- 1Department of Orthopedics, The 960th Hospital of the PLA Joint Logistics Support Force, Jinan, China
- 2First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, China
- 3Department of Radiotherapy, The 960th Hospital of the PLA Joint Logistics Support Force, Jinan, China
Giant cell tumors of the spine have a high recurrence rate owing to their special anatomical site; hence, further treatment after recurrence is very challenging. Achieving effective tumor control and improving the long-term quality of life of the patients are the main treatment purposes to consider for recurrent giant cell tumors of the spine. A patient showing giant cell tumor recurrence of the thoracic spine after curettage received denosumab combined with precision radiotherapy, through which the tumor gained good control and the patient could regain normal functioning. A review of the relevant literature suggested that denosumab combined with radiotherapy is an effective new approach for the treatment of recurrent giant cell tumors of the spine.
1 Introduction
Giant cell tumor (GCT) of the bone is an osteolytic, aggressive primary bone tumor that can manifest in the epiphysis of the limbs, sacrum, spine, and other places, mostly in adults aged 20–45 years (1). GCT comprises mononuclear stromal cells and characteristic multinucleated giant cells exhibiting osteoclastic activity that can modify the appearance of normal bone swelling and the destruction of the bone cortex (2). Although the incidence of spinal GCT is low, accounting for only 3% of all GCT cases (3), the tumor tissues surrounding the spinal cord and nerve roots are not easily accessible owing to their physiological and anatomical structure. It significantly increases the difficulty of extensive resection because it requires the resection of the margin or the inner edge of the lesion, which further contributes to its higher recurrence rate. Long-term follow-up has indicated that surgical treatment alone is associated with a local recurrence of the tumor in 15–50% of the patients (4). For recurrent spinal GCT after surgery, reoperation can result in extensive surgical trauma and functional damage, which is an important factor to consider when selecting the treatment modality. We have hereby presented the report of a patient with recurrent thoracic GCT in our hospital, in whom denosumab combined with precision radiotherapy achieved effective tumor control. Accordingly, based on our successful experience, we have proposed a new treatment concept, reviewed the recent relevant literature on recurrent spinal GCT treatment, and summarized the latest treatment strategies to provide a reference for adaptation in clinical practice.
2 Case report
A 30-year-old woman without a family inherited disease presented to our hospital on 14 April 2020 for the “numbness of both lower extremities since 11 days after a fall.” Her physical examination revealed no pressing pain in the chest or upper back. She experienced hypoesthesia below the navel, on bilateral thighs, the calf front, and the back of the foot skin, especially on the left side. The muscle strength and muscle tension of both lower limbs were found to be normal. The remaining physical examination revealed no evident abnormalities. Thoracic spine X-ray and computed tomography (CT) demonstrated abnormal bone destruction in the T9 vertebral body. Thoracic magnetic resonance imaging (MRI) displayed abnormal signal changes in the T9 vertebral body and space-occupying lesions in the spinal canal. CT-guided downward T9 vertebral tumor puncture biopsy and consideration of the puncture pathology indicated a GCT of the bone (Figures 1A–D). The parents are in good health and have no underlying diseases. After excluding surgical contraindications on 17 April 2020 and after internal fixation of the posterior T9 vertebral tumor microwave with an inactivated curettage graft, the postoperative pathology was the same as puncture pathology (the tumor of the T9 vertebral body was scraped after the microwave, and the titanium cage filled with autogenous bone was supported in the scraped vertebral body). T7, T8, T10, T11 bilateral screws were fixed. After the operation, the patient reported no numbness in either lower limb, a well-healed incision, and no other discomfort. Accordingly, the patient was discharged after removing the stitches (Figures 2A–C). To prevent tumor recurrence, zoledronic acid (4 mg) was administered once a month after the surgery. She was admitted for the eighth sequential postoperative zoledronic acid treatment on 26 January 2021, and no evident abnormalities were detected during her physical examination. A review of the thoracic spine MRI revealed that the spinal cord compression had an irregular signal in the spinal canal. The patient’s past medical history and the postoperative recurrence of GCT were considered. After three treatments with denosumab (120 mg), the thoracic spine MRI was reviewed again. The tumor boundary in the vertebral body was clear, with no invasion of the spinal cord. After consultation in the radiotherapy department, stereotactic radiotherapy was performed for recurrent lesions (cyber knife) with 600 cGy/fraction*6 fractions, 1 fraction/day (Figures 3A–G). Tumor control was achieved after the last radiotherapy, with no evident abnormalities detected. At the latest follow-up in October 2023, no tumor progression was found (Figures 4A–E). The patient has returned to normal life without significant complaints.
