
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 17 January 2024
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1344672
Objective: To evaluate the effectiveness of combined resection and radiotherapy (CRAR) for the treatment of primary pineal malignant melanoma (PPMM).
Methods: Relevant studies were identified through a literature search in PubMed, Embase, and Web of Science from 1899 to September 1, 2023. Then we further screened the literature according to the updated PRISMA 2020 guidelines. The article information, patient information, treatment, and survival rate were analyzed. The primary outcome measures the survival rate of CRAR compared with the overall patients and the patients without treatment. Secondary outcome measures operation methods, radiotherapy methods, and dose.
Results: In total, 28 published articles were recorded. Among them, 35.71% (10/28) articles were on CRAR. The median overall survival, CRAR, and no treatment survival were 65, 88, and 12 weeks, respectively. The median overall survival of CRAR was demonstrably better than that of no treatment (p < 0.0001) and overall survival, even with p = 0.1177. Most of the operations adopted a supracerebellar infratentorial approach, and stereotactic radiation to tumor bed usually ranged between 50 and 60 Gy. Small dose and multiple fractions was the most popular radiotherapy method.
Conclusion: Currently, CRAR, compared with other treatments, is more beneficial to prolonging the survival of PPMM patients. However, many more clinical cases are needed to verify it as the best treatment approach.
Primary malignant melanoma is an uncommon occurrence within the central nervous system, particularly in the pineal region. Primary pineal malignant melanoma (PPMM) poses a significant challenge for neurologists, akin to handling a delicate and complex situation. There is currently no definitive consensus regarding the optimal approach for its treatment. There exists a divergence of opinions among medical professionals regarding the most appropriate course of action, namely surgical intervention, radiotherapy, or chemotherapy.
According to the published literature (1), combined resection and radiotherapy (CRAR) is one of the most effective methods to treat PPMM. However, the effect of CRAR on PPMM compared with other treatment methods is still unknown. Currently, there are no guidelines on the specific operation methods, radiotherapy methods, or dose.
In this study, we aimed to evaluate the effectiveness of combined resection and radiotherapy for primary pineal malignant melanoma, with additional data from our local institution.
This systematic review adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (2). Electronic database searches were performed on PubMed, Embase, and Web of Science. Search keywords included a combination of ‘Pineal’ and ‘Melanoma’. Studies were included if (1) the study was clinical research about humans and (2) the study reported the patient’s treatment and prognosis. Studies were excluded if (1) the intracranial tumors in the study were metastatic tumors or non-pineal region tumors or (2) the study was systematic/narrative review or meta-analysis without reporting new relevant cases.
We recorded the retrieved published articles. The article information, patient information, treatment, and survival rate were analyzed. The primary outcome measures the survival rate of CRAR compared with the overall patients and the patients without treatment. Secondary outcome measures operation methods, radiotherapy methods, and dose.
All analyses were performed with GraphPad Prism V.8.2.1 (GraphPad Software, Inc., La Jolla, CA, United States). Kaplan–Meier survival curves were compared using the log rank test. Results were considered to achieve statistical significance if p < 0.05.
The search queries yielded a total of 479 abstracts from three different databases. After removing the duplicate records and screening according to several criteria, 28 studies were finally included in the study (1, 3–29) (Table 1). Figure 1 presents the search and selection process. The coverage range was 1899–2023, most of which come from Europe and America. The average age of patients was 52.71 (range 20–77, median 52.5) years old. The proportion of men was 53.6% (15/28). Of the articles, 35.71% (10/28) articles were on CRAR and 17.86% (5/28) articles were on no treatment (Figure 2).
In Figure 3, Kaplan–Meier survival curves were compared in overall, CRAR, and no treatment cases. The median overall survival of CRAR was obviously better than that of no treatment (p < 0.0001) and overall survival, even with p = 0.1177. The median overall survival was approximately 65 weeks, ranging from 3 to 280 weeks. Patients who received CRAR treatment had a median survival of 88 weeks. In the absence of treatment, the median survival was reduced to only 12 weeks.
