
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 22 February 2024
Sec. Neurological Biomarkers
Volume 15 - 2024 | https://doi.org/10.3389/fneur.2024.1291950
 Mingzhu Deng1†
Mingzhu Deng1† Kangping Song2†
Kangping Song2† Yangping Tong2
Yangping Tong2 Sufen Chen2
Sufen Chen2 Wei Xu2
Wei Xu2 Guohua He2
Guohua He2 Jue Hu2
Jue Hu2 Hui Xiao2
Hui Xiao2 Changmin Wan2
Changmin Wan2 Zhen Wang2*
Zhen Wang2* Fangyi Li2*
Fangyi Li2*Background: Inflammation and platelet activation play pivotal roles in acute ischemic stroke (AIS) pathogenesis. Early response to thrombolysis is a vital indicator for the long-term prognosis of AIS. However, the correlation between fibrinogen or the neutrophil-to-lymphocyte ratio (NLR) and the early response to intravenous thrombolysis in patients with AIS remains unclear.
Methods: AIS patients undergoing intravenous thrombolysis were enrolled between January 2018 and May 2023. Blood cell counts were sampled before thrombolysis. A good response was defined as a National Institutes of Health Stroke Scale (NIHSS) score decreased ≥4 or complete recovery 24 h after thrombolysis treatment. A poor response was defined as any increase in the NIHSS score or a decrease in the NIHSS score <4 at the 24 h after thrombolysis treatment compared with that at admission. Logistic regression analysis was performed to explore the relationship of the fibrinogen level and NLR with a poor thrombolysis response. Receiver operating characteristic (ROC) analysis was used to assess the ability of the fibrinogen level and NLR to discriminate poor responders.
Results: Among 700 recruited patients, 268 (38.29%) were diagnosed with a good response, and 432 (61.71%) were diagnosed with a poor response to intravenous thrombolysis. A binary logistic regression model indicated that an elevated fibrinogen level (odds ratio [OR], 1.693; 95% confidence interval [CI] 1.325–2.122, P < 0.001) and NLR (OR, 1.253; 95% CI, 1.210–2.005, P = 0.001) were independent factors for a poor response. The area under the curve (AUC) values for the fibrinogen level, NLR and fibrinogen level combined with the NLR for a poor response were 0.708, 0.605, and 0.728, respectively.
Conclusions: Our research indicates that the levels of fibrinogen and NLR at admission can be used as a prognostic factor to predict early poor response to intravenous thrombolysis.
Stroke is a common neurological disease that results in neuronal cell damage due to inadequate blood supply to the brain or a loss of vascular integrity (1, 2). Stroke kills more than 15 million people annually, making it the second most prevalent cause of death globally (3). The major type of stroke is acute ischemic stroke (AIS), which is caused by a sudden arterial blockage (4). A crucial part of the etiology of AIS is played by inflammation and platelet activation (5). Patients with AIS can benefit from intravenous thrombolysis with recombinant tissue plasminogen activator (r-tPA) (6, 7). One cornerstone treatment of AIS is prompt intravenous r-tPA administration in the early phase (≤4.5 h) (6, 8, 9). Nevertheless, r-tPA has numerous drawbacks, including a relatively low recanalization rate among patients with large vessel occlusion, a risk of cerebral bleeding, which is closely related to patients' prognosis. Therefore, it is significant to explore the risk factors and the measurable biomarkers of early poor response to intravenous thrombolysis in AIS patients.
Neuroinflammatory response plays an essential role in the pathophysiology of ischemic stroke (10). Fibrinogen levels and neutrophil-to-lymphocyte ratio (NLR) are two common biomarkers of inflammation (11, 12). A meta-analysis has shown that a high fibrinogen level is an independent predictor for stroke in individuals who appear to be healthy (13). In AIS, fibrinogen has been identified as a biomarker for ischemic lesions (14). Through thrombogenesis (15) and inflammation (16), hyperfibrinogenemia can increase susceptibility to negative outcomes. However, investigations regarding the predictive value of fibrinogen levels after AIS remain contradictory (17–19). The NLR is an easily accessible serum biomarker and may be utilized for assessing systemic inflammation (12). As a prognostic indicator for coronary artery disease, peripheral arterial occlusive disease, and ischemic stroke, an increase in the NLR has been linked to atherosclerotic events (20–22). In particular, it has been found that an elevated NLR in stroke patients was associated with stroke severity, poor functional outcomes, and recurrent ischemic episodes (22, 23). Nevertheless, there is a relative paucity of studies on the correlation between the NLR and the early response to thrombolysis in patients with AIS.