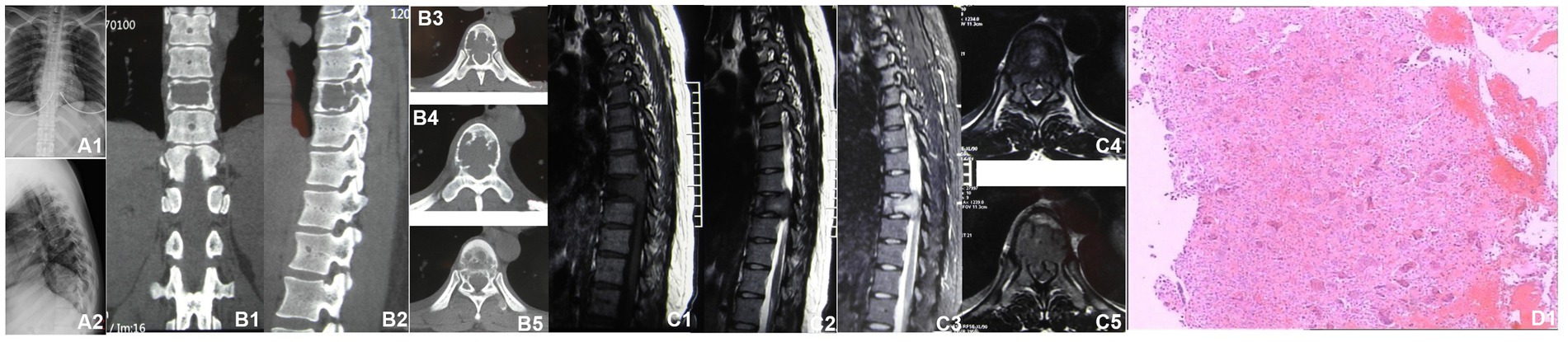
Figure 1. Imaging data at the initial clinic visit (2020-04). (A1,A2) Thoracic vertebral X-ray: The local density of the T9 vertebrae was decreased. (B1–B5) Thoracic vertebral CT: T9 vertebral bone destruction, cortical destruction at the upper, lower, and posterior margins, bilateral bone destruction of the pedicle, an irregular soft tissue density shadow visible in the vertebral body, and the spinal canal were occupied, with a corresponding spinal canal sagittal diameter narrowing. (C1–C5) Thoracic vertebra MRI: T9 vertebral body flattening, and long T1 and long T2 signals appearing within the vertebral body. The fat suppression phase revealed a high signal intensity, bilateral pedicle involvement, bone destruction at the posterior edge of the vertebral body, soft tissue space, and dural compression. (D1) Puncture pathology revealed massive osteoclasts, considering the giant cell tumor of the bone.
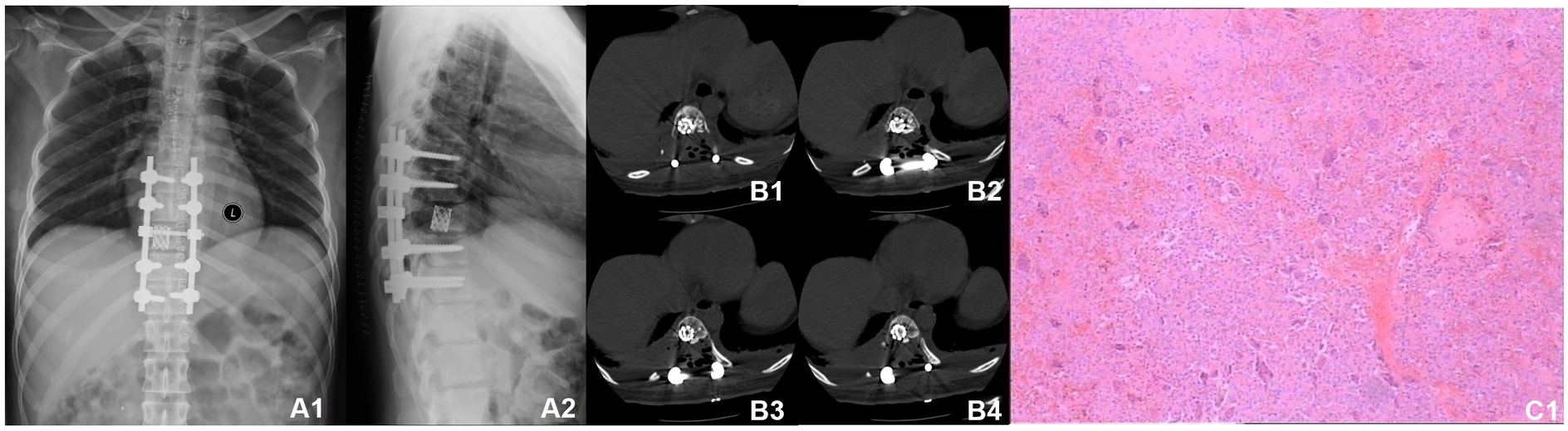
Figure 2. Postoperative radiographic data (2020-04). (A1,A2) Thoracic vertebral X-ray revealed a satisfactory internal fixation position; the placement was visible in the T9 vertebra. (B1–B4) Thoracic vertebral CT showing T9 vertebrae filled with a high-density shadow. (C1) Postoperative pathology revealed a T9 vertebral giant cell tumor of the bone.
