- 1Department of Neurology, Fujian Medical University Union Hospital, Fuzhou, China
- 2Fujian Key Laboratory of Molecular Neurology, Fuzhou, China
- 3Department of Geriatrics, Fujian Medical University Union Hospital, Fuzhou, China
Objective: The objective of this study was to identify the factors that affect the efficacy of added perampanel for the treatment of drug-resistant epilepsy (DRE), and to develop a reliable nomogram to predict the benefit of this addition.
Methods: A retrospective clinical analysis was conducted on DRE patients who received perampanel treatment and who were followed up for at least 6 months from January 2020 and September 2023 at the Epilepsy Center of Fujian Medical University Union Hospital. Data from January 2020 to December 2021 were used as development dataset to build model, while the data from January 2022 to September 2023 were used as validation dataset for internal validation. The predictive factors that affected the efficacy of perampanel as DRE treatment were included in the final multivariate logistic regression model, and a derived nomogram was established.
Results: A total of 119 DRE patients who received perampanel treatment were included in this study (development datasets: n = 76; validation data: n = 43). Among them, 72.3% (n = 86) showed a 50% or greater reduction in seizure frequency after perampanel treatment. Of all the parameters of interest, sex, age, history of generalized tonic-clonic seizures, and the number of antiseizure medications were identified as significant predictors for estimating the benefit of adding perampanel for the treatment of DRE. A model incorporating these four variables was developed, and a nomogram was constructed to calculate the probability of benefit of adding perampanel using the model coefficients. The C-index of the predictive model was 0.838, and the validation C-index was 0.756. The goodness-of-fit test showed good calibration of the model (p = 0.920, 0.752 respectively).
Conclusion: The proposed nomogram has significant clinical potential for predicting the probability of benefit of perampanel as DRE treatment. This nomogram can be used to identify DRE patients who could benefit from the early addition of perampanel to their treatment regimen.
Highlights
- In the present study, a predictive model for predicting the probability of benefit of perampanel for DRE treatment was built based on the clinical factors of patients with epilepsy.
- The model proved to be well discriminated and calibrated, indicating excellent discriminative ability and general applicability.
- This model facilitates the process of distinguishing DRE patients with a high probability of benefit for the addition of perampanel treatment.
Introduction
Epilepsy is a prevalent chronic neurological disorder afflicting over 70 million individuals worldwide (1, 2). This disorder is characterized by recurrent paroxysmal, transient, repetitive, and stereotyped seizures that exhibit notable clinical heterogeneity. Despite the availability of a plethora of antiseizure medications (ASMs), up to 30% of patients with epilepsy exhibit inadequate responses to treatment and develop drug-resistant epilepsy (DRE) (3). According to the International League Against Epilepsy (ILAE) working group, DRE is defined as failure to achieve sustained seizure freedom following the proper administration of two independent ASMs, either as monotherapy or combination therapy (4). Patients with DRE are at risk of premature mortality, injury, psychosocial dysfunction, and reduced quality of life (2). Therefore, there is a pressing need for novel and efficacious treatment modalities.
