
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 05 January 2024
Sec. Headache and Neurogenic Pain
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1323290
This article is part of the Research TopicHeadache and other symptoms in special populationsView all 20 articles
Background: Headache is one of the most common symptoms of acute COVID-19 infection. However, its mechanisms remain poorly understood, and there is a lack of studies investigating changes in the periaqueductal gray (PAG) in COVID-19 patients exhibiting headaches.
Purpose: The study aimed to explore the alterations in metabolites of the PAG pre- and post-COVID-19 infection in individuals who suffered from headaches during the acute phase of the disease using proton magnetic resonance spectroscopy (1H-MRS).
Methods: Fifteen participants who experienced headaches during the acute phase of COVID-19 were recruited. All subjects underwent two proton magnetic resonance spectroscopy (1H-MRS) examinations focusing on the PAG before and after they were infected. Metabolite changes were assessed between the pre- and post-infection groups.
Results: The combined glutamine and glutamate/total creatine ratio (Glx/tCr) was increased in the PAG following COVID-19 infection. The total choline/total creatine ratio (tCho/tCr) in the pre-infection group was negatively correlated with the duration of headache during the COVID-19 acute phase.
Conclusion: The present study indicates that PAG plays a pivotal role in COVID-19 headaches, thereby supporting the involvement of trigeminovascular system activation in the pathophysiology of COVID-19 headaches.
Headache is one of the most prevalent symptoms after infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. It had a high incidence during the acute phase of coronavirus disease 2019 (COVID-19), presenting in up to 50% of patients. Notably, it also emerges as a primary symptom observed in the post-COVID-19, with a prevalence of 10% (1). Interestingly, some patients without a history of previous headache-related disorders experience new-onset headaches following SARS-CoV-2 infection (2). The presence of headache in the acute phase of COVID-19 is associated with a higher prevalence of long-term post-COVID headache (3). In addition, headache is a disabling condition in patients with long COVID-19, leading to a lower quality of life and general fatigue (4, 5). Therefore, it is important to understand the pathophysiology of headache in SARS-CoV-2 infection to facilitate its prevention.
Despite the high frequency of headaches during COVID-19 infection, the exact mechanism by which SARS-CoV-2 can cause headaches remains incompletely understood. Various potential pathophysiological mechanisms of headache in COVID-19 patients have been proposed, such as direct viral invasion of the nervous system, cytokine release syndrome, innate immune response, cerebral bioelectrical dysfunction, and the activation of the trigeminovascular system (6–10). The periaqueductal gray (PAG), a key region of the endogenous analgesia system, exhibits pathological changes in different types of headaches (11–13). Therefore, it is reasonable to posit that the PAG may be involved in the pathophysiology of COVID-19 headaches. However, there is a dearth of research on the PAG in the context of COVID-19 headaches.
Proton magnetic resonance spectroscopy (1H-MRS) is a non-invasive method that allows the in vivo study of tissue metabolism by leveraging the magnetic properties of hydrogen atomic nuclei. It enables the measurement of various metabolites and neurotransmitters, facilitating the investigation of dysregulation in excitatory and inhibitory processes of the brain, altered energy metabolism, changes in neuronal function, variations in cell density, and so on (14, 15). The aim of the present study was to investigate the alterations in metabolites of PAG pre- and post-COVID-19 infection in individuals who suffered from headaches during the acute phase of the disease, utilizing 1H-MRS technology. Furthermore, due to the observed gender disparities in COVID-19 and the greater incidence of headaches in female patients (16–19), the current study selectively enrolled female subjects to mitigate potential sex-related biases and improve the accuracy of the findings.