Previous studies have confirmed that post-thrombolysis early neurological outcomes are relevant to the prognosis of patients treated with intravenous thrombolysis (24, 25). It is important to explore the risk factors of early poor response to intravenous thrombolysis in AIS patients. The correlation between fibrinogen or NLR and the early response to intravenous thrombolysis in patients with AIS remains unclear. In this study, we aimed to investigate whether fibrinogen and NLR can serve as predictive indicators for early response to r-tPA in patients with AIS.
AIS patients who underwent intravenous thrombolysis within 4.5 h were recruited from the Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China. AIS patients were diagnosed with head imaging including CT and MRI, and the diagnostic criteria for AIS is based on Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018 (26). The indications and contraindications for intravenous thrombolysis are also based on Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018 (26). Eligible participants were enrolled in the final analysis if they met the following criteria: (1) admission within 4.5 h after onset; (2) treatment with intravenous thrombolysis with r-tPA; and (3) age 18 years or older. The exclusion criteria included: (1) the presence of severe inflammatory diseases or infectious diseases; (2) had immune system diseases, tumors or hypothyroidism; (3) had complications, such as severe liver and kidney injuries; and (4) incomplete clinical data. Informed consent was obtained from participants or their legal representatives. This study was approved by the Ethics Committee of the Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China. There were 700 AIS patients recruited between January 2018 and May 2023, and a detailed flow diagram for patient enrollment is displayed in Figure 1.
Clinical assessments were performed in a blinded fashion by experienced neurologists. All participants underwent accurate and detailed records of their age, sex, body mass index, risk factors for stroke (hypertension, diabetes mellitus, atrial fibrillation, coronary artery disease, current drinking and smoking) on the day of admission. The lesion site and stroke subtype were determined by computed tomography or magnetic resonance. Stroke subtype was determined with the help of electrocardiography, echocardiography, carotid ultrasonography, and transcranial Doppler. Stroke subtype was classified according to the Trial of Org 10172 in Acute Stroke Treatment criteria (27). The demographic data, baseline clinical parameters, clinical diagnoses, and therapeutic schedules, were carefully extracted using a standardized case-report form. If any information was unclear, the doctors or other healthcare providers who had been in charge were consulted.
Blood samples of all patients were collected at 6–7 a.m the day after fasting for at least 8 h. Two milliliters of EDTA-anticoagulated whole blood were used for routine blood tests (automated hematology analyzer, BZ6800, China). Five milliliters of coagulant-containing blood was used for standard biochemical examination (automatic analyzer, HITACHI 7600, Japan). All the indicators were tested using commercial kits, which were operated by qualified professionals in accordance with the specifications. The counts of white blood cells (WBC), neutrophils, lymphocytes, monocytes, and platelets, as well as the levels of red blood cells (RBC), hemoglobin (Hb), and fibrinogen, were assessed in blood samples. The NLR, PLR, and LMR were calculated. The testing of blood was repeated three times for each.
Patients were classified into two groups, namely, the good response and poor response to intravenous thrombolysis groups. The good response was defined as a National Institutes of Health Stroke Scale (NIHSS) score decreased ≥4 or complete recovery 24 h after thrombolysis treatment. The poor response was defined as any increase in the NIHSS score or a decrease in the NIHSS score <4 at the 24 h after thrombolysis treatment compared with that at admission.
Data analysis was performed by using SPSS 25.0 (IBM SPSS Statistics software, Version 25.0). All the data were tested for a normal distribution using the Kolmogorov–Smirnov test. Continuous variables were presented as mean ± SD, if they were normally distributed; otherwise, they were presented as median (quartile). The results for categorical variables are presented as percentages. Difference in baseline characteristics between groups were analyzed using Student's t test or Mann–Whitney U test for continuous variables as well as the chi-squared test or Fisher's exact test for categorical variables, as appropriate. We used box plots to show the distribution of the NLR, neutrophil count, lymphocyte count and fibrinogen level among the good response group and poor response group. Logistic regression analysis was used to detect risk factors for a poor response. A MedCalc 15.6.0 (MedCalc Software Acacialaan 22, B-8400 Ostend, Belgium) packet program was used to obtain a receiver operating characteristic (ROC) curve to test the overall ability of the NLR and fibrinogen level to discriminate poor responders to thrombolysis. A two-tailed value of P < 0.05 was considered significant.
The demographic and clinical characteristics are comprehensively displayed in Table 1. A good response to thrombolysis was observed in 268 patients (38.29%), and a poor response was observed in 432 patients (61.71%). In poor response group, the age (P = 0.007), NIHSS score after rt-PA 24 h (P < 0.001), hypertension (P = 0.032), diabetes mellitus (P = 0.044), atrial fibrillation (P = 0.013), neutrophil count (P = 0.002), fibrinogen level (P < 0.001), NLR (P < 0.001), and PLR (P < 0.001) were significantly higher than those in good response group, while the lymphocyte count (P = 0.003) and Hb (P = 0.004) were significantly lower than those in good response group. In addition, the proportion of patients with symptomatic intracranial hemorrhage (sICH) was 5.79% for poor response group. Figure 2 shows the box plots of the fibrinogen level, NLR, neutrophil count and lymphocyte count for the two groups. The patients in the poor response group had an elevated fibrinogen level, NLR and neutrophil count compared with those in the good response group; however, the patients in the poor response group had lower lymphocyte counts (P < 0.05).

