
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 08 November 2023
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1286153
 Ryutaro Nakamura1
Ryutaro Nakamura1 Mika Kurihara2
Mika Kurihara2 Shuhei Kobashi1
Shuhei Kobashi1 Yoshitaka Tamaki1
Yoshitaka Tamaki1 Nobuhiro Ogawa1
Nobuhiro Ogawa1 Akihiro Kitamura1
Akihiro Kitamura1 Isamu Yamakawa1
Isamu Yamakawa1 Shigeki Bamba2,3
Shigeki Bamba2,3 Tomoya Terashima1
Tomoya Terashima1 Makoto Urushitani1*
Makoto Urushitani1*Introduction: This study sought to identify the optimal caloric intake to improve function and survival in ALS patients by comparing oral intake per ideal body weight (IBW) and its discrepancy with total energy expenditure (TEE) using the Shimizu formula.
Methods: A retrospective analysis of 104 ALS patients was conducted, categorizing them based on their average intake during the first week after admission using two primary intake cutoffs: 25 kcal/kgIBW and 30 kcal/kgIBW. The variance between oral intake and TEE was also evaluated using −300 kcal and 0 kcal as reference points.
Results: Oral caloric intake per IBW and functional decline rate (rs = −0.35, p < 0.001), but the variance from TEE was not significantly correlated (−0.11, p = 0.27). Survival data showed that patients consuming less than 25 kcal/kgIBW had a median survival of 24 months, increasing to 38 months for those consuming between 25–30 kcal/kgIBW and 63 months for those consuming 30 kcal/kgIBW or more. Deviations from the TEE did not significantly affect survival (p = 0.36). Among patients consuming less than their TEE, those consuming less than 25 kcal/kgIBW had a shorter median survival (24 months) compared to their counterparts (46 months) (p = 0.022). Consumption of less than 25 kcal/kgBW emerged as a significant negative predictor of patient outcome, independent of factors such as age, gender or disease progression.
Discussion: Intakes of 25 kcal/kgIBW or more are correlated with improved ALS outcomes, and larger, multi-regional studies are recommended for deeper insights.
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease characterized by the paralysis of limb and respiratory muscles due to the loss of upper and lower motor neurons. Without ventilator support, patients usually die within 2–4 years of symptom onset (1). Weight loss is observed in approximately 70% of ALS patients, mainly because of loss of oral intake and metabolic alterations (2). A low body mass index (BMI) at diagnosis is an indicator of poor prognosis (3), and post-diagnosis weight gain is reported to be associated with a longer survival (4). Early initiation of gastrostomy contributes to weight maintenance (5), and high-calorie therapy may delay disease progression, particularly in those with rapid progression (6). In this manner, the accumulated evidence underscores the importance of nutritional management.
For maintaining body weight, it is essential that total energy intake exceeds the total energy expenditure (TEE). Typically, TEE is estimated using the Harris-Benedict equation (7). In ALS patients, resting energy expenditure is elevated (8, 9), suggesting the need for a predictive formula for TEE that accounts for this increase. Kasarskis et al. assessed TEE in ALS patients using a double-labeled water method (10), and Shimizu et al. conducted similar research on Japanese ALS patients, and proposed a predictive formula for TEE, referred to here as the “Shimizu formula” (11). TEE measurements by the Shimizu formula incorporate the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) and BMI. This assessment may overwhelm the daily intake of ALS patients, especially in the early phase. Indeed, we occasionally encounter situations where patients face challenges in consuming the estimated amount orally. These situations have prompted us to seek additional calorie indicators to ensure that patients who have difficulty following the Shimizu formula have a favorable prognosis.
In the field of metabolism and nutrition, such as diabetes, energy intake recommendations are based on the ideal body weight (IBW), with an intake of approximately 25-35 kcal/kgIBW recommended depending on activity levels (12). The simplicity of this calculation is its strength, and since it’s a consensus metric for caloric administration in other diseases, it is easy for multi-disciplinary teams to share. However, its utility in the nutritional treatment of ALS patients has not been studied.
In this study, we retrospectively analyzed the relationship between survival duration and oral intake, such as energy intake per IBW and difference from TEE, and proposed a new calorie indicator to be used with the Shimizu formula for ALS patients to avoid poor prognosis in survival.
This single-center retrospective study included patients diagnosed with ALS between March 2016 and July 2023 at Shiga University of Medical Science Hospital, according to either the revised El Escorial criteria (13) being rated as possible or higher, or meeting the Gold Coast criteria (14). The study was approved by the ethics committee of Shiga University of Medical Science Hospital (approval number: R2023-072).
We employed the data gathered during the initial admission for diagnostic purpose or for treatment with edaravone or percutaneous endoscopic gastrostomy (PEG). Data were extracted from medical records, including age at admission, gender, body weight (B.W.; before illness, at first visit, and during caloric assessment), height, the number of months from onset to first consultation and caloric assessment, presence or absence of dysphagia, El Escorial criteria, PEG using during the disease course, and %vital capacity (V.C.). IBW was calculated as follows:
The rate of weight loss was evaluated using the following weight change rate:
The rate of disease progression was evaluated using the following decline in ALSFRS-R:
Oral intake data during the initial hospitalization were evaluated. Upon admission, the ALSFRS-R was initially assessed for the majority of patients. However, data was missing for certain individuals, particularly those for whom the probability of ALS was deemed low at the time of admission. The resting metabolic rate (RMR) was then calculated using the Harris-Benedict equation (7), and TEE was derived using the Shimizu formula (11).