The participants in this study were recruited from local universities. This study employed a longitudinal prospective design, with participants initially scanned before being infected prior to the pandemic. Subsequently, after the pandemic, they were invited back for a second scan. The inclusion criteria were as follows: (1) patients >18 years old; (2) had a positive molecular swab test for SARS-CoV-2; and (3) experienced headaches during the acute phase of COVID-19. The exclusion criteria were as follows: those who (1) had underlying psychiatric disorders; (2) were taking any drugs affecting the central nervous system; and (3) had MRI contraindications such as claustrophobia. Demographic and clinical characteristics, including age, body mass index (BMI), years of education, past medical history, Self-rating Anxiety Scale (SAS), and Self-rating Depression Scale (SDS) prior to infection were recorded. Post-infection clinical data, including symptoms during infection, the duration of headache during the acute phase of COVID-19, and SAS and SDS scores, were documented.
Subjects were scanned using a 3-Tesla GE Discovery MR750 scanner with a 32-channel head coil. Conventional T2-weighted images, T1-FLAIR, and DWI were obtained to ensure the absence of intracranial lesions. High-resolution T1-weighted scan (3D T1-BRAVO, TR = 8.2 ms, TE = 3.2 ms, flip angle = 12, matrix = 256 × 256, slice thickness = 1.0 mm with no gaps) was acquired for accurate voxel placement. For 1H-MRS, a volume of interest (VOI) was placed in the periaqueductal gray (Figure 1), and single-voxel point-resolved spectroscopy (PRESS) was employed with the following parameters: TR = 2000 ms, TE = 35 ms, voxel size = 15 × 15 × 15 mm3, total number of scans = 64, number of excitations (NEX) = 8, water suppressed, with automatic shimming.
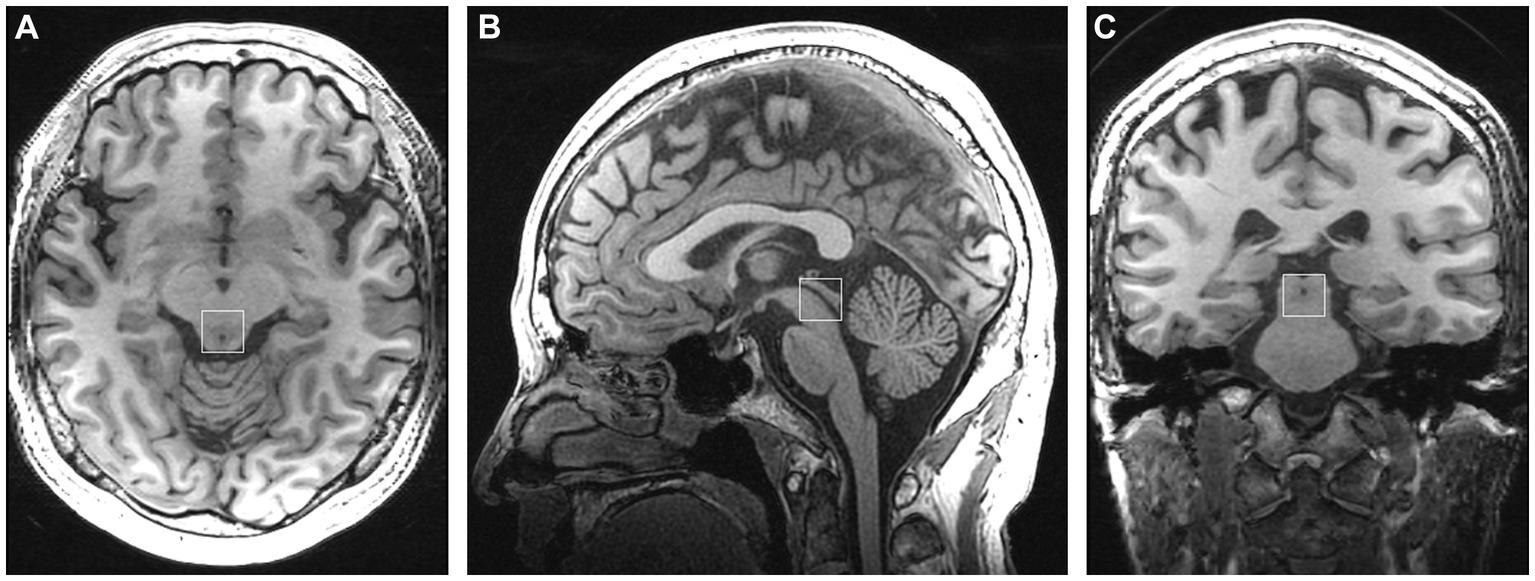
Figure 1. Placement of the single voxel in the axial (A), sagittal (B), and coronal (C) planes in the periaqueductal gray.
