
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 09 January 2024
Sec. Epilepsy
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1307296
Background: The new antiseizure medications (ASMs) and non-invasive brain stimulation (NIBS) are controversial in controlling seizures. So, this network meta-analysis aimed to evaluate the efficacy and safety of five third-generation ASMs and two NIBS therapies for the treatment of refractory epilepsy.
Methods: We searched PubMed, EMBASE, Cochrane Library and Web of Science databases. Brivaracetam (BRV), cenobamate (CNB), eslicarbazepine acetate (ESL), lacosamide (LCM), perampanel (PER), repetitive transcranial magnetic stimulation (rTMS), and transcranial direct current stimulation (tDCS) were selected as additional treatments for refractory epilepsy in randomized controlled studies and other cohort studies. Randomized, double-blind, placebo-controlled, add-on studies that evaluated the efficacy or safety of medication and non-invasive brain stimulation and included patients with seizures were uncontrolled by one or more concomitant ASMs were identified. A random effects model was used to incorporate possible heterogeneity. The primary outcome was the change in seizure frequency from baseline, and secondary outcomes included the proportion of patients with ≥50% reduction in seizure frequency, and the rate of treatment-emergent adverse events.
Results: Forty-five studies were analyzed. The five ASMs and two NIBS decreased seizure frequency from baseline compared with placebo. The 50% responder rates of the five antiseizure drugs were significantly higher than that of placebo, and the ASMs were associated with fewer adverse events than placebo (p < 0.05). The surface under the cumulative ranking analysis revealed that ESL was most effective in decreasing the seizure frequency from baseline, whereas CNB provided the best 50% responder rate. BRV was the best tolerated. No significant publication bias was identified for each outcome index.
Conclusion: The five third-generation ASMs were more effective in controlling seizures than placebo, among which CNB, ESL, and LCM were most effective, and BRV exhibited better safety. Although rTMS and tDCS did not reduce seizure frequency as effectively as the five drugs, their safety was confirmed.
Systematic review registration: PROSPERO, https://www.crd.york.ac.uk/prospero/ (CRD42023441097).
Epilepsy is a long-term neurological condition marked by repeated seizures, and it frequently correlates with irregularities in cognitive function, mental well-being, and social adaptability (1). Epilepsy impacts a minimum of 1.2% of the global population. Most patients with epilepsy can be seizure-free after taking antiseizure medications (ASMs); however, at least one-third of patients develop resistance to ASMs and develop refractory epilepsy, which poses a serious public health problem with high economic costs (2). According to the definition of drug-resistant epilepsy proposed by the International League Against Epilepsy (ILAE) in 2010, the right choice and use of two ASMs cannot achieve sustained seizure freedom which can be considered as drug-resistant epilepsy or refractory epilepsy (3). Refractory epilepsy does not mean that any drug treatment cannot control seizures, but as the course of uncontrollable seizures is prolonged, the responsiveness of new treatments may also be reduced (4). Therefore, it is particularly important to determine the effective treatments in time.
There are several hypotheses about the mechanisms of drug resistance in epilepsy, such as transporter hypothesis, target hypothesis, neural network change hypothesis, neuroinflammation hypothesis, and so on (5, 6). Different patients with refractory epilepsy have personalized resistance mechanisms, and there may be one or more resistance mechanisms at the same time (7). The mechanisms of drug-resistant epilepsy are complex and vary from person to person, so it is necessary to provide individualized antiseizure therapies including drug and non-drug treatments.
Currently, the main treatment for epilepsy is drug therapy. A variety of ASMs are available, among which new ASMs have fewer adverse reactions, while effectively controlling epileptic seizures (8). In the past decade, five “third-generation” ASMs, namely, brivaracetam (BRV), cenobamate (CNB), eslicarbazepine acetate (ESL), lacosamide (LCM) and perampanel (PER) have been approved as adjunctive therapies for adult patients with epilepsy (9). However, the above drugs can only relieve the seizure to a certain extent, but do not reverse the disease. Each type of new ASMs have their own characteristics and scope of application. When selecting ASMs for treatment, physicians must carefully consider and compare the efficacy and safety profiles of the medications in order to provide useful clinical guidance for managing patients with epilepsy.
Surgical intervention is another treatment option for intractable epilepsy. However, surgery is not suitable for all patients with refractory epilepsy due to its high risk, inability to completely control seizures after surgery, and even some sequelae and neurological dysfunction (10). Alternative treatments must be developed. Non-invasive brain stimulation (NIBS) modulates brain excitability and encompasses a range of techniques, including repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) (11). In recent years, a substantial body of evidence has emerged regarding the efficacy of non-invasive brain stimulation (NIBS), particularly repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS). NIBS is painless and non-invasive, and several open-label studies have suggested that rTMS and tDCS exhibit significant antiseizure effect (12–15). tDCS decreases cortical excitability through cathode stimulation, and increases cortical excitability through anode stimulation (16). High-frequency rTMS enhances cortical excitability and may increase the risk of seizures, whereas low-frequency rTMS reduces cortical excitability (17). Some studies have shown that rTMS and tDCS only provide short-term reduction of seizures in patients, whereas other studies demonstrated no significant difference in efficacy compared to placebo treatment (18, 19). NIBS therapies are generally safe and do not cause significant side effects or complications. The treatment usually does not cause pain or discomfort, and patients can be treated in a comfortable environment (20). However, there is currently insufficient data to draw definitive conclusions regarding the antiseizure potential of rTMS or tDCS, thus requiring further research.
Network meta-analyses enable direct and indirect comparisons. Therefore, this systematic review and network meta-analysis aimed to compare the efficacy and safety of different interventions in the treatment of refractory epilepsy through network meta-analysis, in order to identify the interventions with the best clinical outcomes and provide guidance for clinical decision-making. Using placebo as a control, we focused on the efficacy of third-generation ASMs and NIBS as additional treatments to control seizures in patients with refractory epilepsy, as well as the incidence of TEAE during treatment.
We adhered to the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) to conduct this study (21). This review is based on previous research and does not include new research in human participants. This systematic review and network meta-analysis has been registered on PROSPERO (CRD 42023441097).
PubMed, EMBASE, Cochrane Library and Web of Science databases were searched from inception to April 2023. The language of publication is restricted to English. A random effect model was used to incorporate possible heterogeneity. The following search terms were used: [“Seizures” (Mesh) OR “Epilepsy” (Mesh)] AND [“Transcranial Magnetic Stimulation” (Mesh) OR “Transcranial Direct Current Stimulation” (Mesh)] OR [“repetitive transcranial magnetic stimulation” (Title/Abstract) OR “TMS” (Title/Abstract) OR “rTMS” (Title/Abstract) OR “tDCS” (Title/Abstract) OR “brain polarization” (Title/Abstract) OR “galvanic stimulation” (Title/Abstract) OR “eslicarbazepine acetate” (Title/Abstract) OR “perampanel” (Title/Abstract) OR “lacosamide” (Title/Abstract) OR “brivaracetam” (Title/Abstract) OR “cenobamate” (Title/Abstract)].
Randomized, double-blind, placebo-controlled, add-on studies that evaluated the efficacy and safety of medication and non-invasive brain stimulation in the treatment of patients with seizures that was uncontrolled by one or more concomitant ASMs. Concomitant ASMs had been kept stable before trial entry and throughout the treatment periods. The participant agreed to keep the ASMs unchanged throughout the whole study. We included high-quality clinical trials in English, including RCTs and cohort studies with Newcastle–Ottawa scale (NOS) quality scores ≥5.
The exclusion criteria were as follows: (1) reports of reviews or meetings; (2) studies in which the outcome measures did not describe seizure frequency; (3) concomitant ASMs had been kept unstable before trial entry and throughout the treatment periods; (4) studies with no placebo control; and (5) cohort studies with Newcastle–Ottawa scale (NOS) quality scores <5 (22, 23) and RCTs considered to be low quality after Cochrane risk of bias assessment.
The primary outcome was the change in seizure frequency from baseline (seizure response) after treatment. The secondary outcomes included the proportion of patients with ≥50% reduction in seizure frequency (defined as responders), and the rates of treatment-emergent adverse events (TEAEs).
We conducted an extensive literature search to collect research studies that fit our research objectives. The databases we used included PubMed, EMBASE, Cochrane Library and Web of Science databases. During the screening process, three independent researchers conducted an initial screening of the literature, evaluating it based on the relevance of the title and abstract. Subsequently, the full text of the literature meeting the screening criteria was read to finalize the articles included in the study. To extract the data, we designed a standardized data extraction table, as shown in Supplementary Table S1, which was used by three independent investigators to extract data from each included study. If disagreements arose, they were resolved through discussions with other researchers in our team until a consensus was reached. The extracted data included the title, year of publication, author(s), number of participants, study design, intervention, and outcome measures.
The data were synthesized and analyzed using the RevMan 5.4 software to assess the risk of bias. The risk of bias in the included RCTs was assessed according to the recommendations of the Cochrane Collaboration (24). The NOS quality scores was used to evaluate the quality of the cohort studies.
RevMan 5.4.1 and Stata 15.1 were used to analyze and process the data. Measurement data are expressed as the mean difference (MD), and the odds ratio (OR) was adopted for numerical data. We ranked the interventions for each outcome by calculating the surface under the cumulative ranking curve (SUCRA) probabilities.
