
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 08 January 2024
Sec. Neurorehabilitation
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1293153
This article is part of the Research Topic Perioperative neurocognitive disorders View all 5 articles
Background: Postoperative cognitive dysfunction (POCD) and postoperative delirium (POD) are common post-surgical complications that often lead to prolonged hospitalization, reduced quality of life, increased healthcare costs, and increased patient mortality. We conducted a meta-analysis to evaluate the effects of preoperative cognitive function training on postoperative cognitive function.
Methods: PubMed, Cochrane Library, Embase, Web of Science, ClinicalTrials, China National Knowledge Infrastructure, Wanfang Database, VIP Database, and Chinese Biomedical Literature Database were searched for randomized controlled trials comparing the effects of preoperative cognitive function training and conventional preoperative measures on postoperative cognitive function. The search period spanned from the establishment of the databases to March 31, 2023. The primary outcomes were the incidence of POCD and POD.
Results: Eleven randomized controlled trials involving 1,045 patients were included. The results of the meta-analysis showed that, compared to the control group, preoperative cognitive function training significantly reduced the incidence of POCD (RR = 0.38, P < 0.00001), and there was no statistically significant difference in the incidence of POD (P = 0.3). Cognitive function training significantly improved postoperative cognitive function scores compared with the control group (MD = 1.92, P = 0.001). In addition, two studies reported that 10% of the patients in the cognitive training group completed a pre-set training duration.
Conclusion: Cognitive function training significantly reduced the incidence of POCD; however, there was no significant difference in the incidence of POD. Preoperative cognitive function training should be promoted and emphasized as a simple, economical, and practical method of improving postoperative cognitive function.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=396154
Postoperative cognitive dysfunction (POCD) and postoperative delirium (POD) are common complications of surgical procedures that mainly manifest as cognitive dysfunction, especially learning, memory, and attention disorders (1–3). The incidence of POCD ranges from 30 to 41% (4) after non-cardiac surgery and is as high as 43% after cardiac surgery (5). The incidence of POD ranges from 4.7 to 13.7% (6). POCD and POD often result in patient and family suffering, including prolonged hospital stays, reduced quality of life, increased healthcare costs, and even increased mortality rates (7, 8). However, current research on the pathogeneses of POCD and POD is unclear and complex, and common risk factors include the patient's preoperative cognitive functional status, coexisting chronic diseases, nutritional status, anesthesia, surgery, and pain (9–11). Current evidence suggests that drug interventions have an uncertain effect on cognitive impairment (12, 13); therefore, non-drug interventions for POCD and POD can be an effective preventive measure and the focus of future research.
Cognitive function training is the most common non-pharmacological intervention for the clinical improvement of cognitive impairment (14). Cognitive function training refers to programs that encompass structured exercises targeting specific cognitive tasks, aiming to enhance performance in one or multiple cognitive domains, including, but not limited to, memory, attention, and executive function (15). Playing computer games, reading books, exercising, writing, practicing object or word recall, improving spatial memory, and promoting communication and interaction are among the most frequently employed methods of cognitive function training (16, 17). Cognitive function training significantly improves cognitive function in many cognitive disorders, including Parkinson's disease (18), stroke (19), mild cognitive impairment (20), and depression (21). However, whether preoperative cognitive function training can reduce the incidence of POCD and POD remains controversial. Saleh et al. found that preoperative cognitive function training significantly reduced the incidence of POCD after gastrointestinal surgery (16). However, O'Gara et al. found that preoperative and postoperative cognitive function training did not reduce the incidence of POCD or POD. In addition, the cognitive function training group demonstrated lower treatment adherence (22). There have been no meta-analyses of the effects of preoperative cognitive function training on POCD or POD. Therefore, we conducted a meta-analysis to investigate this topic.
This meta-analysis was performed following the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist (23). This study was registered in PROSPERO under the number CRD4202339 6154 (crd.york.ac.uk/prospero/display_record.php?RecordID=396154). Two reviewers (Zhao Li and Guo Yiping) independently searched PubMed, Cochrane Library, Embase, Web of Science, ClinicalTrials, CNKI, Wanfang Database, VIP Database, and Chinese Biomedical Literature Database from the inception of the databases to March 31, 2023. The language used was limited to English or Chinese. The search terms used were as follows: “cognitive function training or cognitive training or cognitive intervention” and “postoperative cognitive dysfunction or POCD or postoperative delirium or POD.” In addition, we searched the reference lists of the identified articles for relevant studies and screened the International Clinical Trials Registry Platform.