The MRS data were processed using the linear combination model (LCModel). Metabolites under investigation were creatine plus phosphocreatine (tCr), N-acetyl-aspartate (NAA), glycerophosphocholine and phosphocholine (total choline, tCho), myo-inositol (MI) and combined glutamine and glutamate (Glx). tCr serves as the reference metabolite and appeared stable in subjects. In this study, the relative concentration of metabolites (expressed as a ratio to tCr) was evaluated. Only metabolites with Cramer–Rao Lower Bounds (CRLB) smaller than 15% SD were considered reliable estimates and were included in further statistical analyses.
Statistical analyses were performed using the Statistical Package for Social Sciences software (SPSS 25.0). To assess the differences in metabolite ratios between post-infection and pre-infection data, as well as explore the relationship between metabolite ratios and clinical data, a paired t-test and Pearson’s correlation test were used for the data conforming to normal distribution. Meanwhile, the rank sum test and Spearman’s correlation test were used for non-normally distributed data. A p-value of less than 0.05 was considered statistically significant.
Initially, sixteen participants with COVID-19-related headaches were recruited for this study. Additionally, five participants who did not experience headaches during the acute phase were also included. One participant with COVID-19 headache and one without headache exhibited unsatisfactory spectral quality, with CRLB exceeding 15%, leading to their exclusion. Consequently, a total of fifteen participants with COVID-19 headaches and four participants without headaches were included in the final analysis. All participants had mild COVID-19 and did not require hospitalization. Demographic and clinical characteristics of the participants are summarized in Table 1. Four participants had a history of migraine before contracting COVID-19; however, they reported no migraine attacks during the infection period and had not received any prophylactic or medications before the scan, as detailed in Supplementary Table S2. There was no statistical difference in SAS and SDS scores among participants before and after infection (p > 0.05). The types and prevalence of COVID-19 symptoms are presented in Table 2.
After infection, a significant elevation in Glx/tCr was observed in the PAG (p = 0.039) (Table 2 and Figure 2). However, this result was not observed in participants who did not experience headaches (Supplementary Table S1). There were no statistical differences in NAA/tCr, tCho/tCr, and MI/tCr between the two groups (all p > 0.05). The tCho/tCr in the pre-infection group was negatively correlated with the duration of headache during the acute phase of COVID-19 (r = −0.617, p = 0.014) (Figure 3).
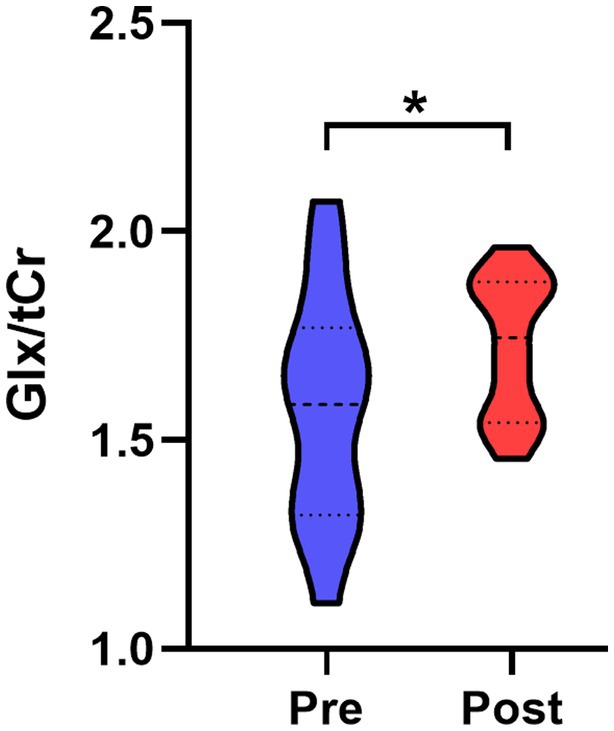
Figure 2. Comparison of PAG Glx/tCr ratio between post- and pre-infection groups. Glx/tCr ratio was significantly increased in the post-infection group relative to the pre-infection group. PAG, periaqueductal gray; Glx, combined glutamine and glutamate; tCr, creatine plus phosphocreatine; Pre, pre-infection group; Post, post-infection group; *, p < 0.05.