Figure 2. Comparisons of the plasma fibrinogen level (A), NLR (B), neutrophil count (C), and lymphocyte count (D) between the good response and poor response groups. **P < 0.01, ***P < 0.001.
Table 2 illustrates the results of crude models for a poor response to thrombolysis. Factors with statistical significance in Table 1 were included in the binary logistic regression model to identify independent risk factors for a poor response to thrombolysis. Notably, the neutrophil and lymphocyte counts were not included in the model due to collinearity with the NLR and PLR. After adjustment for all potential confounders, such as age, hypertension, diabetes mellitus, atrial fibrillation, hemoglobin and PLR, an elevated NLR (OR, 1.253; 95% CI, 1.102–1.423, P = 0.001) and fibrinogen level (OR, 1.693; 95% CI, 1.352–2.122, P < 0.001) were identified as independent factors for a poor response to thrombolysis (Figure 3).

Figure 3. Binary logistic analysis of independent risk factors associated with a poor response to thrombolysis.
We employed ROC curves to test the overall ability of the fibrinogen level and NLR to discriminate poor responders to thrombolysis (Figure 4). We observed that the area under the curve (AUC) of the fibrinogen level to discriminate poor responders to thrombolysis was 0.708 (95% CI, 0.673–0.742, P < 0.001), the optimal cutoff was 3.04, and the sensitivity and specificity were 53.94% and 79.10%, respectively. The AUC of the NLR was 0.605 (95% CI, 0.568–0.642, P < 0.001), the cutoff was 3.25, and the sensitivity and specificity were 39.58% and 80.60%, respectively. In addition, we conducted an ROC curve analysis for the ability of the combination of the fibrinogen level and NLR to discriminate between good responders and poor responders to thrombolysis. The AUC for the combination of the NLR and fibrinogen level was 0.728 (95% CI, 0.693–0.760, P < 0.001), the cutoff was 0.64, and the sensitivity and specificity were 57.41% and 76.49%, respectively.
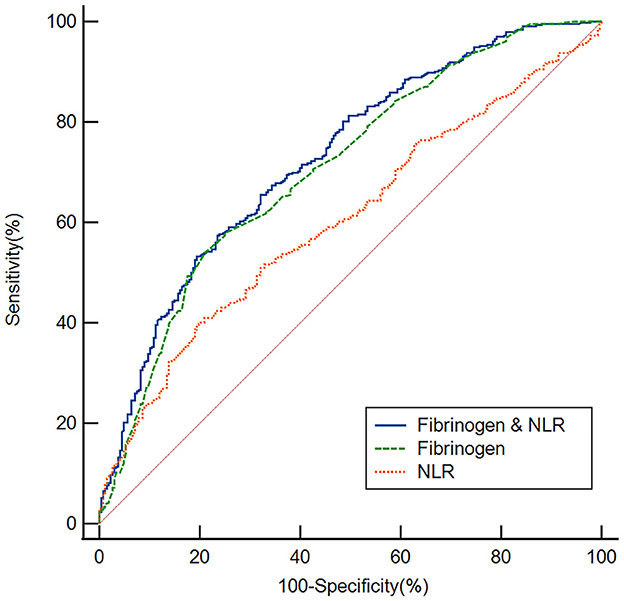
Figure 4. Based on ROC curve analysis, the fibrinogen level, NLR and fibrinogen level & NLR exhibited a respectable power to discriminate responders to thrombolysis, with AUC values of 0.708, 0.605 and 0.728, respectively.
Intravenous r-tPA has been the mainstay therapy for AIS for over 20 years (28). However, findings suggest that although some individuals do not respond to r-tPA, others experience a worsening of their clinical symptoms (such as symptomatic intracranial bleeding) (29, 30). Several studies have examined the long-term prognosis of AIS following intravenous thrombolysis or biomarkers to predict intracranial bleeding, while few studies have examined the early response to r-tPA (31, 32). In our research, a good response to thrombolysis was observed in 268 patients (38.29%), the ratio is also similar to the results of the previous studies (33, 34). Our findings provide several novel observations. First, we note that the plasma fibrinogen level and NLR in AIS patients with a poor thrombolysis response were higher than those in AIS patients with a good thrombolysis response. Second, the binary logistic regression model indicated that an elevated fibrinogen level and NLR were independent factors for a poor response. Finally, this study created fibrinogen level- and NLR-related ROC curves to distinguish AIS patients with a poor response from those with a good response to thrombolysis. Together, these data support that the fibrinogen level and NLR are associated with the response to thrombolysis.