SUCRA is a percentage between 0 and 100 that represents the relative position of each treatment measure in all possible rankings (25). SUCRA values can provide a simple and intuitive way to help decision makers make rational choices between multiple treatment options. Publication bias in the included trials was assessed by generating a funnel plot using the Stata 15 software. Statistical significance was set at p < 0.05.
The literature search using the above English databases retrieved 9,072 studies. After review, 9,027 of these studies were excluded because they did not meet the inclusion criteria, and 45 studies were included in the network meta-analysis. The article retrieval process is illustrated in Figure 1.
The final studies included 28,819 patients (tDCS: 79, rTMS: 47, BRV: 4350, CNB: 674, ESL: 2347, LCM: 3063, PER: 9120, and placebo: 9139). Additional details of the included studies are provided in Supplementary Table S1. The results of the Cochrane risk of bias assessment of the 19 included RCTs using RevMan5.4 software are shown in Figure 2. The NOS scores are presented in Supplementary Table S1 and indicate that the included cohort studies were of high quality.
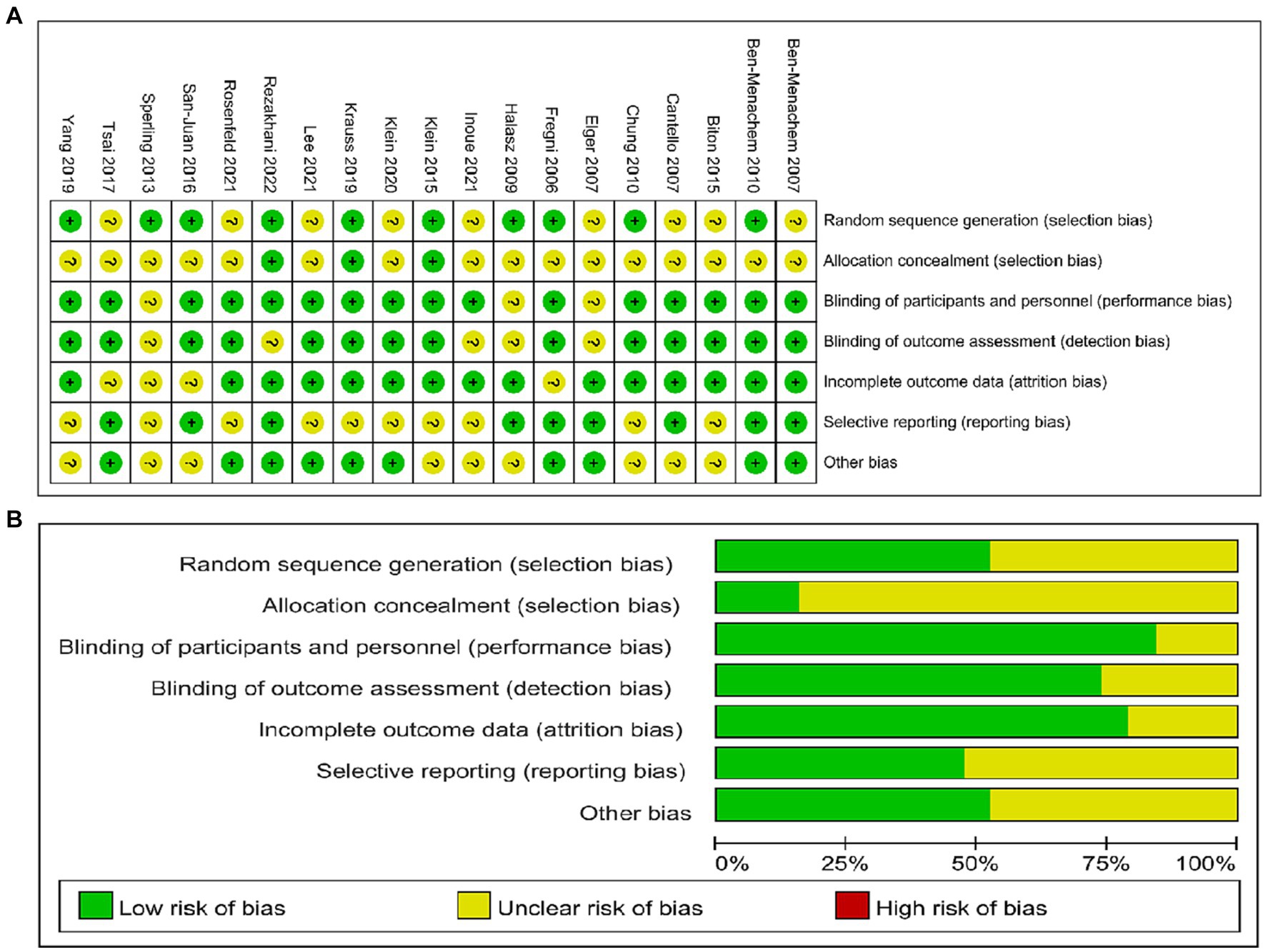
Figure 2. Evaluation of quality of included studies. (A) Review of authors’ judgements for each risk of bias item. (B) Review of authors’ judgements for bias item for each study.
Figure 3A depicts the network geometry of the interactions based on therapeutic evaluations of seizure frequency reduction from baseline. Figure 3B illustrates the network geometry of the interactions based on ≥50% responder rate, and Figure 3C details the network geometry of the interactions based on the rate of TEAEs. The size of the node represents the number of trials per intervention and control group; the larger the node size, the more trials the corresponding node contains. The thickness of the line between the corresponding nodes would indicate the number of comparisons between the two interventions. However, there was no direct comparison between any two interventions, and they were both compared with the placebo group. Hence, this network geometry can be used for direct and indirect evaluation comparisons.
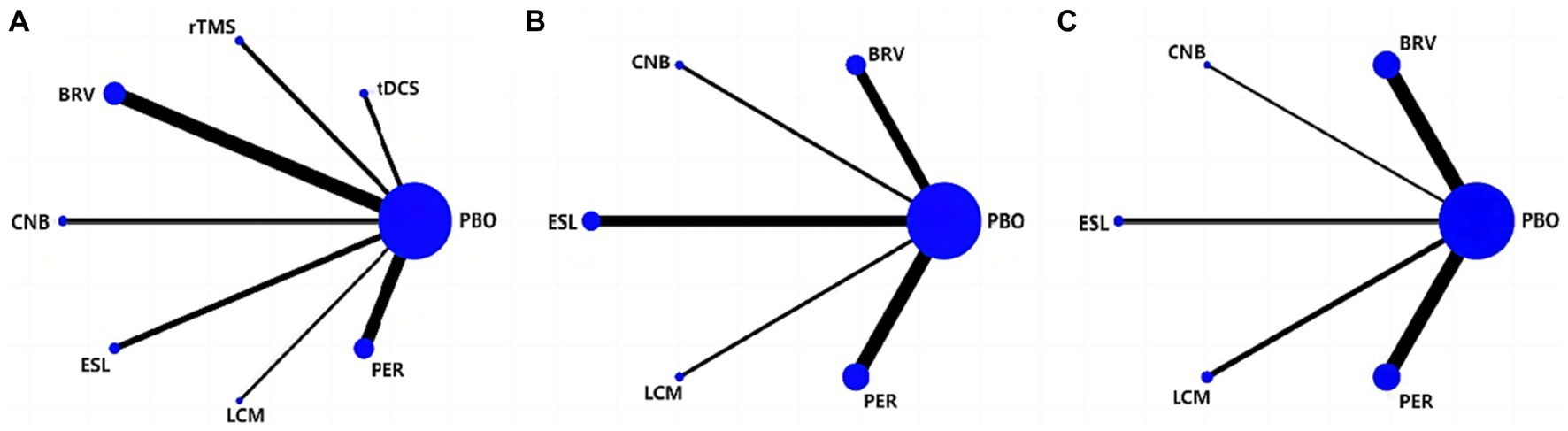
Figure 3. Network of eligible treatment comparisons for seizure frequency reduction from baseline (A), 50% responder rate (B), and TEAEs (C). TEAE, treatment-emergent adverse event; PBO, placebo; BRV, brivaracetam; CNB, cenobamate; ESL, eslicarbazepine acetate; LCM, lacosamide; PER, perampanel; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation.
As shown in Figures 4A, 5A, the ESL group that compared with placebo demonstrated the most favorable treatment effect [MD: −3.11 (95% CI −5.16 to −1.07)], indicating the greatest reduction in seizure frequency from baseline (high-quality evidence, 2,347 participants). For the non-drug therapies, the rTMS group exhibited a worse effect than the tDCS group [0.10 (95% CI −3.56 to 3.76)]. However, this difference was not statistically significant.