The inclusion criteria were as follows: (1) Patients undergoing surgery under general anesthesia without cognitive impairment before surgery, regardless of age, sex, or type of surgery. (2) The experimental group underwent cognitive function training before surgery. (3) Patients in the control group were treated only for the disease itself without cognitive function training. (4) Outcome measures included the incidence of POCD and POD. (5) There was no statistical difference in cognitive function between the cognitive function training and control groups at the time of enrollment. (6) The included studies were randomized controlled trials. We excluded studies in which data could not be extracted or used for analysis.
The primary outcomes were the incidence of POD and POCD. The secondary outcomes were treatment compliance and scores of cognitive function.
Data extraction and quality assessment were performed independently by two authors (Zhao Li and Guo Yiping). Any disagreements were resolved through discussion with the corresponding author (Li Linji). The following information was extracted: the name of the first author, publication year, country, average age of the participants, sample size, type of surgery, and intervention measures. The study quality was assessed using the Cochrane Risk of Bias Tool. Data conversion tools were used to convert the interquartile ranges to means and standard deviations.
Data analysis was conducted using Stata (version 14) and Review Manager (version 5.3). Dichotomous and continuous data were statistically analyzed using the risk ratio (RR) and mean difference (MD) with a 95% confidence interval (CI). A significance level of P < 0.05 was considered statistically significant. Statistical heterogeneity was assessed using the I2 statistic, with I2 > 50% indicating high heterogeneity, and I2 < 50% indicating low heterogeneity. In case of high heterogeneity, a random-effects model was used, whereas a fixed-effects model was employed for low heterogeneity. Sensitivity and subgroup analyses were performed for studies with high levels of heterogeneity. Publication bias was evaluated using Egger's test and a funnel plot.
We initially identified 235 studies using a database search. Eventually, 11 studies were included (16, 17, 24–32), with a sample size of 1,045 cases, including 517 patients in the cognitive function training group and 528 patients in the control group. A flowchart of the study selection process is shown in Figure 1.
The characteristics of the studies are presented in Table 1. Eight and three studies assessed the effects of preoperative cognitive function training on POCD and POD, respectively.
The results of the risk of bias assessment for the included studies are shown in Figure 2. Nine studies were considered to have an unclear risk of allocation concealment (16, 24–31). Three studies (16, 17, 32) were considered to have high risk and six studies (24, 26, 27, 29–31) were considered to have an unclear high risk of participant blinding. Four studies (24, 26, 29, 30) were considered to have unclear risk in outcome assessment blinding. One study (28) did not provide a complete baseline value and another (30) had a small sample size, which may have contributed to other biases.
Eight studies with 682 patients assessed the incidence of POCD (16, 24, 25, 27–31). Five studies reported the incidence of POCD 7 days postoperatively (16, 24, 27, 28, 31). One study (25) reported POCD 1 day after surgery, one study (30) reported POCD 10 days after surgery, and another (29) POCD 14 days after surgery. In four studies (16, 25, 30, 31), POCD was defined when the neuropsychological test battery decreased by one standard deviation. In three studies (27, 28, 31) POCD was defined when Mini-Mental State Examination (MMSE) was < 24 points. One study (29) identified POCD as having a Montreal Cognitive Assessment (MoCA) score of < 26. Because of the low heterogeneity (I2 = 0%) and lack of significant heterogeneity in the Galbraith plot (Figure 3C), a fixed-effects model was used. The result showed that preoperative cognitive training significantly reduced the incidence of POCD compared with the control group (RR = 0.38, 95% CI: 0.28–0.52, P < 0.00001, Figure 3A). Egger's test (P = 0.098) and funnel plot (Figure 3B) did not reveal significant publication bias. Subgroup analysis showed that cognitive training significantly reduced the incidence of POCD in both cardiac and non-cardiac surgeries (P < 0.00001 and P = 0.0006, respectively).
