- 1Institute for Biomechanics, ETH Zürich, Zürich, Switzerland
- 2Department of Neurology, University Hospital Zurich, Zürich, Switzerland
- 3The LOOP Zurich – Medical Research Center, Zürich, Switzerland
- 4Lake Lucerne Institute, Vitznau, Switzerland
- 5creneo Foundation – Center for Interdisciplinary Research, Vitznau, Switzerland
Background: Freezing of Gait (FOG) is a motor symptom frequently observed in advanced Parkinson’s disease. However, due to its paroxysmal nature and diverse presentation, assessing FOG in a clinical setting can be challenging. Before FOG can be fully investigated, it is critical that a reliable experimental setting is established in which FOG can be evoked in a standardized manner, but the efficacy of various gait tasks and triggers for eliciting FOG remains unclear.
Objectives: This study aimed to conduct a systematic review of the existing literature and evaluate the available evidence for the relationship between specific motor tasks, triggers, and FOG episodes in individuals with Parkinson’s disease (PwPD).
Methods: We conducted a literature search on four online databases (PubMed, Web of Science, EMBASE, and Cochrane Library) using the keywords “Parkinson’s disease,” “Freezing of Gait”, “triggers” and “tasks”. A total of 128 articles met the inclusion criteria and were included in our analysis.
Results: The review found that a wide range of gait tasks were employed in studies assessing FOG among PD patients. However, three tasks (turning, dual tasking, and straight walking) emerged as the most frequently used. Turning (28%) appears to be the most effective trigger for eliciting FOG in PwPD, followed by walking through a doorway (14%) and dual tasking (10%).
Conclusion: This review thereby supports the utilisation of turning, especially a 360-degree turn, as a reliable trigger for FOG in PwPD. This finding could be beneficial to clinicians conducting clinical evaluations and researchers aiming to assess FOG in a laboratory environment.
Introduction
Freezing of Gait (FOG) is a common disabling motor symptom that occurs in people with Parkinson’s disease (PwPD). In earlier stages of the disease, FOG is estimated to affect around 50% of PwPD, with the number increasing up to 80% as the disease progresses (1–3). FOG is defined as “a brief, episodic absence or marked reduction of forward progression of the feet despite the intention to walk” (4). In practice, it manifests itself in three different patterns: small ‘shuffling’ steps, alternating leg ‘trembling’ when stationary, and ‘akinesia’ with no discernible forward progression despite movement intention (4, 5). The severity and complexity of these patterns can vary significantly from patient to patient and can even fluctuate within the same individual at different times (6). FOG has a significant impact on mobility, frequently leads to falls, and reduces the quality of life for PwPD and their caregivers (7–10). Additionally, FOG can lead to increased anxiety and insecurity while walking (10–12).
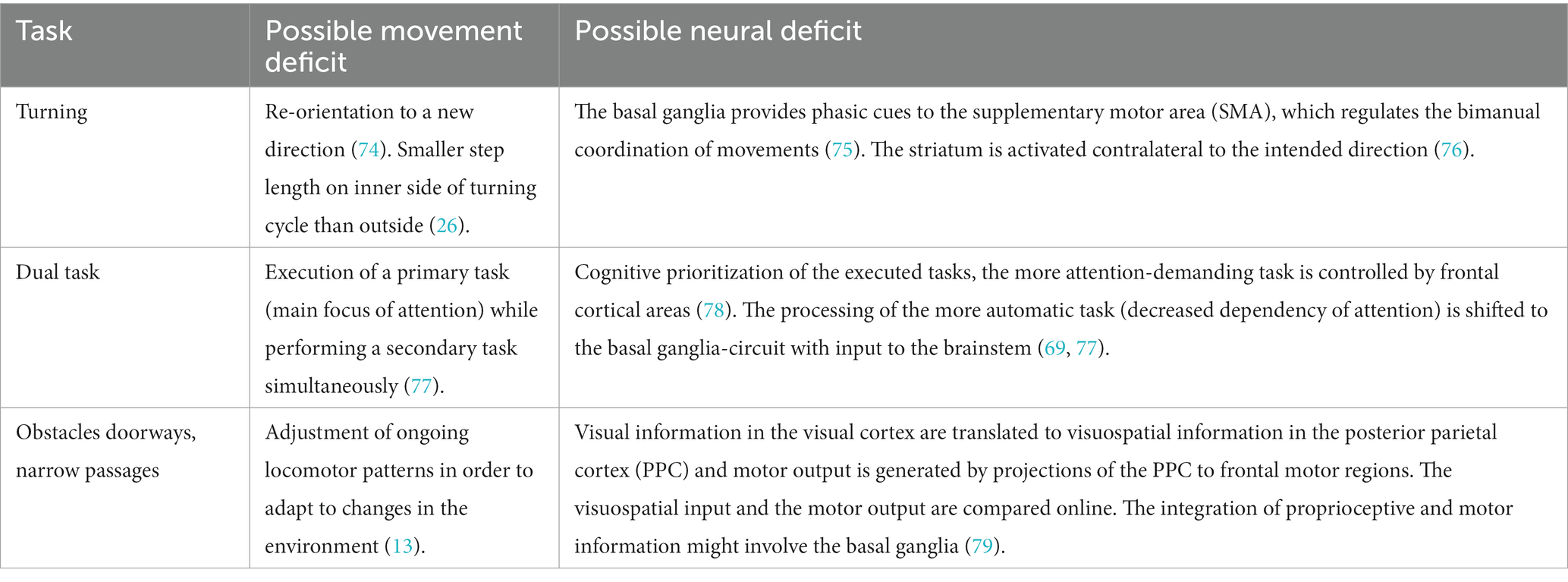
Bardakan and colleagues describe three main pathophysiological models for FOG (13). The interference model suggests that FOG results from impaired crosstalk between cortical and subcortical areas, leading to over-inhibition of brainstem structures and reduced gait automaticity, especially during dual-task situations (14). Freezing episodes frequently observed in the OFF-medication state emphasize the significance of the dopaminergic pathway in relation to FOG (15–17). However, FOG also occurs in the ON medication state, suggesting that a hypodopaminergic state only partially explains FOG (18). Additionally, FOG commonly occurs when passing through doors or when approaching destinations (19). This could be explained by the perceptual dysfunction model, which describes a malfunction in visuomotor processing, resulting in an inability to adapt to dynamic environmental changes. Lastly, the executive dysfunction model accounts for FOG caused by obstacle avoidance. FOG could arise from a disconnection between the frontal lobe and the basal ganglia, as executive functions are called upon to compensate for the loss of automatic movement (13).
The normal control of gait requires coordinated excitation and inhibition of competing motor plans, which is compromised in people with FOG. FOG can occur during ongoing movements as a result of sudden temporal re-inhibition via the indirect pathway and a decrease in the disinhibition of the direct pathway. This leads to an over-inhibition of brainstem structures by the globus pallidus internus (GPi), hindering the initiation and execution of movements, consequently resulting in FOG. Overall, there is partial consensus that connectivity issues between the basal ganglia, the prefrontal cortex, and the frontoparietal areas as potential sources causing FOG (14, 15, 20). The presence of several hypotheses for the causes of FOG demonstrates the complexity of its pathophysiology (5).