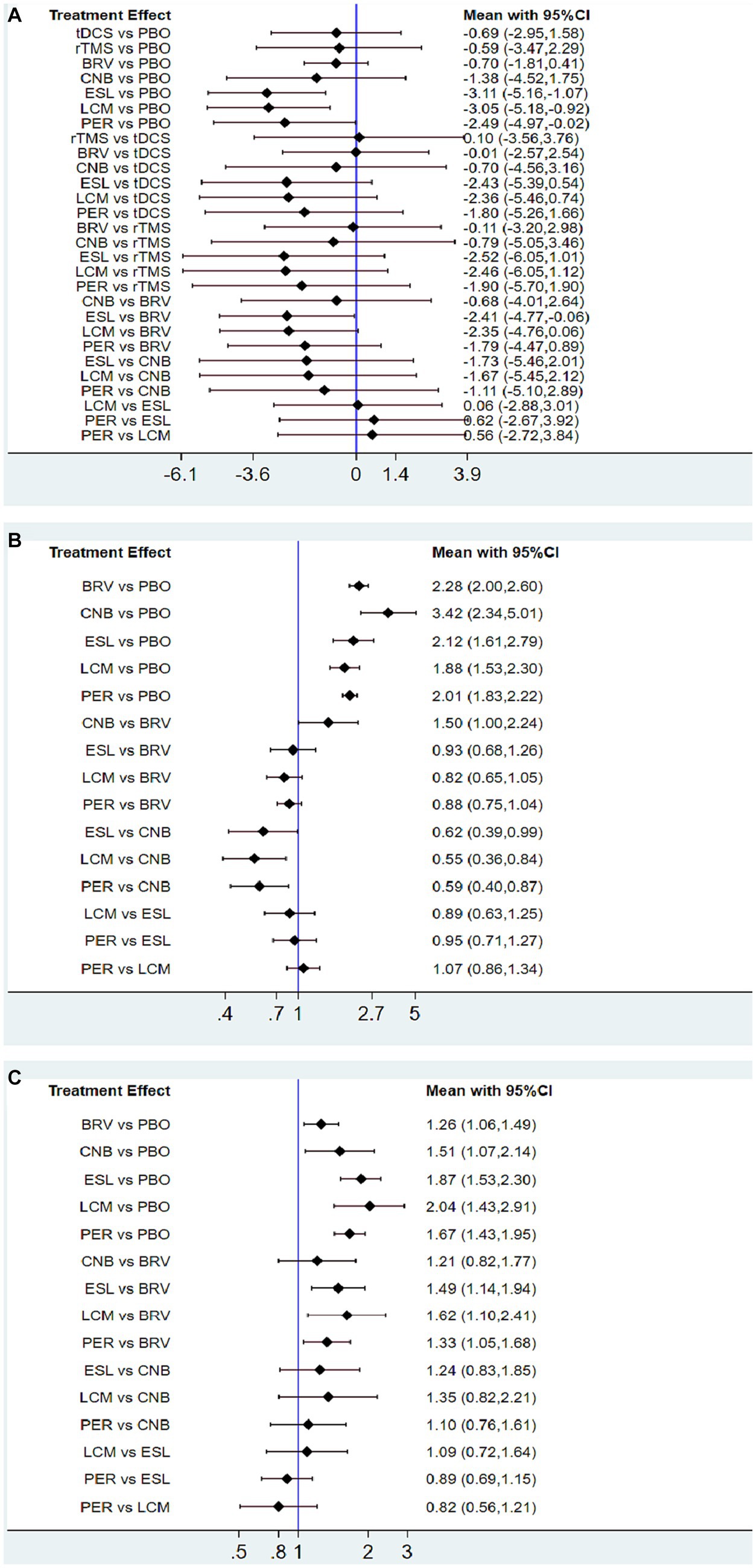
Figure 4. Interval plot for intervention effect size on seizure frequency reduction from baseline (A), 50% responder rate (B), and TEAEs (C). TEAE, treatment-emergent adverse event; PBO, placebo; BRV, brivaracetam; CNB, cenobamate; ESL, eslicarbazepine acetate; LCM, lacosamide; PER, perampanel; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation.
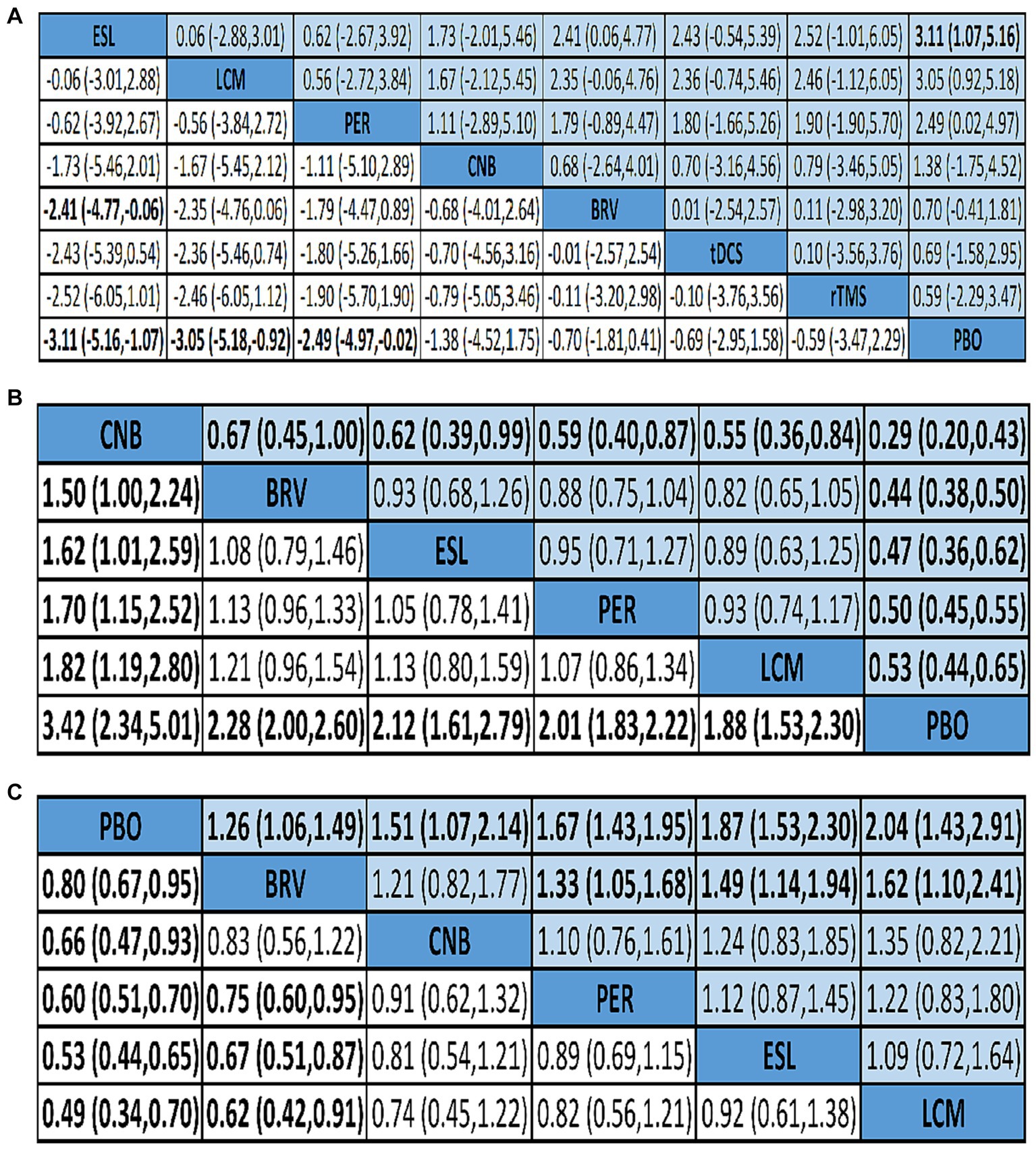
Figure 5. Network meta-analysis league map of outcomes: seizure frequency reduction from baseline (A), 50% responder rate (B), and TEAEs (C). TEAE, treatment-emergent adverse event; PBO, placebo; BRV, brivaracetam; CNB, cenobamate; ESL, eslicarbazepine acetate; LCM, lacosamide; PER, perampanel; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation.
The effects of all treatments were ranked using SUCRA probabilities (Figure 6A), and the ESL achieved the highest probability (SUCRA 83.529%) of being the best treatment to reduce seizure frequency from baseline, followed by LCM (SUCRA 81.157%), PER (SUCRA 71.714%), and placebo (SUCRA 13.914%).
