- 1Department of Rehabilitation Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan
- 2Department of Physical Therapy, Faculty of Health Science, Juntendo University, Tokyo, Japan
- 3Department of Neurology, Juntendo University Hospital, Tokyo, Japan
Objectives: To investigate the construct validity of the Trunk Impairment Scale (TIS), which was developed to assess trunk impairment in patients with stroke, in patients with Parkinson’s disease (PD).
Design: This retrospective, cross-sectional study enrolled consecutive PD inpatients. Correlation analysis was performed to clarify whether the TIS assessment was related to other balance functions, lower extremity muscle strength, or walking ability. Factor analysis was performed to see how the background factors of TIS differ from balance function, lower limb muscle strength, and walking ability.
Results: Examining the data of 471 patients with PD, there were relationships between TIS and the Mini-Balance Evaluation Systems Test (r = 0.67), Barthel Index (r = 0.57), general lower limb extension torque (r = 0.51), two-minute walk test (r = 0.54), Hoehn and Yahr stage (r = −0.61), and Movement Disorder Society Unified Parkinson’s Disease Rating Scale part III total points (r = −0.59). Factor analysis showed that TIS items were divided into three factors (an abdominal muscles and righting reflex component; a perception and verticality component; and a rotational component), differing from other scales that included clinical assessment items.
Conclusion: The TIS can be useful for assessing the underlying trunk impairment as a basis for activities of daily living, gait function, and balance ability in patients with PD.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disease with loss of dopaminergic neurons in the substantia nigra pars compacta that causes bradykinesia, muscle rigidity, and resting tremor. Patients with PD also have axial symptoms, such as postural instability, gait disturbance, and postural abnormalities, during the advanced stages of the disease (1). Factors thought to cause axial symptoms include lower extremity muscle weakness, bradykinesia, rigidity, abnormal pattern of postural reflexes, dystonia, reduced gait automaticity, impaired proprioception, and impaired cognitive function, as well as poor trunk function (2–5). It is also known that trunk impairment is often observed (6).
Previous studies reported that trunk impairment is associated with postural instability, postural abnormality, and gait disturbance that reduce quality of life in patients with PD (3, 7–9). However, a battery of tests for trunk impairment focused on the trunk angle in one plane or the motor function and mobility of the trunk, and these studies separated each other. Moreover, they did not include an assessment of the perceptual aspect (6, 10–13). Proprioception and sensory integration are crucial for trunk function, but they were found to be impaired in patients with PD (14–16). Therefore, a comprehensive evaluation of trunk impairment for patients with PD should be a single assessment battery and include the perceptual aspect, in addition to the motor function and trunk deviation angle.
The Trunk Impairment Scale (TIS) reported by Fujiwara et al. (17) was originally developed to comprehensively assess the trunk impairment of patients with stroke based on the motor and perceptual aspects with seven items (1. perception of trunk verticality; 2, 3. trunk rotation muscle strength on the right and left sides; 4, 5. righting reflex on the right and left sides; 6. verticality; and 7. abdominal muscle strength). The TIS had good reliability and validity in patients with stroke (17). The TIS can predict functional prognosis in patients with early stroke and is an indicator of functional improvement in patients with PD (18, 19). However, the construct validity of TIS reported by Fujiwara has not been examined in patients with PD. This study aimed to assess the trunk impairment of patients with PD using the TIS reported by Fujiwara and to confirm its construct validity. Another objective was to analyze how the TIS is related to disease severity and various physical functions and to determine its clinical usefulness.
Methods
Study design
This retrospective, cross-sectional study was conducted with the approval of the Research Ethics Committee, Faculty of Medicine, Juntendo University (E22-0157-H01). Informed consent was obtained in the form of an opt-out on the website. This study is reported following the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) statement (20).
No previous study reported trunk function in PD including perceptual aspects in its assessment items without TIS reported by Fujiwara; therefore, a sample size calculation for analyzing correlations was considered based on a small effect size (r = 0.2), significance level of 0.01, and power of 0.95 to minimize the probability of committing type II error (21). Sample size calculations were performed with G*Power 3.1 (21). Since the medical record data from regular practice are expected to contain many deficiencies, over 431 patients’ medical record data were included in the study.
Study subjects
A total of 482 consecutive patients with PD of all ages who were admitted to the hospital from January 2019 to April 2022 and underwent rehabilitation were included in the study. The patients had been clinically diagnosed with established and probable Parkinson’s disease according to the Movement Disorder Society Clinical Diagnostic Criteria (1) by neurologists specialized in movement disorders.
The exclusion criteria included patients who were diagnosed with other neurodegenerative diseases (e.g., progressive supranuclear palsy or multiple system atrophy), had complications that severely impaired their physical performance (e.g., orthopedic disease, internal disease, or psychiatric disease), or patients who refused to participate in this research project. In addition, patients with significant missing records were also excluded.
Assessment items
Based on updated medical records when patients with PD visited the hospital, the data of age, sex, duration of disease, duration of levodopa medication, body mass index, Hoehn and Yahr stage, Levodopa Daily Dose (LDD), and Levodopa Equivalent Daily Dose (LEDD) were collected. Part II-Activity Daily living items of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) and Highest ON medication state of part III-Motor items of MDS-UPDRS were collected from updated medical records by neurologists specialized in movement disorders. The physical functions of the TIS, Barthel Index, Mini-Balance Evaluation Systems Test (Mini-BESTest), two-minute walk test, general lower extremity extension torque using the Strength Ergo 240, and handgrip force were assessed by physical therapists. The assessments of physical function were performed 60 to 120 min after oral dosing with confirmed ON-medication status. Each assessment for the physical functions is described in detail below.