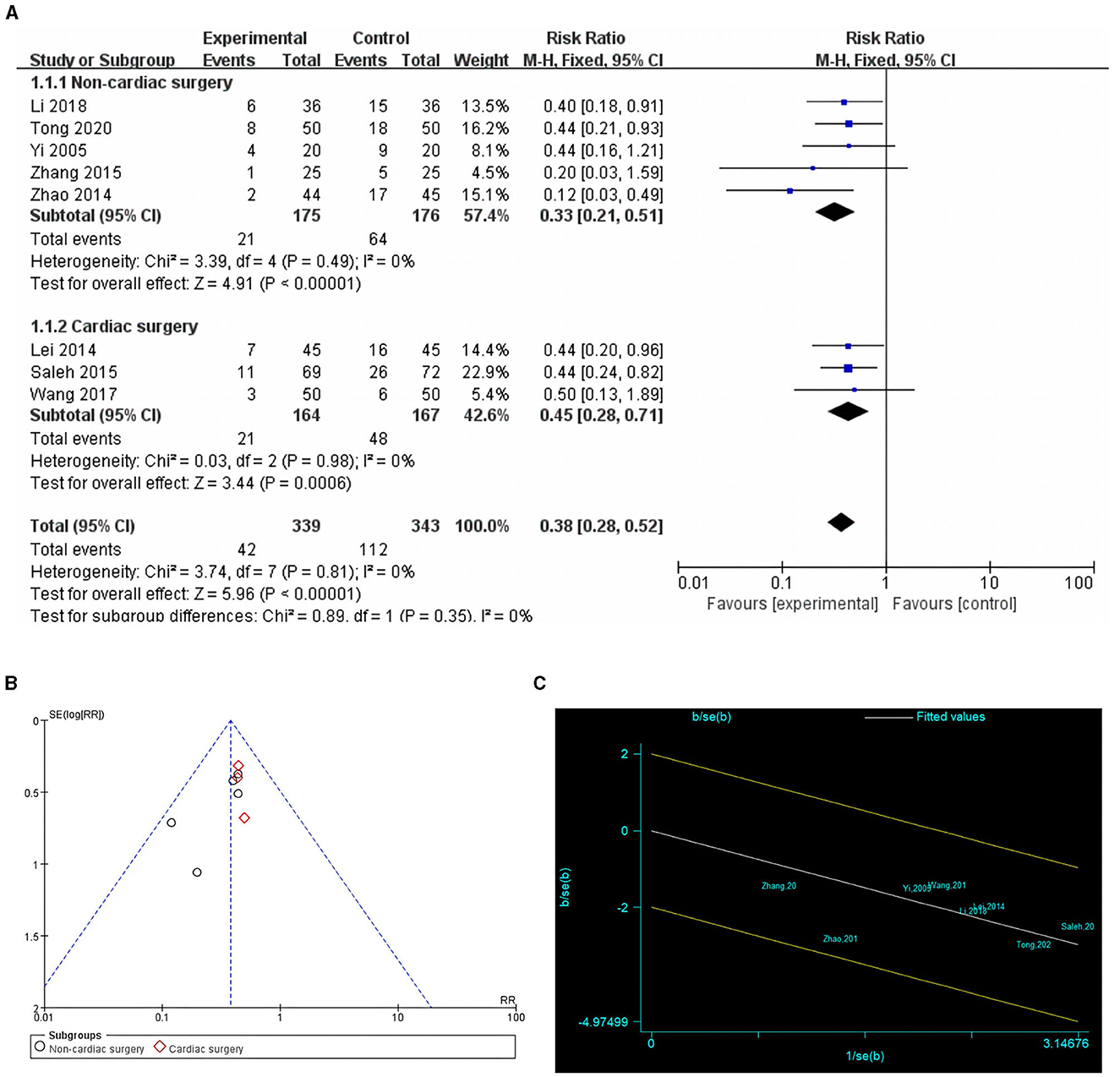
Figure 3. Comparison of POCD incidence in cognitive training and control groups. (A) Forest plot; (B) funnel plot; (C) Galbraith plots.
Three studies assessed the incidence of POD (17, 28, 32). Owing to the high heterogeneity (I2 = 70%), a random-effects model was used. There was no statistically significant difference between the two groups (RR = 0.58, P = 0.30; Figure 4). Sensitivity analysis and publication bias tests were not performed owing to the small amount of research literature.