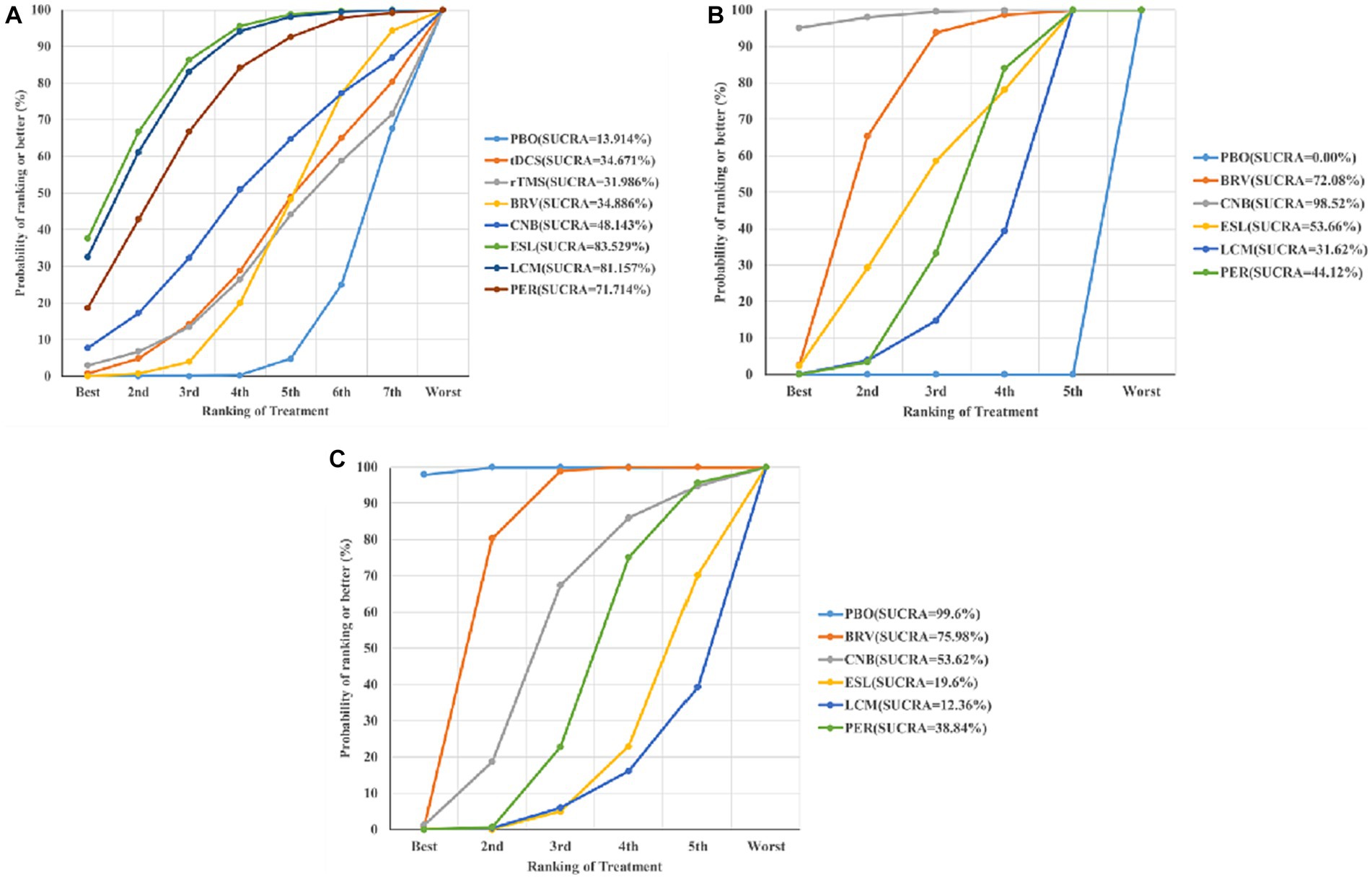
Figure 6. Ranking of treatment strategies according to the SUCRA probabilities: seizure frequency reduction from baseline (A), 50% responder rate (B), and TEAEs (C). SUCRA, surface under the cumulative ranking curve; TEAE, treatment-emergent adverse event; PBO, placebo; BRV, brivaracetam; CNB, cenobamate; ESL, eslicarbazepine acetate; LCM, lacosamide; PER, perampanel; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation.
Twenty-nine studies reported 50% responder rates. Mean values and 95% confidence intervals were used as the aggregate data in the network meta-analysis, and the results revealed that compared with the placebo group, the seizure frequency reduction ≥50% are BRV 2.28 (95% CI 2.00 to 2.60), CNB 3.42 (95% CI 2.34 to 5.01), ESL 2.12 (95% CI 1.61 to 2.79), LCM 1.88 (95% CI 1.53 to 2.30), and PER 2.01 (95% CI 1.83 to 2.22). Therefore, the 50% responder rate of the CNB group was the highest, and that of the LCM group was the lowest. However, the 50% responder rate for the five drugs were significantly higher than that of placebo (Figures 4B, 5B).
Figure 6B shows the seizure response reduction of all treatments ranked by SUCRA probabilities. SUCRA analysis revealed that CNB achieved the best effect on the proportion of patients with ≥50% reduction in seizure frequency (SUCRA 98.52%), followed by BRV (SUCRA 72.08%).
We analyzed the incidence of adverse events after treatment with BRV, CNB, ESL, LCM, PER, and placebo. The mean value in the LCM group was 2.04 (95% CI 1.43 to 2.91), as shown in Figures 4C, 5C, indicating the highest rate of TEAEs. The PER group 1.67 (95% CI 1.43 to 1.95) and the ESL group 1.87 (95% CI 1.53 to 2.30) also produced higher rates of adverse reactions. The mean value in the BRV group was 1.26 (95% CI 1.06 to 1.49), indicating the lowest rate of TEAEs. Based on these two outcome measures, ESL and CNB were ranked first. The rate of TEAEs was higher in ESL than in CNB. Figure 6C shows the rates of TEAE ranked according to SUCRA probabilities. SUCRA analysis revealed that LCM had the highest rate of TEAEs (SUCRA 12.36%), followed by ESL (SUCRA 19.6%).
We assessed publication bias in 32 studies that included seizure frequency (Figure 7A), 29 studies that included a 50% responder rate (Figure 7B), and 32 studies that included TEAEs (Figure 7C). The funnel plots reveal that the scatter was almost symmetrical, suggesting that the included trials had a relatively low publication bias.
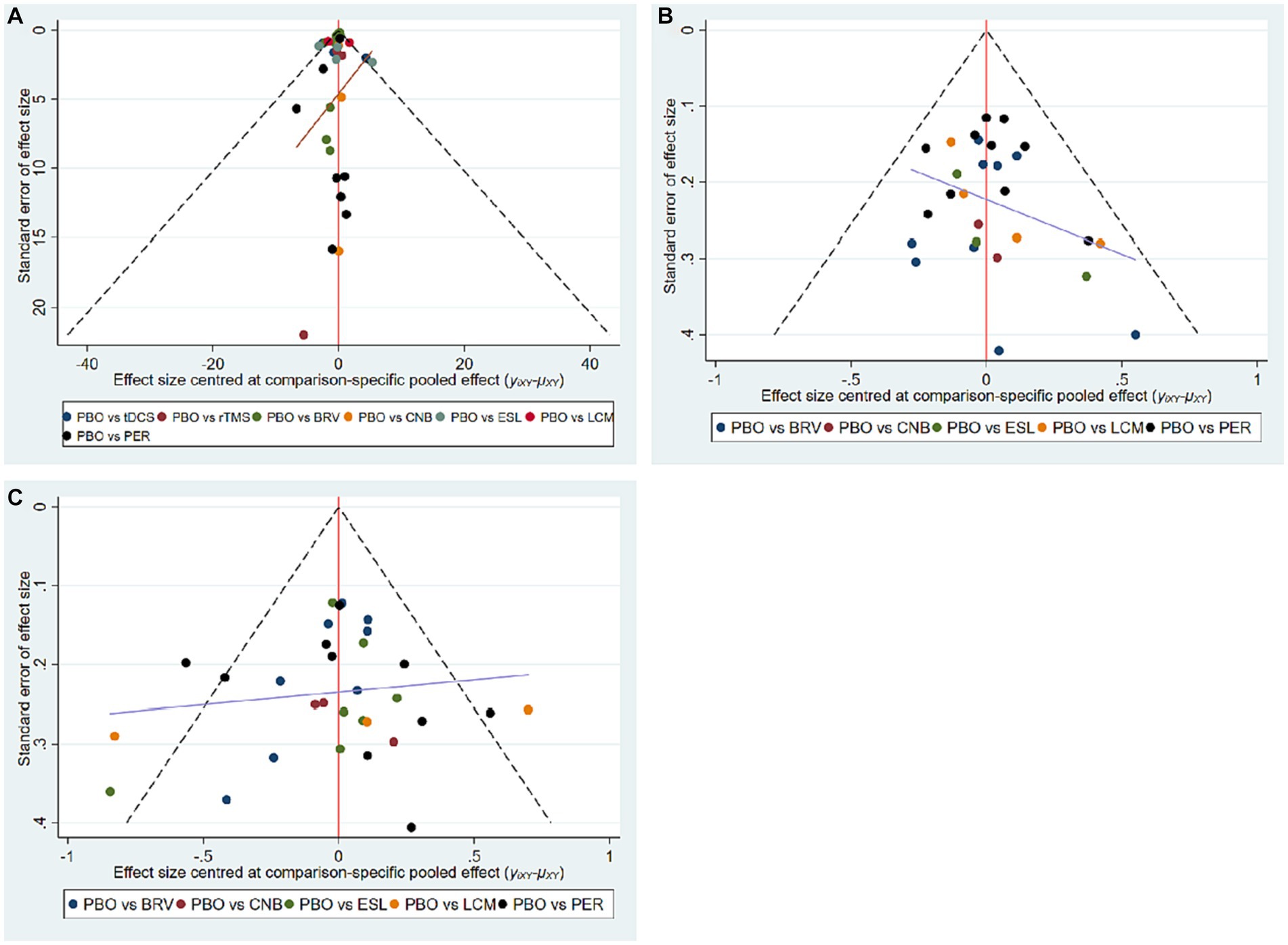
Figure 7. Funnel plots of multiple interventions for seizure frequency reduction from baseline (A), 50% responder rate (B), and TEAE (C). TEAE, treatment-emergent adverse event; PBO, placebo; BRV, brivaracetam; CNB, cenobamate; ESL, eslicarbazepine acetate; LCM, lacosamide; PER, perampanel; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation.