Trunk impairment scale
The TIS consists of seven items: (1) perception of trunk verticality; (2, 3) trunk rotation muscle strength on the right and left sides; (4, 5) righting reflex on the right and left sides; (6) verticality; and (7) abdominal muscle strength. Each item is evaluated on a scale of 0 to 3, with a maximum score of 21 points; a higher score represents better trunk function. The required duration for the completion of the TIS is approximately 5 min. The Supplementary Material shows the details of the TIS’s definition, procedures, and scoring criteria (17).
Postural instability and gait dysfunction
Postural instability and gait dysfunction (PIGD) was assessed with the PIGD sub-score of MDS-UPDRS part III (item 3.9 Arising from chair, 3.10 Gait, 3.11 Freezing of gait, 3.12 Postural stability, 3.13 Posture) (22).
Specific balance abilities were measured with the Mini-Balance Evaluation Systems Test (Mini-BESTest), which consists of four different balance aspects (Anticipatory postural adjustments, Automatic postural responses, Sensory orientation, and Dynamic gait) with the highest score of 28 points (23); higher scores indicate better balance ability. Gait performance was assessed with a two-minute walk test (24, 25).
Strength of the upper and lower extremities and foot bradykinesia
General lower limb extension muscle strength was evaluated with the isokinetic mode of Strength Ergo 240 (SE240: Mitsubishi Electric Engineering Corporation, Tokyo, Japan) (19). The rotational speed was set at 50/min, and the backrest angle was set at 110 degrees during the measurement period. After orientation to strength measurement, the patients kicked pedals with five consecutive drives, and peak torque was calculated.
Handgrip force was measured using the CAMRY dynamometer (CAMRY EH101, Sensun Weighing Apparatus Group Ltd., Guangdong, China) in the sitting position with the humerus vertical and a 90-degree flexed elbow according to previous reports to avoid unexpected falls during the measurement (26, 27).
In addition to the assessments above, MDS-UPDRS part III item 7 toe-tapping was evaluated to clarify foot movement as a distal part of lower bradykinesia (22).
Statistical analysis
All data were tested for normality using the Shapiro–Wilk test. To investigate the construct validity of the TIS, the relationships between TIS and MDS-UPDRS items, Mini-BESTest, BI, Strength of Extremities, and Hoehn and Yahr stage were analyzed using Spearman’s correlation coefficients. To analyze confounding factors within parameters other than the TIS, multiple regression analysis was performed. To analyze the relationships of background factors in each item on the TIS and other physical functions, factor analysis with Promax rotation was used. To determine the number of factors before the factor analysis, the Bayesian information criterion was applied to allow for more objective calculations even with a large sample size. Other determination methods such as the Kaiser-Guttman criterion and scree plot were not used in this study due to the risk of overestimation or uncertain features of visual judgment methods. Randomly occurring missing values were complemented using the multiple imputation method. All statistical analyses were performed with the statistical software R version 4.2.0 for Windows, and the significance level was set at p < 0.05.
Results
After applying the exclusion criteria, data of 471 patients were selected for this study (Figure 1). Data of 11 patients with PD were excluded due to significant missing medical records.
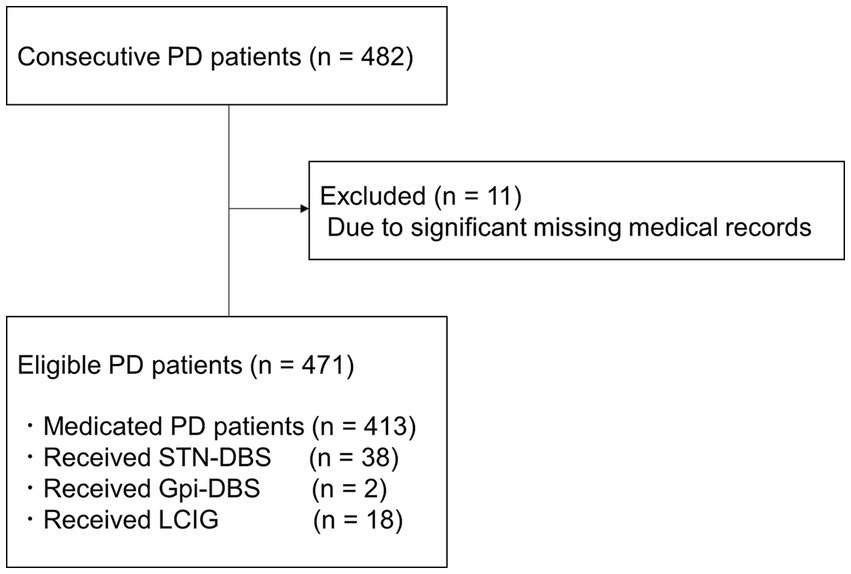
Figure 1. Schematic flow chart of study participants. PD, Parkinson’s disease; STN-DBS, Subthalamic Nucleus Deep Brain Stimulation; Gpi-DBS, Globus Pallidus Internus Deep Brain Stimulation; LCIG, Levodopa-carbidopa intestinal gel therapy.
Fifty-eight patients had previously received device-aided therapy, such as Subthalamic Nucleus Deep Brain Stimulation implantation (38 patients), Globus Pallidus Internus Deep Brain Stimulation implantation (2 patients), and Levodopa-carbidopa intestinal gel therapy (18 patients). Table 1 shows the demographic data of the eligible cases.