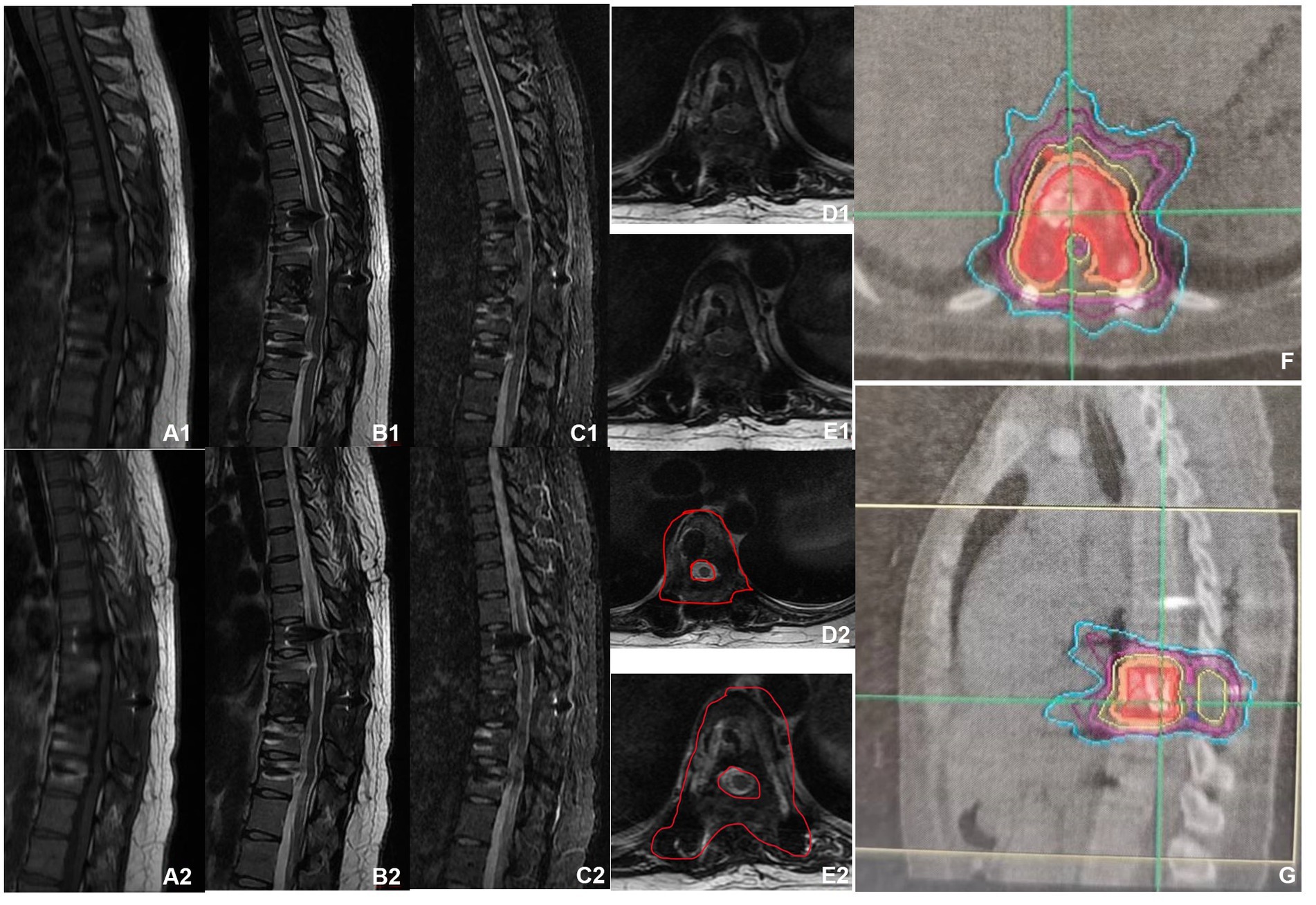
Figure 3. MRI before and after denosumab administration. (A1–E1) MRI before denosumab administration showed uneven bone signals in the T9 vertebrae, with patchy abnormal signals at the posterior margin of the vertebral body. Burst into the spinal canal, and spinal cord compression can be seen. (A2–E2) MRI of the thoracic spine after three doses of denosumab administration (2021-02). The space in the spinal canal is significantly smaller than that at the front, and the boundary with the spinal cord is clear. (F,G) Preoperative planning for cyber knife radiation therapy.
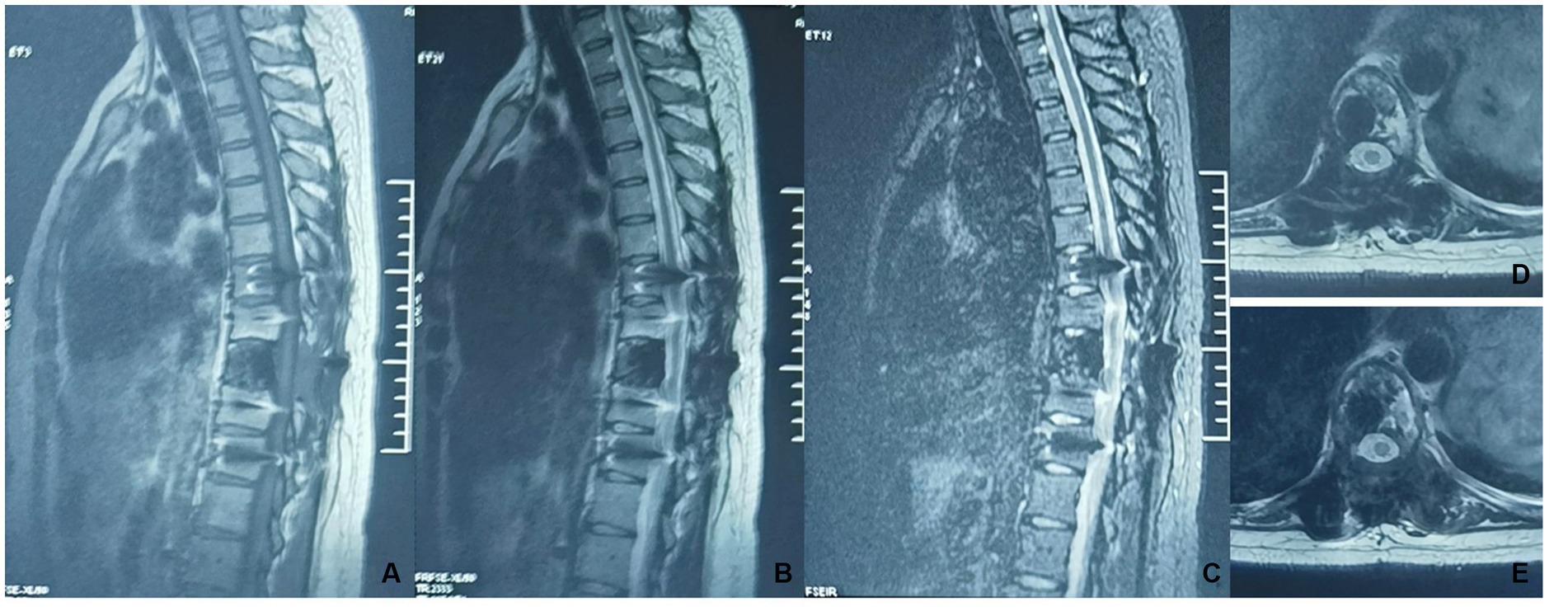
Figure 4. A thoracic MRI was reviewed after radiotherapy (2023-10). (A–E) Non-uniform hypointensity was observed in the T9 vertebrae, with no significant abnormalities in the intraspinal spinal cord.