Epilepsy is a prevalent and intricate disorder of the central nervous system resulting from the extensively synchronized discharge of neurons. About 30%–40% of patients with epilepsy are still unable to effectively control their seizures under the treatment of appropriate ASMs, which is defined as refractory epilepsy. The etiology of intractable epilepsy is multifaceted, encompassing diverse factors associated with the environment, genetics, and medication (7, 26, 27). Pharmacotherapy plays a crucial role in mitigating the severity of epilepsy and enhancing the quality of life for patients (28).
This network meta-analysis updates the currently available correlational studies. We included high-quality RCTs and other cohort studies. Using a Bayesian approach, we conducted a network meta-analysis to comprehensively assess the effectiveness and safety of both drug and non-drug therapies in the treatment of epilepsy. SUCRA was used for ranking. The top three interventions for reducing seizure frequency from baseline were drug therapies. Both tDCS and rTMS were less effective in controlling seizures than the five third-generation ASMs. In terms of the 50% response rate, CNB, BRV, and ESL were ranked highest. Meanwhile, BRV, CNB, and PER were well tolerated; the safety of LCM was lowest among the five third-generation ASMs.
Our findings reveal that ESL and LCM, when used as add-on treatments, could effectively reduce seizure frequency. LCM and ESL are both known to block voltage-gated sodium channels (29). LCM and ESL exert selective effects on pathological currents induced by slow channels, thereby inhibiting the activation of synaptic currents. Therefore, the pathological current can be prevented from spreading and the neural network can be stabilized (29–31). The adverse event profile of LCM presented in this meta-analysis indicates that adverse events are common with LCM therapy, with blurred vision, coordination problems, dizziness, drowsiness, nausea, and vomiting being the most commonly reported adverse effects (32–34). Rosenow et al. (35) included 1,308 patients who were randomly assigned to receive LCM treatment. All patients reported at least one TEAE, but most TEAEs were mild or moderate in intensity. Therapeutic doses of 200–400 mg/day were well tolerated, however severe TEAEs were observed when 600 mg/day was used. During the clinical treatment, the therapeutic dose can be adjusted in a timely manner to the drug responsiveness of the individual patient, which is different from fixed-dose therapy in clinical trials. Therefore, adverse events associated with LCM may be more effectively managed and exhibit a lower occurrence rate in real-world clinical settings. It is well known that ASMs may impair sleep. Liguori et al. (36) summarized the effects of ASMs on sleep in patients with epilepsy and found that LCM was associated with the occurrence of daytime sleepiness. Interestingly, no significant adverse events were observed after short-term use of LCM, suggesting that the adverse effects appear to be related to the duration of the course of treatment (37). ESL shares structural similarities with carbamazepine and oxcarbazepine, while exhibiting a lower propensity for drug interactions (38). The most frequently observed TEAEs included dizziness, drowsiness, headache, and nausea, while serious TEAEs were reported in less than 1% of patients. Adjuvant therapy for ESL is usually well tolerated, with most adverse events being mild to moderate in severity (39–41). We included a total of 7 clinical studies on adjuvant ESL therapy, among which Krauss. et al. (42) included 1,447 patients (ESL: 1021 and placebo: 426) to analyze the TEAE during various doses of 400, 800, or 1,200 mg QD for ESL therapy. The results showed that starting with a lower dose of ESL had a lower incidence of TEAE. In addition, discontinuation due to TEAE was more frequent in patients receiving a maintenance dose of 1,200 mg QD. The tolerance of ESL could be improved by reducing the dose or the titration rate. Because the large sample study has the characteristics of large scale, representativeness, repeatability and high stability, we are more confident in judging the authenticity and importance of the result effect.
For the 50% responder rate, the CNB group showed the highest efficacy. CNB can inhibit voltage-gated sodium currents to decrease the excitation current and target γ-amino butyric acid receptors to enhance inhibitory currents (43–45). The extensive preclinical activity of CNB can be attributed to its dual pharmacodynamic activity, which modulates both excitatory and inhibitory neurotransmission (46). CNB, as a novel pharmacological agent, shows great potential in treating patients with refractory seizures. At the same time, CNB was well tolerated. Studies have reported that the incidence of CNB-associated TEAEs tends to decrease with continued treatment (47, 48). Privitera et al. (49) analyzed the efficacy and safety of CNB and seven other AEDs for the treatment of uncontrolled focal seizures. The results revealed that CNB was more likely to provide ≥50% seizure reduction, without increasing treatment discontinuation due to TEAEs. The dose-related central nervous system adverse effects associated with CNB were primarily drowsiness, dizziness, double vision, and gait and coordination disorders (44). However, studies have found that the incidence of adverse events is higher in patients who use CNB in conjunction with sodium channel blockers (50). The utilization of CNB as an adjunctive therapy raises concerns regarding potential drug interactions. Overall, these findings offer compelling evidence supporting the efficacy of CNB in reducing seizures and its favorable side effect profile, aligning with the outcomes of the current meta-analysis.
In the present study, BRV considerably affected the 50% responder rate and was ranked second to CNB. BRV acts as a potent ligand for synaptic vesicle protein 2A, exerting inhibitory effects on voltage-dependent sodium channels in neurons (51). BRV has a similar chemical structure to levetiracetam (LEV), with a broader antiepileptic spectrum and higher efficacy than LEV. Brandt et al. (52) conducted a comprehensive analysis of safety data related to BRV as an adjunct therapy for the treatment of focal seizures. The results showed that the incidence of TEAE during treatment in the BRV group was not significantly different from that in the placebo group. Additionally, our meta-analysis demonstrated that BRV exhibited the highest tolerability among the drugs assessed, with the lowest incidence of TEAEs, corroborating previous research findings (53, 54).
PER, a noncompetitive α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist, has been considered as a first-in-class ASM. PER reduces neuronal excitability by inhibiting the AMPA receptor-mediated synaptic transmission (55). In this network meta-analysis, PER showed moderate efficacy in reducing seizure frequency. Further, the TEAE rate was low. Dizziness was the most common adverse reaction and may exhibit a dose-dependent response. In addition, PER may increase the incidence of somnolence, fatigue, and irritability (56).
At present, a wide range of studies have confirmed the efficacy and safety of NIBS in central nervous system diseases such as depression (57), Parkinson’s disease (58), stroke (59), Alzheimer’s Disease (60) and so on. Owing to its effects on modulating neuronal excitability and its high tolerability, NIBS has garnered increasing attention for the treatment of epilepsy (18). Six studies were analyzed which included 206 patients (tDCS: 79, rTMS: 47 and placebo: 80). Most patients had a slight itching sensation at the beginning of tDCS stimulation, and this discomfort disappeared immediately after the stimulation ended. In few patients, headache after tDCS treatment is of short duration and can be resolved on its own (61). Yang et al. (61) and San-Juan et al. (16) reported that even patients with refractory epilepsy who had a history of craniocerebral injury or surgery were able to tolerate tDCS intervention well. Rezakhani et al. (62) demonstrated that the quality of life of patients with refractory epilepsy improved significantly after 3 months of tDCS intervention compared with sham group, and that tDCS can improved cognition as well. We included a total of 3 studies on rTMS (rTMS: 47 and placebo: 41). A randomized, sham-controlled study used cognitive assessment as a secondary outcome measure for rTMS intervention to initially evaluate the safety of rTMS. The results showed that rTMS intervention group improved working memory, reactivity, attention and so on (63). Cantello et al. (64) evaluated the efficacy of rTMS in controlling seizures by seizure frequency and EEG changes. In this study, no significant or persistent side effects were reported, and medical and neurological examinations were unchanged. The included studies in our network meta-analysis study verified the safety of NIBS through some subjective scales and objective tests, but more high-quality clinical studies with larger sample size and more objective indicators are needed for further research in the future.
Transcranial magnetic stimulation (TMS) is a well-tolerated technique that effectively stimulates both excitatory and inhibitory neurons within the cerebral cortex without causing discomfort (65). rTMS has been widely shown to induce long-lasting effects after consecutive sessions (66, 67). When an electric current passes through the coil, it generates a magnetic field that has the potential to induce a localized intracranial electric current within the brain, effectively reaching and stimulating the desired brain tissue (68). rTMS produces long-term inhibitory effects on synaptic potentials and focal cortical excitability, which may reduce the rate of seizures (69). The application of rTMS in the treatment of central nervous system diseases holds promise, but it is important to acknowledge its limitations. It is worth noting that rTMS can induce seizures when the frequency is high and the stimulation interval is short (70). The bidirectional regulation of human cortical excitability can be achieved by adjusting the stimulation rate (71). Further studies focusing on personalized rTMS parameters may be required to maximize the therapeutic outcomes of this technique for brain stimulation in clinical settings. A small number of people had mild dizziness or headaches during treatment, and no significant or persistent side effects were reported.
tDCS, through the application of direct currents on the intact scalp, has the ability to induce enduring changes in cortical excitability in the human brain. The stimulation is released by placing a relatively large area of electrodes on the scalp area of interest (72). The flow of current through the targeted neuronal tissue in a specific direction leads to a polarity-dependent alteration in the resting membrane potential (73). Like rTMS, tDCS can modulate neuronal excitability in both directions as well. The effect of cathode tDCS is similar to that of low frequency rTMS, which is conducive to enhancing inhibition (74). Compared to adult patients, inpatient children appear to have higher 50% responder rates after tDCS treatment (75, 76). However, larger studies are required to confirm whether younger patients should preferentially receive tDCS as a treatment for epilepsy. No serious adverse events related to the application of tDCS have been reported. Minor local skin itching and tingling are common TEAEs, and these adverse reactions can be cured by themselves (77).