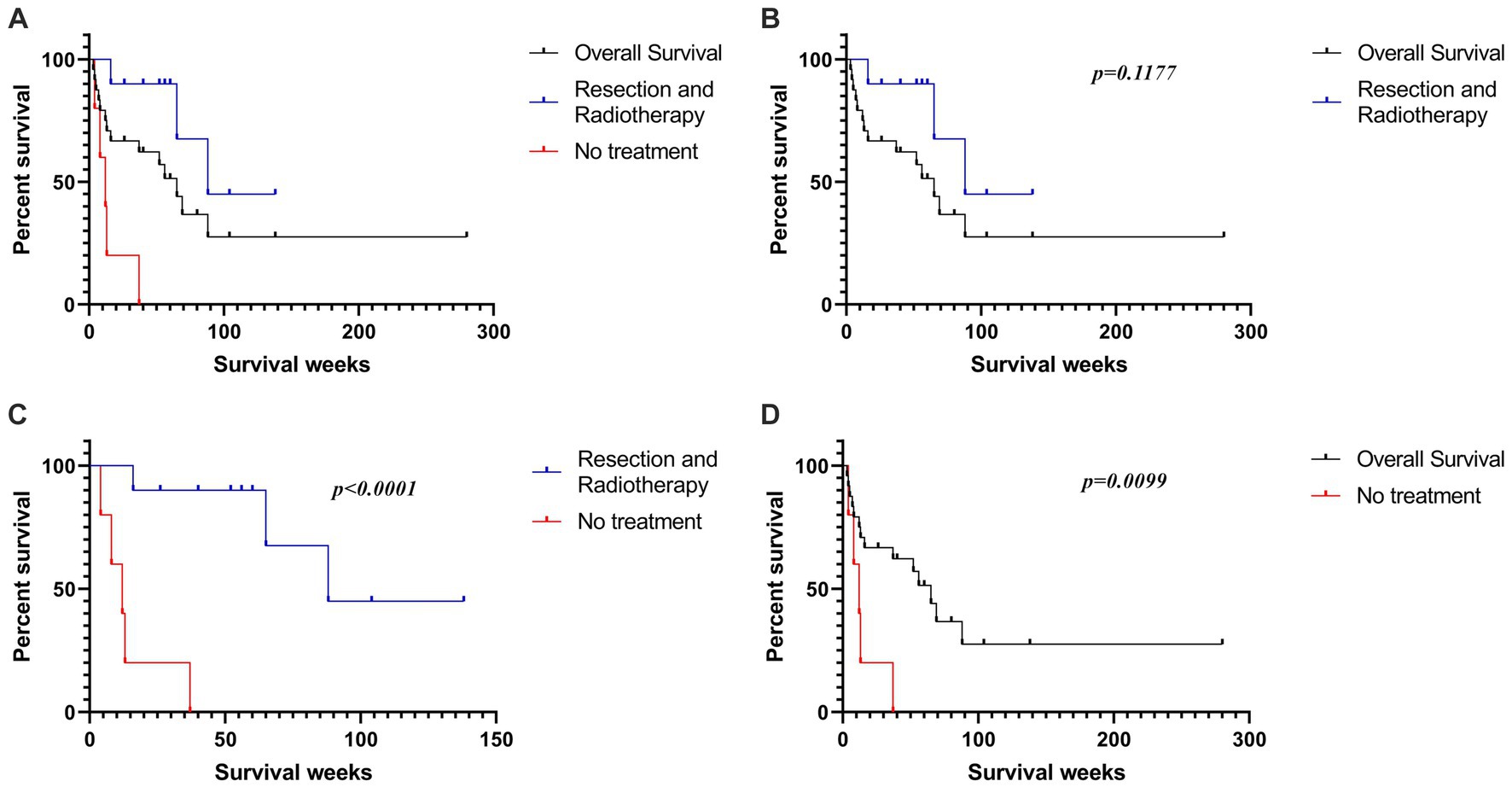
Figure 3. (A) The Kaplan-Meier survival curves of all reported PPMM patients, as well as the patients who received CRAR and those who did not receive treatment. (B) Patients who received CRAR showed a trend of better median overall survival than all reported PPMM patients (p = 0.1177). (C) The median overall survival of untreated patients was significantly lower than that of patients who underwent surgical resection combined with radiotherapy (p < 0.0001). (D) The median overall survival of untreated patients was significantly lower than the overall median survival of all reported PPMM patients (p = 0.0099).
In Table 2, we demonstrate that most of the operations adopted a supracerebellar infratentorial approach, a common approach for the treatment of pineal region tumors. As for the method of radiotherapy, whole-brain radiotherapy was most common in the past, while stereotactic radiation to tumor bed is more commonly used now. Tumor control doses usually range between 50 and 60 Gy. Small dose and multiple fractions was the most popular radiotherapy method. Local data were similar to the above treatment methods and have received good results.
The aim of the review was to evaluate the effectiveness of CRAR for PPMM. The included 28 studies reported PPMM cases, including their treatment and prognosis. Of the studies, 35.71% focused on CRAR. Our study found that CRAR has a good effect compared with the overall survival rate of patients without treatment.