Spearman’s correlation coefficients showed moderate positive correlations between the TIS and the Mini-BESTest (r = 0.66, p < 0.001), Barthel Index (r = 0.57, p < 0.001), general lower limb extension torque (r = 0.51, p < 0.001), and the two-minute walk test (r = 0.54, p < 0.001). There were moderate negative correlations between the TIS and Hoehn and Yahr stage (r = −0.58, p < 0.001), MDS-UPDRS part III total points (r = −0.55, p < 0.001), and part III Postural stability (r = −0.51, p < 0.001) (Figure 2). In the other functions or general state, the TIS was weakly correlated with handgrip force (r = 0.46, p < 0.001), age (r = −0.37, p < 0.001), MDS-UPDRS part III toe-tapping (r = −0.34, p < 0.001), Arising from chair (r = −0.48, p < 0.001), Gait (r = −0.49, p < 0.001), Freezing of gait (r = −0.42, p < 0.001), and Posture (r = −0.45, p < 0.001). There were negligible correlations between the TIS and MDS-UPDRS part II Turning in bed (r = −0.29, p < 0.001), body mass index (r = 0.16, p = 0.007), duration of disease (r = −0.16, p < 0.001), duration of levodopa medication (r = −0.16, p < 0.001), and LEDD (r = 0.15, p = 0.0014).
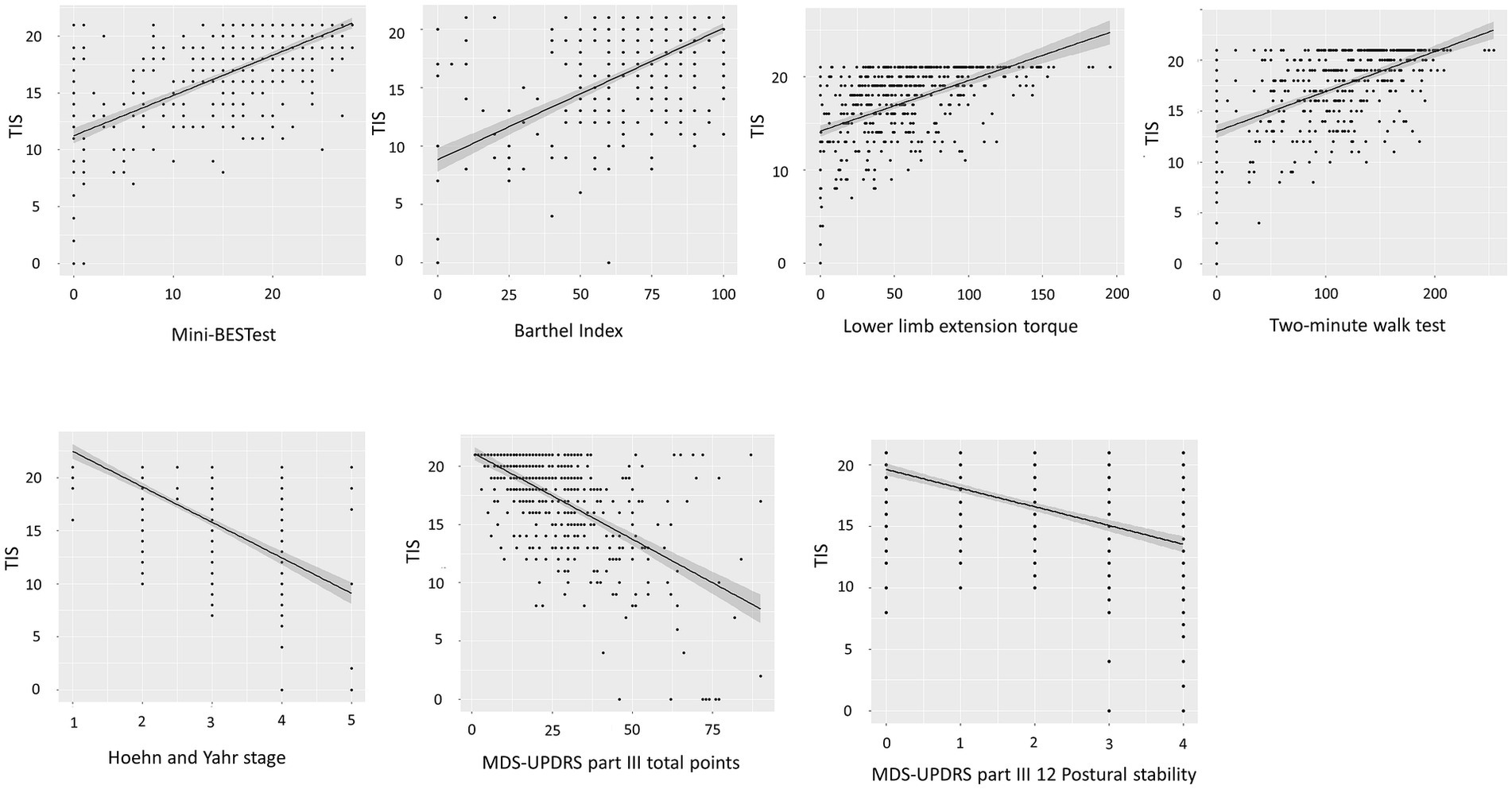
Figure 2. Scatter plots depicting the relationship between the TIS and associated assessment parameters. Spearman‘s correlation coefficients show a positive association of the TIS (range 0–21 points) and Mini-BESTest (range 0–28 points). Spearman’s correlation coefficients show negative associations of the TIS (range 0–21 points) and Hoehn and Yahr stage (range 1–5) and MDS-UPDRS part III total points (range 0–132 points). TIS, Trunk Impairment Scale; Mini-BESTest, Mini-Balance Evaluation Systems Test; MDS-UPDRS, Movement Disorders Society-unified Parkinson’s disease rating scale.