ASMs with innovative mechanisms of action represent potential avenues for seizure control in drug-refractory epilepsy. Activation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors has been implicated in seizure induction (5, 6). Preclinical research has demonstrated that AMPA receptor antagonists reduce or eliminate epileptiform activity in vitro, whereas blocking NMDA receptors is insufficient to abolish epileptiform discharges (7). Moreover, AMPA receptor activation is thought to participate in seizure synchronization and to facilitate the transition from epileptiform discharges to seizure activity (8). Consequently, the blockade of AMPA receptors may suppress seizures. Based on these premises, perampanel, a novel drug targeting AMPA receptors, has entered clinical trials (9). Perampanel is a highly selective and non-competitive antagonist of AMPA. In 2012, perampanel received approval for the adjunctive treatment of focal and generalized seizures in patients aged 12 years and older in Germany (10). In September 2019, it was approved as an adjunctive therapy for focal seizures (with or without secondary generalized seizures) in patients aged 12 years and older in China (11). The principal mechanism of action of perampanel is non-competitive binding to AMPA receptors on postsynaptic membranes, selectively inhibiting these receptors to decrease glutamatergic neurotransmission (5). As such, it inhibits glutamate-induced excitatory neurotransmission and exerts antiepileptic effects. Several randomized controlled trials of perampanel (including multiple phase II and III clinical trials) have demonstrated its superior efficacy compared to a placebo as an adjunctive therapy for refractory focal seizures, with a safe and well-tolerated dose range (4, 8, and 12 mg/day) (12–14). Nevertheless, a subset of DRE patients fail to improve their seizures following perampanel administration and experience adverse psychiatric effects, such as dizziness and drowsiness. Therefore, developing reliable methods to predict which patients with DRE may benefit from perampanel is of paramount importance.
Nomograms have recently been recognized as dependable instruments to create intuitive and simple graphical models that quantitatively predict the risk of clinical events. The need for integrated models to promote personalized medicine has been met, making it more convenient for clinicians to make prognosis predictions (15, 16). Nomogram models generate more precise and intuitive predictions than traditional assessment methods. These models are graphical tools based on regression models that quantify the risk of an event through various predictive factors. Nomograms are primarily employed to establish prediction models. By converting conventional statistical prediction models into visual graphics, nomograms can accurately predict the relationship between several variables and outcome indicators (17, 18). Presently, nomograms have been extensively employed in clinical settings. However, there is no nomogram model to predict the potential benefits of adding perampanel to the treatment of DRE patients.
In this study, our objective was to determine the clinical variables that significantly enhance seizure outcomes in patients after the administration of perampanel. Therefore, we constructed a nomogram to predict the probability of significant improvement in seizure outcomes following perampanel administration to support clinical decision-making.
Materials and methods
Study participants
This retrospective study aimed to collect the medical records of DRE patients who received adjunctive perampanel treatment at the Epilepsy Center of Fujian Medical University Union Hospital between January 2020 and September 2023. The inclusion criteria were as follows: (1) DRE patients who met the ILAE definition and received perampanel treatment; (2) patients with complete baseline seizure frequency records for at least 6 months before perampanel treatment initiation; (3) patients aged >14 years; (4) Patients with complete medical records; and (5) patients who were willing to participate in follow-up. The exclusion criteria were as follows: (1) irregular ASM intake; (2) patients with severe liver or kidney dysfunction; (3) patients with unclear medical records; (4) patients who underwent epilepsy-related surgery, vagus nerve stimulation, or ketogenic diet during perampanel treatment; and (5) patients with a follow-up time of <6 months.
In total of 119 DRE patients receiving adjunctive perampanel treatment met the eligibility criteria for this study. The Ethics Committee of Fujian Medical University Union Hospital approved this study, and informed consent for the use of medical records was obtained from all participants in compliance with the Helsinki Declaration. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Perampanel treatment regimen
Perampanel dose titration was conducted based on the clinical practice of our epilepsy center. The patients received once-daily perampanel before bedtime, starting at 2 mg/day. The dose was gradually increased by 2 mg every 2–4 weeks, based on the individual patient's clinical response and tolerability. The maintenance dose was determined according to the guidelines, drug interactions, and patient drug response. Physicians adjusted the dose at their discretion based on the patient's clinical response.
Data collection
Data were obtained from the clinical records of patients and involved a baseline evaluation as well as follow-up assessments every 1–2 months following the administration of perampanel treatment. The following parameters were collected at baseline: demographic variables (including sex, age, body weight, body mass index [BMI], education years); clinical features (including seizure type, seizure frequency, video-EEG [VEEG], course of epilepsy); epilepsy type categorized by the origin of the epileptic focus in the brain (based on previous EEG, neuroimaging, and VEEG monitoring results); etiology; age of onset; medical history of generalized tonic-clonic seizures (GTCS), febrile seizures, traumatic brain injury, previous neurological disease; existence of psychiatric comorbidities, cognitive impairment (prior to the initiation of perampanel treatment); number of antiseizure medications (ASMs); and baseline seizure frequency. Baseline seizure frequency was defined as the frequency of seizures during the 6-month period before the initiation of perampanel.