For men:
For women:
The amount of food provided during hospitalization was determined based on the Shimizu formula since the publication. The caloric needs of patients admitted prior to formula adaptation were determined using the Harris-Benedict equation. All the patients had been informed about the importance of food consumption, and food intake was visually evaluated and documented by ward nurses. To ensure a stable intake assessment, the average intake over seven days post-admission was considered the daily oral intake. For patients undergoing gastrostomy within one week of admission, the average intake up to the day before the procedure was used.
To evaluate the relationship between oral intake and metabolism, we measured the resting energy expenditure (mREE; kcal) using an indirect calorimeter (Aeromonitor® AE310S, Minato Medical Science Co., Osaka, Japan). The examination was conducted for 10 min in the morning after an overnight fast with patients in a supine position. Before starting, participants rested for 30 min (15). The Respiratory Quotient (R.Q. = VCO2/VO2) was calculated, and the mREE was determined using the Weir formula (16). The evaluation of hypermetabolism varies across races (17), and there is no consensus for Japanese patients. In this study, similar to our previous research (18), we used the REE per lean soft tissue mass (LSTM; kg) as the main metabolic indicator. LSTM was measured through a bioelectrical impedance analysis with a body composition analyzer (InBody S10; InBody, Tokyo, Japan), conducted consecutively with mREE measurement. Other metabolic markers included data on body temperature measured in the axilla in the morning during the hospitalization. From the medical records of the first week after admission, we obtained the average body temperature of five days, excluding the highest and lowest values. We also collected data on High-density lipoprotein cholesterol (HDL) (19, 20) and Low-density lipoprotein cholesterol (LDL) (20, 21) from blood tests taken at the first visit, including non-fasting outpatient blood tests because HDL and LDL tend to decrease over time in patients with ALS (20).
Survival time was the time since the onset to death or tracheotomy invasive ventilation (TIV), with a cutoff point of 31st July 2023. To mitigate the influence of the life-prolonging effect of TIV on survival length, we considered both TIV and death as endpoints. For those who survived past this date, their survival time was censored.
Statistical analysis was performed using EZR version 1.53 (Saitama Medical Center, Jichi Medical University, Tochigi, Japan) (22). Continuous variables were presented as median and quartiles. Comparisons between two groups were made using the Mann–Whitney U test and Fisher’s exact probability test. Correlations were assessed using the Spearman correlation coefficient. The endpoints were defined as death or tracheostomy. Survival duration from onset was evaluated using Kaplan–Meier curves and the Log-rank test. For oral intake per IBW, we referred to the recommended amount for type 2 diabetes patients performing light work (23). We divided the patients into three groups using 25 kcal/kgIBW and 30 kcal/kgIBW as cutoff values and examined their relationships with survival duration. Since there is no precedent study about the cutoff value for the difference from TEE, we divided the patients into three groups using −300 kcal and 0 kcal as the cutoff values, considering the amount that can be supplemented with supplementary food. Furthermore, to evaluate the independence of the risk of less than 25 kcal/kgIBW and the benefit of calorie intake more than the Shimizu formula, we performed a Cox multivariate analysis including age, ΔALSFRS-R, ΔBW, presence or absence of dysphagia, the time from onset to calorie evaluation, % V.C., and gender as variables. All tests were deemed significant at p ≤ 0.05.
Of the 105 patients initially admitted, one patient was excluded from the study due to inability to consume orally at the time. The demographic information is shown in Table 1. In 5 cases, the gastrostomy was performed within one week after admission. The median oral intake per IBW was 28.1 kcal/kgIBW. Among the participants, 29 patients consumed less than 25 kcal/kgIBW, 44 consumed equal to or more than 25 but less than 30 kcal/kgIBW, and 31 consumed equal to or more than 30 kcal/kgIBW. The intake was significantly higher in men at 28.8 kcal/kgIBW compared to women at 27.6 kcal/kgIBW (p = 0.047). For 94 patients whose required calorie intake was calculated using the Shimizu method, the median difference between oral intake and TEE was −172 kcal. Despite encouragement to increase caloric intake, only 24 of the 94 patients (25.5%) consumed equal to or more than their TEE. We investigated potential differences in the frequency of dysphagia and the factors encompassed by the Shimizu formula between two groups: those with oral intake equal to or exceeding the Total Energy Expenditure (TEE) and those with intake below TEE (Table 2). The group with an oral intake at or above TEE was notably older and exhibited a lower body weight. When stratified by oral intake per IBW, only 1 out of 25 patients (4.0%) consuming less than 25 kcal/kgIBW had this surplus, compared to 23 out of 69 (33.3%) in the group consuming equal to or more than 25 kcal/kgIBW, which was significantly higher (p = 0.003). There was a positive correlation between oral intake per IBW and the difference between TEE and oral intake (Figure 1, rs = 0.63, p < 0.001).