3 Literature review
3.1 Criteria for literature selection
Inclusion criteria: (1) GCT of the bone was diagnosed; (2) GCT development in the spine and recurrence after initial treatment; (3) systematic diagnosis after recurrence and treatment was performed, and the prognosis was clearly observed; (4) retrospective analyses or individual case reports.
Exclusion criteria: (1) case reports without a systematic diagnosis or treatment process; (2) reports of malignant tumor changes before recurrence; (3) literature review and meta-analysis; (4) literature of repeatedly reported cases.
3.2 Literature search strategy
The search terms “giant cell tumor of bone,” “spine or spinal,” and “recurrent or recurrences” were used for literature published in PubMed and the Web of Science from 2010 to 2022 to search for relevant studies.
3.3 Literature search results
After screening by applying the inclusion and exclusion criteria, a total of 9 articles were shortlisted (Table 1) (5–13), which included 7 case reports and 2 case series reports, totaling 21 patients (7 male patients, 14 female patients; age: 11–64 years); presenting with cervical vertebrae (n = 2); thoracic vertebrae (n = 10); lumbar vertebrae (n = 7); sacral vertebrae (n = 2); treatment modalities included sodium ibandronate, radiotherapy, surgical resection, chemotherapy, interferon administration, and denosumab administration.
4 Discussion
Spinal GCT can involve the adjacent vertebrae through adjacent joints (14). When the tumor expands into the spinal canal, the spinal cord and the associated nerve roots and blood vessels often get compressed, resulting in a free degree of lower back pain or even paraplegia (15). Generally, curettage or partial or total vertebral resection is selected for spinal GCT based on the Enneking staging of the tumor (16). Previous studies have reported postoperative local recurrence rates of 20–50% with spinal GCT, with the maximum rate reaching 70% due to the difficulties encountered in complete resection (17, 18). The recurrence rate of local tumors is closely correlated with the site of the tumor and the degree of primary surgical intervention (6). Curettage may cause minor tissue damage but a relatively high local recurrence rate. Resection poses a lower risk of local recurrence but can result in relatively severe tissue damage and serious complications (19, 20). It is important to consider that the primary therapeutic goal of GCT is to provide long-term symptom relief, especially from pain, as well as tumor control to maintain the long-term good functional status of the patient (21). Therefore, seeking an approach with a low recurrence rate and good functional retention is an important choice in surgery.
Cervical spinal tumors, curettage, intralesional curettage, and non-intact tumors are the risk factors associated with local recurrence (22). The use of adjuvant therapy during and after surgery can reduce the risk of recurrence of GCTs from 45–65% to 12–18% (23). For example, the application of high-speed burring facilitates the selection of tumor curettage and ensures the adequacy and quality of curettage (24). According to the literature, tumor inactivation was performed using frozen, phenol, alcohol, and phenol–alcohol combinations. The scraped cavity was filled with poly(methyl methacrylate) (PMMA) and acrylic cement, and the heat released by the polymerization was applied to induce tissue necrosis, and the resultant cytotoxicity was used to create hypoxia in the cells. Long-term postoperative use of bisphosphonates (25–28) is believed to significantly reduce the local recurrence rate while preserving the neurological functions of the patient (29). Yu et al. (30) and Zheng et al. (31) also indicated that the use of cementation after curettage shows promise in limiting early postoperative complications, lower recurrence, and easier usage in general.
Regular re-examination is critical to detecting tumor recurrence over time. Asymptomatic recurrent spinal GCT is uncommon. The lower back pain and neurological dysfunction of recurrent spinal GCTs are mostly caused by advanced lesions with intraspinal tumor spread. A recent study (32) showed that in spinal tumors, the most common cause of revision was tumor progression (66.7%). More aggressive surgeries (en bloc or gross total) are considered the best option for the treatment of a recurrent primary tumor (33). The feasibility and applicability of reoperation for recurrent spinal GCT are extremely limited; it is inoperable owing to the location of the tumor, and secondary surgery can result in unacceptable functional defects. Even an apparently appropriate en bloc resection can be unsuccessful (34). As the literature points out (1, 5, 6, 12, 13), direct reoperation alone is the only way to remove recurrent tumors. En bloc resection requires sacrificing not only the affected bone but also almost all connecting elements, creating full instability (35). A contemporary series of GCTs in the spine reported a perioperative death after neurologic decline postoperatively, which highlights the risks involved with these surgeries (36). Therefore, non-surgical treatment or combination therapy may be considered a better alternative.