Assessing FOG in a clinical setting can be challenging due to the paroxysmal nature and diverse presentation of symptoms (21–23). Multiple FOG-triggering settings have been associated with the condition, such as gait initiation (24, 25), turning (26–28), anxiety (11, 29), and walking in narrow spaces (30–32), among others. Three distinct phenomenological types of FOG have been identified in previous studies (5, 33): asymmetric-motor, sensory-attention, and anxious freezing. Asymmetric motor freezing occurs mainly during turning, movement initiation, or when walking through narrow passages (5, 33–35). Sensory attentive freezing is often a result of walking in the dark, walking through an unorganized space, or when the surface is sloped (5, 33). Proprioceptive disturbances such as in concomitant polyneuropathy can contribute to freezing or mask it. Lastly, anxious freezing is triggered in stressful situations, such as when under time pressure or dual tasking (5, 21, 29, 36).
Most FOG episodes occur outside of a clinical environment as the awareness of being observed (Hawthorne effect) may enhance walking performance (37), making it difficult to study the symptom during a doctor’s visit or in clinical studies (36, 38–42). Questionnaires such as the Movement Disorder Society – Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) (43), the Freezing of Gait Questionnaire (FOG-Q) (44), or the New Freezing of Gait Questionnaire (NFOG-Q) (45) are available to confirm and determine the presence, frequency, and severity of FOG retrospectively (46). However, due to recall bias accompanied by a cognitive decline patients experience difficulties when self-assessing FOG based on their perception (47). Furthermore, minor differences in freezing severity may not be reliably detected by the NFOG-Q as it is a self-rated questionnaire and awareness of the freezing behaviour might change over time (47). As a result, videotaped gait analysis is currently the standard for FOG detection in clinics (15). During these analyses, patients often perform different gait tasks like turning or dual tasking, but there is a wide variation regarding the protocol and the tasks included in different studies and clinical examinations.
In order to better understand FOG and find effective therapies, many studies have attempted to recreate scenarios where FOG can be elicited in a reliable manner. Despite the availability of several questionnaires and analysis techniques to capture the appearance of freezing, the lack of coherent recommendations for reliably eliciting freezing episodes in both observational and interventional research has impeded progress in understanding FOG. This has led to sparse objective information regarding the detection and effectiveness of treatments in clinical practice (5, 14, 15, 34, 48). Some experimental studies have found certain walking tasks to be more effective in eliciting FOG, such as turning with a small radius or walking through doors (21, 49, 50). Based on these findings, some research groups have developed obstacle courses that combine various triggers, such as turns and narrow passages, as well as straight walking or dual tasking elements (51, 52). Additionally, virtual, or augmented reality technologies have been used to elicit FOG by providing individualized triggers and the ability to scale the difficulty and complexity of tasks (53–57). Although there is a large collection of gait tasks used to assess FOG, the effectiveness of these individual triggers in eliciting FOG remains elusive (29, 58, 59). Various studies, using various aforementioned triggers, were not able to elicit any freezing episodes during their protocol (19, 60, 61). As a first step to guide further development of triggering paradigms, now one of the challenges is to ascertain which motor task serves as the most efficient trigger for FOG.
Therefore, to address this challenge, the aim of this study was to conduct a systematic review of the existing literature to evaluate the evidence linking specific motor tasks and triggers to FOG episodes in PwPD. By gaining a comprehensive understanding of these triggers, we aim to lay the foundations for advancing current knowledge of the pathophysiological mechanisms underlying FOG, optimizing treatments, and enabling the development of new therapies.
Methods
Search strategy and selection criteria
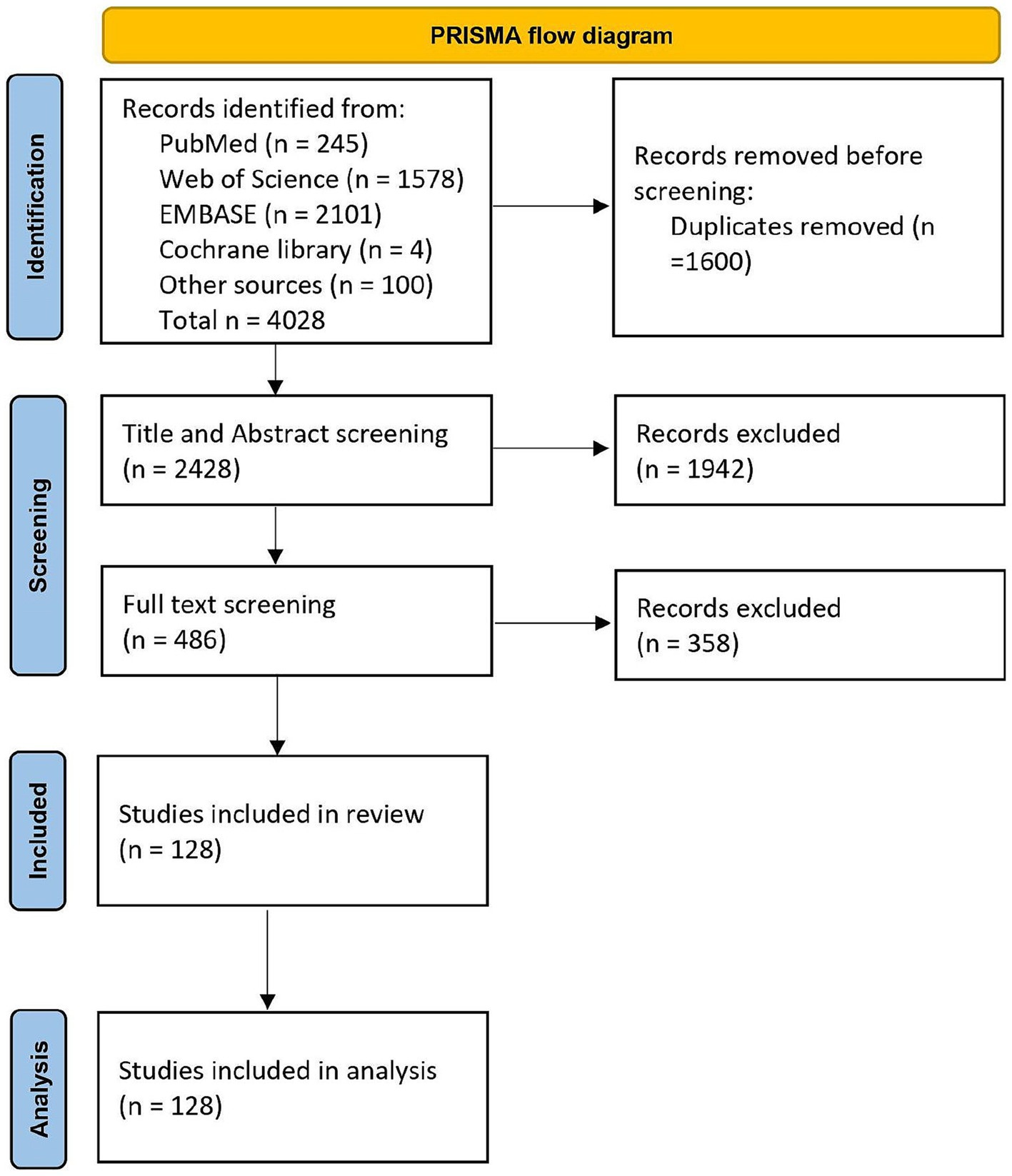
In October 2021, a literature search was carried out following the guidelines of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) (62). Four online databases, namely PubMed, Web of Science, EMBASE, and Cochrane Library, were searched. In addition, articles that were identified prior to the commencement of the formal review were also included for screening (“other sources” in Figure 1). The search was conducted on articles published between 1998 and 2021 and was restricted to original articles published in English in a peer-reviewed journal. The complete search string is available as a Supplementary Methods 1. Furthermore, the complete review protocol has been registered in the PROSPERO database (CRD42022330511).