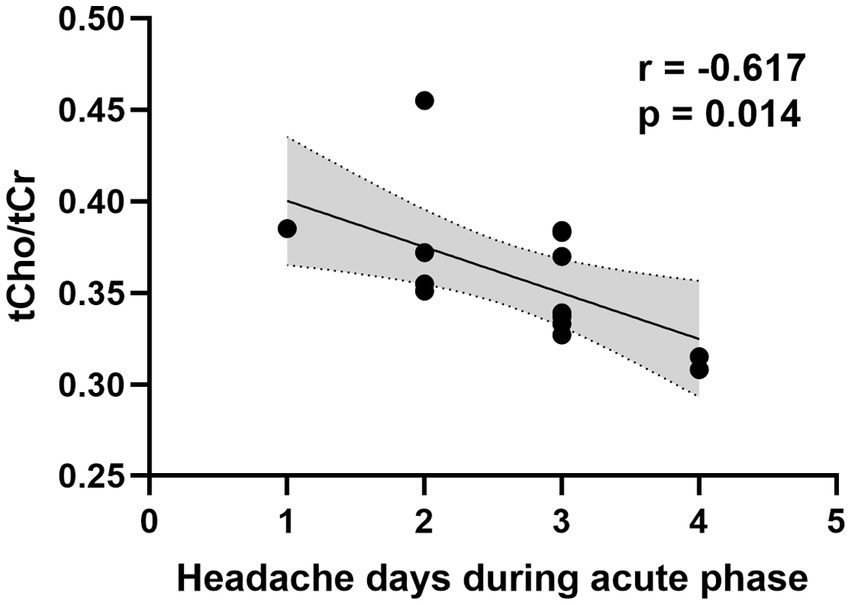
Figure 3. Correlation between PAG tCho/tCr ratio in the pre-infection group and duration of headache during the acute phase of COVID-19. PAG, periaqueductal gray; tCho, total choline; tCr, creatine plus phosphocreatine.
To the best of our knowledge, this study is the first to focus on PAG changes in patients who experienced COVID-19 headaches. The results revealed an elevated Glx/tCr ratio in the PAG following infection. Additionally, the tCho/tCr ratio in the pre-infection group was negatively correlated with the duration of headache during the acute phase of COVID-19.