Denosumab has been formally applied in the treatment of patients with unresectable GCT of the bone, indicating promising efficacy and biological integrity. It controls the progression of GCT by inhibiting osteoclast-mediated bone destruction and reducing the tumor blood supply (37). Denosumab is a fully human monoclonal antibody to the receptor activator of the nuclear factor kappa B ligand (RANKL). Presently, preoperative denosumab is not recommended as it can result in local bone sclerosis, unclear tumor boundaries, and insufficient curettage of tumors, thereby contributing to a high tumor recurrence rate. However, it has achieved important efficacy for recurrent or inoperable GCT (38–40), which can significantly reduce the tumor size and protect the integrity of the adjacent bone tissues. Boriani et al. (41) also demonstrated that denosumab can be considered an excellent solution in spine GCTs whose surgical treatment cannot be Enneking appropriate or is associated with unacceptable morbidity or loss of function. There is evidence that the discontinuation of the treatment can be associated with tumor progression. Because it is still unclear at what minimum effective dose and time interval this drug can be safely injected, it is still impossible to state when to safely stop the treatment (42, 43). As Luo et al. (8) said, an 11-year-old patient achieved tumor control but was unable to stop denosumab. Therefore, denosumab is a more beneficial and rational application that deserves further consideration by our clinicians.
GCTs are highly sensitive to radiotherapy, and local radiotherapy has demonstrated good outcomes in long-term local tumor control and the incidence of adverse events (44). Previous studies have reported serious complications from reoperation, such as resident tumors from surgical margin incision or recurrence; hence, radiotherapy should be considered, which has been associated with controllable postoperative complications (45). In addition, the response rate of radiotherapy is 100%, with an overall survival rate of 98% and an overall local control rate of 79% (44). A recurrent tumor is an indication for radiotherapy (46). According to past studies, radiotherapy at a dose of 40–45 GY is highly effective, although better outcomes have been achieved with a total dose of GCT >45 GY while considering the special anatomy of the spinal cord. Considering the specific anatomical structure of the spinal cord, no local control rate was found to improve despite increasing the total radiation dose (47, 48). However, the local benefits of radiotherapy are debatable, and the risk of secondary malignancy cannot be excluded (49, 50). Nevertheless, with the advancements in radiotherapy technology, such as the development of 3-dimensional conformal radiotherapy and intensity-modulated radiotherapy, an adequate radiation dose can be produced with lower radiation toxicity, and the key anatomical structures and important tissues can be safeguarded. When the tumor cannot be completely excised or subjected to curettage in patients presenting with multiple recurrences, radiation therapy can be considered to achieve effective control of the tumor. However, when recurrent tumors have invaded the neurospinal cord, the use of radiotherapy is limited. As reported in the literature (6), even if tumor control is achieved, paralysis of the patient cannot be avoided.
Selective arterial embolization (SAE) is also an effective approach to reducing or ossifying the tumor, which can alleviate pain, stabilize lesions, and improve survival in the presence of adequate blood supply to spinal GCT (51). N-butyl 2-cyanoacrylate (NBCA), as a new embolic agent for preoperative endovascular embolization and vascular embolization of recurrent cervical GCT, can not only significantly reduce postoperative bleeding but also reduce the chance of recurrence (52). Literature reports the application of doxycycline sclerotherapy in the treatment of axial skeleton cases of postoperative recurrence and the inability to undergo surgery (53). Interferon alfa-2b (IFNɑ-2a) achieves good tumor control via its anti-tumor and angiogenic effects (11). There is a lack of reports with a high level of evidence.
In the present case, after curettage of thoracic GCT, the continuous application of bisphosphonates continued to reduce the chance of recurrence. After 9 months of the operation, the tumor recurred. Although the patient did not complain of any obvious discomfort and showed no positive signs after physical examination, the imaging indicated the invasion of the recurrent tumor into the spinal canal and the adjacent running nerve. This event highlights the need to conduct a timely intervention to avoid further tumor progression and serious complications. Although the efficacy of radiotherapy and denosumab for recurrent GCT has been fully verified, the recurrent tumor in this patient has invaded the spinal canal and nerve. Thus, it is evident that the blind use of radiotherapy can damage the important tissues surrounding the tumor. Therefore, after multidisciplinary consultation and discussion, denosumab was used first in the present case, which significantly narrowed the tumor and showed clear boundaries with the surrounding dural and nerves. Accordingly, local radiotherapy was performed six times to achieve good tumor control. There has no tumor progression in the 33 months follow-up.