Selection of studies and data extraction
The process of selecting studies was aided by the EPPI-Reviewer 4 (V.4.12.5.0, EPPI-Centre, UCL Institute of Education, University of London, London, UK) and EndNote (EndNote 20, Clarivate, Philadelphia, United States) software programs. Once duplicates were eliminated, two reviewers (CC, DKR) independently screened the titles, abstracts, and full texts, resolving any discrepancies through consensus. Studies were considered eligible if they included adult patients with PD and reported on FOG during a walking task. Studies were excluded if they reported FOG outcomes during stepping or turning in place tasks, gait initiation, unsupervised daily-life environments, or if participants walked using walking aids.
A spreadsheet (MS Excel, version 2018, Microsoft Corporation, Washington, United States) was used by one reviewer (CC) to extract the following information:
• Publication details such as author names, title, and publication year
• Study information including the number of individuals in freezing and non-freezing, gait tasks performed, and the technology / assessment scores used for FOG assessment
• Participant demographics such as age, gender, disease duration, disease stage, medication, and clinical assessments [MDS-UPDRS score (51) and Hoehn and Yahr score (60)]
• FOG outcomes such as total FOG count, participants with FOG, percentage of time spent frozen, trigger, gait task, etc.
If any information was missing or needed clarification, the authors of the included studies were contacted for additional details.
Data synthesis
In order to assess the most effective triggers, the data of all studies that stated the gait task triggering FOG were extracted and normalized by the number of studies reporting the same trigger. For every activity, the numbers of FOG episodes (“Total FOG count”, Supplementary Table S1) were summed and normalized by the number of studies reporting FOG episodes triggered by the respective task. Multiple studies conducted measurements while participants were both ON and OFF medication states or involved different participant groups, such as freezers and non-freezers, among others (“Cohort”, Supplementary Table S1). In such cases, reported outcomes, such as the number of FOG episodes for all states and groups, were extracted and utilized for the analysis. Plots were created in the RStudio Software (R version 4.1.3, R Core Team, Vienna, Austria) using the ggplot2 package (version 3.4.2) (63).
Results
Study selection and characteristics
After an initial search that yielded almost 4,000 articles, 1,600 were identified as duplicates. Following a screening of titles and abstracts, 486 articles remained eligible, and 128 of them met the inclusion criteria (Figure 1).
Among the 128 studies included, 96 reported either the number or the percentage of participants who experienced FOG during the gait trials. In total, these studies included 2,804 patients, of whom 2,156 (77%) were categorized as ‘freezers’, either based on clinical assessments conducted through physical examinations or through self-reporting. In 116 out of the 128 studies included, 11,599 FOG episodes were reported. The mean age of all PD participants was 66.9 years, ranging from 51.0 to 76.8 years. Furthermore, the average disease severity was determined based on Hoehn and Yahr scale, was available in 81 out of the 128 studies, resulting in an average disease stage of 2.5, indicative of mild bilateral disease (64). Overall, more male participants (64%) were included in the studies compared to female participants (36%). Two studies reported only the median value for FOG outcomes (average FOG count, FOG duration and percentage time with FOG). For the analysis in this study, the median value from both studies was utilized as the mean value due to sufficient sample sizes in both studies.
Patients with FOG
The patient cohort sample size, as well as the percentage of participants who experienced FOG, varied greatly between studies from 4 to 305 subjects and 0% to 100%, respectively (Figure 2). Looking at all participants with PD, 12 studies were not able to elicit any FOG in their test population, while 9 studies triggered FOG in all their participants. A total of 316 participants (average 26 participants per study) belongs to the group of studies with no FOG while the number of participants in studies with 100% FOG sums up to 121 (average 13). Out of the previously mentioned 80% of participants that were categorized as freezers, 54% experienced freezing in the course of the gait experiment.
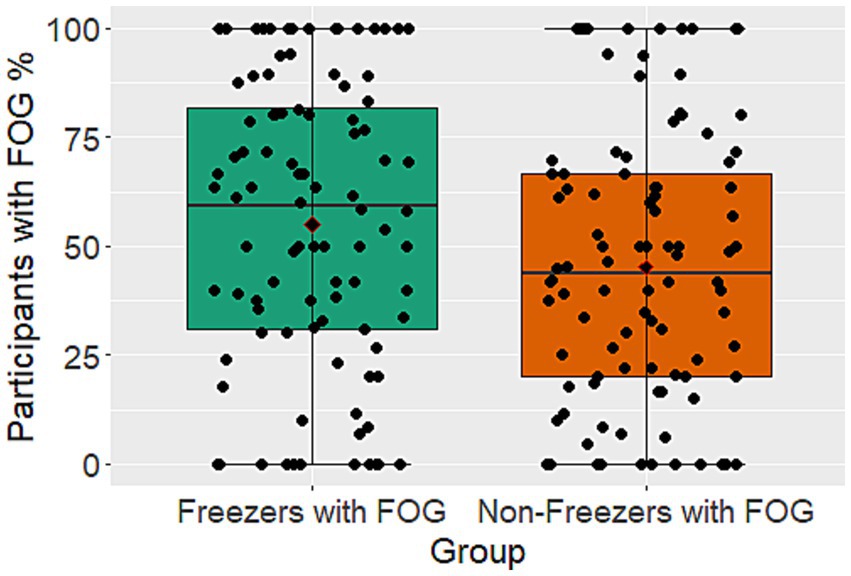
Figure 2. Boxplots showing the percentage of participants with FOG during gait trials for PwPD that are not known as freezers and the known freezers subgroup. Illustrated is the median with 25% and 75% confidence intervals.