Five studies reported postoperative cognitive function scores (24, 26–29), with four using the MMSE, and one using the MoCA (29). The difference between the postoperative cognitive function scores and pre-intervention baseline values was called the postoperative cognitive function score difference. This difference was used to indicate the degree of postoperative cognitive decline due to the effects of anesthesia and surgery. Owing to the high heterogeneity (I2 = 73%), a random-effects model was chosen. The results showed that preoperative cognitive function training significantly improved postoperative cognitive function scores compared to the control group (MD = 1.92, P = 0.0001, Figure 5A). Galbraith plots suggested that there was significant heterogeneity between the study by Wang et al. and the other studies (Figure 5C). Sensitivity analyses excluding the included studies one by one showed no significant changes in the results of the studies, indicating that the results were stable. The results of the subgroup analyses showed statistically significant differences between the results of the cognitive function training group and the control group in studies using the MMSE and MoCA scales (P = 0.002 and P = 0.003, respectively). Egger's test (P = 0.617) and funnel plot (Figure 5B) did not reveal significant publication bias.
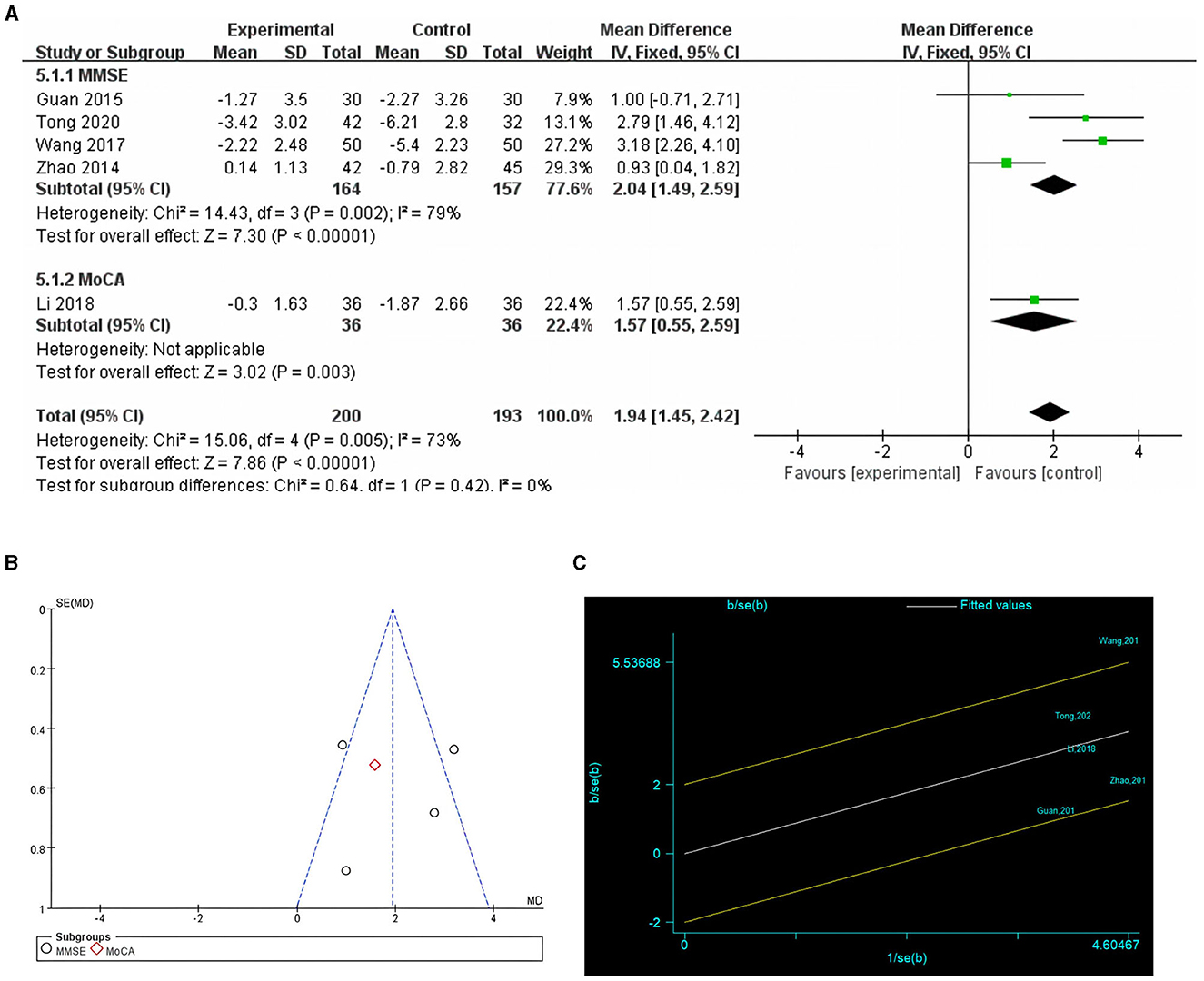
Figure 5. Comparison of cognitive function scores in cognitive training and control groups. (A) Forest plot; (B) funnel plot; (C) Galbraith plots.