Patients with epilepsy in special situations, such as pregnant individuals, require tailored considerations during their treatment due to the unique challenges posed by their condition. The high safety profile of NIBS therapy, a non-surgical and non-drug treatment, renders it potentially significant for addressing the unique needs of epilepsy patients in special populations. During pregnancy, the pharmacokinetics of ASMs change. These changes may potentially affect seizure frequency and fetal exposure to ASMs, and even carry the risk of teratogenic effects (78, 79). Laurin et al. (80) presented three case reports demonstrating that tDCS appears to be a safe and effective treatment for many mental disorders in the perinatal period, including depression and post-traumatic stress disorder. Pregnant patients with treatment-resistant depression exhibited favorable tolerability to rTMS, with more than 50% of patients in the intervention group showing improved mood after the treatment period ended (81). Multiple clinical studies and high-quality systematic reviews have provided evidence regarding the efficacy and safety of NIBS in pregnant patients with depression (82–84). rTMS and tDCS, which can influence synaptic transmission to alter neuronal excitability (82, 83), appear to be a potential additional approach for patients with epilepsy during pregnancy to reduce the use of drugs and thus reduce drug-related risks. However, there is currently a lack of studies investigating the use of NIBS in pregnant patients with epilepsy, and more evidence is needed to verify the feasibility of this hypothesis in the future.
In summary, both rTMS and tDCS show great potential as therapeutic approaches for individuals with epilepsy. Nevertheless, the clinical advantages of these techniques should be validated through larger-scale, double-blind, randomized trials.
This study has some limitations. The RCTs and other cohort studies included were placebo-controlled; hence direct comparison of the different treatments was not possible. The network meta-analysis lacked adequate dose limitations in the included trials, which may have limited the comprehensive evaluation of therapeutic effects for the interventions. Hence, additional studies are necessary to address these limitations and facilitate the derivation of more precise and specific conclusions.
This study revealed that CNB, ESL, and LCM are more effective in controlling seizures, among the five third-generation antiseizure medications. BRV exhibited the lowest occurrence rate of adverse events. Moreover, rTMS and tDCS exhibit satisfactory safety profiles. In the future, it is necessary to conduct high-quality randomized controlled trials and other cohort studies to validate the findings of this study.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
YY: Writing – original draft, Writing – review & editing. YFS: Writing – original draft, Writing – review & editing. XMW: Data curation, Funding acquisition, Investigation, Writing – review & editing. RHL: Formal analysis, Methodology, Software, Writing – review & editing. ZYS: Data curation, Investigation, Writing – review & editing. MT: Formal analysis, Methodology, Software, Writing – review & editing. GHJ: Funding acquisition, Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by funding from the Science Foundation of China (No. 81971220) and the Natural Science Foundation of Sichuan Province (No. 2023NSFSC0622).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1307296/full#supplementary-material
1. Hermann, BP, Struck, AF, Busch, RM, Reyes, A, Kaestner, E, and McDonald, CR. Neurobehavioural comorbidities of epilepsy: towards a network-based precision taxonomy. Nat Rev Neurol. (2021) 17:731–46. doi: 10.1038/s41582-021-00555-z
2. Niesvizky-Kogan, I, Bass, M, Goldenholz, SR, and Goldenholz, DM. Focal cooling for drug-resistant epilepsy: a review. JAMA Neurol. (2022) 79:937–44. doi: 10.1001/jamaneurol.2022.1936
3. Kwan, P, Arzimanoglou, A, Berg, AT, Brodie, MJ, Allen Hauser, W, Mathern, G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. (2010) 51:1069–77. doi: 10.1111/j.1528-1167.2009.02397.x
4. Perucca, E, Perucca, P, White, HS, and Wirrell, EC. Drug resistance in epilepsy. Lancet Neurol. (2023) 22:723–34. doi: 10.1016/S1474-4422(23)00151-5
5. Servilha-Menezes, G, and Garcia-Cairasco, N. A complex systems view on the current hypotheses of epilepsy pharmacoresistance. Epilepsia Open. (2022) 7:S8–S22. doi: 10.1002/epi4.12588
6. Tang, F, Hartz, AMS, and Bauer, B. Drug-resistant epilepsy: multiple hypotheses, few answers. Front Neurol. (2017) 8:301. doi: 10.3389/fneur.2017.00301
7. Löscher, W, Potschka, H, Sisodiya, SM, and Vezzani, A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev. (2020) 72:606–38. doi: 10.1124/pr.120.019539
8. Nevitt, SJ, Sudell, M, Cividini, S, Marson, AG, and Tudur Smith, C. Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data. Cochrane Database Syst Rev. (2022) 4:CD011412. doi: 10.1002/14651858.CD011412.pub4
9. Lattanzi, S, Trinka, E, Zaccara, G, Striano, P, Russo, E, Del Giovane, C, et al. Third-generation antiseizure medications for adjunctive treatment of focal-onset seizures in adults: a systematic review and network meta-analysis. Drugs. (2022) 82:199–218. doi: 10.1007/s40265-021-01661-4
10. Engel, J. The current place of epilepsy surgery. Curr Opin Neurol. (2018) 31:192–7. doi: 10.1097/WCO.0000000000000528
11. Ziemann, U, Paulus, W, Nitsche, MA, Pascual-Leone, A, Byblow, WD, Berardelli, A, et al. Consensus: motor cortex plasticity protocols. Brain Stimul. (2008) 1:164–82. doi: 10.1016/j.brs.2008.06.006
12. The Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology. (1995) 45:224–30. doi: 10.1212/WNL.45.2.224
13. Velasco, M, Velasco, F, Velasco, AL, Boleaga, B, Jimenez, F, Brito, F, et al. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures and paroxysmal EEG activities. Epilepsia. (2000) 41:158–69. doi: 10.1111/j.1528-1157.2000.tb00135.x
14. Chen, R, Classen, J, Gerloff, C, Celnik, P, Wassermann, EM, Hallett, M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. (1997) 48:1398–403. doi: 10.1212/WNL.48.5.1398
15. Begemann, MJ, Brand, BA, Ćurčić-Blake, B, Aleman, A, and Sommer, IE. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol Med. (2020) 50:2465–86. doi: 10.1017/S0033291720003670
16. San-Juan, D, Espinoza López, DA, Vázquez Gregorio, R, Trenado, C, Fernández-González Aragón, M, Morales-Quezada, L, et al. Transcranial direct current stimulation in mesial temporal lobe epilepsy and hippocampal sclerosis. Brain Stimul. (2017) 10:28–35. doi: 10.1016/j.brs.2016.08.013
17. Tergau, F, Naumann, U, Paulus, W, and Steinhoff, BJ. Low-frequency repetitive transcranial magnetic stimulation improves intractable epilepsy. Lancet. (1999) 353:2209. doi: 10.1016/S0140-6736(99)01301-X
18. Mosilhy, EA, Alshial, EE, Eltaras, MM, Rahman, MMA, Helmy, HI, Elazoul, AH, et al. Non-invasive transcranial brain modulation for neurological disorders treatment: a narrative review. Life Sci. (2022) 307:120869. doi: 10.1016/j.lfs.2022.120869
19. Yap, JYY, Keatch, C, Lambert, E, Woods, W, Stoddart, PR, and Kameneva, T. Critical review of transcutaneous vagus nerve stimulation: challenges for translation to clinical practice. Front Neurosci. (2020) 14:284. doi: 10.3389/fnins.2020.00284
20. Guidetti, M, Bertini, A, Pirone, F, Sala, G, Signorelli, P, Ferrarese, C, et al. Neuroprotection and non-invasive brain stimulation: facts or fiction? Int J Mol Sci. (2022) 23:13775. doi: 10.3390/ijms232213775
21. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol. (2021) 74:790–9. doi: 10.1016/j.recesp.2021.06.016