Table 2 shows the results of multiple regression analysis between the TIS and age, body height, body weight, duration of disease, years, Barthel Index, LEDD, MDS-UPDRS II Turning in bed, MDS-UPDRS III total score, Hoehn and Yahr stage, Mini-BESTest total score, general lower extremity extension torque, two-minute walk test, and handgrip force. The analysis showed that the independent variables Barthel Index (β = 0.14, p = 0.01), MDS-UPDRS II Turning in bed (β = −0.12, p = 0.002), Hoehn and Yahr stage (β = −0.16, p = 0.003), and Mini-BESTest total score (β = 0.27, p = 0.00003) contributed significantly to the dependent variable TIS.
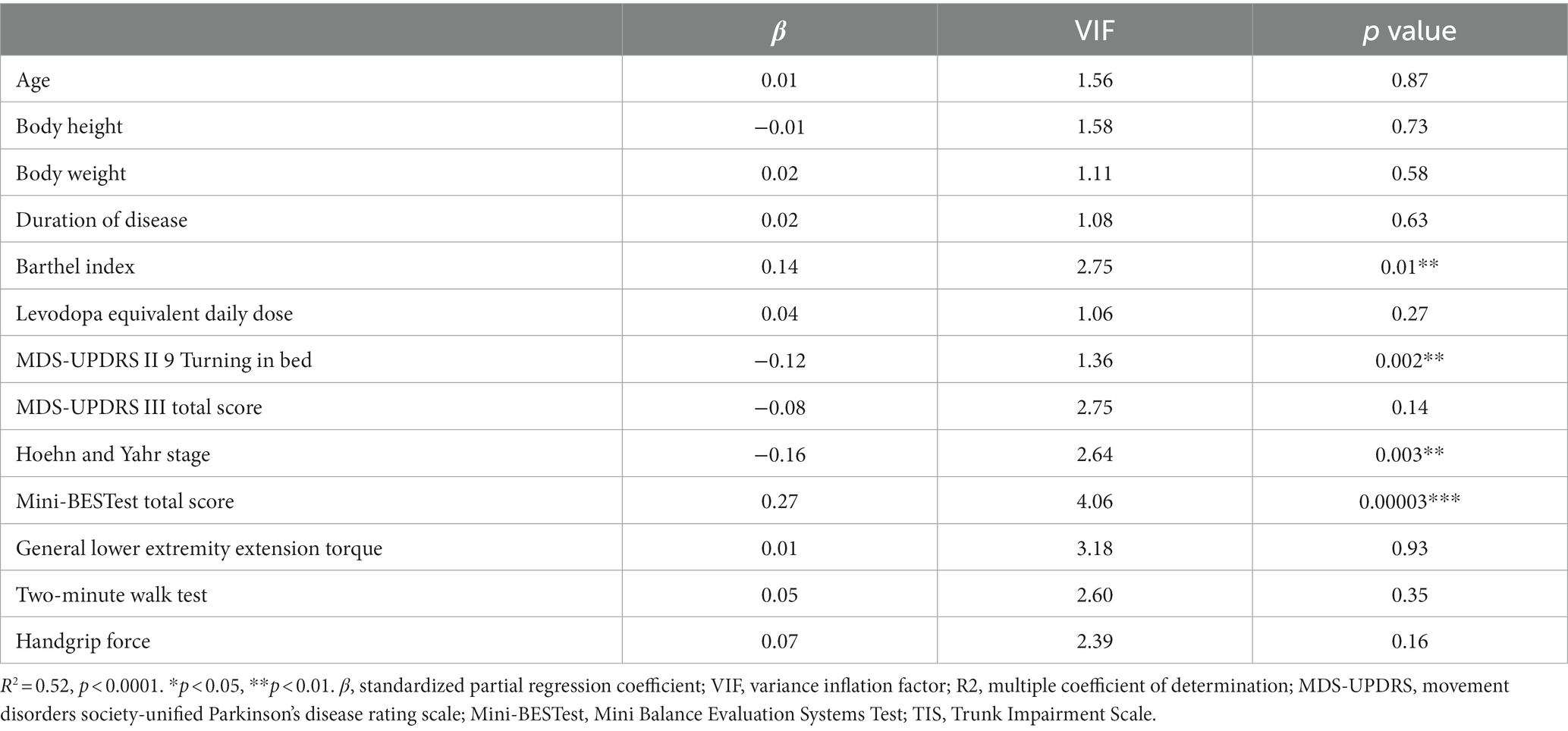
Table 2. Multiple regression analysis between the TIS total score and demographic and other clinical assessments.