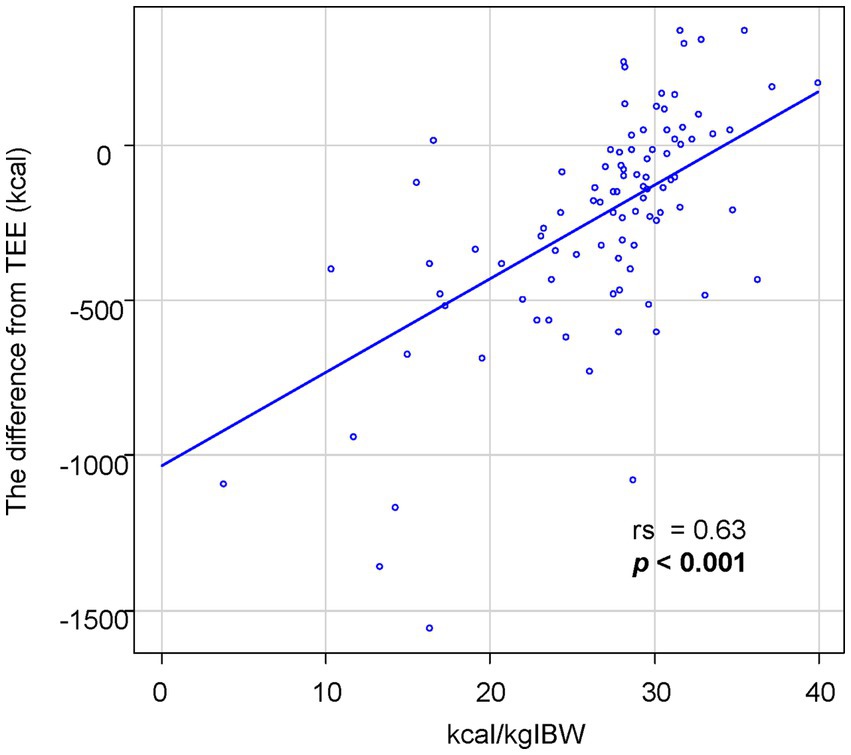
Figure 1. The association between oral intake per IBW and the difference from TEE. The oral intake per IBW is significantly correlated with the difference between oral intake and TEE.
Next, we studied the relationship between oral intake per IBW and metabolic factors, progression rate, and rate of weight loss (Table 3). Positive correlations were found with LDL (rs = 0.21, p = 0.039), LDL/HDL (rs = 0.29, p = 0.003), and %VC (rs = 0.27, p = 0.009). Negative correlations were observed with body temperature (rs = −0.28, p = 0.004), ΔALSFRS-R (rs = −0.39, p < 0.001), and ΔBW (rs = −0.34, p < 0.001). HDL (rs = −0.17, p = 0.080) and LSTM (rs = 0.23, p = 0.052) also seemed to be associated with oral intake per IBW, although insignificant. There was no significant correlation with mREE or mREE/LSTM. The difference from TEE did not show any significant correlations. Body temperature positively correlated with mREE/LSTM (rs = 0.25, p = 0.022).
Furthermore, we investigated the relationship between survival duration and oral intake. The median survival time for patients consuming less than 25 kcal/kgIBW was 20 months, 38 months for those consuming 25-30 kcal/kgIBW, and 63 months for those consuming equal to or more than 30 kcal/kgIBW, showing a significant difference (Figure 2A, p < 0.001). Even after excluding 17 patients with extremely low dietary intake of less than 20 kcal/kgIBW, the group consuming less than 25 kcal/kgIBW had a significantly shorter median survival time as long as 19 months (Supplemental Figure, p < 0.001). There was no significant difference in survival duration between groups, stratified by the difference from TEE (Figure 2B, p = 0.36). Among the 70 patients who consumed less than their TEE, a significant difference in median survival was observed between those who consumed less than 25 kcal/kgIBW (25 months) and those who consumed equal to more (45 months) (Figure 3, p = 0.022). Among the 24 patients who consumed more than their