PAG is an essential region of the endogenous analgesia system. Both take part in ascending and descending projections and modulate the ‘ON’ (facilitating the activity of neurons responding to noxious stimuli) and ‘OFF’ (ceasing firing immediately, inhibiting nociceptive responses) cells in the rostral ventromedial medulla to control nociceptive processing (20–22). Stimulation of the PAG can induce analgesic effects, while lesions in the PAG have been associated with causing headaches (13, 23). Glutamate and glutamine are typically quantified together as the glutamate/glutamine complex (Glx) due to their spectral overlap, making it challenging to separate their individual signals. Glutamate, the most abundant excitatory neurotransmitter in the brain, and glutamine, a precursor of glutamate, play a crucial role in signal transmission to and from the PAG (15). A glutamatergic pathway connecting the PAG with the rostral ventromedial medulla, specifically targeting OFF-cells, may exist (24). Activation of glutamatergic neurons in the PAG has been shown to suppress nociception, while inhibition of these neurons facilitates nociception (25). Therefore, the elevated Glx/tCr observed in the PAG after infection in our study may suggest an increased analgesic effect. Notably, no significant alteration in Glx/tCr was observed between pre- and post-infection in individuals who did not experience headaches. This finding may indicate a self-protective response against headaches. Moreover, PAG is involved in the descending control of trigeminovascular nociceptive traffic, with trigeminally evoked craniovascular pain being inhibited by PAG stimulation. Conversely, PAG activation has been observed following trigeminally evoked craniovascular pain (21, 22, 26, 27). As such, these results support the hypothesis that the pathophysiological mechanisms underlying headaches in COVID-19 patients may be linked to trigeminovascular activation.
The present study also unveiled a negative correlation between the tCho/tCr ratio in the pre-infection group and the duration of headaches during the acute phase of COVID-19. The tCho signal detected by MRS in the brain primarily originates from phosphocholine and glycerophosphocholine. Consequently, changes in the tCho signal reflect altered membrane turnover (increased membrane synthesis or breakdown) or changes in cell density, suggesting potential alterations in neuronal integrity and function (15). Therefore, the correlation results in this study suggest that the function of PAG before infection is related to the duration of headaches during the acute phase of COVID-19. Importantly, dysfunction in the PAG may contribute to prolonged headache duration. This discovery holds the potential to inform innovative clinical strategies for managing COVID-19-related headaches. Enhancing PAG function may reduce the likelihood and duration of headaches, thereby lowering the incidence of long COVID-19 headaches and improving patients’ overall quality of life.
The current study has certain limitations that warrant consideration. First, despite its longitudinal design, the sample size is relatively small, emphasizing the need for further validation with a larger cohort to enhance the generalizability of the results. Second, this study specifically focuses on patients who experienced headaches during the acute phase of COVID-19. Future research could extend its focus to individuals with long COVID-19 headaches to provide a more comprehensive understanding of the role of the PAG in this context. Third, it is important to note that four participants had a history of migraine before contracting COVID-19. While the longitudinal study design may mitigate the impact of migraine on the results to some extent, the potential influence of migraine on the outcomes cannot be completely disregarded.
To the best of our knowledge, this study represents the first exploration of PAG changes in patients with COVID-19 headaches. The findings underscore the significant role of the PAG in the pathophysiology of COVID-19 headaches. Notably, PAG activation post-infection appears to enhance the analgesic effect, and its pre-infection function correlates with the duration of headaches during the acute phase of COVID-19. Moreover, these results provide support for the hypothesis that trigeminovascular system activation contributes to the pathophysiology of COVID-19 headaches. These insights may pave the way for innovative approaches to the clinical management of COVID-19 headaches.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ethics Committee of Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
PJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Validation, Visualization, Writing – original draft. FC: Data curation, Formal analysis, Methodology, Software, Writing – original draft. LZ: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Zhejiang Province Traditional Chinese Medicine Science and Technology Plan [grant number 2022ZA103]; Hangzhou Agricultural and Social Development Research Project [grant number 202204B07]; Bio-medicine and health industry development support science and technology project of Hangzhou [grant number 2021WJCY197].
The authors would like to thank the volunteers for their involvement in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1323290/full#supplementary-material
coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; 1H-MRS: proton magnetic resonance spectroscopy; PAG: periaqueductal gray; tCr: creatine plus phosphocreatine; Glx: combined glutamine and glutamate; tCho: glycerophosphocholine and phosphocholine; NAA: N-acetyl-aspartate; MI: myo-inositol.