5 Conclusion
The successful application of denosumab combined with radiotherapy implies that this new treatment modality can be applied to relapsed spinal GCT in order to achieve maximum control of tumors with minimal damage. Therefore, a combination of multiple methods is deemed optimal to achieve better outcomes. We believe that the proposed therapeutic approach can serve as a reference for future development and application. For recurrent GCT in spine, radiotherapy may be useful in order to avoid denosumab dependence.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the 960th Hospital of the PLA Joint Logistics Support Force. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZM: Data curation, Investigation, Resources, Writing – original draft. MX: Investigation, Resources, Supervision, Writing – review & editing. KZ: Investigation, Resources, Supervision, Writing – review & editing. HG: Investigation, Resources, Writing – review & editing. NY: Investigation, Resources, Writing – review & editing. QC: Investigation, Resources, Writing – review & editing. XY: Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Basu Mallick, A, and Chawla, SP. Giant cell tumor of bone: an update. Curr Oncol Rep. (2021) 23:51. doi: 10.1007/s11912-021-01047-5
2. Chakarun, CJ, Forrester, DM, Gottsegen, CJ, Patel, DB, White, EA, and Matcuk, GR Jr. Giant cell tumor of bone: review, mimics, and new developments in treatment. Radiographics. (2013) 33:197–211. doi: 10.1148/rg.331125089
3. Mendenhall, WM, Zlotecki, RA, Scarborough, MT, Gibbs, CP, and Mendenhall, NP. Giant cell tumor of bone. Am J Clin Oncol. (2006) 29:96–9. doi: 10.1097/01.coc.0000195089.11620.b7
4. Chawla, S, Blay, JY, Rutkowski, P, le Cesne, A, Reichardt, P, Gelderblom, H, et al. Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol. (2019) 20:1719–29. doi: 10.1016/S1470-2045(19)30663-1
5. Zhang, W, Zhang, Y, Li, P, Rhodesm, SD, Wang, Y, Xue, X, et al. Administration of sodium ibandronate in the treatment of complicated giant cell tumor of the spine. Spine (Phila Pa 1976). (2011) 36:E1166–72. doi: 10.1097/BRS.0b013e3182127f91
6. Meyer, A, Bastian, L, and Bruns, F. Benign giant cell tumor of the spine: an unusual indication for radiotherapy. Arch Orthop Trauma Surg. (2006) 126:517–21. doi: 10.1007/s00402-006-0174-x
7. Agarwal, A, Larsen, BT, Buadu, LD, Dunn, J, Crawford, R, Daniel, J, et al. Denosumab chemotherapy for recurrent giant-cell tumor of bone: a case report of neoadjuvant use enabling complete surgical resection. Case Rep Oncol Med. (2013) 2013:496351. doi: 10.1155/2013/496351
8. Luo, Y, Xiu, P, Chen, H, Zeng, J, Song, Y, and Li, T. Denosumab salvage therapy in an 11-year-old boy with locally recurrent un-resectable giant cell tumor of the lumbar spine after surgery. Neurochirurgie. (2023) 69:101427. doi: 10.1016/j.neuchi.2023.101427
9. Duan, PG, Sheng, YH, Deng, CH, Tang, BY, and Yao, HQ. Recurrent giant cell tumour of the thoracic spine managed by total en bloc spondylectomy and denosumab therapy: a case report. BMC Musculoskelet Disord. (2020) 21:105. doi: 10.1186/s12891-020-3129-4
10. Shirzadi, A, Drazin, D, Bannykh, S, and Danielpour, M. Giant cell tumor of the odontoid in an adolescent male: radiation, chem-otherapy, and resection for recurrence with 10-year follow-up. J Neurosurg Pediatr. (2011) 8:367–71. doi: 10.3171/2011.7.PEDS10566
11. Wei, F, Liu, X, Liu, Z, Jiang, L, Dang, G, Ma, Q, et al. Interferon alfa-2b for recurrent and metastatic giant cell tumor of the spine: report of two cases. Spine (Phila Pa 1976). (2010) 35:E1418–22. doi: 10.1097/BRS.0b013e3181e7bf5a
12. Lin, P, Lin, N, Teng, W, Wang, SD, Pan, WB, Huang, X, et al. Recurrence of Giant cell tumor of the spine after resection: a report of 10 cases. Orthop Surg. (2018) 10:107–14. doi: 10.1111/os.12375
13. Chin, BZ, Ji, T, Tang, X, Yang, R, and Guo, W. Three-level lumbar En bloc Spondylectomy with three-dimensional-printed vertebrae reconstruction for recurrent Giant cell tumor. World Neurosurg. (2019) 129:531–537.e1. doi: 10.1016/j.wneu.2019.06.056
14. Si, MJ, Wang, CG, Wang, CS, Du LJ, DXY, Zhang, WB, et al. Giant cell tumours of the mobile spine: char-acteristic imaging features and differential diagnosis. Radiol Med. (2014) 119:681–93. doi: 10.1007/s11547-013-0352-1
15. Inoue, G, Imura, T, Miyagi, M, Saito, W, Tazawa, R, Nakazawa, T, et al. Total en bloc spondylectomy of the eleventh thoracic vertebra following denosumab therapy for the treatment of a giant cell tumor. Oncol Lett. (2017) 14:4005–10. doi: 10.3892/ol.2017.6655
16. Enneking, WF, Spanier, SS, and Goodman, MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. (1980) 153:106???120–07. doi: 10.1097/00003086-198011000-00013
17. Xu, K, Wan, W, Li, B, Li, J, Huang, Q, Liu, Y, et al. Prognostic significance of preoperative plasma D-dimer level and clinical factors in patients with spinal Giant cell tumor: retrospective analysis of 153 patients in a single center. World Neurosurg. (2019) 122:e872–80. doi: 10.1016/j.wneu.2018.10.169
18. Martin, C, and McCarthy, EF. Giant cell tumor of the sacrum and spine: series of 23 cases and a review of the literature. Iowa Orthop J. (2010) 30:69–75.