Tasks
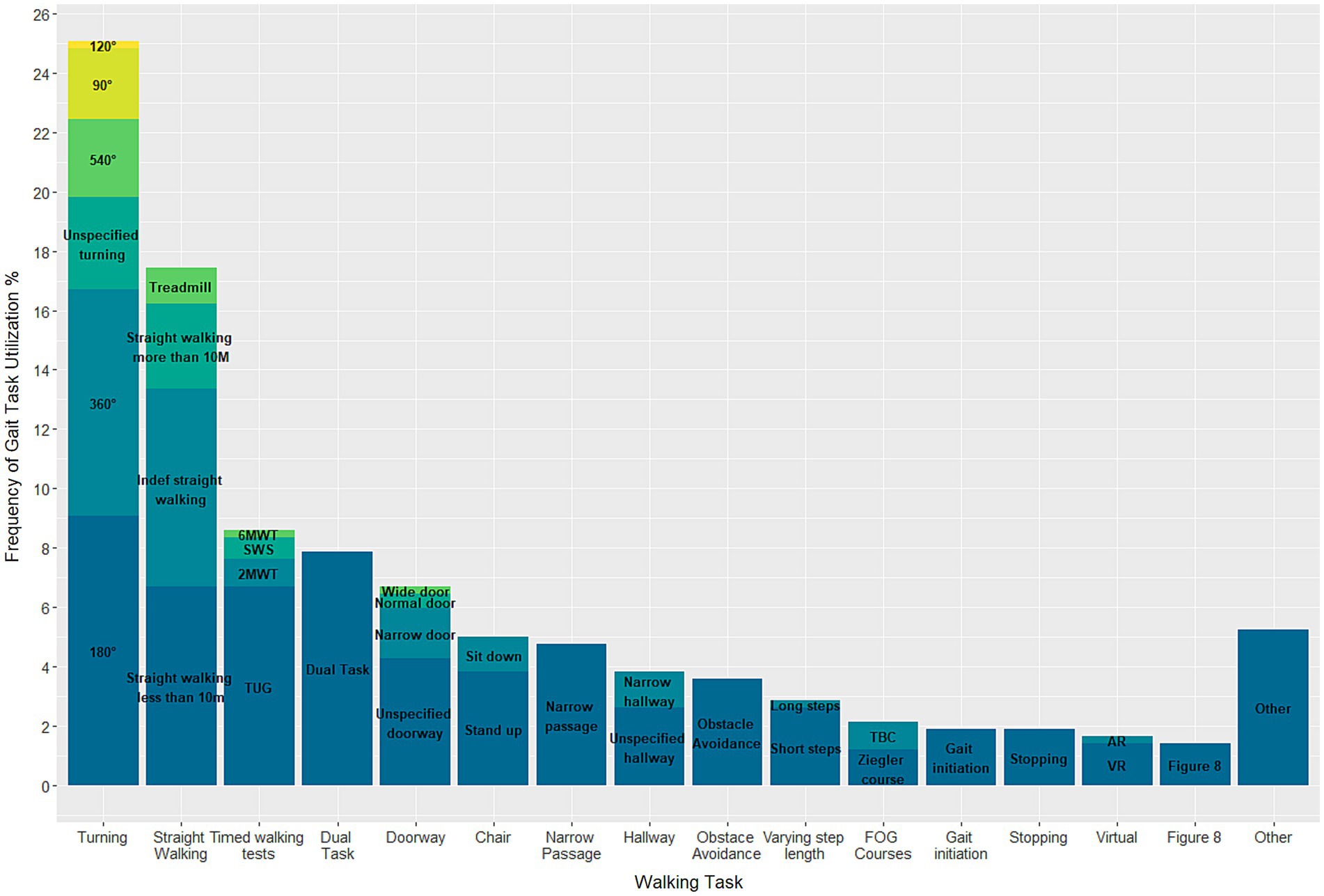
In total, 40 different gait tasks were reported and grouped into 16 categories based on higher-level tasks. Of the 128 included articles, only 22 studies exposed their participants to a single walking task, while the remainder requested their participants to perform at least two tasks. Turning of 180° (n = 38 studies) was the most used task, followed by 360° turning (n = 32 studies) (Figure 3). A few tasks were only conducted in a singular study, namely: long steps, augmented reality, turning of 120°, passing a wide door, and a Six Minute Walking Test. For the Turning and Barrier Course (TBC) subjects were “instructed to stand up, walk around the dividers twice in an ellipse, and then walk in a ‘figure eight’, around and through the opening between the dividers, twice, before sitting down again” (65). Execution of the Ziegler course included a “Stand Up and Go test crossing through a doorway and then, turning back” (66). Details on the study designs and execution of the other gait tasks can be found in the Supplementary material (Supplementary Table S1).
Trigger
Among the included studies, 26 specified the gait tasks that triggered FOG. This includes studies that examine participants in both ON- and OFF-medication condition. In total 24 different triggers were identified. Of these, the most effective trigger was 360° turning, which was responsible for 15.3% of FOG episodes recorded in the aforementioned studies (Figure 4). In addition, unspecified turning (10.1%), dual tasking (9.9%), stepping in place (9.5%), and passing a doorway (7.6%) were all activities with relatively high rates of eliciting FOG.
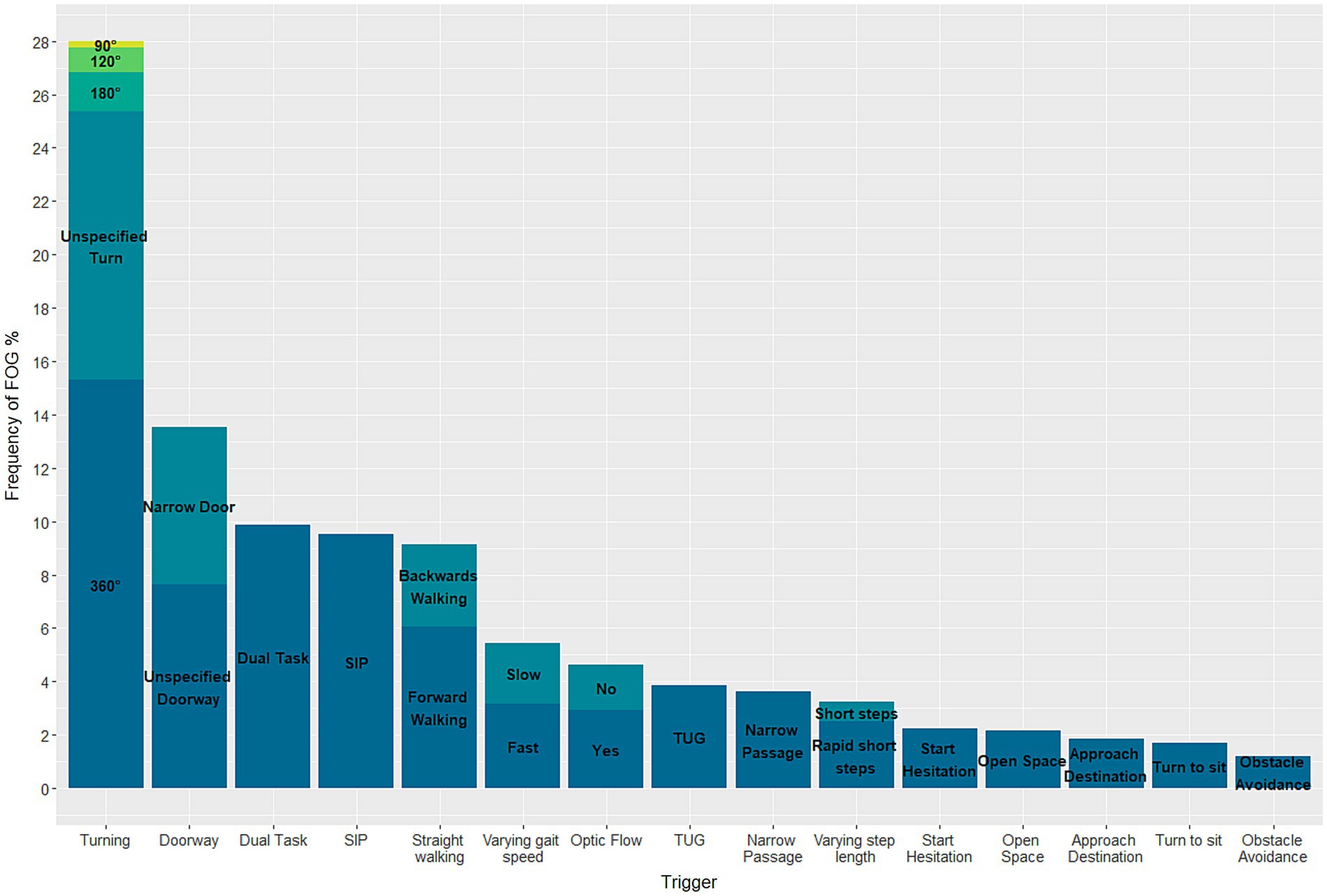
Figure 4. Stacked barplot demonstrating the triggers eliciting FOG in known freezers during gait trials.