Two studies reported treatment compliance of cognitive function training (17, 32). The proportion of patients who completed the predetermined training duration was referred to as treatment compliance. A fixed-effects model was chosen because of the presence of low heterogeneity (I2 = 21.9%) and the results showed that the completion rate of cognitive function training was 10% (Figure 6).
As the demand for perioperative comfort treatment increases, more studies have begun to focus on perioperative neurocognitive disorders (PND) (33, 34). In this meta-analysis, we evaluated the effects of preoperative cognitive function training on postoperative neurocognitive function. This is the first meta-analysis to assess the effect of preoperative cognitive function training on POCD and POD.
The 2018 guidelines established a definition for PND that encompassed preoperative cognitive impairment, POD, delayed neurocognitive recovery, and cognitive decline identified within 2–12 months after surgery (35). As most previous studies have used POD and POCD as outcome indicators of postoperative cognitive function, we used POCD and POD to assess postoperative cognitive function.
This meta-analysis showed that preoperative cognitive function training significantly reduced the incidence of POCD compared to the control group (RR = 0.38, P < 0.00001), and the difference was not statistically significant in the incidence of POD (P = 0.30). Preoperative cognitive function training significantly improved postoperative cognitive function scores compared with the control group (MD = 1.92, P = 0.0001). In addition, treatment compliance with cognitive function training was 10%.
This meta-analysis showed that preoperative cognitive function training significantly reduced the incidence of POCD (P < 0.00001) with low heterogeneity (I2 = 0%) compared with conventional preoperative measures. The mechanism by which cognitive function training improves cognitive function may be as follows: first, cognitive function training may increase the density of cortical dopamine D1 receptors, which depend on adequate dopamine neurotransmission for key functions of human cognition (36, 37). Second, cognitive function training has been found to potentially enhance cognitive reserve. Some studies have found that patients with greater cognitive reserve, such as those with higher education, have a lower incidence of POCD (38). Therefore, cognitive function training may improve cognitive functions in patients by enhancing cognitive reserve (39). Third, cognitive function, perception, and memory function have been shown to decline progressively with age; however, the brain retains lifelong plasticity and adaptive reorganization ability. Therefore, some cognitive functions of the brain can be improved by using appropriately designed training programs (40, 41).
Anxiety is a risk factor of POCD (9). Since most studies did not achieve patient blinding, this may have led to some preoperative anxiety in patients in the preoperative routine treatment group compared to those in the cognitive training group, which may have biased the results. In most included studies, the control group did not receive any cognitive intervention. Assuming active control is used, such as a simple cognitive intervention. It can help in more accurately assessing the true effects of cognitive training and its cost-effectiveness ratio. Among the included studies, we assessed only the effects of preoperative cognitive training on postoperative cognitive outcomes. However, these studies lacked the transfer effect of preoperative cognitive training in everyday life or real-life scenarios. Future research could further explore the effects of cognitive training on real-life transfers and better understand the potential benefits of cognitive training in improving cognitive function.
This meta-analysis showed no statistically significant difference between the preoperative cognitive function training and control groups in terms of the incidence of POD (P = 0.30). The quality of evidence for this outcome metric was low, as only three studies reported POD.
Two studies reported a 10% rate of people completing the scheduled training duration (17, 32). One of these articles reported that the reasons for low adherence were lack of computer access, time constraints, and feeling overwhelmed (32). Therefore, simplification of training methods and provision of computer assistance are necessary to avoid low adherence to cognitive function training. As compliance was reported in only two studies, the quality of evidence for the outcome of treatment compliance was low. In addition, In O'Gara et al.'s study, it was found that most people completed >40% of their cognitive training duration (22).