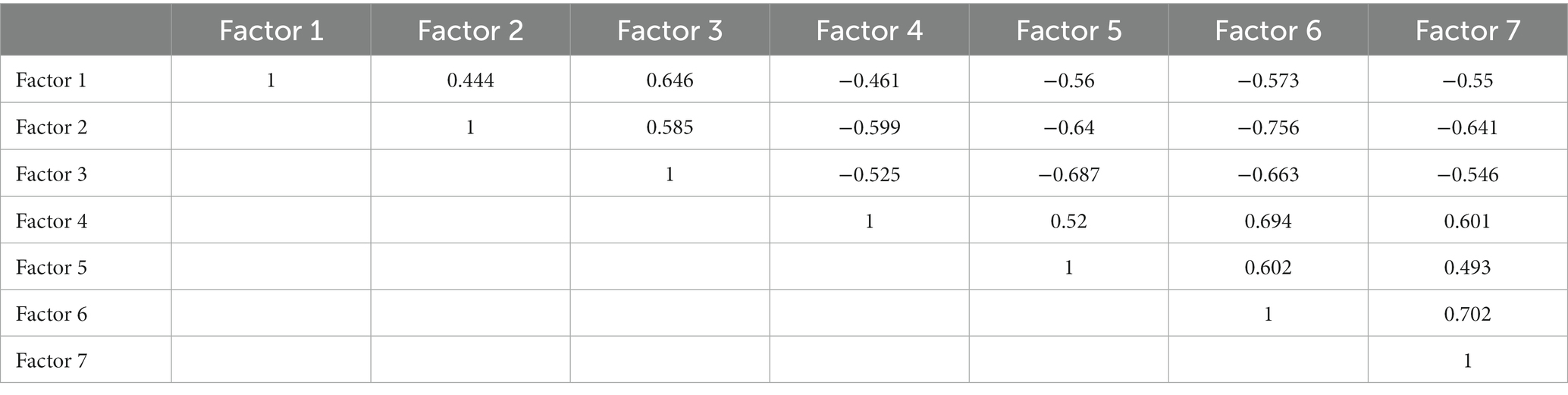
The Bayesian information criterion analysis yielded seven factors in the MDS-UPDRS part II Turning in bed, MDS-UPDRS III Toe tapping, PIGD score (Arising from chair, Gait, Freezing of gait, Postural stability, Posture), Mini-BESTest sub-score (Anticipatory postural adjustments, Automatic postural responses, Sensory orientation, and Dynamic gait), TIS sub-score (Perception of trunk verticality, Trunk rotation muscle strength on the right and left side, Righting reflex on the left and right side, Verticality, Abdominal muscle strength), general lower extremity extension torque, two-minute walk test, and handgrip force. The factor analysis with Promax rotation indicated that the first factor included the MDS-UPDRS II Turning in bed, MDS-UPDRS III Toe tapping, and PIGD score (Arising from chair, Gait, Freezing of gait, Postural stability, Posture). The second factor consisted of the Mini-BESTest sub-score (Anticipatory postural adjustments, Automatic postural responses, Sensory orientation, and Dynamic gait), and the two-minute walk test. The third factor comprised the TIS righting reflex on the left side, TIS righting reflex on the right side, and TIS abdominal muscle strength. The fourth factor included the TIS trunk rotation muscle strength on the right side and TIS trunk rotation muscle strength on the left side. The fifth factor involved the TIS perception of trunk verticality and TIS verticality. The sixth factor covered the general lower extremity extension torque, two-minute walk test, and handgrip force. The seventh factor included the MDS-UPDRS III Postural stability and Mini-BESTest Automatic postural responses (Table 3). All 7 factors showed moderate or low correlations with each other (Table 4).
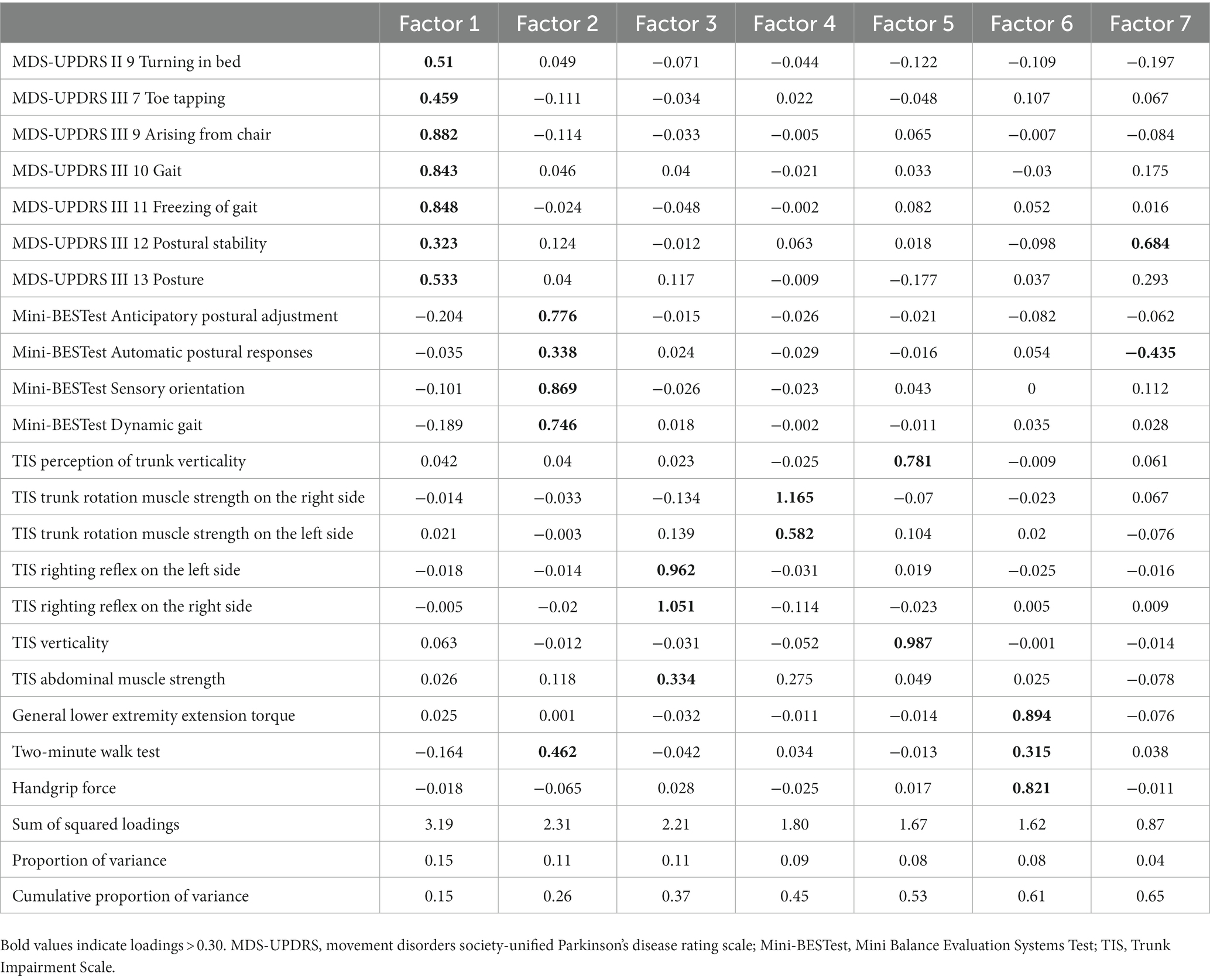
Table 3. Factor loading matrix for 21 variables after promax rotation for the physical performance data.