Discussion
Freezing of Gait is a debilitating symptom experienced by many people with Parkinson’s disease. However, due to challenges in eliciting FOG in controlled laboratory or clinical settings, there has been a relative lack of research focused specifically on this phenomenon. Here, the challenge was to identify gait tasks most effective at triggering FOG. To address this, a systematic review of the literature was conducted, aiming to provide valuable insights into the tasks most likely to elicit FOG. The review revealed that turning is not only the most frequently studied gait task but also the most effective gait task for inducing FOG in PwPD (28%), followed by passing a doorway and dual tasking. This effort supports researchers in studying the underlying mechanisms of the symptom and developing effective interventions to improve the quality of life of PwPD.
Several theories exist surrounding the potent induction of FOG by turning. One hypothesis posited by previous studies suggests that FOG is caused by a delay in maximum head-pelvis separation, resulting in inadequate preparation for directional changes (27, 59). Moreover, the asymmetrical stepping pattern induced by turning or the reduced ability of PwPD to adapt to a new gait pattern may also contribute to this phenomenon (67).
Cowie et al. (49) reported a study that observed a slowing of walking in PwPD when approaching doorways, which they attributed to impaired visuomotor processing. However, an alternative explanation could be that attention is diverted from walking when approaching a doorway, leading to reliance on automatic movement, which can be disrupted in some PwPD (68, 69). Previous studies have demonstrated that dual tasking can lead to increased gait arrhythmicity and unsteadiness, as well as reduced step length and walking speed in PwPD (61, 70, 71). This suggests that dual tasking may contribute to the occurrence of FOG, as patients divert their attention away from the gait task towards the secondary task, thereby increasing cognitive demand. Although several other gait tasks elicited FOG to a lesser extent, the diversity of triggers suggests that various brain areas and mechanisms are involved in the occurrence of FOG. Human movement involves a complex interplay of several brain areas (14, 72, 73), and any damage along the neural chain could therefore influence movement in different ways (Table 1) (14).
In this systematic review, it was found that three gait tasks, namely turning, dual tasking, and straight walking, were performed in at least half of the 128 studies analysed. Other gait tasks were performed much less frequently. The reasons for this preference for certain gait tasks can only be speculated on. It is possible that the choice of tasks was influenced by previous studies reporting successful triggering with a specific task or the ease of preparation and infrastructure required for the task. For instance, tests such as the TUG can be performed with minimal preparation, and adding a dual task to a pre-existing gait task can also be relatively simple.
A wide variation was observed in the percentage of participants experiencing FOG during gait assessment, ranging from 0 to 100% for both known freezers and PwPD (Figure 4). This variability is likely due to differences in study protocols, number of walking trials, medication status, and disease severity. However, the paroxysmal and unpredictable nature of FOG, influenced by environmental, emotional, and cognitive factors, is also a contributing factor (4, 21). In the studies with no FOG episodes the most commonly examined task was straight walking. Nonetheless, all of the studies encompassed a diverse range of additional tasks, rendering it unfeasible to reach any definitive conclusions. In comparison to a previous study of almost a thousand participants, which reported a FOG prevalence of around one-third (80), the current study, involving a larger population of over 95 studies with exposure to a variety of tasks, showed a higher prevalence of around 50% for both PwPD and the subgroup of only Freezers (76). This could enable a more enhanced exploration of the underlying mechanisms by the use of more effective triggers.
The male predominance in our sample is consistent with the fact that males are twice as likely to be affected by the disease than women (81). The broad range of disease severity from I to almost IV further complicates comparison of study outcomes. Despite these limitations, this study provides a valuable contribution to the literature on FOG in PwPD and highlights the need for further investigation and standardization of FOG assessment protocols. With the study’s broad inclusion criteria, this is the first survey to comprehensively examine activities that elicit FOG by reviewing a large number of studies. However, the diverse nature of the studies included with varying execution of the conducted gait tasks posed a challenge in comparing outcomes. Additionally, a limitation of the study is the absence of a bias assessment to evaluate the quality of the included studies. Nonetheless, the study’s strengths, such as its comprehensive review and broad inclusion criteria, outweigh its limitations and contribute significantly to the current understanding of FOG in PwPD.
A noteworthy discovery from this study was the relatively low number of studies that focused on the triggers of FOG. The reason for this may be attributed to the challenge of accurately determining the cause of FOG episodes, which can be influenced by various emotional, environmental, and other factors. Interestingly, the frequency of FOG episodes did not significantly differ between the Freezers group and the entire PD patient population. This could be due to the unpredictable nature of FOG (10), or even the feeling of being observed and therefore increased attention while undertaking functional assessments in clinical and laboratory settings (34).
In order to systematically study the underlying mechanisms of FOG, it is important to know which gait tasks act as the most efficient triggers for FOG. Current research is moving increasingly towards real-life assessment using IMUs (82). However, this approach only informs about behavioural changes and does not provide information about the specific triggers that cause FOG. Therefore, more studies that focus specifically on the triggers of FOG are needed. Based on the analysis of 26 studies that specified the gait tasks triggering FOG, this review provides evidence suggesting that turning is currently the most prominent trigger of FOG. Nevertheless, several other gait tasks also possess the capability to elicit FOG to some extent. Based on these findings, turning might be the most effective task for FOG assessment in clinical examinations, but future studies should continue to cover a broad range of potential triggers in their protocol, as this can facilitate inference from phenomenological observation to underlying mechanisms. Using different gait tasks separately according to a standardized protocol could simplify the process of precisely identifying and distinguishing the exact triggers of a FOG episode. However, the identification and categorization of triggers for FOG can be a complex task due to their subjective nature. Importantly, researchers should systematically annotate the cause of the FOG episode. This is particularly noteworthy as, until now, only a minority of studies explicitly report the actual triggers of FOG.
As the population continues to age, the prevalence of neurodegenerative diseases like PD is expected to rise. Thus, it is crucial to fully comprehend the symptoms, diagnosis, and treatment options associated with this condition. However, despite significant advancements in this area, research on the occurrence, severity, epidemiology, and underlying causes of FOG remains scarce. Therefore, this systematic review’s findings on triggers for FOG in PwPD will be valuable in guiding future research and clinical applications, aiding in the selection of assessments for FOG. Emerging technologies such as augmented or virtual reality hold promise in this field, as they can be utilized to evaluate, diagnose, and cue FOG effectively. These technologies can incorporate individual triggers as building blocks, allowing for the creation of personalized walking courses tailored to an individual’s preferred difficulty level.
Conclusion
This review offers a significant contribution to the understanding of FOG triggers in PwPD by providing a comprehensive overview. The results indicate that turning is the most effective trigger for FOG in PwPD, followed by walking through a doorway and dual tasking. These findings have potential applications for researchers designing studies on FOG, clinicians evaluating patients with PD, and the development of interventions to manage or prevent FOG. Implementing the results to design coherent recommendations in research as well as clinical evaluation can lead to a better understanding of FOG and therefore an improvement of current treatment.
Author contributions
CC: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing, Methodology. CL: Formal analysis, Visualization, Writing – review & editing. CB: Writing – review & editing. CE: Writing – review & editing. WT: Funding acquisition, Resources, Writing – review & editing. DR: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by grants from The LOOP Zürich and the Vontobel Foundation. Open access funding by ETH Zurich.