Currently, various methods of cognitive training are available, including application software for electronic equipment, reading and writing training, mental activity, and compound cognitive training. More large-sample randomized controlled trials are needed to determine which of these training modalities is more helpful in improving postoperative cognitive function. A large number of current studies have used electronic devices for cognitive function training as a simple, economical, practical, and significant improvement in postoperative cognitive function, especially POCD, and should, therefore, be worthy of attention and promotion.
This meta-analysis revealed high heterogeneity in the pooled results. These heterogeneities may be due to differences in the duration and manner of cognitive function training. Furthermore, the diagnostic tools for cognitive function and the time of assessment were not consistent.
This study had some limitations. First, the sample size is relatively small. Second, some of the pooled results of this study suggest high heterogeneity. This heterogeneity may be due to differences in the duration and modality of cognitive function training, diagnostic tools for cognitive function, and inconsistencies in the timing of assessments. Third, because the incidence of POCD and POD was the primary outcome, we excluded studies that did not include data on POCD and POD; therefore, the application of secondary outcomes may be limited. Fourth, the main intervention in this study was preoperative cognitive function training; however, postoperative cognitive function training has also been reported to improve cognitive function (42). Fifth, the included studies only investigated the POCD incidence up to 14 days after surgery and lacked efficacy data beyond 1 month. Therefore, additional randomized controlled trials are required to verify the comprehensive effects of perioperative cognitive function training.
Our meta-analysis found that preoperative cognitive function training significantly reduced the incidence of POCD and improved postoperative cognitive function scores. However, the difference in the incidence of POD was not statistically significant. In addition, two studies showed low treatment compliance for cognitive function training. Preoperative cognitive function training should be promoted and emphasized as a simple, economical, and practical method of improving postoperative cognitive function.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
LZ: Writing—original draft, Writing—review & editing. YG: Writing—original draft, Writing—review & editing. XZ: Data curation, Formal analysis, Methodology, Software, Writing—original draft. WM: Data curation, Formal analysis, Methodology, Software, Writing—original draft. LL: Conceptualization, Investigation, Project administration, Supervision, Writing—review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by research funds from Nanchong Science and Technology Bureau (22SXQT0293).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Terrando N, Brzezinski M, Degos V, Eriksson LI, Kramer JH, Leung JM, et al. Perioperative cognitive decline in the aging population. Mayo Clin Proc. (2011) 86:885–93. doi: 10.4065/mcp.2011.0332
2. Evered L, Silbert B, Knopman DS, Scott DA, DeKosky ST, Rasmussen LS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. (2018) 121:1005–12. doi: 10.1016/j.bja.2017.11.087
3. Subramaniyan S, Terrando N. Neuroinflammation and perioperative neurocognitive disorders. Anesth Analg. (2019) 128:781–8. doi: 10.1213/ANE.0000000000004053
4. Monk TG, Weldon BC, Garvan CW Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. (2008) 108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e
5. Greaves D, Psaltis PJ, Ross TJ, Davis D, Smith AE, Boord MS, et al. Cognitive outcomes following coronary artery bypass grafting: a systematic review and meta-analysis of 91,829 patients. Int J Cardiol. (2019) 289:43–9. doi: 10.1016/j.ijcard.2019.04.065
6. Berian JR, Zhou L, Russell MM, Hornor MA, Cohen ME, Finlayson E, et al. Postoperative delirium as a target for surgical quality improvement. Ann Surg. (2018) 268:93–9. doi: 10.1097/SLA.0000000000002436
7. Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. (2009) 110:548–55. doi: 10.1097/ALN.0b013e318195b569
8. Nakamura R, Miyamoto K, Tsuji K, Ozaki K, Kunimoto H, Honda K, et al. The impact of a preoperative nurse-led orientation program on postoperative delirium after cardiovascular surgery: a retrospective single-center observational study. J Intensive Care. (2023) 11:20. doi: 10.1186/s40560-023-00666-3