Neuroinflammation has received increasing attention in recent years, and multiple studies have demonstrated that inflammatory processes are essential for the pathophysiology and development of ischemic stroke (35, 36). Fibrinogen can increase during any inflammatory event, where it serves to control systemic inflammatory signals (37, 38), and it is a major driver of blood viscosity involved in primary hemostasis, platelet aggregation, and interactions between leukocytes and endothelial cells (39). According to one study, fibrinogen might accumulate in inflammatory foci, and extravascular deposits worsen inflammation (40). Our study showed that fibrinogen levels were significantly higher in the poor response group than in the good response group. In addition, after adjustment for potential confounders, an elevated fibrinogen level was identified as an independent factor for a poor response to thrombolysis. Prior research has demonstrated an association between baseline elevated fibrinogen levels and an increased probability of short-term poor functional outcomes in AIS patients (41). Additionally, some research revealed that hyperfibrinogenemia was related to a poor functional result (42, 43). These results suggest that an elevated fibrinogen level is associated with a poor early response to thrombolysis in patients with AIS. Interestingly, as a defibrinogenating agent, the use of batroxobin alone decreased fibrinogen concentrations and erythrocyte aggregability, reduced stroke recurrence rates, and produced more significant improvements in neurological assessments (44). Thus, we speculate that drugs targeting fibrinogen may be valuable in improving the prognosis of AIS among specific patients. However, other studies showed no connection between fibrinogen levels and outcome (19, 45). We believe that the variances in the ethnicity of the research populations, the small sample sizes, the medication status, and the severity of the condition may be the causes of the discrepancies between various studies.
Neutrophils accumulate in the ischemic region during the early stages of stroke and produce inflammatory mediators, which disrupt the blood–brain barrier and cause an increase in the infarct volume, hemorrhagic transformation, and poor neurological outcomes (46, 47). Reducing the inflammatory response of peripheral neutrophils can improve regional cerebral blood flow and improve short-term and long-term functional outcomes after stroke (48). The production of matrix metalloproteinase-9 by peripheral neutrophils and monocytes might result in hemorrhagic transformation and a worsening of symptoms (49, 50). In contrast, lymphocytes, which are the brain's main regulators, can aid in both the healing of inflammatory damage and the restoration of brain function (51). In the present study, neutrophil counts were significantly higher in the thrombolytic poor response group than in the thrombolytic good response group; conversely, lymphocyte levels were decreased, which suggests the existence of peripheral inflammatory immune dysregulation in AIS. The NLR could serve as a peripheral indicator of the level of systemic inflammation (52), as this ratio incorporates information from both leukocyte subsets and supplementary immunological pathways. The NLR was shown to be able to predict the clinical prognosis in AIS patients in earlier research (34, 53, 54). It can predict hemorrhagic transformation following an ischemic stroke (55). Furthermore, studies have shown that an elevated NLR may indicate pneumonia associated with stroke (56). Our study reports a significantly higher NLR in the thrombolytic poor response group patients with AIS than in the thrombolytic good response group. Finally, after adjustment for potential confounders, an elevated NLR was identified as an independent factor for a poor response to thrombolysis. The results of our study have expanded on the role of the NLR in cerebrovascular illness and offer new viewpoints on therapeutic treatment.
In this study, we also discovered that age, hypertension, diabetes mellitus, atrial fibrillation, hemoglobin, and the PLR were related to a poor response to thrombolysis. Age and high baseline blood glucose levels were found to be risk factors for neurological recovery in earlier studies (49, 57, 58). AIS may lead to aberrant platelet function, and excessive platelet activation and buildup may impede stroke recovery (59). The PLR was shown to be able to predict the clinical prognosis in AIS patients in earlier research (60). Furthermore, the proportion of patients with sICH was 3.57% (25/700) for all AIS patients undergoing intravenous thrombolysis with r-tPA, which is consistent with a previous study (61).
According to estimates, AIS kills 1.9 million neurons every minute, and the concept that time is the brain is deeply rooted among the people. The earlier intervention may bring better outcome. Therefore, it is significant to explore the risk factors and the measurable biomarkers of early poor response to intravenous thrombolysis in AIS patients, it may help to save the neurological function of patient with AIS. The results of our ROC curve analysis showed that the fibrinogen level and NLR had appropriate sensitivity and specificity in distinguishing patients in the good response group from those in the poor response group. Clearly, the fibrinogen level is more discriminating than the NLR, suggesting that the fibrinogen level at admission may be a useful tool to predict a poor response to thrombolysis. Finally, we found that the combination of the fibrinogen level and NLR had a better ability to discriminate poor responders to thrombolysis, with an AUC of 0.728, which was higher than that of the individual markers, suggesting the use of markers in combination to predict a poor response to thrombolysis.