Seizure frequency and response rates were assessed at every outpatient visit, typically every 1–2 months, based on clinical evaluations. Seizure frequency was evaluated based on patient and caregiver reports, as well as seizure records in a seizure diary collected during each clinical visit. Response was assessed at the final visit by comparing the seizure frequency to that at baseline; the patients who experienced a reduction in seizure frequency of 50% or more were classified as responders, while those with a reduction in seizure frequency of <50% or no improvement were categorized as non-responders.
Comorbidities assessment
Cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) and Mini-Mental State Examination (MMSE). Participants with MoCA scores ≥26 and MMSE scores ≥24 were classified as cognitively normal, whereas those with MoCA scores ≤25 or MMSE scores <24 were considered to have cognitive impairment. Anxiety and depression levels were estimated using the Self-rating Anxiety Scale (SAS) and Self-rating Depression Scale (SDS). To examine the influence of anxiety and depression on the study findings, the patients were stratified into two subgroups: those without anxiety or depressive symptoms (SAS < 50 and SDS < 53) and those with anxiety or depressive symptoms (SAS ≥ 50 or SDS ≥ 53), designated as no SAS/SDS and SAS/SDS, respectively.
VEEG analysis
The VEEG data were scrutinized and classified as “normal EEG,” “abnormal background without epileptiform discharges,” or “epileptiform discharges” based on the original VEEG recordings or reports. The interictal VEEGs of all patients were recorded before perampanel administration.
Brain MRI analysis
Magnetic resonance imaging (MRI) was performed for all patients to rule out structural abnormalities. The MRI reports were reviewed and categorized as “Negative” or “Positive.” “Positive” indicated that the MRI revealed structural abnormalities, including cerebral arteriovenous malformation, aneurysms, brain malformation, encephalomalacia and gliosis, partial cerebral parenchyma, hyperintense hippocampi, and focal cortical dysplasia. “Negative” referred to MRI findings without structural abnormalities.
Statistical analysis
The data were analyzed using SPSS 26.0 software (IBM Corp.) and STATA 16 software. Numerical data are expressed as percentages, and continuous data are presented as mean ± standard deviation (SD). Univariate and multivariate logistic regression analyses were performed to identify factors that may influence DRE.
Nomogram model construction and validation
Data from January 2020 to December 2021 were used as development dataset to build model, while the data from January 2022 to September 2023 were used as validation dataset for internal validation. Univariate logistic regression analysis was performed to identify the factors affecting the potential benefits of perampanel treatment for patients with DRE. Those factors with a p-value < 0.10 were included in the multivariate analysis. The independent factors were then determined using multivariate logistic regression analysis, with a backward stepwise approach based on the Akaike Information Criterion (AIC), to identify the most precise combination of useful factors for predicting the benefits of perampanel treatment for DRE.
Subsequently, a nomogram model was constructed based on the multivariate logistic regression model, with the aim of predicting the benefit probability of perampanel treatment. The performance of the nomogram model was evaluated using two main parameters: discrimination and calibration. Discrimination refers to the ability of a model to differentiate between patients who will and will not experience an event. The concordance index (C-index) and receiver operating characteristic (ROC) curve were used to evaluate the discriminative ability of the nomogram.
Calibration was used to assess the consistency between predicted and observed survival. A calibration plot was constructed to evaluate the calibration of the nomogram model. Finally, decision curve analysis (DCA) was applied to calculate the net benefits, thereby enabling an assessment of the performance of the model.