Discussion
The TIS was developed specifically to assess trunk function in patients with stroke. This is the first study to show that the TIS based on the motor function of the trunk and perceptual aspects had high construct validity. The present results showed that the TIS was correlated to disease severity, activities of daily living, limb strength, gait, and balance ability in patients with PD. Based on the results of correlation and multiple regression analysis, TIS may evaluate the trunk function related to balance function, disease severity, ADL function, lower limb strength, and which is different from the effects of age and BMI. The factors of the TIS were constructed with three different aspects (abdominal muscles and righting reflex component, perception and verticality component, and rotational component), and it differed from other factors that included clinical assessment items of Mini-BESTest, MDS-UPDRS part II Turning in bed, part III Postural instability and gait disturbance scores, two-minute walk test, and limb muscle strength. The TIS can be used to understand trunk impairment itself in patients with PD.
According to the previous studies, the factor analysis of TIS in patients with stroke did not divide the number of factors into multiple numbers, whereas multiple numbers were seen in patients with PD. The trunk impairment in stroke presents mainly with hypotonic hemiparesis, whereas PD presents with a variety of trunk impairments, including rigidity of trunk muscles (7), reduced righting reflexes (5), and altered vertical axis perception (5, 28). Although the results of this factor analysis might reflect the difference in the number of samples covered, there might be differences in background characteristics between stroke and PD.
After analyzing various factors such as MDS-UPDRS, Mini-BESTest, upper and lower extremity muscle strength, balance ability, and gait function, only the TIS was divided into three factors, even though these factors were correlated. Given that the Mini-BESTest is tailored explicitly to assess equilibrium in both standing and ambulatory scenarios, it was likely subjected to factor analysis as a single domain. The TIS administered in a supine or seated posture, and its broader assessment beyond mere motoric capacities of achievable or unattainable, might reflect why it was stratified into distinct domains during factor analysis. The elements that the TIS could capture separately were the [1] trunk righting reflex and abdominal muscle strength component, [2] the trunk rotation component, and [3] the verticality and vertical axis perception component, and it has been reported that, in PD, the trunk righting reflex and muscle strength (5), trunk rotation (9, 12), and vertical axis perception (5, 28) were decreased. The TIS in the present study is considered to be an assessment scale that is constructed to reflect these factors. The results of the factor analysis in the present study also suggest that the TIS may be an assessment index that can provide a more detailed evaluation of trunk dysfunction in patients with PD, which is difficult to obtain with other assessments.
In the clinical setting, trunk function is considered to be important for rolling over, but the TIS was not related to the MDS-UPDRS part II Turning in bed item in the present study. A previous study recruited only patients with PD with mild disease severity and showed that bed roll ability was related to trunk motor function, which was different from the result of the present study (6). One possible explanation is that these results might reflect the assessment method differences between the TIS, which reflects trunk dysfunction accurately by physical examination, and the MDS-UPDRS part II Turning in bed item, which allows turning movements even with compensatory movements, by questionnaire evaluation.
Previous studies have reported that trunk function in patients with PD affects ADL performance (6, 12), balance function, and gait function (7, 16). In the present study, the direct impact on trunk function on multiple regression analysis resulted in a low contribution rate, as indicated by the standard partial regression coefficients, although there were significant differences. Therefore, it is unlikely that ADL, balance function, and walking ability have a strong influence on trunk function due to these factors. From a clinical perspective, although ADL, balance function, and walking ability are correlated with trunk function, it is unlikely that they are confounding factors that influence trunk function. Rather, it is possible that trunk function influences ADLs, balance function, and walking ability.
Evaluation scales that separately quantify trunk function, balance function, and gait function in patients with PD have been scarce, making it difficult to capture the relationships among trunk function, balance function, and gait function in patients with PD, as well as trunk function itself. Since the TIS can comprehensively capture the trunk function of patients with PD from the motor and perceptual aspects, the clinical application of this assessment is considered highly meaningful.