TEE, only one whose TEE was about 820 kcal consumed less than 25 kcal/kgIBW. Multivariate Cox analysis showed that an oral intake of less than 25 kcal/kgIBW was an independent poor prognostic factor (Table 4, hazard ratio; H.R.: 3.45; p = 0.012). A tendency for a favorable prognosis was observed with consumption greater than TEE, though it wasn’t significant (Supplemental Table, H.R.: 0.51; p = 0.062).
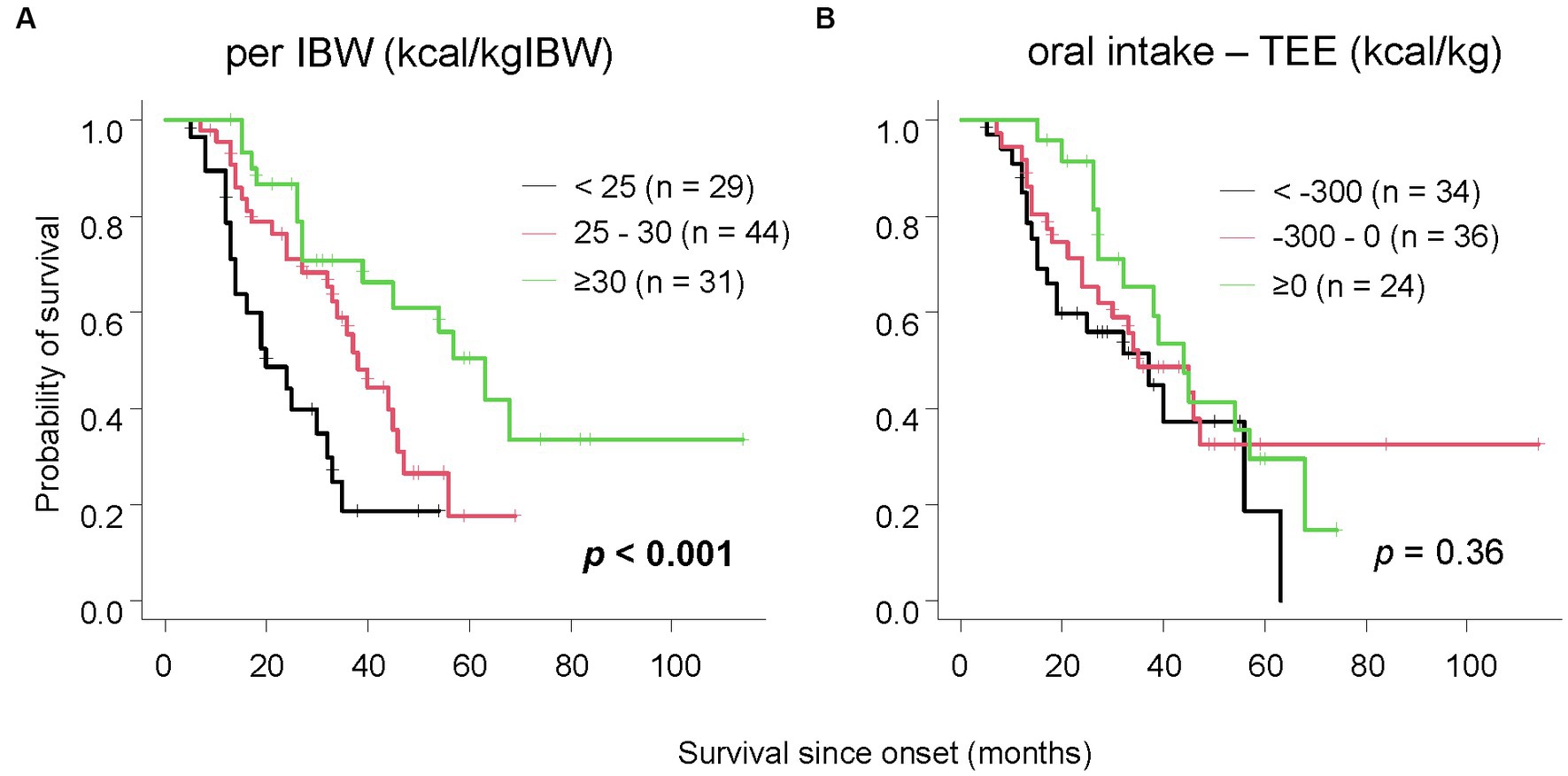
Figure 2. The relationship between survival time and oral intake. Patients with lower oral intake per IBW had significantly shorter survival (A). When classified by the difference between oral intake and TEE, no significant difference in survival was observed (B).
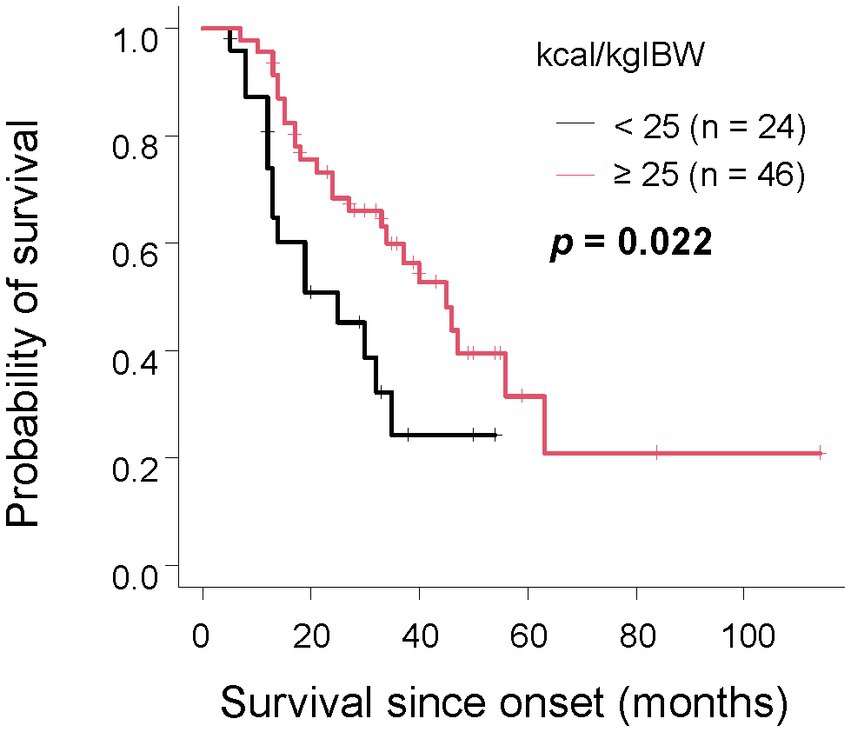
Figure 3. The relationship between survival time and oral intake per IBW in the group with unmet TEE In the group where TEE was not met, patients with an oral intake per IBW of less than 25 kcal/kgIBW had a significantly shorter survival compared to those with the same or more.
Given the significant finding that patients with an oral intake of less than 25 kcal/kgIBW had shorter survival durations, we performed subgroup analyses considering the time from onset to calorie evaluation, presence or absence of dysphagia, progression rate differences, and gender (Figure 4). Based on prior research, we categorized the time from onset as less than or more than 1 year (24) and defined rapid progression as ΔALSFRS-R≧0.62 and slow progression as less (6). Groups with onset time equal or less than 1 year (p = 0.003), more than 1 year (p = 0.006), rapid progression (p = 0.006), men (p = 0.004), and women (p = 0.013), and without dysphagia (p = 0.016) all showed that patients with an oral intake of less than 25 kcal/kgIBW had significantly shorter survival durations than other groups. Similar tendencies, though not significant, were observed in patients with dysphagia (p = 0.058). No significant relationship was observed in the slow progression group (p = 0.78).