Results
Patient characteristics
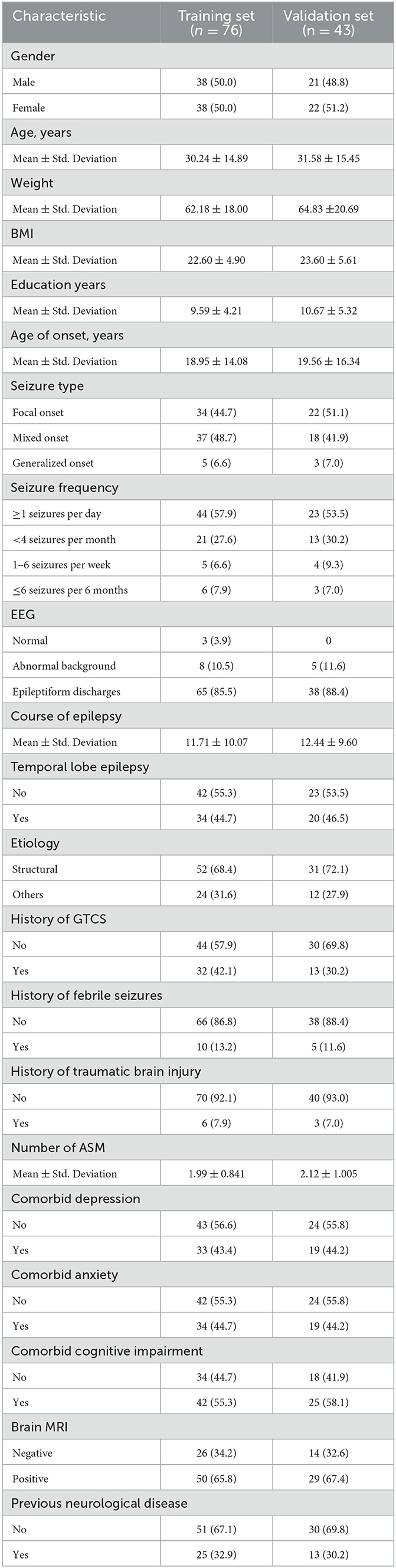
In total of 119 patients diagnosed with DRE and treated with perampanel at our epilepsy center between January 2020 and September 2023 were included in this study after meeting the predetermined inclusion and exclusion criteria. The patients were an average age of 30.24 and 31.58 years, with a mean age of onset of 18.95 and 19.56 years, a mean disease duration of 11.71 and 12.44 years, and a mean education duration of 9.59 and 10.67 years. Of the patients enrolled in this study, 73.7 and 69.77% (56/76 and 30/43) experienced a significant reduction of 50% or more in seizure frequency after receiving perampanel treatment in the development and validation dataset. Additionally, 43.4% (33/76) and 44.2% (19/43) of patients had comorbid depression, 44.7% (34/76) and 44.2% (19/43) had comorbid anxiety, and 55.3% (42/76) and 58.1% (25/43) had comorbid cognitive impairment in the development and validation dataset. Table 1 presents the patient characteristics.
In our cohort, 14 patients had adverse reactions, including vertigo (n = 6), irritability (n = 2), weight gain (n = 2), somnolence (n = 2), and digestive system symptoms (n = 2). Most patients had no or could tolerate mild adverse reactions.