The present study has some limitations. First, because this was a cross-sectional study, predictive factors of TIS itself or effect for the other physical functions such as balance function, and minimum clinically important difference could not be analyzed. Therefore, an additional cohort study should be planned. Second, the subjects in this study were only clinically diagnosed with PD, not pathologically confirmed. Third, this study did not contain the aspect of nonmotor symptoms and fluctuations of dopaminergic medications. A future study should include the assessment of nonmotor features and fluctuations of dopaminergic medications. In addition, it is necessary to conduct randomized, controlled studies to clarify the causal relationship to determine whether the improvement in trunk function captured by the TIS analyzed in the present study leads to an improvement in physical function in patients with PD.
Conclusion
The TIS can evaluate three different components including motor and perceptual aspects of trunk function separately from limb muscle strength, balance function, and parkinsonism. These findings suggest that the TIS can be useful for exploring the underlying trunk impairment as a basis for activities of daily living, gait function, and balance ability in patients with PD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Research Ethics Committee, Faculty of Medicine, Juntendo University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because informed consent was obtained in the form of an opt-out on the website.
Author contributions
KS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. YY: Data curation, Investigation, Writing – review & editing. YK: Data curation, Investigation, Writing – review & editing. KW: Data curation, Investigation, Writing – review & editing. EK: Data curation, Investigation, Writing – review & editing. KHar: Writing – review & editing. YTa: Writing – review & editing. YF: Writing – review & editing. TY: Writing – review & editing. TM: Writing – review & editing. HM: Writing – review & editing. RI: Writing – review & editing. YM: Writing – review & editing. MT: Writing – review & editing. KHo: Writing – review & editing. KHat: Writing – review & editing. YO: Writing – review & editing. YTo: Writing – review & editing. TH: Writing – review & editing. NH: Project administration, Supervision, Writing – review & editing. TF: Methodology, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank the medical staff of the Laboratory of Rehabilitation Medicine, Juntendo University Graduate School of Medicine for their support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1303215/full#supplementary-material
References
1. Postuma, RB, Berg, D, Stern, M, Poewe, W, Olanow, CW, Oertel, W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
2. Mak, MK, Pang, MY, and Mok, V. Gait difficulty, postural instability, and muscle weakness are associated with fear of falling in people with Parkinson's disease. Parkinsons Dis. (2012) 2012:901721. doi: 10.1155/2012/901721
3. Artigas, NR, Franco, C, Leao, P, and Rieder, CR. Postural instability and falls are more frequent in Parkinson's disease patients with worse trunk mobility. Arq Neuropsiquiatr. (2016) 74:519–23. doi: 10.1590/0004-282X20160074
4. Fasano, A, Canning, CG, Hausdorff, JM, Lord, S, and Rochester, L. Falls in Parkinson's disease: a complex and evolving picture. Mov Disord. (2017) 32:1524–36. doi: 10.1002/mds.27195
5. Bloem, BR . Postural instability in Parkinson's disease. Clin Neurol Neurosurg. (1992) 94:41–5. doi: 10.1016/0303-8467(92)90018-x
6. Verheyden, G, Willems, AM, Ooms, L, and Nieuwboer, A. Validity of the trunk impairment scale as a measure of trunk performance in people with Parkinson's disease. Arch Phys Med Rehabil. (2007) 88:1304–8. doi: 10.1016/j.apmr.2007.06.772
7. Cano-de-la-Cuerda, R, Vela-Desojo, L, Miangolarra-Page, JC, Macías-Macías, Y, and Muñoz-Hellín, E. Axial rigidity and quality of life in patients with Parkinson's disease: a preliminary study. Qual Life Res. (2011) 20:817–23. doi: 10.1007/s11136-010-9818-y
8. Spildooren, J, Vercruysse, S, Heremans, E, Galna, B, Vandenbossche, J, Desloovere, K, et al. Head-pelvis coupling is increased during turning in patients with Parkinson's disease and freezing of gait. Mov Disord. (2013) 28:619–25. doi: 10.1002/mds.25285
9. Yang, WC, Hsu, WL, Wu, RM, Lu, TW, and Lin, KH. Motion analysis of axial rotation and gait stability during turning in people with Parkinson's disease. Gait Posture. (2016) 44:83–8. doi: 10.1016/j.gaitpost.2015.10.023
10. Wright, WG, Gurfinkel, VS, Nutt, J, Horak, FB, and Cordo, PJ. Axial hypertonicity in Parkinson's disease: direct measurements of trunk and hip torque. Exp Neurol. (2007) 208:38–46. doi: 10.1016/j.expneurol.2007.07.002
11. Huxham, F, Baker, R, Morris, ME, and Iansek, R. Head and trunk rotation during walking turns in Parkinson's disease. Mov Disord. (2008) 23:1391–7. doi: 10.1002/mds.21943