19. Turcotte, RE, Wunder, JS, Isler, MH, Bell, RS, Schachar, N, Masri, BA, et al. Canadian sarcoma group. Giant cell tumor of long bone: a Canadian sarcoma group study. Clin Orthop Relat Res. (2002) 397:248–58. doi: 10.1097/00003086-200204000-00029
20. Boriani, S, Gasbarrini, A, Bandiera, S, Ghermandi, R, and Lador, R. Predictors for surgical complications of en bloc resections in the spine: review of 220 cases treated by the same team. Eur Spine J. (2016) 25:3932–41. doi: 10.1007/s00586-016-4463-y
21. Tsukamoto, S, Mavrogenis, AF, Kido, A, and Errani, C. Current concepts in the treatment of Giant cell tumors of bone. Cancers (Basel). (2021) 13:3647. doi: 10.3390/cancers13153647
22. Ouyang, HQ, Jiang, L, Liu, XG, Wei, F, Yang, SM, Meng, N, et al. Recurrence factors in Giant cell tumors of the spine. Chin Med J. (2017) 130:1557–63. doi: 10.4103/0366-6999.208239
23. Balke, M, Schremper, L, Gebert, C, Ahrens, H, Streitbuerger, A, Koehler, G, et al. Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol. (2008) 134:969–78. doi: 10.1007/s00432-008-0370-x
24. Gouin, F, and Dumaine, V, French Sarcoma and Bone Tumor Study Groups GSF-GETO. Local recurrence after curettage treatment of giant cell tumors in peripheral bones: a retrospective study by the GSF-GETO (French sarcoma and bone tumor study groups). Orthop Traumatol Surg Res. (2013) 99:S313–8. doi: 10.1016/j.otsr.2013.07.006
25. Oh, JH, Yoon, PW, Lee, SH, Cho, HS, Kim, WS, and Kim, HS. Surgical treatment of giant cell tumour of long bone with an-hydrous alcohol adjuvant. Int Orthop. (2006) 30:490–4. doi: 10.1007/s00264-006-0154-3
26. Meller, I, Weinbroum, A, Bickels, J, Dadia, S, Nirkin, A, Merimsky, O, et al. Fifteen years of bone tumor cryosurgery: a single-center experience of 440 procedures and long-term follow-up. Eur J Surg Oncol. (2008) 34:921–7. doi: 10.1016/j.ejso.2007.11.001
27. Kivioja, AH, Blomqvist, C, Hietaniemi, K, Trovik, C, Walloe, A, Bauer, HC, et al. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian sarcoma group study of 294 patients followed for a median time of 5 years. Acta Orthop. (2008) 79:86–93. doi: 10.1080/17453670710014815
28. Xu, W, Li, X, Huang, W, Wang, Y, Han, S, Chen, S, et al. Factors affecting prognosis of patients with giant cell tumors of the mobile spine: retrospective analysis of 102 patients in a single center. Ann Surg Oncol. (2013) 20:804–10. doi: 10.1245/s10434-012-2707-6
29. Xu, W, Wang, Y, Wang, J, Yang, X, Liu, W, Zhou, W, et al. Long-term administration of bisphosphonate to reduce local recurrence of sacral giant cell tumor after nerve-sparing surgery. J Neurosurg Spine. (2017) 26:716–21. doi: 10.3171/2016.10.SPINE151197
30. Yu, X, Xu, M, Xu, S, and Su, Q. Clinical outcomes of giant cell tumor of bone treated with bone cement filling and internal fixation, and oral bisphosphonates. Oncol Lett. (2013) 5:447–51. doi: 10.3892/ol.2012.1036
31. Zheng, K, Yu, XC, Hu, YC, Wang, Z, Wu, SJ, and Ye, ZM. How to fill the cavity after curettage of Giant cell tumors around the knee? A multicenter analysis. Chin Med J (Engl). (2017) 130:2541–6. doi: 10.4103/0366-6999.217093
32. Alamanda, VK, Robinson, MM, Kneisl, JS, Spector, LR, and Patt, JC. Survival outcomes and factors associated with revision surgery for metastatic disease of the spine. J Oncol. (2018) 2018:6140381:1–6. doi: 10.1155/2018/6140381
33. Ailon, T, Torabi, R, Fisher, CG, Rhines, LD, Clarke, MJ, Bettegowda, C, et al. Management of Locally Recurrent Chordoma of the Mobile spine and sacrum: a systematic review. Spine (Phila Pa 1976). (2016) 41 Suppl 41 Suppl 20:S193–8. doi: 10.1097/BRS.0000000000001812
34. Berjano, P, Cecchinato, R, Pun, A, and Boriani, S. Revision surgery for tumors of the thoracic and lumbar spine: causes, prevention, and treatment strategy. Eur Spine J. (2020) 29:66–77. doi: 10.1007/s00586-019-06276-8
35. Boriani, S. En bloc resection in the spine: a procedure of surgical oncology. J Spine Surg. (2018) 4:668–76. doi: 10.21037/jss.2018.09.02
36. Junming, M, Cheng, Y, Dong, C, Jianru, X, Xinghai, Y, Quan, H, et al. Giant cell tumor of the cervical spine: a series of 22 cases and outcomes. Spine (Phila Pa 1976). (2008) 33:280–8. doi: 10.1097/BRS.0b013e318162454f
37. Niu, X, Yang, Y, Wong, KC, Huang, Z, Ding, Y, and Zhang, W. Giant cell tumour of the bone treated with denosumab: how has the blood supply and oncological prognosis of the tumour changed? J Orthop Translat. (2018) 18:100–8. doi: 10.1016/j.jot.2018.10.003