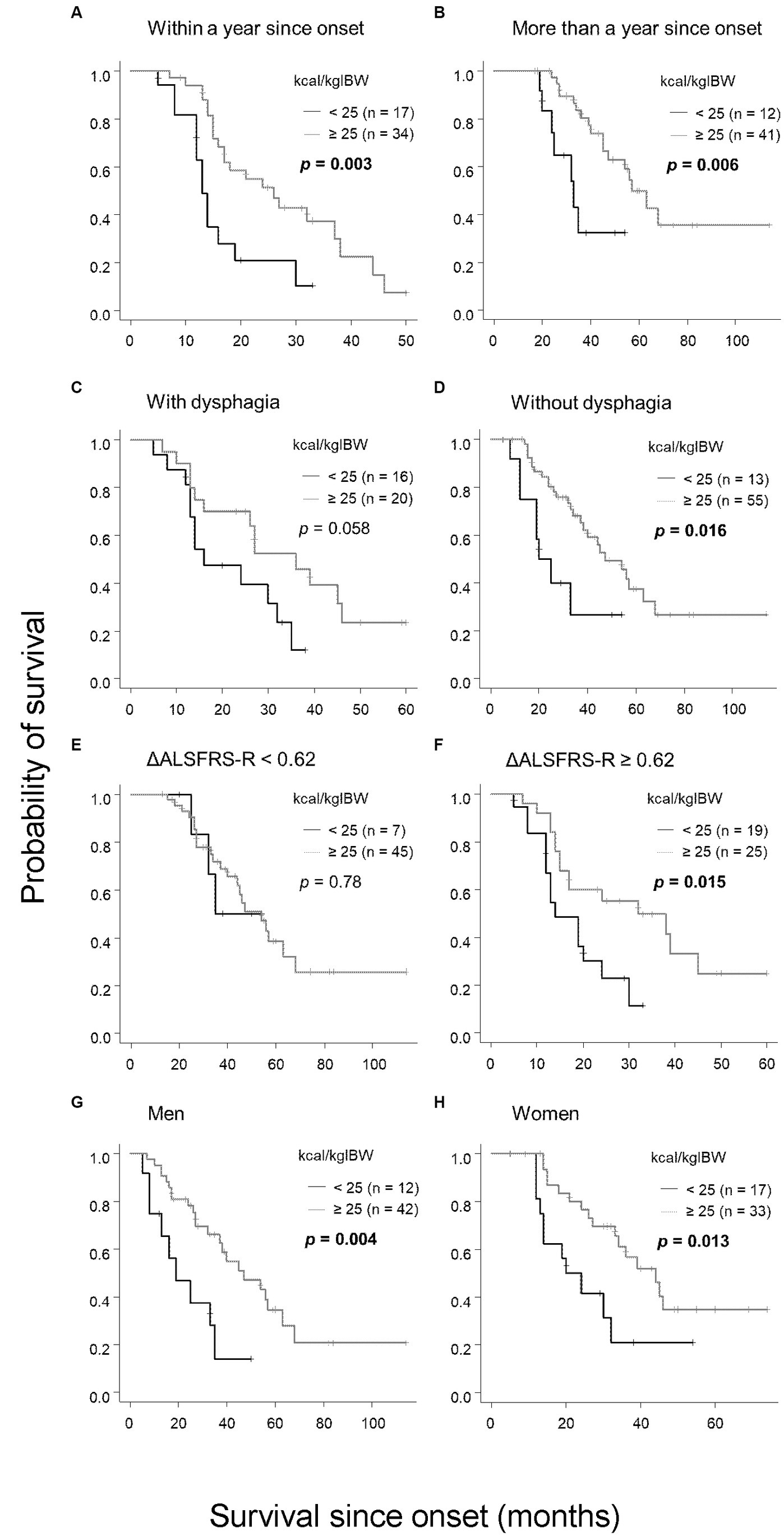
Figure 4. Subgroup analysis of the relationship between oral intake per IBW and survival. Patients with an oral intake per IBW of less than 25 kcal/kgIBW had significantly shorter survival compared with those with 25 kcal/kgIBW or more in the following categories: (A) within 1 year of onset, (B) more than 1 year from onset, (D) absence of dysphagia, (F) ΔALSFRS-R≧0.62, (G) male, and (H) female. In patients with dysphagia (C), the trend was the same, although no significant difference was observed. In the group with ΔALSFRS-R < 0.62, no significant relationship between oral intake and survival was observed (E).
This study investigated the previously undetailed relationship between oral intake and prognosis in ALS. Patients with lower intake per IBW, particularly under 25 kcal/kgIBW, had notably shorter survival, consistent across disease duration from onset and gender. However, the difference derived from the Shimizu formula for TEE did not significantly affect survival.
The results of this study suggest that oral intake per IBW can be useful in predicting prognosis. Of course, in the group where dietary intake had already decreased at the time of first admission, this may be due to the inclusion of patients with rapid progression, severe dysphagia, or those who have progressed to the point where they are unable to eat. However, subgroup analysis showed that groups with low oral intake per IBW tended to have poor prognosis, even when stratified by the disease progression rate or presence or absence of dysphagia (Figure 4). Furthermore, in the Cox multivariate analysis, an oral intake of less than 25 kcal/kgIBW was an independent poor prognostic factor, regardless of age, progression rate, weight loss rate, presence of dysphagia, and % V.C. (Table 4). Even excluding patients with an extremely low dietary intake below 20 kcal/kgIBW, the correlation between oral intake per IBW and prognosis remained unchanged. This suggests that having a low oral intake per IBW itself is a significant issue related to disease progression in ALS patients. Considering the positive correlation of this index with LDL, LDL/HDL ratio, and LSTM, and a negative correlation with weight loss rate (Table 3), a decrease in oral intake per IBW may directly lead to malnutrition and affect prognosis.