Risk factors for DRE
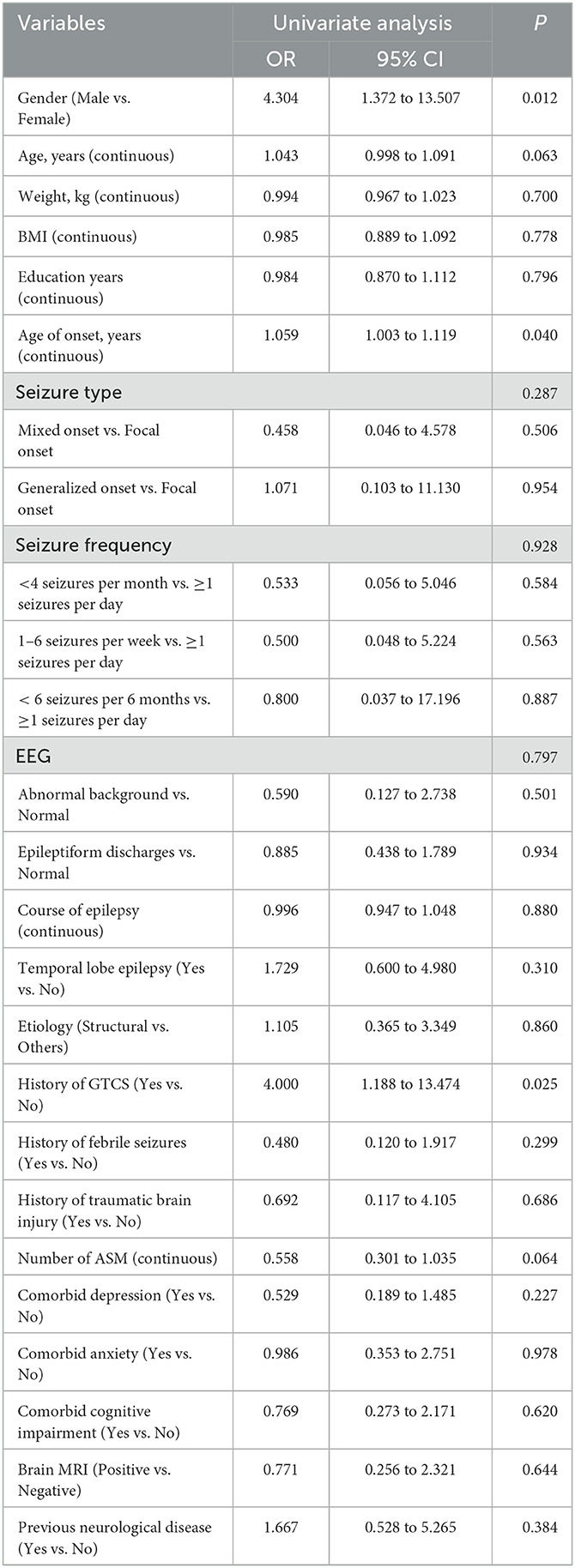
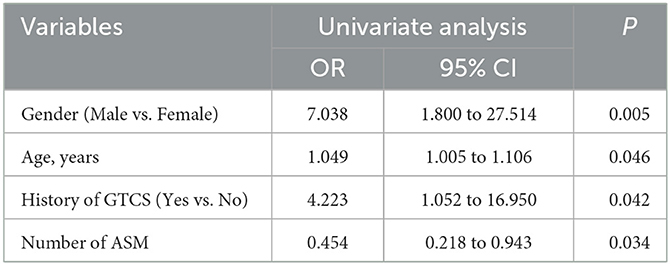
To determine the potential factors that may influence the efficacy of perampanel treatment in patients with DRE, we performed univariate logistic regression analyses for each variable. Variables with a p-value <0.10 included sex, age, age at onset of first seizure, history of GTCS, and the number of antiseizure medications (ASMs) used (Table 2). These five variables with a p-value <0.10 were entered into the initial multivariable logistic regression analysis, and after eliminating irrelevant factors, four variables remained in the final logistic regression model: sex, age, history of GTCS, and number of ASMs (Table 3).
Nomogram model development and validation
We developed a model based on the results of multivariate logistic regression, which incorporated four key features. Subsequently, we plotted a nomogram (Figure 1) using the coefficients derived from the model to calculate the likelihood of benefit from adding perampanel for DRE treatment. The number of ASMs was allocated the highest weighting in the nomogram, followed by patient age, while the history of GTCS had the smallest impact on the benefit probability.