Primary intracranial malignant melanoma (PIMM) accounts for only 1% of all melanoma cases (30). Due to the limitations of the blood–brain barrier on the efficacy of radiotherapy and chemotherapy, previous literature has recommended surgical resection as the main treatment for PIMM (31). Our study similarly suggests that resection plays a crucial role in treating PPMM. We should make every effort to completely remove the tumors. The supracerebellar infratentorial approach, which offers optimal tumor exposure, is considered the most favorable option. The combined use of a microscope and endoscopy technique has proven to be effective in treating pineal region tumors. The maximum extent of tumor removal can be guaranteed. In this approach, the tumor is initially removed through surgery using a microscope. Any remaining tumor is then identified and removed using endoscopy. Endoscopy is also used to observe whether the mesencephalic aqueduct is obstructed by blood clots and to exclude the occurrence of hydrocephalus after operation.
For radiotherapy, so far, stereotactic radiation to tumor bed for PPMM usually ranges between 50 and 60 Gy. Small dose and multiple fractions was the most popular radiotherapy method. Radiotherapy has been reported to increase the permeability of the blood–brain barrier and is believed to delay the recurrence of metastatic malignant melanoma (32, 33). Therefore, radiotherapy remains one of the primary treatments for intracranial malignant melanoma. Furthermore, the utilization of radioenhancers to enhance the effectiveness of radiation therapy and the advancement of stereotactic radiosurgery, specifically gamma knife radiosurgery and cyber knife radiosurgery (which can now be administered stereotactically, thereby reducing the undesired exposure of the chiasm and pituitary stalk to gamma knife and other forms of radiation therapy), have the potential to enhance the prognosis of patients (34, 35). Our results demonstrated the effectiveness of CRAR in prolonging the survival period of patients with PPMM, which also supported the potential role of radiotherapy in the treatment of PIMM. However, due to the very few cases of PPMM who only received radiotherapy, our study could not clearly define the value of radiotherapy in the treatment for PIMM.
Obviously, there are few reports about the therapeutic effect of chemotherapy, which means a clear conclusion cannot be drawn yet. Yamane et al. (12) conducted a case study on a patient who underwent resection and chemotherapy and achieved a survival time of over 280 weeks. This represents the longest reported survival data for patients with PPMM. Martin-Blondel et al. (18) reported a patient who survived for 52 weeks after undergoing chemotherapy and radiotherapy. Jetschke et al. (25) reported a case where a patient who received chemotherapy alone had a survival time of only 3 weeks. Wendel et al. (1) reported that chemotherapy administration could potentially be delayed until recurrence occurs. Regardless of the surgical procedures and additional therapies used, it is widely believed that achieving complete tumor removal is extremely important. Maybe in the future, PPMM research on cells and animals in laboratory can improve the treatment of the disease in humans. The tumor cells are treated with radiotherapy and / or chemotherapy in vitro. And after the cells are injected into the pineal region of mice, they are treated with surgery and / or radiotherapy and / or chemotherapy in vivo.
In addition to radiotherapy and chemotherapy, emerging adjuvant therapies such as targeted therapy and immunotherapy have demonstrated clear effectiveness in managing melanoma brain metastases. Approximately 50% of melanomas have a mutation in the serine–threonine protein kinase B-RAF (BRAF) gene, leading to activation of the MAPK pathway. About 90% of BRAF mutations occur at codon 600, with BRAFV600E (substituting valine with glutamic acid) being the most common mutation (36). To date, three BRAF inhibitors have been approved for treating metastatic melanoma. The identification of BRAF mutations in PPMM is gaining more attention (1, 25). Only one case of a BRAFV600E mutation in a PPMM has been documented. In this case, the patient’s condition worsened 2 weeks after surgery and resulted in death 26 days after the operation. Nevertheless, this finding suggests that some PPMM patients may respond well to BRAF-targeted therapy (25). Immunotherapy is mainly used to treat patients with multiple melanoma brain metastases, with drugs like ipilimumab and pembrolizumab shown to be effective (37). Famoso et al. reported a case of a PPMM with positive PD-L1 expression. After partial tumor removal and radiotherapy, the patient was treated with pembrolizumab and remained recurrence-free for 138 weeks (27). This finding confirms the potential of immunotherapy in treating PPMM.
Most of the previous reports were from America or Europe. Our previous report is the first study in China (28). In fact, we also searched the Chinese database for PPMM reports, but found nothing. We do not know whether there is any possibility of different incidence rates of disease between different countries and regions. This needs more data to verify in the future.
