, R, Saber Tehrani, AS, et al. HINTS outperforms ABCD2 to screen for stroke in acute continuous vertigo and dizziness. Acad Emerg Med. (2013) 20:986–96. doi: 10.1111/acem.12223
21. Korda, A, Wimmer, W, Zamaro, E, Wagner, F, Sauter, TC, Caversaccio, MD, et al. Videooculography “HINTS” in acute vestibular syndrome: a prospective study. Front Neurol. (2022) 13:920357. doi: 10.3389/fneur.2022.920357
22. Le, TN, Westerberg, BD, and Lea, J. Vestibular neuritis: recent advances in Etiology, diagnostic evaluation, and treatment. Adv Otorhinolaryngol. (2019) 82:87–92. doi: 10.1159/000490275
23. Kattah, JC. Use of HINTS in the acute vestibular syndrome. An Overview Stroke. Vasc Neurol. (2018) 3:190–6. doi: 10.1136/svn-2018-000160
24. Gufoni, M. Uphill/downhill nystagmus Nistagmo in salita e nistagmo in discesa. Acta Otorhinolaryngol Ital. (2017) 37:513–8. doi: 10.14639/0392-100X-1403
25. Lee, JY, Kim, CH, Park, JS, and Kim, MB. Peripheral vestibulopathy presenting as acute vertigo and spontaneous nystagmus with negative video head impulse test. Otolaryngol Head Neck Surg. (2019) 160:894–901. doi: 10.1177/0194599818825458
26. Kattah, JC, Talkad, AV, Wang, DZ, Hsieh, YH, and Newman-Toker, DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. (2009) 40:3504–10. doi: 10.1161/STROKEAHA.109.551234
27. Thomas, DB, and Newman-Toker, DE. Avoiding “HINTS positive/negative” to minimize diagnostic confusion in acute vertigo and dizziness. J Acute Care Phys Ther. (2016) 7:129–31. doi: 10.1097/JAT.0000000000000042
28. Hotson, JR, and Baloh, RW. Acute vestibular syndrome. N Engl J Med. (1998) 339:680–5. doi: 10.1056/NEJM199809033391007
29. Ataç, C, Kısabay, A, Akkoç, CÇ, Saruhan, G, and Çelebisoy, N. Vestibular nuclear infarction: case series and review of the literature. J Stroke Cerebrovasc Dis. (2020) 29:104937. doi: 10.1016/j.jstrokecerebrovasdis.2020.104937
30. Kim, HJ, Lee, SH, Park, JH, Choi, JY, and Kim, JS. Isolated vestibular nuclear infarction: report of two cases and review of the literature. J Neurol. (2014) 261:121–9. doi: 10.1007/s00415-013-7139-0
31. Choi, JY, Kim, HJ, and Kim, JS. Recent advances in head impulse test findings in central vestibular disorders. Neurology. (2018) 90:602–12. doi: 10.1212/WNL.0000000000005206
32. Yacovino, DA, Akly, MP, Luis, L, and Zee, DS. The floccular syndrome: dynamic changes in eye movements and vestibulo-ocular reflex in isolated infarction of the cerebellar flocculus. Cerebellum. (2018) 17:122–31. doi: 10.1007/s12311-017-0878-1
33. Eggers, SD, and Kattah, JC. Approaching acute vertigo with diplopia: a rare skew deviation in vestibular neuritis. Mayo Clin Proc Innov Qual Outcomes. (2020) 4:216–22. doi: 10.1016/j.mayocpiqo.2019.12.003
34. Safran, AB, Vibert, D, Issoua, D, and Häusler, R. Skew deviation after vestibular neuritis. Am J Ophthalmol. (1994) 118:238–45. doi: 10.1016/S0002-9394(14)72904-6
35. Sankalia, D, Kothari, S, and Phalgune, DS. Diagnosing stroke in acute vertigo: sensitivity and specificity of HINTS battery in Indian population. Neurol India. (2021) 69:97–101. doi: 10.4103/0028-3886.310089
36. Guan, Q, Zhang, L, Hong, W, Yang, Y, Chen, Z, Lu, P, et al. Video head impulse test for early diagnosis of vestibular neuritis among acute vertigo. Can J Neurol Sci. (2017) 44:556–61. doi: 10.1017/cjn.2017.202
37. Ooi, S, Phillips, G, Tang, T, Chen, L, Fok, A, Ly, J, et al. Meta-analysis of the use of head impulse test and head impulse test with direction changing nystagmus and test of skew deviation in the diagnosis of peripheral vertigo and stroke. Cerebrovasc Dis. (2023) 52:184–93. doi: 10.1159/000526331
38. Machner, B, Erber, K, Choi, JH, Sprenger, A, Helmchen, C, and Trillenberg, P. A simple gain-based evaluation of the video head impulse test reliably detects normal vestibulo-ocular reflex indicative of stroke in patients with acute vestibular syndrome. Front Neurol. (2021) 12:741859. doi: 10.3389/fneur.2021.741859
39. Korda, A, Wimmer, W, Wyss, T, Michailidou, E, Zamaro, E, Wagner, F, et al. Artificial intelligence for early stroke diagnosis in acute vestibular syndrome. Front Neurol. (2022) 13:919777. doi: 10.3389/fneur.2022.919777
40. Siniscalchi, A, Sztajzel, R, Malferrari, G, and Gallelli, L. The National Institutes of Health stroke scale: its role in patients with posterior circulation stroke. Hosp Top. (2017) 95:79–81. doi: 10.1080/00185868.2017.1322888
41. Thomas, JO, Sharobeam, A, Venkat, A, Blair, C, Ozalp, N, Calic, Z, et al. Video head impulse testing to differentiate vestibular neuritis from posterior circulation stroke in the emergency department: a prospective observational study. BMJ Neurol Open. (2022) 4:e000284. doi: 10.1136/bmjno-2022-000284