On the other hand, the difference between TEE and oral intake showed no significant relationship with survival period and was not correlated with lipid profile, progression rate, or weight loss rate. In this cohort, although meals were provided to aim for a TEE, only 25.5% of the total could achieve oral intake above TEE. Patients who were younger and had higher body weights demonstrated a decreased likelihood of achieving TEE. Deciding whether to increase nutritional intake in these patients or use a gastrostomy to exceed the TEE is a significant clinical challenge. Patients with an oral intake of 25 kcal/kgIBW or more, even if below the TEE, had survival rates similar to average ALS patients, suggesting this intake level may be adequate. It should be noted that the Shimizu formula, using the double-label method to estimate energy expenditure for Japanese ALS patients, is highly accurate. Despite the advantages of high-calorie therapy (6, 25), the main objective is to match or surpass the TEE from the Shimizu formula, setting a baseline of 25 kcal/kgIBW for oral intake.
Larger energy intake to maintain energy balance is crucial, particularly in the light of the observed hypermetabolism and increased physical activity-energy expenditure in ALS patients compared to controls (26). Altered metabolism is also evident in the presymptomatic stage, demonstrated by studies indicating reduced lean body mass in presymptomatic ALS gene carriers (27). Moreover, BMI has shown a decline in the 5 years prior to diagnosis (28). These findings highlight the potential need for gastrostomy in individuals with inadequate caloric intake. Consistent with this, multiple observational studies have indicate that gastrostomy helps maintain weight in ALS patients (29) and prolongs survival (30–32); the American Neurological Association also endorses it for ALS patients (5). For patients with dysphagia, survival is longer with gastrostomy (33–36), and it is advised for those with over 10% weight loss (37) or BMI below 18.5 (2). However, it’s impractical to immediately use gastrostomy upon detecting dysphagia, and its efficacy may be diminished in instances of low BMI. The optimal timing remains undefined in both scenarios. Future multi-center studies should explore if initiating gastrostomy based on an IBW oral intake of 25 kcal/kgIBW indicator can extend survival.
This study also explored the uncharted link between oral intake and metabolism. While no direct correlation emerged between oral intake per IBW and mREE or mREE/LSTM (Table 3), a notable negative association was found between oral intake per IBW and body temperature. Intriguingly, body temperature exhibited a positive correlation with mREE/LSTM. To the best of our knowledge, the connection between body temperature and hypermetabolism has not been previously documented in the context of ALS. Our results suggest a potential link between body temperature and resting energy expenditure, shedding light on a novel aspect of ALS metabolism. Additionally, the negative correlation between oral intake and body temperature suggests an underlying link between oral intake and metabolism in ALS patients.
This study bears limitations. Firstly, this retrospective, single-center study was conducted solely on Japanese subjects. The Shimizu formula, which was developed based on data from Japanese patients—typically exhibiting lower body weights than Caucasians—necessitates careful interpretation of results with consideration for regional variations. Secondly, the assessment relied on a 7-day dietary intake visually evaluated by ward nurses during diagnostic hospital stays, which might not reflect typical home consumption. However, patients and their families were instructed on how to assess calorie intake at home to make the results of this study useful in regular healthcare. Thirdly, differing PEG timings precluded us from examining its effect on survival length. Fourthly, measuring body temperature in the axillary area can be affected by external conditions like room temperature. Fifthly, pre-morbid body weight may not be entirely accurate as patients might not remember their former weight precisely. Finally, we informed all the patients of the significance of caloric intake, and urged them to adhere; yet, the reactions and effectiveness might have differed among individuals, potentially influencing the results.
In conclusion, the study shows that ALS patients consuming less than 25 kcal/kgIBW have shorter survival spans, which may be associated with malnutrition. Notably, even if patients did not achieve the TEE as per the Shimizu formula, outcomes were favorable as long as oral intake matched or surpassed this benchmark. Proactive nutritional interventions, like gastrostomy, using the 25 kcal/kgIBW marker might aid in enhancing prognosis where reaching the full TEE is difficult.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Shiga University of Medical Science Research Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
RN: Conceptualization, Formal analysis, Project administration, Writing – original draft. MK: Data curation, Methodology, Supervision, Writing – review & editing. SK: Investigation, Supervision, Writing – review & editing. YT: Investigation, Supervision, Writing – review & editing. NO: Investigation, Supervision, Writing – review & editing. AK: Conceptualization, Methodology, Supervision, Writing – review & editing. IY: Methodology, Supervision, Writing – review & editing. SB: Conceptualization, Formal analysis, Methodology, Supervision, Writing – review & editing. TT: Investigation, Supervision, Writing – review & editing. MU: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Intramural research fund from Shiga University of Medical Science (SUMS).