Currently, CRAR, compared with other treatments, is more beneficial to prolonging the survival of PPMM patients. However, many more clinical cases are needed to verify it as the best treatment approach.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
JZ: Writing – original draft, Data curation, Formal analysis, Methodology. ZW: Writing – original draft, Data curation, Formal analysis. JC: Writing – review & editing, Funding acquisition.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wendel, C, Kaech, DL, and Woodtli, M. Primary malignant melanoma in the pineal region: case report and literature review. J Neurol Surg A Cent Eur Neurosurg. (2018) 79:344–52. doi: 10.1055/s-0038-1639504
2. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
5. Foot, NC, and Zeek, P. Two cases of melanoma of the meninges with autopsy. Am J Pathol. (1931) 7:605–618 5.
6. Gibson, JB, Burrows, D, and Weir, W. Primary melanoma of the meninges. J Pathol Bacteriol. (1957) 74:419–38. doi: 10.1002/path.1700740220
7. Enriquez, R, Egbert, B, and Bullock, J. Primary malignant melanoma of central nervous system. Pineal involvement in a patient with nevus of ota and multiple pigmented skin nevi. Arch Pathol. (1973) 95:392–5.
8. Arlant, PA, Grunnet, ML, and Heilbrun, MP. Primary malignant melanoma of the pineal region. Surg Neurol. (1977) 7:121–3.
9. Carlson, BR, Glick, AD, and Cushman, AR. Primary malignant melanoma of pineal region. J Tenn Med Assoc. (1987) 80:597–9.
10. Weindling, SM, Press, GA, and Hesselink, JR. MR characteristics of a primary melanoma of the quadrigeminal plate. AJNR Am J Neuroradiol. (1988) 9:214–5.
11. Rubino, GJ, King, WA, Quinn, B, Marroquin, CE, and Verity, MA. Primary pineal melanoma: case report. Neurosurgery. (1993) 33:511–5; discussion 515. doi: 10.1227/00006123-199309000-00024
12. Yamane, K, Shima, T, Okada, Y, Nishida, M, Okita, S, Hatayama, T, et al. Primary pineal melanoma with long-term survival: case report. Surg Neurol. (1994) 42:433–7. doi: 10.1016/0090-3019(94)90353-0
13. Mitchell, PJ, Funt, SA, Gonzales, MF, and Popovic, EA. Primary pineal and meningeal malignant melanomatosis. J Clin Neurosci. (1998) 5:353–6. doi: 10.1016/S0967-5868(98)90078-9
14. Czirják, S, Vitanovic, D, Slowik, F, and Magyar, A. Primary meningeal melanocytoma of the pineal region. Case report. J Neurosurg. (2000) 92:461–5. doi: 10.3171/jns.2000.92.3.0461
15. Suzuki, T, Yasumoto, Y, Kumami, K, Matsumura, K, Kumami, M, Mochizuki, M, et al. Primary pineal melanocytic tumor. Case report. J Neurosurg. (2001) 94:523–7. doi: 10.3171/jns.2001.94.3.0523
16. Bookland, M, Anderson, WS, Biser-Rohrbaugh, A, and Jallo, GI. Primary pineal malignant melanoma. Pediatr Neurosurg. (2007) 43:303–8. doi: 10.1159/000103311
17. Barron, J, Morris-Larkin, C, Finch, T, Maroun, F, Hache, N, and Yousef, GM. Long survival of primary pineal melanoma with radiation treatment only. Can J Neurol Sci. (2007) 34:251–3. doi: 10.1017/S0317167100006156
18. Martin-Blondel, G, Rousseau, A, Boch, AL, Cacoub, P, and Sène, D. Primary pineal melanoma with leptomeningeal spreading: case report and review of the literature. Clin Neuropathol. (2009) 28:387–94.