9. Kong H, Xu LM, Wang DX. Perioperative neurocognitive disorders: a narrative review focusing on diagnosis, prevention, and treatment. Cns Neurosci Ther. (2022) 28:1147–67. doi: 10.1111/cns.13873
10. Xu X, Hu Y, Yan E, Zhan G, Liu C, Yang C. Perioperative neurocognitive dysfunction: thinking from the gut? Aging. (2020) 12:15797–817. doi: 10.18632/aging.103738
11. Evered L, Atkins K, Silbert B, Scott DA. Acute peri-operative neurocognitive disorders: a narrative review. Anaesthesia. (2022) 77(Suppl.1):34–42. doi: 10.1111/anae.15613
12. Siddiqi N, Harrison JK, Clegg A, Teale EA, Young J, Taylor J, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochr Database Syst Rev. (2016) 3:CD5563. doi: 10.1002/14651858.CD005563.pub3
13. Li L, Wang C, Fang M, Xu H, Lu H, Zhang H. Effects of dexamethasone on post-operative cognitive dysfunction and delirium in adults following general anaesthesia: a meta-analysis of randomised controlled trials. BMC Anesthesiol. (2019) 19:113. doi: 10.1186/s12871-019-0783-x
14. Burton JK, Craig L, Yong SQ, Siddiqi N, Teale EA, Woodhouse R, et al. Non-pharmacological interventions for preventing delirium in hospitalised non-ICU patients. Cochr Database Syst Rev. (2021) 11:CD13307. doi: 10.1002/14651858.CD013307.pub3
15. Guglietti B, Hobbs D, Collins-Praino LE. Optimizing cognitive training for the treatment of cognitive dysfunction in Parkinson's disease: current limitations and future directions. Front Aging Neurosci. (2021) 13:709484. doi: 10.3389/fnagi.2021.709484
16. Saleh AJ, Tang GX, Hadi SM, Yan L, Chen MH, Duan KM, et al. Preoperative cognitive intervention reduces cognitive dysfunction in elderly patients after gastrointestinal surgery: a randomized controlled trial. Med Sci Monit. (2015) 21:798–805. doi: 10.12659/MSM.893359
17. Humeidan ML, Reyes JC, Mavarez-Martinez A, Roeth C, Nguyen CM, Sheridan E, et al. Effect of cognitive prehabilitation on the incidence of postoperative delirium among older adults undergoing major noncardiac surgery: the neurobics randomized clinical trial. J Am Med Assoc Surg. (2021) 156:148–56. doi: 10.1001/jamasurg.2020.4371
18. Walton CC, Naismith SL, Lampit A, Mowszowski L, Lewis SJG. Cognitive training in Parkinson's disease. Neurorehabil Neural Repair. (2017) 31:207–16. doi: 10.1177/1545968316680489
19. Xuefang L, Guihua W, Fengru M. The effect of early cognitive training and rehabilitation for patients with cognitive dysfunction in stroke. Int J Methods Psychiatr Res. (2021) 30:e1882. doi: 10.1002/mpr.1882
20. Hu M, Wu X, Shu X, Hu H, Chen Q, Peng L, et al. Effects of computerised cognitive training on cognitive impairment: a meta-analysis. J Neurol. (2021) 268:1680–8. doi: 10.1007/s00415-019-09522-7
21. Woolf C, Lampit A, Shahnawaz Z, Sabates J, Norrie LM, Burke D, et al. A systematic review and meta-analysis of cognitive training in adults with major depressive disorder. Neuropsychol Rev. (2022) 32:419–37. doi: 10.1007/s11065-021-09487-3
22. O'Gara BP, Mueller A, Gasangwa D, Patxot M, Shaefi S, Khabbaz K, et al. Prevention of early postoperative decline: a randomized, controlled feasibility trial of perioperative cognitive training. Anesth Analg. (2020) 130:586–95. doi: 10.1213/ANE.0000000000004469
23. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. Br Med J. (2009) 339:b2535. doi: 10.1136/bmj.b2535
24. Qiaoyan Z. Psychological Effects of Health Education on Patients After Coronary Artery Bypass Grafting Operation. Xinxiang Medical College (2014).
25. Zhang C, Fatuan D, Weiqing M, Yunli Y, Huiming W. Effects of psycho-behavioral intervention on early postoperative cognitive dysfunction of young army patients. Med J Natl Defend Forc Southw China. (2015) 25:9–11. doi: 10.3969/j.issn.1004-0188.2015.01.004
26. Guan X, Longyan W. Effect of preoperative cognitive intervention on the postoperative delirium in elderly patients after spinal surgery. J Qilu Nurs. (2015) 25:29–30. doi: 10.3969/j.issn.1006-7256.2015.04.012
27. Wang Y, Ying W. Effect of preoperative cognitive function training on early postoperative cognitive dysfunction in elder patients undergoing general anesthesia. J Harbin Med Univ. (2017) 51:183–5. doi: 10.3969/CNKI:SUN:HYDX.0.2017-02-024
28. Tong NN, An-Ping Y. Preoperative cognitive function training reduces the postoperative cognitive dysfunction in patients undergoing cardiac surgery. J Harbin Med Univ. (2020) 54:215–7.