This study has the following limitations: (1) this is a retrospective study, and there may be potential inherent biases (such as selection bias); (2) this was a cross-sectional study, and all the participants enrolled were Chinese patients treated with intravenous thrombolysis, so our results need to be tested in non-Chinese populations, and longitudinal cohort studies with larger populations are required in the future; (3) there is a lack of other inflammatory markers to further assess whether the fibrinogen level and NLR are correlated with other inflammatory markers, which may hinder us from obtaining a comprehensive understanding of the role that inflammation plays in AIS; (4) the fibrinogen level and NLR are recognized as markers of inflammation in the peripheral circulation; they are not specific neuroinflammatory markers and can be influenced by various factors, thus making it challenging to draw conclusions about their specific impact on certain neuroinflammatory pathways; and (5) despite our study provided some evidence that higher fibrinogen and neutrophil-to-lymphocyte ratio may be a predictor for poor response to intravenous thrombolysis among AIS patients, no treatment strategies were changed accordingly. To explore additional appropriate treatment among AIS patients with higher fibrinogen and neutrophil-to-lymphocyte ratio is what we will study in the near future.
In conclusion, our research indicates that the higher fibrinogen level and NLR are associated with a poor early response to thrombolysis. Furthermore, combining the fibrinogen level and NLR may better predict the response to intravenous thrombolysis in patients with AIS. However, future studies are necessary to extend the results and to explore corresponding treatment strategies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the Affiliated Changsha Central Hospital, Hengyang Medical School, University of South China. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of our previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
MD: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. KS: Data curation, Investigation, Writing – original draft, Writing – review & editing. YT: Data curation, Funding acquisition, Investigation, Writing – review & editing. SC: Conceptualization, Investigation, Software, Writing – review & editing. WX: Data curation, Methodology, Supervision, Writing – review & editing. GH: Investigation, Software, Visualization, Writing – review & editing. JH: Data curation, Methodology, Supervision, Writing – review & editing. HX: Project administration, Validation, Writing – review & editing. CW: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. ZW: Conceptualization, Data curation, Methodology, Software, Supervision, Visualization, Writing – review & editing. FL: Data curation, Formal analysis, Methodology, Project administration, Software, Supervision, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Medical Clinical Research 4310 Project of University of South China (No. KJZX2021078).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zille M, Farr TD, Keep RF, Römer C, Xi G, Boltze J. Novel targets, treatments, and advanced models for intracerebral haemorrhage. EBioMedicine. (2022) 76:103880. doi: 10.1016/j.ebiom.2022.103880
3. Bonkhoff AK, Schirmer MD, Bretzner M, Hong S, Regenhardt RW, Brudfors M, et al. Outcome after acute ischemic stroke is linked to sex-specific lesion patterns. Nat Commun. (2021) 12:3289. doi: 10.1038/s41467-021-23492-3
4. Yousufuddin M, Young N. Aging and ischemic stroke. Aging. (2019) 11:2542–4. doi: 10.18632/aging.101931
5. Shehjar F, Maktabi B, Rahman ZA, Bahader GA, James AW, Naqvi A, et al. Stroke: molecular mechanisms and therapies: update on recent developments. Neurochem Int. (2023) 162:105458. doi: 10.1016/j.neuint.2022.105458
6. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