19. Cedeno Diaz, OM, Leal, RG, and La Cruz Pelea, C. Primary pineal malignant melanoma. Clin Pract. (2011) 1:e31. doi: 10.4081/cp.2011.e31
20. Arantes, M, Castro, AF, Romão, H, Meireles, P, Garcia, R, Honavar, M, et al. Primary pineal malignant melanoma: case report and literature review. Clin Neurol Neurosurg. (2011) 113:59–64. doi: 10.1016/j.clineuro.2010.08.003
21. Shinsato, Y, Hanada, T, Kisanuki, T, Yonezawa, H, Yunoue, S, Yoshioka, T, et al. Primary malignant melanoma in the pineal region treated without chemotherapy. Surg Neurol Int. (2012) 3:123. doi: 10.4103/2152-7806.102348
22. Azimi, P, Mohmmadi, HR, and Refiezadeh, M. Primary pineal melanoma presenting with leptomeningeal spreading in a 22-year-old woman: a case report. J Med Case Rep. (2012) 6:165. doi: 10.1186/1752-1947-6-165
23. Park, JH, and Hong, YK. Primary malignant melanoma in the pineal region. J Korean Neurosurg Soc. (2014) 56:504–8. doi: 10.3340/jkns.2014.56.6.504
24. Biswas, A, Chaudhari, PB, M, S, Sigamani, E, Sharma, MC, Kalra, SK, et al. Primary pineal malignant melanoma - illustrated review. Turk Neurosurg. (2015) 25:201–9. doi: 10.5137/1019-5149.JTN.6568-12.1
25. Jetschke, K, Viehweger, H, Freesmeyer, M, Warnke, JP, and Mawrin, C. Primary pineal malignant melanoma with B-Raf V600E mutation: a case report and brief review of the literature. Acta Neurochir. (2015) 157:1267–70. doi: 10.1007/s00701-015-2427-3
26. Hajhouji, F, Ganau, M, Helene, C, Romano, A, Gubian, A, Proust, F, et al. Rare encounters: primary pineal malignant melanoma with lepto-meningeal spread. Case report and literature review on management challenges and outcomes. J Clin Neurosci. (2019) 65:161–5. doi: 10.1016/j.jocn.2019.03.029
27. Famoso, J, Lemole, G, Sundararajan, S, and Stea, B. Targeting PD-L1 after adjuvant radiation in subtotally resected primary pineal melanoma: a case report and literature review. J Natl Compr Cancer Netw. (2019) 17:1148–53. doi: 10.6004/jnccn.2019.7336
28. Zhang, J, Xiong, Z, and Chen, J. Combined microscope and endoscopy total resection of primary pineal malignant melanoma: case report and literature review. J Cancer Res Clin Oncol. (2020) 146:2589–94. doi: 10.1007/s00432-020-03328-1
29. Aaroe, AE, Glitza Oliva, IC, al-Zubidi, N, Nader, ME, Kaya, D, Ferguson, SD, et al. Pearls & oy-sters: primary pineal melanoma with leptomeningeal carcinomatosis. Neurology. (2021) 97:248–50. doi: 10.1212/WNL.0000000000012121
30. Shah, I, Imran, M, Akram, R, Rafat, S, Zia, K, and Emaduddin, M. Primary intracranial malignant melanoma. J Coll Physicians Surg Pak. (2013) 23:157–9.
31. Byun, J, Park, ES, Hong, SH, Cho, YH, Kim, YH, Kim, CJ, et al. Clinical outcomes of primary intracranial malignant melanoma and metastatic intracranial malignant melanoma. Clin Neurol Neurosurg. (2018) 164:32–8. doi: 10.1016/j.clineuro.2017.11.012
32. Gibney, GT, Forsyth, PA, and Sondak, VK. Melanoma in the brain: biology and therapeutic options. Melanoma Res. (2012) 22:177–83. doi: 10.1097/CMR.0b013e328352dbef
33. Kocher, M, Soffietti, R, Abacioglu, U, Villà, S, Fauchon, F, Baumert, BG, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. (2011) 29:134–41. doi: 10.1200/JCO.2010.30.1655
34. Ganau, M, Foroni, RI, Gerosa, M, Zivelonghi, E, Longhi, M, and Nicolato, A. Radiosurgical options in neuro-oncology: a review on current tenets and future opportunities. Part I: therapeutic strategies. Tumori. (2014) 100:459–65. doi: 10.1700/1636.17912
35. Ganau, M, Foroni, RI, Gerosa, M, Ricciardi, GK, Longhi, M, and Nicolato, A. Radiosurgical options in neuro-oncology: a review on current tenets and future opportunities. Part II: adjuvant radiobiological tools. Tumori. (2015) 101:57–63. doi: 10.5301/tj.5000215
36. Rishi, A, and Yu, HM. Current treatment of melanoma brain metastasis. Curr Treat Options Oncol. (2020) 21:45. doi: 10.1007/s11864-020-00733-z
Keywords: primary pineal malignant melanoma, PPMM, resection, radiotherapy, total resection
Citation: Zhang J, Wei Z and Chen J (2024) The effectiveness of combined resection and radiotherapy for primary pineal malignant melanoma: a systematic review. Front. Neurol. 14:1344672. doi: 10.3389/fneur.2023.1344672
Received: 26 November 2023; Accepted: 21 December 2023;
Published: 17 January 2024.
Edited by:
Salvatore Chibbaro, Strasbourg University Hospital, FranceReviewed by:
Mario Ganau, Oxford University Hospitals NHS Trust, United KingdomCopyright © 2024 Zhang, Wei and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jincao Chen, Y2hlbmppbmNhbzg4QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.