29. Li Q-C, Zhong-Hua F, Shu-Juan W, Chao-Liang L. Effect of early cognitive behavior intervention on cognitive function in elderly patients after coronary artery bypass grafting. Chin J Front Med Sci. (2018) 10:96–9. doi: 10.12037/YXQY.2018.07-18
30. Yi S, Yanli Q, Le Y, Zhongliang T, Ling X. The effect of psychological and behavioral intervention on early postoperative cognitive dysfunction after heart valve replacements. Chin J Behav Med Brain Sci. (2005) 10:502–3. doi: 10.3760/cma.j.issn.1674-6554.2005.06.010
31. Lie J, Guanxiu T, Saleh AJ, Jia C, Linying P, Zhifang C, et al. Effect of memory training using the “method of loci” before operation on early postoperative cognitive function in elderly patients undergoing laparotomy under general anesthesia. Chin J Anesthesiol. (2014) 34:525–8. doi: 10.3760/cma.j.issn.0254-1416.2014.05.001
32. Vlisides PE, Das AR, Thompson AM, Kunkler B, Zierau M, Cantley MJ, et al. Home-based cognitive prehabilitation in older surgical patients: a feasibility study. J Neurosurg Anesthesiol. (2019) 31:212–7. doi: 10.1097/ANA.0000000000000569
33. Stenberg E, Dos RFL, O'Kane M, Liem R, Pournaras DJ, Salminen P, et al. Guidelines for perioperative care in bariatric surgery: enhanced recovery after surgery (eras) society recommendations: a 2021 update. World J Surg. (2022) 46:729–51. doi: 10.1007/s00268-021-06394-9
34. Engelman DT, Ben AW, Williams JB, Perrault LP, Reddy VS, Arora RC, et al. Guidelines for perioperative care in cardiac surgery: enhanced recovery after surgery society recommendations. J Am Med Assoc Surg. (2019) 154:755–66. doi: 10.1001/jamasurg.2019.1153
35. Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. Anesth Analg. (2018) 127:496–505. doi: 10.1213/ANE.0000000000003514
36. Bäckman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev. (2010) 34:670–7. doi: 10.1016/j.neubiorev.2009.12.008
37. McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, et al. Changes in cortical dopamine d1 receptor binding associated with cognitive training. Science. (2009) 323:800–2. doi: 10.1126/science.1166102
38. Feinkohl I, Winterer G, Spies CD, Pischon T. Cognitive reserve and the risk of postoperative cognitive dysfunction. Dtsch Arztebl Int. (2017) 114:110–7. doi: 10.3238/arztebl.2017.0110
39. Petrelli A, Kaesberg S, Barbe MT, Timmermann L, Rosen JB, Fink GR, et al. Cognitive training in Parkinson's disease reduces cognitive decline in the long term. Eur J Neurol. (2015) 22:640–7. doi: 10.1111/ene.12621
40. Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proc Natl Acad Sci USA. (2006) 103:12523–8. doi: 10.1073/pnas.0605194103
41. Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, et al. A cognitive training program based on principles of brain plasticity: results from the improvement in memory with plasticity-based adaptive cognitive training (impact) study. J Am Geriatr Soc. (2009) 57:594–603. doi: 10.1111/j.1532-5415.2008.02167.x
Keywords: perioperative neurocognitive disorders, cognitive training, cognitive function training, postoperative cognitive dysfunction, postoperative delirium, meta-analysis
Citation: Zhao L, Guo Y, Zhou X, Mao W and Li L (2024) Preoperative cognitive training improves postoperative cognitive function: a meta-analysis and systematic review of randomized controlled trials. Front. Neurol. 14:1293153. doi: 10.3389/fneur.2023.1293153
Received: 12 September 2023; Accepted: 15 December 2023;
Published: 08 January 2024.
Edited by:
Daniele Corbo, University of Brescia, ItalyReviewed by:
Marius Butz, Kerckhoff Klinik, GermanyCopyright © 2024 Zhao, Guo, Zhou, Mao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linji Li, bGxqLXN0ZXBoZW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.