22. Zhang, Y, Huang, L, Wang, D, Ren, P, Hong, Q, and Kang, D. The ROBINS-I and the NOS had similar reliability but differed in applicability: a random sampling observational studies of systematic reviews/meta-analysis. J Evid Based Med. (2021) 14:112–22. doi: 10.1111/jebm.12427
23. Lo, CK-L, Mertz, D, and Loeb, M. Newcastle–Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:45. doi: 10.1186/1471-2288-14-45
24. Higgins, JP, Altman, DG, Gotzsche, PC, Juni, P, Moher, D, Oxman, AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
25. Farag, HM, Yunusa, I, Goswami, H, Sultan, I, Doucette, JA, and Eguale, T. Comparison of amitriptyline and US Food and Drug Administration-approved treatments for fibromyalgia: a systematic review and network meta-analysis. JAMA Netw Open. (2022) 5:e2212939. doi: 10.1001/jamanetworkopen.2022.12939
26. Smolarz, B, Makowska, M, and Romanowicz, H. Pharmacogenetics of drug-resistant epilepsy (review of literature). Int J Mol Sci. (2021) 22:11696. doi: 10.3390/ijms222111696
27. Łukawski, K, and Czuczwar, SJ. Understanding mechanisms of drug resistance in epilepsy and strategies for overcoming it. Expert Opin Drug Metab Toxicol. (2021) 17:1075–90. doi: 10.1080/17425255.2021.1959912
28. Pong, AW, Xu, KJ, and Klein, P. Recent advances in pharmacotherapy for epilepsy. Curr Opin Neurol. (2023) 36:77–85. doi: 10.1097/WCO.0000000000001144
29. Carona, A, Bicker, J, Silva, R, Fonseca, C, Falcão, A, and Fortuna, A. Pharmacology of lacosamide: from its molecular mechanisms and pharmacokinetics to future therapeutic applications. Life Sci. (2021) 275:119342. doi: 10.1016/j.lfs.2021.119342
30. Babar, RK, Bresnahan, R, Gillespie, CS, and Michael, BD. Lacosamide add-on therapy for focal epilepsy. Cochrane Database Syst Rev. (2021) 5:CD008841. doi: 10.1002/14651858.CD008841.pub3
31. Chang, X-C, Yuan, H, Wang, Y, Xu, HQ, Hong, WK, Zheng, RY, et al. Eslicarbazepine acetate add-on for drug-resistant focal epilepsy. Cochrane Database Syst Rev. (2018) 2018:CD008907. doi: 10.1002/14651858.CD008907.pub3
32. Vossler, DG, Knake, S, O’Brien, TJ, Watanabe, M, Brock, M, Steiniger-Brach, B, et al. Efficacy and safety of adjunctive lacosamide in the treatment of primary generalised tonic-clonic seizures: a double-blind, randomised, placebo-controlled trial. J Neurol Neurosurg Psychiatry. (2020) 91:1067–75. doi: 10.1136/jnnp-2020-323524
33. Li, J, Sun, M, and Wang, X. The adverse-effect profile of lacosamide. Expert Opin Drug Saf. (2020) 19:131–8. doi: 10.1080/14740338.2020.1713089
34. Pozzi, M, Zanotta, N, Epifanio, R, Baldelli, S, Cattaneo, D, Clementi, E, et al. Lacosamide effectiveness and tolerability in patients with drug-resistant epilepsy and severe disability under polytherapy: therapy optimization as emerging from an observational study. Epilepsy Behav. (2022) 128:108598. doi: 10.1016/j.yebeh.2022.108598
35. Biton, V, Gil-Nagel, A, Isojarvi, J, Doty, P, Hebert, D, and Fountain, NB. Safety and tolerability of lacosamide as adjunctive therapy for adults with partial-onset seizures: analysis of data pooled from three randomized, double-blind, placebo-controlled clinical trials. Epilepsy Behav. (2015) 52:119–27. doi: 10.1016/j.yebeh.2015.09.006
36. Liguori, C, Toledo, M, and Kothare, S. Effects of anti-seizure medications on sleep architecture and daytime sleepiness in patients with epilepsy: a literature review. Sleep Med Rev. (2021) 60:101559. doi: 10.1016/j.smrv.2021.101559
37. Rosenow, F, Kelemen, A, Ben-Menachem, E, McShea, C, Isojarvi, J, Doty, P, et al. Long-term adjunctive lacosamide treatment in patients with partial-onset seizures. Acta Neurol Scand. (2016) 133:136–44. doi: 10.1111/ane.12451
38. Almeida, L, and Soares-da-Silva, P. Eslicarbazepine acetate (BIA 2-093). Neurotherapeutics. (2007) 4:88–96. doi: 10.1016/j.nurt.2006.10.005
39. Gama, H, Vieira, M, Costa, R, Graça, J, Magalhães, LM, and Soares-da-Silva, P. Safety profile of eslicarbazepine acetate as add-on therapy in adults with refractory focal-onset seizures: from clinical studies to 6 years of post-marketing experience. Drug Saf. (2017) 40:1231–40. doi: 10.1007/s40264-017-0576-4
40. Magalhães, LM, Costa, R, Vieira, M, Moreira, J, Gama, H, and Soares-da-Silva, P. Safety of eslicarbazepine acetate in elderly versus non-elderly patients with focal seizures: from pooled data of clinical studies to 8 years of post-marketing experience. Drug Saf. (2021) 44:1099–107. doi: 10.1007/s40264-021-01097-5
41. Altalib, H, Grinnell, T, Cantu, D, Ikedo, F, Vieira, M, Zhang, Y, et al. Psychiatric adverse events in three phase III trials of eslicarbazepine acetate for focal seizures. Epilepsia Open. (2022) 7:616–32. doi: 10.1002/epi4.12635
42. Krauss, G, Biton, V, Harvey, JH, Elger, C, Trinka, E, Soares da Silva, P, et al. Influence of titration schedule and maintenance dose on the tolerability of adjunctive eslicarbazepine acetate: an integrated analysis of three randomized placebo-controlled trials. Epilepsy Res. (2018) 139:1–8. doi: 10.1016/j.eplepsyres.2017.10.021
43. Nakamura, M, Cho, JH, Shin, H, and Jang, IS. Effects of cenobamate (YKP3089), a newly developed anti-epileptic drug, on voltage-gated sodium channels in rat hippocampal CA3 neurons. Eur J Pharmacol. (2019) 855:175–82. doi: 10.1016/j.ejphar.2019.05.007
44. Roberti, R, de Caro, C, Iannone, LF, Zaccara, G, Lattanzi, S, and Russo, E. Pharmacology of cenobamate: mechanism of action, pharmacokinetics, drug-drug interactions and tolerability. CNS Drugs. (2021) 35:609–18. doi: 10.1007/s40263-021-00819-8
45. Sharma, R, Nakamura, M, Neupane, C, Jeon, BH, Shin, H, Melnick, SM, et al. Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur J Pharmacol. (2020) 879:173117. doi: 10.1016/j.ejphar.2020.173117
46. Guignet, M, Campbell, A, and White, HS. Cenobamate (XCOPRI): can preclinical and clinical evidence provide insight into its mechanism of action? Epilepsia. (2020) 61:2329–39. doi: 10.1111/epi.16718
47. Vossler, DG. Remarkably high efficacy of cenobamate in adults with focal-onset seizures: a double-blind, randomized, placebo-controlled trial. Epilepsy Curr. (2020) 20:85–7. doi: 10.1177/1535759720903032
48. French, JA. Cenobamate for focal seizures—a game changer? Nat Rev Neurol. (2020) 16:133–4. doi: 10.1038/s41582-019-0309-7
49. Privitera, M, Richy, FF, and Schabert, VF. Indirect treatment comparison of cenobamate to other ASMs for the treatment of uncontrolled focal seizures. Epilepsy Behav. (2022) 126:108429. doi: 10.1016/j.yebeh.2021.108429
50. Krauss, GL, Klein, P, Brandt, C, Lee, SK, Milanov, I, Milovanovic, M, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. (2020) 19:38–48. doi: 10.1016/S1474-4422(19)30399-0
51. Kasteleijn-Nolst Trenité, DGA, Genton, P, Parain, D, Masnou, P, Steinhoff, BJ, Jacobs, T, et al. Evaluation of brivaracetam, a novel SV2A ligand, in the photosensitivity model. Neurology. (2007) 69:1027–34. doi: 10.1212/01.wnl.0000271385.85302.55
52. Brandt, C, Klein, P, Badalamenti, V, Gasalla, T, and Whitesides, J. Safety and tolerability of adjunctive brivaracetam in epilepsy: in-depth pooled analysis. Epilepsy Behav. (2020) 103:106864. doi: 10.1016/j.yebeh.2019.106864
53. Lattanzi, S, Cagnetti, C, Foschi, N, Provinciali, L, and Silvestrini, M. Brivaracetam add-on for refractory focal epilepsy: a systematic review and meta-analysis. Neurology. (2016) 86:1344–52. doi: 10.1212/WNL.0000000000002545
54. Ma, J, Huang, S, and You, C. Adjunctive brivaracetam for patients with refractory partial seizures: a meta-analysis of randomized placebo-controlled trials. Epilepsy Res. (2015) 114:59–65. doi: 10.1016/j.eplepsyres.2015.04.017
55. Bresnahan, R, Hill, RA, and Wang, J. Perampanel add-on for drug-resistant focal epilepsy. Cochrane Database Syst Rev. (2023) 2023:CD010961. doi: 10.1002/14651858.CD010961.pub2