1. Fernandez-de-Las-Penas, C, Navarro-Santana, M, Gomez-Mayordomo, V, Cuadrado, ML, Garcia-Azorin, D, Arendt-Nielsen, L, et al. Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: a meta-analysis of the current literature. Eur J Neurol. (2021) 28:3820–5. doi: 10.1111/ene.15040
2. Torrente, A, Alonge, P, Di Stefano, V, Baschi, R, Ornello, R, Correnti, E, et al. New-onset headache following COVID-19: an Italian multicentre case series. J Neurol Sci. (2023) 446:120591. doi: 10.1016/j.jns.2023.120591
3. Fernandez-de-Las-Penas, C, Gomez-Mayordomo, V, Cuadrado, ML, Palacios-Cena, D, Florencio, LL, Guerrero, AL, et al. The presence of headache at onset in SARS-CoV-2 infection is associated with long-term post-COVID headache and fatigue: a case-control study. Cephalalgia. (2021) 41:1332–41. doi: 10.1177/03331024211020404
4. Fujita, K, Otsuka, Y, Sunada, N, Honda, H, Tokumasu, K, Nakano, Y, et al. Manifestation of headache affecting quality of life in long COVID patients. J Clin Med. (2023) 12:3533. doi: 10.3390/jcm12103533
5. Rodrigues, AN, Dias, ARN, Paranhos, ACM, Silva, CC, Bastos, TDR, de Brito, BB, et al. Headache in long COVID as disabling condition: a clinical approach. Front Neurol. (2023) 14:1149294. doi: 10.3389/fneur.2023.1149294
6. Bobker, SM, and Robbins, MS. COVID-19 and headache: a primer for trainees. Headache. (2020) 60:1806–11. doi: 10.1111/head.13884
7. Bolay, H, Gul, A, and Baykan, B. COVID-19 is a Real headache! Headache. (2020) 60:1415–21. doi: 10.1111/head.13856
8. Garate, G, Toriello, M, Gonzalez-Quintanilla, V, Perez-Pereda, S, Madera, J, Pascual, M, et al. Serum alpha-CGRP levels are increased in COVID-19 patients with headache indicating an activation of the trigeminal system. BMC Neurol. (2023) 23:109. doi: 10.1186/s12883-023-03156-z
9. Straburzynski, M, Kuca-Warnawin, E, and Waliszewska-Prosol, M. COVID-19-related headache and innate immune response – a narrative review. Neurol Neurochir Pol. (2023) 57:43–52. doi: 10.5603/PJNNS.a2022.0049
10. Waliszewska-Prosol, M, and Budrewicz, S. The unusual course of a migraine attack during COVID-19 infection – case studies of three patients. J Infect Public Health. (2021) 14:903–5. doi: 10.1016/j.jiph.2021.04.013
11. Welch, KM, Nagesh, V, Aurora, SK, and Gelman, N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. (2001) 41:629–37. doi: 10.1046/j.1526-4610.2001.041007629.x
12. Chen, Z, Chen, X, Liu, M, Liu, S, Ma, L, and Yu, S. Volume gain of periaqueductal gray in medication-overuse headache. J Headache Pain. (2017) 18:12. doi: 10.1186/s10194-016-0715-9
13. Fiore, A, Tsantes, E, Curti, E, Bazzurri, V, and Granella, F. Secondary cluster headache due to a contralateral demyelinating periaqueductal gray matter lesion. Headache. (2021) 61:1136–9. doi: 10.1111/head.14180
14. Younis, S, Hougaard, A, Vestergaard, MB, Larsson, HBW, and Ashina, M. Migraine and magnetic resonance spectroscopy: a systematic review. Curr Opin Neurol. (2017) 30:246–62. doi: 10.1097/WCO.0000000000000436
15. Rae, CD . A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochem Res. (2014) 39:1–36. doi: 10.1007/s11064-013-1199-5