7. Jin C, Huang RJ, Peterson ED, Laskowitz DT, Hernandez AF, Federspiel JJ, et al. Intravenous tPA (tissue-type plasminogen activator) in patients with acute ischemic stroke taking non-vitamin k antagonist oral anticoagulants preceding stroke. Stroke. (2018) 49:2237–40. doi: 10.1161/STROKEAHA.118.022128
8. Xiong Y, Manwani B, Fisher M. Management of acute ischemic stroke. Am J Med. (2019) 132:286–91. doi: 10.1016/j.amjmed.2018.10.019
9. Marko M, Posekany A, Szabo S, Scharer S, Kiechl S, Knoflach M, et al. Trends of r-tPA (recombinant tissue-type plasminogen activator) treatment and treatment-influencing factors in acute ischemic stroke. Stroke. (2020) 51:1240–7. doi: 10.1161/STROKEAHA.119.027921
10. Parikh NS, Merkler AE, Iadecola C. Inflammation, autoimmunity, infection, and stroke: epidemiology and lessons from therapeutic intervention. Stroke. (2020) 51:711–8. doi: 10.1161/STROKEAHA.119.024157
11. Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. (2012) 34:43–62. doi: 10.1007/s00281-011-0290-8
12. Novellino F, Donato A, Malara N, Madrigal JL, Donato G. Complete blood cell count-derived ratios can be useful biomarkers for neurological diseases. Int J Immunopathol Pharmacol. (2021) 35:20587384211048264. doi: 10.1177/20587384211048264
13. Danesh J, Lewington S, Thompson SG, Lowe GD, Collins R, Kostis JB, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. Jama. (2005) 294:1799–809. doi: 10.1001/jama.294.14.1799
14. Peycheva M, Deneva T, Zahariev Z. The role of fibrinogen in acute ischaemic stroke. Neurol Neurochir Pol. (2021) 55:74–80. doi: 10.5603/PJNNS.a2020.0094
15. de Moerloose P, Boehlen F, Neerman-Arbez M. Fibrinogen and the risk of thrombosis. Semin Thromb Hemost. (2010) 36:7–17. doi: 10.1055/s-0030-1248720
16. Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory mechanisms in ischemic stroke: focus on cardioembolic stroke, background, and therapeutic approaches. Int J Mol Sci. (2020) 21:6454. doi: 10.3390/ijms21186454
17. Swarowska M, Ferens A, Pera J, Slowik A, Dziedzic T. Can prediction of functional outcome after stroke be improved by adding fibrinogen to prognostic model? J Stroke Cerebrovasc Dis. (2016) 25:2752–5. doi: 10.1016/j.jstrokecerebrovasdis.2016.07.029
18. You S, Yin X, Liu H, Zheng D, Zhong C, Du H, et al. Hyperfibrinogenemia is significantly associated with an increased risk of in-hospital mortality in acute ischemic stroke patients. Curr Neurovasc Res. (2017) 14:242–9. doi: 10.2174/1567202614666170621103604
19. Zhou X, Yu F, Feng X, Wang J, Li Z, Zhan Q, et al. Immunity and inflammation predictors for short-term outcome of stroke in young adults. Int J Neurosci. (2018) 128:634–9. doi: 10.1080/00207454.2017.1408614
20. Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. (2008) 395:27–31. doi: 10.1016/j.cca.2008.04.019
21. Erturk M, Cakmak HA, Surgit O, Celik O, Aksu HU, Akgul O, et al. Predictive value of elevated neutrophil to lymphocyte ratio for long-term cardiovascular mortality in peripheral arterial occlusive disease. J Cardiol. (2014) 64:371–6. doi: 10.1016/j.jjcc.2014.02.019
22. Wang L, Song Q, Wang C, Wu S, Deng L, Li Y, et al. Neutrophil to lymphocyte ratio predicts poor outcomes after acute ischemic stroke: a cohort study and systematic review. J Neurol Sci. (2019) 406:116445. doi: 10.1016/j.jns.2019.116445
23. Xue J, Huang W, Chen X, Li Q, Cai Z, Yu T, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:650–7. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010
24. Yeo LL, Paliwal P, Teoh HL, Seet RC, Chan BP, Wakerley B, et al. Early and continuous neurologic improvements after intravenous thrombolysis are strong predictors of favorable long-term outcomes in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2013) 22:e590–596. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.024
25. Mori M, Naganuma M, Okada Y, Hasegawa Y, Shiokawa Y, Nakagawara J, et al. Early neurological deterioration within 24 hours after intravenous rt-PA therapy for stroke patients: the stroke acute management with urgent risk factor assessment and improvement rt-PA registry. Cerebrovasc Dis. (2012) 34:140–6. doi: 10.1159/000339759