Acknowledgments
We thank for Pino Wüest for his assistance in data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1326300/full#supplementary-material
References
1. Tan, DM, McGinley, JL, Danoudis, ME, Iansek, R, and Morris, ME. Freezing of gait and activity limitations in people with Parkinson's disease. Arch Phys Med Rehabil. (2011) 92:1159–65. doi: 10.1016/j.apmr.2011.02.003
2. Hely, MA, Reid, WGJ, Adena, MA, Halliday, GM, and Morris, JGL. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. (2008) 23:837–44. doi: 10.1002/mds.21956
3. Perez-Lloret, S, Negre-Pages, L, Damier, P, Delval, A, Derkinderen, P, Destée, A, et al. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol. (2014) 71:884–90. doi: 10.1001/jamaneurol.2014.753
4. Nutt, JG, Bloem, BR, Giladi, N, Hallett, M, Horak, FB, and Nieuwboer, A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. (2011) 10:734–44. doi: 10.1016/S1474-4422(11)70143-0
5. Falla, M, Cossu, G, and Di Fonzo, A. Freezing of gait: overview on etiology, treatment, and future directions. Neurol Sci. (2022) 43:1627–39. doi: 10.1007/s10072-021-05796-w
6. Uehara, A, Kawamoto, H, Imai, H, Shirai, M, Sone, M, Noda, S, et al. Author correction: gait improvement with wearable cyborg HAL trunk unit for parkinsonian patients: five case reports. Sci Rep. (2023) 13:8247. doi: 10.1038/s41598-023-35421-z
7. Moore, O, Peretz, C, and Giladi, N. Freezing of gait affects quality of life of peoples with Parkinson's disease beyond its relationships with mobility and gait. Mov Disord. (2007) 22:2192–5. doi: 10.1002/mds.21659
8. Okuma, Y, Silva de Lima, AL, Fukae, J, Bloem, BR, and Snijders, AH. A prospective study of falls in relation to freezing of gait and response fluctuations in Parkinson's disease. Parkinsonism Relat Disord. (2018) 46:30–5. doi: 10.1016/j.parkreldis.2017.10.013
9. Walton, CC, Shine, JM, Hall, JM, O’Callaghan, C, Mowszowski, L, Gilat, M, et al. The major impact of freezing of gait on quality of life in Parkinson's disease. J Neurol. (2015) 262:108–15. doi: 10.1007/s00415-014-7524-3
10. Bloem, BR, Hausdorff, JM, Visser, JE, and Giladi, N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord. (2004) 19:871–84. doi: 10.1002/mds.20115
11. Pimenta, M, Moreira, D, Nogueira, T, Silva, C, Pinto, EB, Valenca, GT, et al. Anxiety independently contributes to severity of freezing of gait in people with Parkinson’s disease. J Neuropsychiatry Clin Neurosci. (2018) 31:80–5. doi: 10.1176/appi.neuropsych.17090177
12. Giladi, N, and Hausdorff, JM. The role of mental function in the pathogenesis of freezing of gait in Parkinson's disease. J Neurol Sci. (2006) 248:173–6. doi: 10.1016/j.jns.2006.05.015
13. Bardakan, MM, Fink, GR, Zapparoli, L, Bottini, G, Paulesu, E, and Weiss, PH. Imaging the neural underpinnings of freezing of gait in Parkinson's disease. Neuroimage Clin. (2022) 35:103123. doi: 10.1016/j.nicl.2022.103123
14. Lewis, SJ, and Shine, JM. The next step: a common neural mechanism for freezing of gait. Neuroscientist. (2016) 22:72–82. doi: 10.1177/1073858414559101
15. Cucca, A, Biagioni, MC, Fleisher, JE, Agarwal, S, Son, A, Kumar, P, et al. Freezing of gait in Parkinson's disease: from pathophysiology to emerging therapies. Neurodegenerative Dis Manag. (2016) 6:431–46. doi: 10.2217/nmt-2016-0018
16. Amboni, M, Barone, P, Picillo, M, Cozzolino, A, Longo, K, Erro, R, et al. A two-year follow-up study of executive dysfunctions in parkinsonian patients with freezing of gait at on-state. Mov Disord. (2010) 25:800–2. doi: 10.1002/mds.23033
18. Schaafsma, JD, Balash, Y, Gurevich, T, Bartels, AL, Hausdorff, JM, and Giladi, N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson's disease. Eur J Neurol. (2003) 10:391–8. doi: 10.1046/j.1468-1331.2003.00611.x
19. Almeida, QJ, and Lebold, CA. Freezing of gait in Parkinson's disease: a perceptual cause for a motor impairment? J Neurol Neurosurg Psychiatry. (2010) 81:513–8. doi: 10.1136/jnnp.2008.160580
20. Snijders, AH, Takakusaki, K, Debu, B, Lozano, AM, Krishna, V, Fasano, A, et al. Physiology of freezing of gait. Ann Neurol. (2016) 80:644–59. doi: 10.1002/ana.24778
21. Spildooren, J, Vercruysse, S, Desloovere, K, Vandenberghe, W, Kerckhofs, E, and Nieuwboer, A. Freezing of gait in Parkinson's disease: the impact of dual-tasking and turning. Mov Disord. (2010) 25:2563–70. doi: 10.1002/mds.23327
22. Sawada, M, Wada-Isoe, K, Hanajima, R, and Nakashima, K. Clinical features of freezing of gait in Parkinson's disease patients. Brain Behav. (2019) 9:e01244. doi: 10.1002/brb3.1244
23. Barthel, C, Mallia, E, Debû, B, Bloem, BR, and Ferraye, MU. The practicalities of assessing freezing of gait. J Parkinsons Dis. (2016) 6:667–74. doi: 10.3233/JPD-160927
24. Fasano, A, and Lang, AE. Unfreezing of gait in patients with Parkinson's disease. Lancet Neurol. (2015) 14:675–7. doi: 10.1016/S1474-4422(15)00053-8
25. Nieuwboer, A, and Giladi, N. Characterizing freezing of gait in Parkinson's disease: models of an episodic phenomenon. Mov Disord. (2013) 28:1509–19. doi: 10.1002/mds.25683
26. Spildooren, J, Vinken, C, van Baekel, L, and Nieuwboer, A. Turning problems and freezing of gait in Parkinson’s disease: a systematic review and meta-analysis. Disabil Rehabil. (2019) 41:2994–3004. doi: 10.1080/09638288.2018.1483429
27. Janssen, S, de Ruyter van Steveninck, J, Salim, HS, Cockx, HM, Bloem, BR, Heida, T, et al. The effects of augmented reality visual cues on turning in place in Parkinson's disease patients with freezing of gait. Front Neurol. (2020) 11:185. doi: 10.3389/fneur.2020.00185
28. Mancini, M, Weiss, A, Herman, T, and Hausdorff, JM. Turn around freezing: community-living turning behavior in people with Parkinson's disease. Front Neurol. (2018) 9:18. doi: 10.3389/fneur.2018.00018
29. Martens, KAE, Ellard, CG, and Almeida, QJ. Does anxiety cause freezing of gait in Parkinson's disease? PLoS One. (2014) 9:e106561. doi: 10.1371/journal.pone.0106561
30. Silveira, CRA, Ehgoetz Martens, KA, Pieruccini-Faria, F, Bell-Boucher, D, Roy, EA, and Almeida, QJ. Disentangling perceptual judgment and online feedback deficits in Parkinson's freezing of gait. J Neurol. (2015) 262:1629–36. doi: 10.1007/s00415-015-7759-7