42. Robinson, DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. (1963) 10:137–45.
43. Boleas-Aguirre, M, Migliaccio, AA, and Carey, JP. Vestibulo-oculomotor reflex recording using the scleral search coil technique. Review of peripheral vestibular disorders. Acta Otorrinolaringol. (2007) 58:321–6. doi: 10.1016/S2173-5735(07)70358-7
44. Chen, L, Todd, M, Halmagyi, GM, and Aw, S. Head impulse gain and saccade analysis in pontine-cerebellar stroke and vestibular neuritis. Neurology. (2014) 83:1513–22. doi: 10.1212/WNL.0000000000000906
45. Lee, JY, Kwon, E, Kim, HJ, Choi, JY, Oh, HJ, Koo, JW, et al. Dissociated results between caloric and video head impulse tests in dizziness: prevalence, pattern, lesion location, and etiology. J Clin Neurol. (2020) 16:277–84. doi: 10.3988/jcn.2020.16.2.277
46. Newman-Toker, DE, Tehrani, ASS, Mantokoudis, G, Pula, JH, Guede, CI, Kerber, KA, et al. Quantitative video-oculography to help diagnose stroke in acute vertigo and dizziness: toward an ECG for the eyes. Stroke. (2013) 44:1158–61. doi: 10.1161/STROKEAHA.111.000033
47. Newman-Toker, DE, Curthoys, IS, and Halmagyi, GM. Diagnosing stroke in acute vertigo: the HINTS family of eye movement tests and the future of the “eye ECG.”. Semin Neurol. (2015) 35:506–21. doi: 10.1055/s-0035-1564298
48. Mantokoudis, G, Tehrani, ASS, Wozniak, A, Eibenberger, K, Kattah, JC, Guede, CI, et al. VOR gain by head impulse video-oculography differentiates acute vestibular neuritis from stroke. Otol Neurotol. (2015) 36:457–65. doi: 10.1097/MAO.0000000000000638
49. Alhabib, SF, and Saliba, I. Video head impulse test: a review of the literature. Eur Arch Otorrinolaringol. (2016) 274:1215–22. doi: 10.1007/s00405-016-4157-4
50. Ha, SH, Lee, DK, Park, G, Kim, BJ, Chang, JY, Kang, DW, et al. Prospective analysis of video head impulse tests in patients with acute posterior circulation stroke. Front Neurol. (2023) 14:826. doi: 10.3389/fneur.2023.1256826
51. Nam, GS, Shin, HJ, Kang, JJ, Lee, NR, and Oh, SY. Clinical implication of corrective saccades in the video head impulse test for the diagnosis of posterior inferior cerebellar artery infarction. Front Neurol. (2021) 12:605040. doi: 10.3389/fneur.2021.605040
52. Lee, SH, Kim, JM, Kim, JT, and Tarnutzer, AA. Video head impulse testing in patients with isolated (hemi) nodular infarction. Front Neurol. (2023) 14:1124217. doi: 10.3389/fneur.2023.1124217
53. Day, GS, Swartz, RH, Chenkin, J, Shamji, AI, and Frost, DW. Lateral medullary syndrome: a diagnostic approach illustrated through case presentation and literature review. Can J Emerg Med. (2014) 16:164–70. doi: 10.2310/8000.2013.131059
54. Lee, H. Neuro-Otological aspects of cerebellar stroke syndrome. J Clin Neurol. (2009) 5:65–73. doi: 10.3988/jcn.2009.5.2.65
Keywords: video head impulse test, posterior circulation stroke, acute vestibular syndrome, vestibular neuritis, vestibulo-ocular reflex
Citation: Jaganathan N, Mohamed MH, Md Pauzi AL, Mahayidin H, Hanapai AF, Wan Sulaiman WA, Basri H and Inche Mat L (2024) Video head impulse test in stroke: a review of published studies. Front. Neurol. 15:1339039. doi: 10.3389/fneur.2024.1339039
Edited by:
Shinichiro Uchiyama, Sanno Medical Center, JapanReviewed by:
Jorge Kattah, University of Illinois at Chicago, United StatesLuke Chen, Monash University, Australia
Copyright © 2024 Jaganathan, Mohamed, Md Pauzi, Mahayidin, Hanapai, Wan Sulaiman, Basri and Inche Mat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyana Inche Mat, bGl5YW5hbmFqd2FAdXBtLmVkdS5teQ==
 Niranjana Jaganathan1
Niranjana Jaganathan1 Wan Aliaa Wan Sulaiman
Wan Aliaa Wan Sulaiman Hamidon Basri
Hamidon Basri Liyana Inche Mat
Liyana Inche Mat