26. Neurology C, Society C. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol. (2018) 51:666–82.
27. Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
28. Karaszewski B, Wyszomirski A, Jabłoński B, Werring DJ, Tomaka D. Efficacy and safety of intravenous rTPA in ischemic strokes due to small-vessel occlusion: systematic review and meta-analysis. Transl Stroke Res. (2021) 12:406–15. doi: 10.1007/s12975-021-00890-9
29. Liu C, Xie J, Sun S, Li H, Li T, Jiang C, et al. Hemorrhagic transformation after tissue plasminogen activator treatment in acute ischemic stroke. Cell Mol Neurobiol. (2022) 42:621–46. doi: 10.1007/s10571-020-00985-1
30. Yaghi S, Eisenberger A, Willey JZ. Symptomatic intracerebral hemorrhage in acute ischemic stroke after thrombolysis with intravenous recombinant tissue plasminogen activator: a review of natural history and treatment. JAMA Neurol. (2014) 71:1181–5. doi: 10.1001/jamaneurol.2014.1210
31. Zhou Z, Delcourt C, Xia C, Yoshimura S, Carcel C, Torii-Yoshimura T, et al. Low-dose vs standard-dose alteplase in acute lacunar ischemic stroke: the ENCHANTED trial. Neurology. (2021) 96:e1512–26. doi: 10.1212/WNL.0000000000011598
32. Tian M, Li Y, Wang X, Tian X, Pei LL, Wang X, et al. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is associated with poor outcome of acute ischemic stroke. Front Neurol. (2020) 11:610318. doi: 10.3389/fneur.2020.610318
33. Zhu B, Zhang L, Du W, Yang J, Tian Y, Wu M, et al. Levels of fibrin degradation products at admission with acute ischemic stroke correlate with the NIH stroke scale score 1 h after intravenous thrombolysis. Front Neurol. (2021) 12:651867. doi: 10.3389/fneur.2021.651867
34. Gong P, Liu Y, Gong Y, Chen G, Zhang X, Wang S, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. (2021) 18:51. doi: 10.1186/s12974-021-02090-6
35. Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. (2016) 15:869–81. doi: 10.1016/S1474-4422(16)00114-9
36. Shi K, Tian DC Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. (2019) 18:1058–66. doi: 10.1016/S1474-4422(19)30078-X
37. Huang W, Wang S, Zhang H, Zhang B, Wang C. Prognostic significance of combined fibrinogen concentration and neutrophil-to-lymphocyte ratio in patients with resectable non-small cell lung cancer. Cancer Biol Med. (2018) 15:88–96. doi: 10.20892/j.issn.2095-3941.2017.0124
38. Ubaldo OGV, Lazaro MAE, Aventura ET, Cinco JE. Can serum fibrinogen predict ARDS? Infect Dis. (2020) 13:1178633720943505. doi: 10.1177/1178633720943505
39. Weisel JW. Fibrinogen and fibrin. Adv Protein Chem. (2005) 70:247–99. doi: 10.1016/S0065-3233(05)70008-5
40. Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood. (2019) 133:511–20. doi: 10.1182/blood-2018-07-818211
41. Xu J, Sun Y, Gong D, Fan Y. Utility of fibrinogen levels for predicting survival and functional outcomes in patients with acute ischemic stroke: a meta-analysis. Curr Med Chem. (2023) 30:481–91. doi: 10.2174/0929867329666220728125624
42. Hou HQ, Xiang XL, Pan YS, Zhang QH, Li H, Meng X, et al. Baseline or 90-day fibrinogen levels and long-term outcomes after ischemic stroke or TIA: results from the China national stroke registry III. Atherosclerosis. (2021) 337:35–41. doi: 10.1016/j.atherosclerosis.2021.10.002
43. Li D, Xing C, Li Y, Zhu X. Elevated plasma fibrinogen indicates short-term poor outcome in patients with acute ischemic stroke after intravenous thrombolysis. J Stroke Cerebrovasc Dis. (2020) 29:104991. doi: 10.1016/j.jstrokecerebrovasdis.2020.104991
44. Lan D, Song S, Liu Y, Jiao B, Meng R. Use of batroxobin in central and peripheral ischemic vascular diseases: a systematic review. Front Neurol. (2021) 12:716778. doi: 10.3389/fneur.2021.716778
45. Zeng L, Liu J, Wang Y, Wang L, Weng S, Chen S, et al. Cocktail blood biomarkers: prediction of clinical outcomes in patients with acute ischemic stroke. Eur Neurol. (2013) 69:68–75. doi: 10.1159/000342896
46. Kim JY, Park J, Chang JY, Kim SH, Lee JE. Inflammation after ischemic stroke: the role of leukocytes and glial cells. Exp Neurobiol. (2016) 25:241–51. doi: 10.5607/en.2016.25.5.241
47. Otxoa-de-Amezaga A, Gallizioli M, Pedragosa J, Justicia C, Miró-Mur F, Salas-Perdomo A, et al. Location of neutrophils in different compartments of the damaged mouse brain after severe ischemia/reperfusion. Stroke. (2019) 50:1548–1557. doi: 10.1161/STROKEAHA.118.023837