We thank all the patients with ALS who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1286153/full#supplementary-material
1. Chiò, A, Logroscino, G, Hardiman, O, Swingler, R, Mitchell, D, Beghi, E, et al. Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler. (2009) 10:310–23. doi: 10.3109/17482960802566824
2. Dupuis, L, Pradat, PF, Ludolph, AC, and Loeffler, JP. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. (2011) 10:75–82. doi: 10.1016/S1474-4422(10)70224-6
3. Shimizu, T, Nagaoka, U, Nakayama, Y, Kawata, A, Kugimoto, C, Kuroiwa, Y, et al. Reduction rate of body mass index predicts prognosis for survival in amyotrophic lateral sclerosis: a multicenter study in Japan. Amyotroph Lateral Scler. (2012) 13:363–6. doi: 10.3109/17482968.2012.678366
4. Shimizu, T, Nakayama, Y, Matsuda, C, Haraguchi, M, Bokuda, K, Ishikawa-Takata, K, et al. Prognostic significance of body weight variation after diagnosis in ALS: a single-Centre prospective cohort study. J Neurol. (2019) 266:1412–20. doi: 10.1007/s00415-019-09276-2
5. Miller, RG, Jackson, CE, Kasarskis, EJ, England, JD, Forshew, D, Johnston, W, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the quality standards Subcommittee of the American Academy of neurology. Neurology. (2009) 73:1218–26. doi: 10.1212/WNL.0b013e3181bc0141
6. Ludolph, AC, Dorst, J, Dreyhaupt, J, Weishaupt, JH, Kassubek, J, Weiland, U, et al. Effect of high-caloric nutrition on survival in amyotrophic lateral sclerosis. Ann Neurol. (2020) 87:206–16. doi: 10.1002/ana.25661
7. Harris, JA, and Benedict, FG. A biometric study of human basal metabolism. Proc Natl Acad Sci U S A. (1918) 4:370–3. doi: 10.1073/pnas.4.12.370
8. Bouteloup, C, Desport, JC, Clavelou, P, Guy, N, Derumeaux-Burel, H, Ferrier, A, et al. Hypermetabolism in ALS patients: an early and persistent phenomenon. J Neurol. (2009) 256:1236–42. doi: 10.1007/s00415-009-5100-z
9. Steyn, FJ, Ioannides, ZA, van Eijk, RPA, Heggie, S, Thorpe, KA, Ceslis, A, et al. Hypermetabolism in ALS is associated with greater functional decline and shorter survival. J Neurol Neurosurg Psychiatry. (2018) 89:1016–23. doi: 10.1136/jnnp-2017-317887
10. Kasarskis, EJ, Mendiondo, MS, Matthews, DE, Mitsumoto, H, Tandan, R, Simmons, Z, et al. Estimating daily energy expenditure in individuals with amyotrophic lateral sclerosis. Am J Clin Nutr. (2014) 99:792–803. doi: 10.3945/ajcn.113.069997
11. Shimizu, T, Ishikawa-Takata, K, Sakata, A, Nagaoka, U, Ichihara, N, Ishida, C, et al. The measurement and estimation of total energy expenditure in Japanese patients with ALS: a doubly labelled water method study. Amyotroph Lateral Scler Frontotemporal Degener. (2017) 18:37–45. doi: 10.1080/21678421.2016.1245756
12. Nakajima, Y, Sato, K, Sudo, M, Nagao, M, Kano, T, Harada, T, et al. Practical dietary calorie management, body weight control and energy expenditure of diabetic patients in short-term hospitalization. J Atheroscler Thromb. (2010) 17:558–67. doi: 10.5551/jat.3806
13. Brooks, BR, Miller, RG, Swash, M, and Munsat, TL, World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. (2000) 1:293–9. doi: 10.1080/146608200300079536
14. Hannaford, A, Pavey, N, van den Bos, M, Geevasinga, N, Menon, P, Shefner, JM, et al. Diagnostic utility of Gold Coast criteria in amyotrophic lateral sclerosis. Ann Neurol. (2021) 89:979–86. doi: 10.1002/ana.26045
15. Takaoka, A, Sasaki, M, Kurihara, M, Iwakawa, H, Inoue, M, Bamba, S, et al. Comparison of energy metabolism and nutritional status of hospitalized patients with Crohn's disease and those with ulcerative colitis. J Clin Biochem Nutr. (2015) 56:208–14. doi: 10.3164/jcbn.14-95
16. WEIR JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. (1949) 109:1–9. doi: 10.1113/jphysiol.1949.sp004363
17. Ludolph, A, Dupuis, L, Kasarskis, E, Steyn, F, Ngo, S, and McDermott, C. Nutritional and metabolic factors in amyotrophic lateral sclerosis. Nat Rev Neurol. (2023) 19:511–24. doi: 10.1038/s41582-023-00845-8
18. Nakamura, R, Kurihara, M, Ogawa, N, Kitamura, A, Yamakawa, I, Bamba, S, et al. Prognostic prediction by hypermetabolism varies depending on the nutritional status in early amyotrophic lateral sclerosis. Sci Rep. (2021) 11:17943. doi: 10.1038/s41598-021-97196-5
19. Janse van Mantgem, MR, van Rheenen, W, Hackeng, AV, van Es, MA, Veldink, JH, van den Berg, LH, et al. Association between serum lipids and survival in patients with amyotrophic lateral sclerosis: a Meta-analysis and population-based study. Neurology. (2023) 100:e1062–71. doi: 10.1212/WNL.0000000000201657
20. Nakamura, R, Kurihara, M, Ogawa, N, Kitamura, A, Yamakawa, I, Bamba, S, et al. Investigation of the prognostic predictive value of serum lipid profiles in amyotrophic lateral sclerosis: roles of sex and hypermetabolism. Sci Rep. (2022) 12:1826. doi: 10.1038/s41598-022-05714-w
21. Ingre, C, Chen, L, Zhan, Y, Termorshuizen, J, Yin, L, and Fang, F. Lipids, apolipoproteins, and prognosis of amyotrophic lateral sclerosis. Neurology. (2020) 94:e1835–44. doi: 10.1212/WNL.0000000000009322