31. Plotnik, M, Shema, S, Dorfman, M, Gazit, E, Brozgol, M, Giladi, N, et al. A motor learning-based intervention to ameliorate freezing of gait in subjects with Parkinson's disease. J Neurol. (2014) 261:1329–39. doi: 10.1007/s00415-014-7347-2
32. Zhao, J, Zhang, L, Li, P, Liu, S, Yu, S, Chen, Z, et al. Prediction of freezing of gait in Parkinson's disease using a random Forest model based on an orthogonal experimental design: a pilot study. Front Hum Neurosci. (2021) 15:636414. doi: 10.3389/fnhum.2021.636414
33. Ehgoetz Martens, KA, Shine, JM, Walton, CC, Georgiades, MJ, Gilat, M, Hall, JM, et al. Evidence for subtypes of freezing of gait in Parkinson's disease. Mov Disord. (2018) 33:1174–8. doi: 10.1002/mds.27417
34. Mancini, M, Bloem, BR, Horak, FB, Lewis, SJG, Nieuwboer, A, and Nonnekes, J. Clinical and methodological challenges for assessing freezing of gait: future perspectives. Mov Disord. (2019) 34:783–90. doi: 10.1002/mds.27709
35. Mohammadi, F, Bruijn, SM, Vervoort, G, van Wegen, EE, Kwakkel, G, Verschueren, S, et al. Motor switching and motor adaptation deficits contribute to freezing of gait in Parkinson's disease. Neurorehabil Neural Repair. (2015) 29:132–42. doi: 10.1177/1545968314545175
36. Nieuwboer, A, and Giladi, N. The challenge of evaluating freezing of gait in patients with Parkinson's disease. Br J Neurosurg. (2008) 22:S16–8. doi: 10.1080/02688690802448376
37. Hafer, JF, Vitali, R, Gurchiek, R, Curtze, C, Shull, P, and Cain, SM. Challenges and advances in the use of wearable sensors for lower extremity biomechanics. J Biomech. (2023) 157:111714. doi: 10.1016/j.jbiomech.2023.111714
38. Nonnekes, J, Snijders, AH, Nutt, JG, Deuschl, G, Giladi, N, and Bloem, BR. Freezing of gait: a practical approach to management. Lancet Neurol. (2015) 14:768–78. doi: 10.1016/S1474-4422(15)00041-1
39. Nieuwboer, A, Weerdt, W, Dom, R, and Lesaffre, E. A frequency and correlation analysis of motor deficits in Parkinson patients. Disabil Rehabil. (1998) 20:142–50. doi: 10.3109/09638289809166074
40. Snijders, AH, Haaxma, CA, Hagen, YJ, Munneke, M, and Bloem, BR. Freezer or non-freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord. (2012) 18:149–54. doi: 10.1016/j.parkreldis.2011.09.006
41. Snijders, AH, Nijkrake, MJ, Bakker, M, Munneke, M, Wind, C, and Bloem, BR. Clinimetrics of freezing of gait. Mov Disord. (2008) 23:S468–74. doi: 10.1002/mds.22144
42. Bekkers, EMJ, van Rossom, S, Heremans, E, Dockx, K, Devan, S, Verschueren, SMP, et al. Adaptations to postural perturbations in patients with freezing of gait. Front Neurol. (2018) 9:540. doi: 10.3389/fneur.2018.00540
43. Goetz, CG, Tilley, BC, Shaftman, SR, Stebbins, GT, Fahn, S, Martinez-Martin, P, et al. Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. (2008) 23:2129–70. doi: 10.1002/mds.22340
44. Giladi, N, Tal, J, Azulay, T, Rascol, O, Brooks, DJ, Melamed, E, et al. Validation of the freezing of gait questionnaire in patients with Parkinson's disease. Mov Disord. (2009) 24:655–61. doi: 10.1002/mds.21745
45. Nieuwboer, A, Rochester, L, Herman, T, Vandenberghe, W, Emil, GE, Thomaes, T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait Posture. (2009) 30:459–63. doi: 10.1016/j.gaitpost.2009.07.108
46. Giladi, N, and Nieuwboer, A. Understanding and treating freezing of gait in parkinsonism, proposed working definition, and setting the stage. Mov Disord. (2008) 23:S423–5. doi: 10.1002/mds.21927
47. Hulzinga, F, Nieuwboer, A, Dijkstra, BW, Mancini, M, Strouwen, C, Bloem, BR, et al. The new freezing of gait questionnaire: unsuitable as an outcome in clinical trials? Mov Disord Clin Pract. (2020) 7:199–205. doi: 10.1002/mdc3.12893
48. Lord, SR, Bindels, H, Ketheeswaran, M, Brodie, MA, Lawrence, AD, Close, JCT, et al. Freezing of gait in people with Parkinson's disease: nature, occurrence, and risk factors. J Parkinsons Dis. (2020) 10:631–40. doi: 10.3233/JPD-191813
49. Cowie, D, Limousin, P, Peters, A, Hariz, M, and Day, BL. Doorway-provoked freezing of gait in Parkinson's disease. Mov Disord. (2012) 27:492–9. doi: 10.1002/mds.23990
50. Bluett, B, Bayram, E, and Litvan, I. The virtual reality of Parkinson's disease freezing of gait: a systematic review. Parkinsonism Relat Disord. (2019) 61:26–33. doi: 10.1016/j.parkreldis.2018.11.013
51. Ziegler, K, Schroeteler, F, Ceballos-Baumann, AO, and Fietzek, UM. A new rating instrument to assess festination and freezing gait in parkinsonian patients. Mov Disord. (2010) 25:1012–8. doi: 10.1002/mds.22993
52. O’Day, JJ, Syrkin-Nikolau, J, Anidi, CM, Kidzinski, L, Delp, SL, and Bronte-Stewart, HM. The turning and barrier course: a standardized tool for identifying freezing of gait and demonstrating the efficacy of deep brain stimulation. bioRxiv. (2019):671479. doi: 10.1101/671479
53. Gómez-Jordana, LI, Stafford, J, CLE, P, and Craig, CM. Crossing virtual doors: a new method to study gait impairments and freezing of gait in Parkinson's disease. Parkinson's Disease. (2018) 2018:2957427. doi: 10.1155/2018/2957427
54. Geerse, DJ, Coolen, B, van Hilten, JJ, and Roerdink, M. Holocue: a wearable holographic cueing application for alleviating freezing of gait in Parkinson's disease. Front Neurol. (2022) 12:628388. doi: 10.3389/fneur.2021.628388
55. Shine, JM, Matar, E, Bolitho, SJ, Dilda, V, Morris, TR, Naismith, SL, et al. Modeling freezing of gait in Parkinson's disease with a virtual reality paradigm. Gait Posture. (2013) 38:104–8. doi: 10.1016/j.gaitpost.2012.10.026
56. Janssen, S, Bolte, B, Nonnekes, J, Bittner, M, Bloem, BR, Heida, T, et al. Usability of three-dimensional augmented visual cues delivered by smart glasses on (freezing of) gait in Parkinson's disease. Front Neurol. (2017) 8:279. doi: 10.3389/fneur.2017.00279
57. Janssen, S, Ruyter Steveninck, J, Salim, HS, Bloem, BR, Heida, T, and RJA, W. The beneficial effects of conventional visual cues are retained when augmented reality glasses are worn. Parkinsons Dis. (2020):4104712. doi: 10.1155/2020/4104712