12. Franco, CR, Leão, P, Townsend, R, and Rieder, CR. Reliability and validity of a scale for measurement of trunk mobility in Parkinson's disease: trunk mobility scale. Arq Neuropsiquiatr. (2011) 69:636–41. doi: 10.1590/s0004-282x2011000500012
13. Bridgewater, KJ, and Sharpe, MH. Trunk muscle performance in early Parkinson's disease. Phys Ther. (1998) 78:566–76. doi: 10.1093/ptj/78.6.566
14. Carpenter, MG, and Bloem, BR. Postural control in Parkinson patients: a proprioceptive problem? Exp Neurol. (2011) 227:26–30. doi: 10.1016/j.expneurol.2010.11.007
15. Vaugoyeau, M, Hakam, H, and Azulay, JP. Proprioceptive impairment and postural orientation control in Parkinson's disease. Hum Mov Sci. (2011) 30:405–14. doi: 10.1016/j.humov.2010.10.006
16. Wright, WG, Gurfinkel, VS, King, LA, Nutt, JG, Cordo, PJ, and Horak, FB. Axial kinesthesia is impaired in Parkinson's disease: effects of levodopa. Exp Neurol. (2010) 225:202–9. doi: 10.1016/j.expneurol.2010.06.016
17. Fujiwara, T, Liu, M, Tsuji, T, Sonoda, S, Mizuno, K, Akaboshi, K, et al. Development of a new measure to assess trunk impairment after stroke (trunk impairment scale): its psychometric properties. Am J Phys Med Rehabil. (2004) 83:681–8. doi: 10.1097/01.phm.0000137308.10562.20
18. Ishiwatari, M, Honaga, K, Tanuma, A, Takakura, T, Hatori, K, Kurosu, A, et al. Trunk impairment as a predictor of activities of daily living in acute stroke. Front Neurol. (2021) 12, 12:665592. doi: 10.3389/fneur.2021.665592
19. Sato, K, Hokari, Y, Kitahara, E, Izawa, N, Hatori, K, Honaga, K, et al. Short-term motor outcomes in Parkinson's disease after subthalamic nucleus deep brain stimulation combined with post-operative rehabilitation: a pre-post comparison study. Parkinsons Dis. (2022) 2022:8448638. doi: 10.1155/2022/8448638
20. von Elm, E, Altman, DG, Egger, M, Pocock, SJ, Gøtzsche, PC, and Vandenbroucke, JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. (2007) 335:806–8. doi: 10.1136/bmj.39335.541782.AD
21. Faul, F, Erdfelder, E, Lang, AG, and Buchner, A. G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/bf03193146
22. Goetz, CG, Tilley, BC, Shaftman, SR, Stebbins, GT, Fahn, S, Martinez-Martin, P, et al. Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. (2008) 23:2129–70. doi: 10.1002/mds.22340
23. Franchignoni, F, Horak, F, Godi, M, Nardone, A, and Giordano, A. Using psychometric techniques to improve the balance evaluation systems test: the mini-BESTest. J Rehabil Med. (2010) 42:323–31. doi: 10.2340/16501977-0537
24. Bohannon, RW, Bubela, D, Magasi, S, McCreath, H, Wang, YC, Reuben, D, et al. Comparison of walking performance over the first 2 minutes and the full 6 minutes of the six-minute walk test. BMC Res Notes. (2014) 7:269. doi: 10.1186/1756-0500-7-269
25. Bohannon, RW, Wang, YC, and Gershon, RC. Two-minute walk test performance by adults 18 to 85 years: normative values, reliability, and responsiveness. Arch Phys Med Rehabil. (2015) 96:472–7. doi: 10.1016/j.apmr.2014.10.006
26. Vargas-Pinilla, OC, and Rodríguez-Grande, EI. Reproducibility and agreement between three positions for handgrip assessment. Sci Rep. (2021) 11:12906. doi: 10.1038/s41598-021-92296-8
27. Huang, L, Liu, Y, Lin, T, Hou, L, Song, Q, Ge, N, et al. Reliability and validity of two hand dynamometers when used by community-dwelling adults aged over 50 years. BMC Geriatr. (2022) 22:580. doi: 10.1186/s12877-022-03270-6
Keywords: Parkinson’s disease, trunk impairment, rehabilitation, physical therapy, factor analysis
Citation: Sato K, Yamazaki Y, Kameyama Y, Watanabe K, Kitahara E, Haruyama K, Takahashi Y, Fujino Y, Yamaguchi T, Matsuda T, Makabe H, Isayama R, Murakami Y, Tani M, Honaga K, Hatori K, Oji Y, Tomizawa Y, Hatano T, Hattori N and Fujiwara T (2024) Factor analysis for construct validity of a trunk impairment scale in Parkinson’s disease: a cross-sectional study. Front. Neurol. 14:1303215. doi: 10.3389/fneur.2023.1303215
Edited by:
Claudio Belvedere, Rizzoli Orthopedic Institute (IRCCS), ItalyReviewed by:
Giorgio Cassiolas, Rizzoli Orthopedic Institute (IRCCS), ItalyAnjali Sivaramakrishnan, The University of Texas Health Science Center at San Antonio, United States
Copyright © 2024 Sato, Yamazaki, Kameyama, Watanabe, Kitahara, Haruyama, Takahashi, Fujino, Yamaguchi, Matsuda, Makabe, Isayama, Murakami, Tani, Honaga, Hatori, Oji, Tomizawa, Hatano, Hattori and Fujiwara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazunori Sato, a3NhdG9oQGp1bnRlbmRvLmFjLmpw
 Kazunori Sato
Kazunori Sato Yuta Yamazaki
Yuta Yamazaki Yoshihiro Kameyama1
Yoshihiro Kameyama1 Yoko Takahashi
Yoko Takahashi Tomofumi Yamaguchi
Tomofumi Yamaguchi Tadamitsu Matsuda
Tadamitsu Matsuda Hitoshi Makabe
Hitoshi Makabe Reina Isayama
Reina Isayama Mami Tani
Mami Tani Kaoru Honaga
Kaoru Honaga Yutaka Oji
Yutaka Oji Yuji Tomizawa
Yuji Tomizawa Taku Hatano
Taku Hatano Nobutaka Hattori
Nobutaka Hattori Toshiyuki Fujiwara
Toshiyuki Fujiwara