22. Kanda, Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. (2013) 48:452–8. doi: 10.1038/bmt.2012.244
23. Haneda, M, Noda, M, Origasa, H, Noto, H, Yabe, D, Fujita, Y, et al. Japanese clinical practice guideline for diabetes 2016. Diabetol Int. (2018) 9:1–45. doi: 10.1007/s13340-018-0345-3
24. Oki, R, Izumi, Y, Fujita, K, Miyamoto, R, Nodera, H, Sato, Y, et al. Efficacy and safety of ultrahigh-dose Methylcobalamin in early-stage amyotrophic lateral sclerosis: a randomized clinical trial. JAMA Neurol. (2022) 79:575–83. doi: 10.1001/jamaneurol.2022.0901
25. Dorst, J, Schuster, J, Dreyhaupt, J, Witzel, S, Weishaupt, JH, Kassubek, J, et al. Effect of high-caloric nutrition on serum neurofilament light chain levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. (2020) 91:1007–9. doi: 10.1136/jnnp-2020-323372
26. Tandan, R, Howard, D, and Matthews, DE. Increased total daily energy expenditure in mild to moderate ALS: greater contribution from physical activity energy expenditure than hyper-metabolism. Amyotroph lateral Scler frontotemporal Degener. (2023):1–8. doi: 10.1080/21678421.2023.2240377
27. Dorst, J, Weydt, P, Brenner, D, Witzel, S, Kandler, K, Huss, A, et al. Metabolic alterations precede neurofilament changes in presymptomatic ALS gene carriers. EBioMedicine. (2023) 90:104521. doi: 10.1016/j.ebiom.2023.104521
28. Goutman, SA, Boss, J, Iyer, G, Habra, H, Savelieff, MG, Karnovsky, A, et al. Body mass index associates with amyotrophic lateral sclerosis survival and metabolomic profiles. Muscle Nerve. (2023) 67:208–16. doi: 10.1002/mus.27744
29. López-Gómez, JJ, Ballesteros-Pomar, MD, Torres-Torres, B, Pintor-De la Maza, B, Penacho-Lázaro, MA, Palacio-Mures, JM, et al. Impact of percutaneous endoscopic gastrostomy (PEG) on the evolution of disease in patients with amyotrophic lateral sclerosis (ALS). Nutrients. (2021) 13:2765. doi: 10.3390/nu13082765
30. Chiò, A, Mora, G, Leone, M, Mazzini, L, Cocito, D, Giordana, MT, et al. Early symptom progression rate is related to ALS outcome: a prospective population-based study. Neurology. (2002) 59:99–103. doi: 10.1212/WNL.59.1.99
31. Mazzini, L, Corrà, T, Zaccala, M, Mora, G, Del Piano, M, and Galante, M. Percutaneous endoscopic gastrostomy and enteral nutrition in amyotrophic lateral sclerosis. J Neurol. (1995) 242:695–8. doi: 10.1007/BF00866922
32. Shaw, AS, Ampong, MA, Rio, A, Al-Chalabi, A, Sellars, ME, Ellis, C, et al. Survival of patients with ALS following institution of enteral feeding is related to pre-procedure oximetry: a retrospective review of 98 patients in a single Centre. Amyotroph Lateral Scler. (2006) 7:16–21. doi: 10.1080/14660820510012013
33. Bokuda, K, Shimizu, T, Imamura, K, Kawata, A, Watabe, K, Hayashi, M, et al. Predictive factors for prognosis following unsedated percutaneous endoscopic gastrostomy in ALS patients. Muscle Nerve. (2016) 54:277–83. doi: 10.1002/mus.25051
34. Gorrie, GH, Chandran, S, Colville, S, Newton, J, Leighton, D, Mcdonald, M, et al. Improved survival and 30-day mortality after gastrostomy in Scottish motor neurone disease patients: evidence from a national retrospective cohort study using STROBE criteria. Amyotroph Lateral Scler Frontotemporal Degener. (2019) 20:165–71. doi: 10.1080/21678421.2019.1570271
35. Spataro, R, Ficano, L, Piccoli, F, and La Bella, V. Percutaneous endoscopic gastrostomy in amyotrophic lateral sclerosis: effect on survival. J Neurol Sci. (2011) 304:44–8. doi: 10.1016/j.jns.2011.02.016
36. Van Eenennaam, RM, Kruithof, WJ, Kruitwagen-Van Reenen, ET, van den Berg, LH, Visser-Meily, JMA, and Beelen, A. Current practices and barriers in gastrostomy indication in amyotrophic lateral sclerosis: a survey of ALS care teams in the Netherlands. Amyotroph Lateral Scler Frontotemporal Degener. (2021) 23:242–51. doi: 10.1080/21678421.2021.1973505
37. Jackson-Tarlton, CS, Benstead, TJ, and Doucette, S, ON BEHALF OF THE CNDR INVESTIGATOR NETWORK. Correlating factors in the recommendation of feeding tubes in the nutritional management of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. (2016) 17:515–21. doi: 10.1080/21678421.2016.1213851
Keywords: amyotrophic lateral sclerosis, nutritional therapy, ideal body weight, metabolism, prognosis
Citation: Nakamura R, Kurihara M, Kobashi S, Tamaki Y, Ogawa N, Kitamura A, Yamakawa I, Bamba S, Terashima T and Urushitani M (2023) Ideal body weight-based determination of minimum oral calories beneficial to function and survival in ALS. Front. Neurol. 14:1286153. doi: 10.3389/fneur.2023.1286153
Received: 06 September 2023; Accepted: 23 October 2023;
Published: 08 November 2023.
Edited by:
Jens Schmidt, Immanuel Klinik Rüdersdorf, GermanyReviewed by:
Angela Rosenbohm, University of Ulm, GermanyCopyright © 2023 Nakamura, Kurihara, Kobashi, Tamaki, Ogawa, Kitamura, Yamakawa, Bamba, Terashima and Urushitani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Makoto Urushitani, dXJ1QGJlbGxlLnNoaWdhLW1lZC5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.