58. Gilat, M, Lígia Silva de Lima, A, Bloem, BR, Shine, JM, Nonnekes, J, and Lewis, SJG. Freezing of gait: promising avenues for future treatment. Parkinsonism Relat Disord. (2018) 52:7–16. doi: 10.1016/j.parkreldis.2018.03.009
59. Spildooren, J, Vercruysse, S, Heremans, E, Galna, B, Vandenbossche, J, Desloovere, K, et al. Head-pelvis coupling is increased during turning in patients with Parkinson's disease and freezing of gait. Mov Disord. (2013) 28:619–25. doi: 10.1002/mds.25285
60. Nanhoe-Mahabier, W, Snijders, AH, Delval, A, Weerdesteyn, V, Duysens, J, Overeem, S, et al. Split-belt locomotion in Parkinson's disease with and without freezing of gait. Neuroscience. (2013) 236:110–6. doi: 10.1016/j.neuroscience.2013.01.038
61. Pieruccini-Faria, F, Jones, JA, and Almeida, QJ. Motor planning in Parkinson's disease patients experiencing freezing of gait: the influence of cognitive load when approaching obstacles. Brain Cogn. (2014) 87:76–85. doi: 10.1016/j.bandc.2014.03.005
62. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. (2021) 18:e1003583. doi: 10.1371/journal.pmed.1003583
64. Goetz, CG, Poewe, W, Rascol, O, Sampaio, C, Stebbins, GT, Counsell, C, et al. Movement Disorder Society task force report on the Hoehn and Yahr staging scale: status and recommendations the Movement Disorder Society task force on rating scales for Parkinson's disease. Mov Disord. (2004) 19:1020–8. doi: 10.1002/mds.20213
65. O'Day, J, Syrkin-Nikolau, J, Anidi, C, Kidzinski, L, Delp, S, and Bronte-Stewart, H. The turning and barrier course reveals gait parameters for detecting freezing of gait and measuring the efficacy of deep brain stimulation. PLoS One. (2020) 15:e0231984. doi: 10.1371/journal.pone.0231984
66. Rodriguez-Martin, D, Samà, A, Pérez-López, C, Català, A, Moreno Arostegui, JM, Cabestany, J, et al. Home detection of freezing of gait using support vector machines through a single waist-worn triaxial accelerometer. PLoS One. (2017) 12:e0171764. doi: 10.1371/journal.pone.0171764
67. Mancini, M, Smulders, K, Cohen, RG, Horak, FB, Giladi, N, and Nutt, JG. The clinical significance of freezing while turning in Parkinson's disease. Neuroscience. (2017) 343:222–8. doi: 10.1016/j.neuroscience.2016.11.045
68. Cowie, D, Limousin, P, Peters, A, and Day, BL. Insights into the neural control of locomotion from walking through doorways in Parkinson's disease. Neuropsychologia. (2010) 48:2750–7. doi: 10.1016/j.neuropsychologia.2010.05.022
69. Heremans, E, Nieuwboer, A, and Vercruysse, S. Freezing of gait in Parkinson's disease: where are we now? Curr Neurol Neurosci Rep. (2013) 13:350. doi: 10.1007/s11910-013-0350-7
70. Yogev, G, Giladi, N, Peretz, C, Springer, S, Simon, ES, and Hausdorff, JM. Dual tasking, gait rhythmicity, and Parkinson's disease: which aspects of gait are attention demanding? Eur J Neurosci. (2005) 22:1248–56. doi: 10.1111/j.1460-9568.2005.04298.x
71. Beck, EN, Martens, KAE, and Almeida, QJ. Freezing of gait in Parkinson's disease: an overload problem? PLoS One. (2015) 10:e0144986. doi: 10.1371/journal.pone.0144986
72. Takakusaki, K . Forebrain control of locomotor behaviors. Brain Res Rev. (2008) 57:192–8. doi: 10.1016/j.brainresrev.2007.06.024
73. Takakusaki, K, Oohinata-Sugimoto, J, Saitoh, K, and Habaguchi, T. Role of basal ganglia-brainstem systems in the control of postural muscle tone and locomotion. Prog Brain Res. (2004) 143:231–7. doi: 10.1016/S0079-6123(03)43023-9
74. Mellone, S, Mancini, M, King, LA, Horak, FB, and Chiari, L. The quality of turning in Parkinson's disease: a compensatory strategy to prevent postural instability? J Neuroeng Rehabil. (2016) 13:39. doi: 10.1186/s12984-016-0147-4
75. Plotnik, M, Giladi, N, Balash, Y, Peretz, C, and Hausdorff, JM. Is freezing of gait in Parkinson's disease related to asymmetric motor function? Ann Neurol. (2005) 57:656–63. doi: 10.1002/ana.20452
76. Wagner, J, Stephan, T, Kalla, R, Brückmann, H, Strupp, M, Brandt, T, et al. Mind the bend: cerebral activations associated with mental imagery of walking along a curved path. Exp Brain Res. (2008) 191:247–55. doi: 10.1007/s00221-008-1520-8
77. O'Shea, S, Morris, ME, and Iansek, R. Dual task interference during gait in people with Parkinson disease: effects of motor versus cognitive secondary tasks. Phys Ther. (2002) 82:888–97. doi: 10.1093/ptj/82.9.888
78. Yogev-Seligmann, G, Rotem-Galili, Y, Mirelman, A, Dickstein, R, Giladi, N, and Hausdorff, JM. How does explicit prioritization Alter walking during dual-task performance? Effects of age and sex on gait speed and variability. Phys Ther. (2010) 90:177–86. doi: 10.2522/ptj.20090043
79. Shafer, RL, Solomon, EM, Newell, KM, Lewis, MH, and Bodfish, JW. Visual feedback during motor performance is associated with increased complexity and adaptability of motor and neural output. Behav Brain Res. (2019) 376:112214. doi: 10.1016/j.bbr.2019.112214
80. Giladi, N, McMahon, D, Przedborski, S, Flaster, E, Guillory, S, Kostic, V, et al. Motor blocks in Parkinson's disease. Neurology. (1992) 42:333. doi: 10.1212/WNL.42.2.333
81. Cerri, S, Mus, L, and Blandini, F. Parkinson's disease in women and men: What's the difference? J Parkinsons Dis. (2019) 9:501–15. doi: 10.3233/JPD-191683
Keywords: Parkinson’s disease, freezing, gait, triggers, tasks
Citation: Conde CI, Lang C, Baumann CR, Easthope CA, Taylor WR and Ravi DK (2023) Triggers for freezing of gait in individuals with Parkinson’s disease: a systematic review. Front. Neurol. 14:1326300. doi: 10.3389/fneur.2023.1326300
Edited by:
Mya C. Schiess, University of Texas Health Science Center at Houston, United StatesReviewed by:
Jacky Ganguly, Institute of Neurosciences, Kolkata (I-NK), IndiaGuillermo De Arcas, Polytechnic University of Madrid, Spain
Copyright © 2023 Conde, Lang, Baumann, Easthope, Taylor and Ravi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deepak K. Ravi, ZGVlcGFrLnJhdmlAaGVzdC5ldGh6LmNo
 Carolina I. Conde1
Carolina I. Conde1 Charlotte Lang
Charlotte Lang Christian R. Baumann
Christian R. Baumann Chris A. Easthope
Chris A. Easthope William R. Taylor
William R. Taylor Deepak K. Ravi
Deepak K. Ravi