16. Grisold, W, Moro, E, Teresa Ferretti, M, Hege Aamodt, A, Arabia, G, Lebedeva, ER, et al. Gender issues during the times of COVID-19 pandemic. Eur J Neurol. (2021) 28:e73–7. doi: 10.1111/ene.14815
17. Garcia-Azorin, D, Layos-Romero, A, Porta-Etessam, J, Membrilla, JA, Caronna, E, Gonzalez-Martinez, A, et al. Post-COVID-19 persistent headache: a multicentric 9-months follow-up study of 905 patients. Cephalalgia. (2022) 42:804–9. doi: 10.1177/03331024211068074
18. Vasilevskaya, A, Mushtaque, A, Tsang, MY, Alwazan, B, Herridge, M, Cheung, AM, et al. Sex and age affect acute and persisting COVID-19 illness. Sci Rep. (2023) 13:6029. doi: 10.1038/s41598-023-33150-x
19. Bechmann, N, Barthel, A, Schedl, A, Herzig, S, Varga, Z, Gebhard, C, et al. Sexual dimorphism in COVID-19: potential clinical and public health implications. Lancet Diabetes Endocrinol. (2022) 10:221–30. doi: 10.1016/S2213-8587(21)00346-6
20. Benarroch, EE . Descending monoaminergic pain modulation: bidirectional control and clinical relevance. Neurology. (2008) 71:217–21. doi: 10.1212/01.wnl.0000318225.51122.63
21. Akerman, S, Romero-Reyes, M, and Holland, PR. Current and novel insights into the neurophysiology of migraine and its implications for therapeutics. Pharmacol Ther. (2017) 172:151–70. doi: 10.1016/j.pharmthera.2016.12.005
22. Akerman, S, Holland, PR, and Goadsby, PJ. Diencephalic and brainstem mechanisms in migraine. Nat Rev Neurosci. (2011) 12:570–84. doi: 10.1038/nrn3057
23. Zhang, T, Wang, Z, Liang, H, Wu, Z, Li, J, Ou-Yang, J, et al. Transcranial focused ultrasound stimulation of periaqueductal gray for analgesia. IEEE Trans Biomed Eng. (2022) 69:3155–62. doi: 10.1109/TBME.2022.3162073
24. Taylor, NE, Pei, J, Zhang, J, Vlasov, KY, Davis, T, Taylor, E, et al. The role of glutamatergic and dopaminergic neurons in the periaqueductal gray/dorsal raphe: separating analgesia and anxiety. eNeuro. (2019) 6:ENEURO.0018–18.2019. doi: 10.1523/ENEURO.0018-18.2019
25. Samineni, VK, Grajales-Reyes, JG, Copits, BA, O'Brien, DE, Trigg, SL, Gomez, AM, et al. Divergent modulation of nociception by glutamatergic and GABAergic neuronal subpopulations in the periaqueductal gray. eNeuro. (2017) 4:ENEURO.0129–16.2017. doi: 10.1523/ENEURO.0129-16.2017
26. Hoskin, KL, Bulmer, DC, Lasalandra, M, Jonkman, A, and Goadsby, PJ. Fos expression in the midbrain periaqueductal grey after trigeminovascular stimulation. J Anat. (2001) 198:29–35. doi: 10.1046/j.1469-7580.2001.19810029.x
Keywords: COVID-19, headache, periaqueductal gray, magnetic resonance spectroscopy, metabolites
Citation: Jin P, Cui F and Zhang L (2024) Altered metabolites in the periaqueductal gray of COVID-19 patients experiencing headaches: a longitudinal MRS study. Front. Neurol. 14:1323290. doi: 10.3389/fneur.2023.1323290
Received: 17 October 2023; Accepted: 15 December 2023;
Published: 05 January 2024.
Edited by:
Deena E. Kuruvilla, Yale University, United StatesReviewed by:
Marta Waliszewska-Prosół, Wroclaw Medical University, PolandCopyright © 2024 Jin, Cui and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luping Zhang, emxwMTIyOEBmb3htYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.