56. Tsai, J-J, Ikeda, A, Hong, SB, Likasitwattanakul, S, and Dash, A. Efficacy, safety, and tolerability of perampanel in Asian and non-Asian patients with epilepsy. Epilepsia. (2019) 60:37–46. doi: 10.1111/epi.14642
57. Vigod, SN, Murphy, KE, Dennis, CL, Oberlander, TF, Ray, JG, Daskalakis, ZJ, et al. Transcranial direct current stimulation (tDCS) for depression in pregnancy: a pilot randomized controlled trial. Brain Stimul. (2019) 12:1475–83. doi: 10.1016/j.brs.2019.06.019
58. Brabenec, L, Klobusiakova, P, Simko, P, Kostalova, M, Mekyska, J, and Rektorova, I. Non-invasive brain stimulation for speech in Parkinson’s disease: a randomized controlled trial. Brain Stimul. (2021) 14:571–8. doi: 10.1016/j.brs.2021.03.010
59. Morone, G, Capone, F, Iosa, M, Cruciani, A, Paolucci, M, Martino Cinnera, A, et al. May dual transcranial direct current stimulation enhance the efficacy of robot-assisted therapy for promoting upper limb recovery in chronic stroke? Neurorehabil Neural Repair. (2022) 36:800–9. doi: 10.1177/15459683221138743
60. Gangemi, A, Colombo, B, and Fabio, RA. Effects of short- and long-term neurostimulation (tDCS) on Alzheimer’s disease patients: two randomized studies. Aging Clin Exp Res. (2021) 33:383–90. doi: 10.1007/s40520-020-01546-8
61. Yang, D, Wang, Q, Xu, C, Fang, F, Fan, J, Li, L, et al. Transcranial direct current stimulation reduces seizure frequency in patients with refractory focal epilepsy: a randomized, double-blind, sham-controlled, and three-arm parallel multicenter study. Brain Stimul. (2020) 13:109–16. doi: 10.1016/j.brs.2019.09.006
62. Rezakhani, S, Amiri, M, Weckhuysen, S, and Keliris, GA. Therapeutic efficacy of seizure onset zone-targeting high-definition cathodal tDCS in patients with drug-resistant focal epilepsy. Clin Neurophysiol. (2022) 136:219–27. doi: 10.1016/j.clinph.2022.01.130
63. Fregni, F, Otachi, PTM, do Valle, A, Boggio, PS, Thut, G, Rigonatti, SP, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol. (2006) 60:447–55. doi: 10.1002/ana.20950
64. Cantello, R, Rossi, S, Varrasi, C, Ulivelli, M, Civardi, C, Bartalini, S, et al. Slow repetitive TMS for drug-resistant epilepsy: clinical and EEG findings of a placebo-controlled trial. Epilepsia. (2007) 48:366–74. doi: 10.1111/j.1528-1167.2006.00938.x
65. Hallett, M. Transcranial magnetic stimulation: a primer. Neuron. (2007) 55:187–99. doi: 10.1016/j.neuron.2007.06.026
66. Yang, Y-W, Pan, W-X, and Xie, Q. Combined effect of repetitive transcranial magnetic stimulation and physical exercise on cortical plasticity. Neural Regen Res. (2020) 15:1986–94. doi: 10.4103/1673-5374.282239
67. Aloizou, A-M, Pateraki, G, Anargyros, K, Siokas, V, Bakirtzis, C, Liampas, I, et al. Transcranial magnetic stimulation (TMS) and repetitive TMS in multiple sclerosis. Rev Neurosci. (2021) 32:723–36. doi: 10.1515/revneuro-2020-0140
68. Jannati, A, Oberman, LM, Rotenberg, A, and Pascual-Leone, A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology. (2023) 48:191–208. doi: 10.1038/s41386-022-01453-8
69. Somaa, FA, de Graaf, TA, and Sack, AT. Transcranial magnetic stimulation in the treatment of neurological diseases. Front Neurol. (2022) 13:793253. doi: 10.3389/fneur.2022.793253
70. Zhou, X, Li, K, Chen, S, Zhou, W, Li, J, Huang, Q, et al. Clinical application of transcranial magnetic stimulation in multiple sclerosis. Front Immunol. (2022) 13:902658. doi: 10.3389/fimmu.2022.902658
71. Jiang, X, Yan, W, Wan, R, Lin, Y, Zhu, X, Song, G, et al. Effects of repetitive transcranial magnetic stimulation on neuropathic pain: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2022) 132:130–41. doi: 10.1016/j.neubiorev.2021.11.037
72. Gschwind, M, and Seeck, M. Transcranial direct-current stimulation as treatment in epilepsy. Expert Rev Neurother. (2016) 16:1427–41. doi: 10.1080/14737175.2016.1209410
73. Salazar, CA, Feng, W, Bonilha, L, Kautz, S, Jensen, JH, George, MS, et al. Transcranial direct current stimulation for chronic stroke: is neuroimaging the answer to the next leap forward? J Clin Med. (2023) 12:2601. doi: 10.3390/jcm12072601
74. D’Urso, G, Toscano, E, Sanges, V, Sauvaget, A, Sheffer, CE, Riccio, MP, et al. Cerebellar transcranial direct current stimulation in children with autism spectrum disorder: a pilot study on efficacy, feasibility, safety, and unexpected outcomes in tic disorder and epilepsy. J Clin Med. (2021) 11:143. doi: 10.3390/jcm11010143
75. San-Juan, D, del Castillo Calcáneo, JD, González-Aragón, MF, Bermúdez Maldonado, L, Moreno Avellán, Á, Gómez Argumosa, EV, et al. Transcranial direct current stimulation in adolescent and adult Rasmussen’s encephalitis. Epilepsy Behav. (2011) 20:126–31. doi: 10.1016/j.yebeh.2010.10.031
76. Auvichayapat, N, Rotenberg, A, Gersner, R, Ngodklang, S, Tiamkao, S, Tassaneeyakul, W, et al. Transcranial direct current stimulation for treatment of refractory childhood focal epilepsy. Brain Stimul. (2013) 6:696–700. doi: 10.1016/j.brs.2013.01.009
77. Sudbrack-Oliveira, P, Barbosa, MZ, Thome-Souza, S, Razza, LB, Gallucci-Neto, J, da Costa Lane Valiengo, L, et al. Transcranial direct current stimulation (tDCS) in the management of epilepsy: a systematic review. Seizure. (2021) 86:85–95. doi: 10.1016/j.seizure.2021.01.020
78. Vegrim, HM, Dreier, JW, Alvestad, S, Gilhus, NE, Gissler, M, Igland, J, et al. Cancer risk in children of mothers with epilepsy and high-dose folic acid use during pregnancy. JAMA Neurol. (2022) 79:1130–8. doi: 10.1001/jamaneurol.2022.2977
79. Arfman, IJ, Wammes-van der Heijden, EA, ter Horst, PGJ, Lambrechts, DA, Wegner, I, and Touw, DJ. Therapeutic drug monitoring of antiepileptic drugs in women with epilepsy before, during, and after pregnancy. Clin Pharmacokinet. (2020) 59:427–45. doi: 10.1007/s40262-019-00845-2
80. Laurin, A, Nard, N, Dalmont, M, Bulteau, S, Bénard, C, Bonnot, O, et al. Efficacy and safety of transcranial electric stimulation during the perinatal period: a systematic literature review and three case reports. J Clin Med. (2022) 11:4048. doi: 10.3390/jcm11144048
81. Hızlı Sayar, G, Ozten, E, Tufan, E, Cerit, C, Kağan, G, Dilbaz, N, et al. Transcranial magnetic stimulation during pregnancy. Arch Womens Ment Health. (2014) 17:311–5. doi: 10.1007/s00737-013-0397-0
82. Konstantinou, GN, Vigod, SN, Mehta, S, Daskalakis, ZJ, and Blumberger, DM. A systematic review of non-invasive neurostimulation for the treatment of depression during pregnancy. J Affect Disord. (2020) 272:259–68. doi: 10.1016/j.jad.2020.03.151
83. Pacheco, F, Guiomar, R, Brunoni, AR, Buhagiar, R, Evagorou, O, Roca-Lecumberri, A, et al. Efficacy of non-invasive brain stimulation in decreasing depression symptoms during the peripartum period: a systematic review. J Psychiatr Res. (2021) 140:443–60. doi: 10.1016/j.jpsychires.2021.06.005
Keywords: third-generation antiseizure medications, epilepsy, non-invasive brain stimulation, network meta-analysis, refractory epilepsy
Citation: Yang Y, Shangguan Y, Wang X, Liu R, Shen Z, Tang M and Jiang G (2024) The efficacy and safety of third-generation antiseizure medications and non-invasive brain stimulation to treat refractory epilepsy: a systematic review and network meta-analysis study. Front. Neurol. 14:1307296. doi: 10.3389/fneur.2023.1307296
Received: 05 October 2023; Accepted: 13 December 2023;
Published: 09 January 2024.
Edited by:
Luisa Lilia Rocha, National Polytechnic Institute of Mexico (CINVESTAV), MexicoReviewed by:
Walter Besio, University of Rhode Island, United StatesCopyright © 2024 Yang, Shangguan, Wang, Liu, Shen, Tang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guohui Jiang, bmV1cm9kb2N0b3JAMTYzLmNvbQ==
†These authors have contributed equally to this work
‡ORCID: Guohui Jiang, https://orcid.org/0000-0002-1267-2221
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.