38. Thomas, D, Henshaw, R, Skubitz, K, Chawla, S, Staddon, A, Blay, JY, et al. Deno-sumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. (2010) 11:275–80. doi: 10.1016/S1470-2045(10)70010-3
39. Agarwal, MG, Gundavda, MK, Gupta, R, and Reddy, R. Does Denosumab change the Giant cell tumor treatment strategy? Lessons learned from early experience. Clin Orthop Relat Res. (2018) 476:1773–82. doi: 10.1007/s11999.0000000000000243
40. Akel, U, Robinson, ME, Werier, J, Rampersaud, R, Rakhra, K, Johnston, D, et al. Local tumor recurrence and escape from suppression of bone resorption with Denosumab treatment in two adolescents with Giant cell tumors of bone. JBMR Plus. (2019) 3:e10196. doi: 10.1002/jbm4.10196
41. Boriani, S, Cecchinato, R, Cuzzocrea, F, Bandiera, S, Gambarotti, M, and Gasbarrini, A. Denosumab in the treatment of giant cell tumor of the spine. Preliminary report, review of the literature and protocol proposal. Eur Spine J. (2020) 29:257–71. doi: 10.1007/s00586-019-05997-0
42. Arefpour, A, Shafieesabet, M, Chehrassan, M, Ahmadzadehnanva, A, and Ghandhari, H. Effect of denosumab in treatment of unresectable spine and sacrum giant cell tumor of bone. Musculoskelet Surg. (2023). doi: 10.1007/s12306-023-00799-6
43. Matcuk, GR Jr, Patel, DB, Schein, AJ, White, EA, and Menendez, LR. Giant cell tumor: rapid recurrence after cessation of long-term denosumab therapy. Skelet Radiol. (2015) 44:1027–31. doi: 10.1007/s00256-015-2117-5
44. Ma, Y, Xu, W, Yin, H, Huang, Q, Liu, T, Yang, X, et al. Therapeutic radiotherapy for giant cell tumor of the spine: a systemic review. Eur Spine J. (2015) 24:1754–60. doi: 10.1007/s00586-015-3834-0
45. Bennett, CJ, Marcus, RB, Million, RR, and Enneking, WF. Radiation therapy for giant cell tumor of bone. Int J Radiat Oncol Biol Phys. (1993) 26:299–304. doi: 10.1016/0360-3016(93)90210-m
46. Noh, SH, Ha, Y, Cho, PG, Kim, KN, Shin, DA, and Kim, SH. The effect of Denosumab and risk factors for recurrence in spinal Giant cell tumors: a systematic review and Meta-analysis. Yonsei Med J. (2022) 63:834–41. doi: 10.3349/ymj.2022.63.9.834
47. Hug, EB, Muenter, MW, Adams, JA, de Vries, A, Rosenberg, AE, and Munzenrider, JE. 3-D-conformal radiation therapy for pediatric giant cell tumors of the skull base. Strahlenther Onkol. (2002) 178:239–44. doi: 10.1007/s00066-002-0931-x
48. Miszczyk, L, Wydmański, J, and Spindel, J. Efficacy of radiotherapy for giant cell tumor of bone: given either postoperatively or as sole treatment. Int J Radiat Oncol Biol Phys. (2001) 49:1239–42. doi: 10.1016/s0360-3016(00)01520-0
49. Palmerini, E, Picci, P, Reichardt, P, and Downey, G. Malignancy in Giant cell tumor of bone: a review of the literature. Technol Cancer Res Treat. (2019) 18:1533033819840000. doi: 10.1177/1533033819840000
50. Skubitz, KM. Giant cell tumor of bone: current treatment options. Curr Treat Options in Oncol. (2014) 15:507–18. doi: 10.1007/s11864-014-0289-1
51. He, SH, Xu, W, Sun, ZW, Liu, WB, Liu, YJ, Wei, HF, et al. Selective arterial embolization for the treatment of sacral and pelvic Giant cell tumor: a systematic review. Orthop Surg. (2017) 9:139–44. doi: 10.1111/os.12336
52. Mindea, SA, Eddleman, CS, Hage, ZA, Batjer, HH, Ondra, SL, and Bendok, BR. Endovascular embolization of a recurrent cervical giant cell neoplasm using N-butyl 2-cyanoacrylate. J Clin Neurosci. (2009) 16:452–4. doi: 10.1016/j.jocn.2008.03.017
Keywords: giant cell tumor of bone, spine, local recurrence, radiotherapy, denosumab
Citation: Miao Z, Xu M, Zheng K, Gong H, Yan N, Chen Q and Yu X (2024) Denosumab combined with precision radiotherapy for recurrent giant cell tumor of the thoracic spine: a case report and literature review. Front. Neurol. 14:1308600. doi: 10.3389/fneur.2023.1308600
Edited by:
Shinji Kawabata, Osaka Medical and Pharmaceutical University, JapanReviewed by:
Raees Tonse, Baptist Hospital of Miami, United StatesFulvio Tartara, Istituto Clinico Città Studi (ICCS), Italy
Copyright © 2024 Miao, Xu, Zheng, Gong, Yan, Chen and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuchun Yu, MTM5NjkxMzIxOTBAMTYzLmNvbQ==
 Zukang Miao
Zukang Miao Ming Xu
Ming Xu Kai Zheng
Kai Zheng Hai Gong3
Hai Gong3 Qian Chen
Qian Chen Xiuchun Yu
Xiuchun Yu