48. Dhanesha N, Patel RB, Doddapattar P, Ghatge M, Flora GD, Jain M, et al. PKM2 promotes neutrophil activation and cerebral thromboinflammation: therapeutic implications for ischemic stroke. Blood. (2022) 139:1234–45. doi: 10.1182/blood.2021012322
49. Gong P, Xie Y, Jiang T, Liu Y, Wang M, Sun H, et al. Neutrophil-lymphocyte ratio predicts post-thrombolysis early neurological deterioration in acute ischemic stroke patients. Brain Behav. (2019) 9:e01426. doi: 10.1002/brb3.1426
50. Duan Z, Wang H, Wang Z, Hao Y, Zi W, Yang D, et al. Neutrophil-lymphocyte ratio predicts functional and safety outcomes after endovascular treatment for acute ischemic stroke. Cerebrovasc Dis. (2018) 45:221–7. doi: 10.1159/000489401
51. Li S, Huang Y, Liu Y, Rocha M, Li X, Wei P, et al. Change and predictive ability of circulating immunoregulatory lymphocytes in long-term outcomes of acute ischemic stroke. J Cereb Blood Flow Metab. (2021) 41:2280–94. doi: 10.1177/0271678X21995694
52. Muñoz-Delgado L, Macías-García D, Jesús S, Martín-Rodríguez JF, Labrador-Espinosa M, Jiménez-Jaraba MV, et al. Peripheral immune profile and neutrophil-to-lymphocyte ratio in Parkinson's disease. Mov Disord. (2021) 36:2426–30. doi: 10.1002/mds.28685
53. Lux D, Alakbarzade V, Bridge L, Clark CN, Clarke B, Zhang L, et al. The association of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio with 3-month clinical outcome after mechanical thrombectomy following stroke. J Neuroinflammation. (2020) 17:60. doi: 10.1186/s12974-020-01739-y
54. Wang C, Zhang Q, Ji M, Mang J, Xu Z. Prognostic value of the neutrophil-to-lymphocyte ratio in acute ischemic stroke patients treated with intravenous thrombolysis: a systematic review and meta-analysis. BMC Neurol. (2021) 21:191. doi: 10.1186/s12883-021-02222-8
55. Zhang WB, Zeng YY, Wang F, Cheng L, Tang WJ, Wang XQ, et al. high neutrophil-to-lymphocyte ratio predicts hemorrhagic transformation of large atherosclerotic infarction in patients with acute ischemic stroke. Aging (Albany NY). (2020) 12:2428–39. doi: 10.18632/aging.102752
56. Cheng W, Chen L, Yu H, Lu D, Yu R, Chen J. Value of combining of the NLR and the fibrinogen level for predicting stroke-associated pneumonia. Neuropsychiatr Dis Treat. (2021) 17:1697–705. doi: 10.2147/NDT.S311036
57. Zhang X, Gong P, Sheng L, Lin Y, Fan Q, Zhang Y, et al. Prognostic value of subclinical thyroid dysfunction in ischemic stroke patients treated with intravenous thrombolysis. Aging (Albany NY). (2019) 11:6839–50. doi: 10.18632/aging.102215
58. Gong P, Zhang X, Gong Y, Liu Y, Wang S, Li Z, et al. A novel nomogram to predict early neurological deterioration in patients with acute ischaemic stroke. Eur J Neurol. (2020) 27:1996–2005. doi: 10.1111/ene.14333
59. Xu XR, Zhang D, Oswald BE, Carrim N, Wang X, Hou Y, et al. Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin Lab Sci. (2016) 53:409–30. doi: 10.1080/10408363.2016.1200008
60. Xu JH, He XW, Li Q, Liu JR, Zhuang MT, Huang FF, et al. Higher platelet-to-lymphocyte ratio is associated with worse outcomes after intravenous thrombolysis in acute ischaemic stroke. Front Neurol. (2019) 10:1192. doi: 10.3389/fneur.2019.01192
61. Warach SJ, Ranta A, Kim J, Song SS, Wallace A, Beharry J, et al. Symptomatic intracranial hemorrhage with tenecteplase vs alteplase in patients with acute ischemic stroke: the comparative effectiveness of routine tenecteplase vs alteplase in acute ischemic stroke (CERTAIN) collaboration. JAMA Neurol. (2023) 80:732–8. doi: 10.1001/jamaneurol.2023.1449
Keywords: acute ischemic stroke, intravenous thrombolysis, fibrinogen, neutrophil-to-lymphocyte ratio, response
Citation: Deng M, Song K, Tong Y, Chen S, Xu W, He G, Hu J, Xiao H, Wan C, Wang Z and Li F (2024) Higher fibrinogen and neutrophil-to-lymphocyte ratio are associated with the early poor response to intravenous thrombolysis in acute ischemic stroke. Front. Neurol. 15:1291950. doi: 10.3389/fneur.2024.1291950
Received: 18 September 2023; Accepted: 06 February 2024;
Published: 22 February 2024.
Edited by:
Pradeep Kumar, All India Institute of Medical Sciences, IndiaReviewed by:
Yubin Liang, University of Jinan, ChinaCopyright © 2024 Deng, Song, Tong, Chen, Xu, He, Hu, Xiao, Wan, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Wang, ZG9jd3oyMDA3QDE2My5jb20=; Fangyi Li, NDc1MDQwOTQyQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.