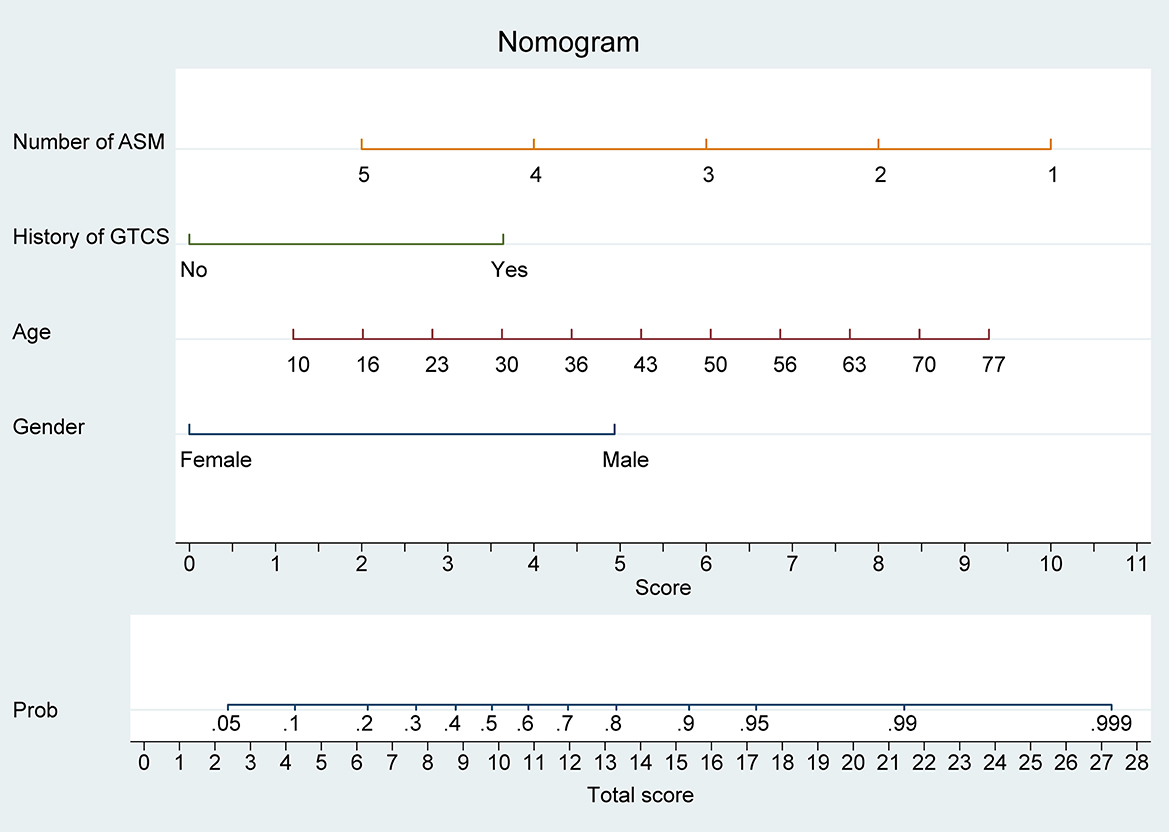
Figure 1. Nomogram model for predicting the efficacy of perampanel treatment in drug-resistant epilepsy (DRE) patients. The nomogram model integrates four predictive factors, including the number of anti-seizure medications (ASMs), history of generalized tonic-clonic seizures (GTCS), age, and sex. Each predictive factor is assigned a score based on its contribution to the overall model and the sum of these scores is used to calculate the predicted probability of benefiting from perampanel treatment.
Our nomogram provides a convenient and accurate tool for predicting the likelihood of benefit from adding perampanel treatment in patients with DRE. For individual DRE patients, the position of each variable on the corresponding axis was determined, and the scores for each variable were summed to obtain a total score. The total score axis was used to estimate the benefit likelihood.
The nomogram demonstrated excellent accuracy in predicting the likelihood of benefit from adding perampanel treatment in DRE patients, with a C-index of 0.838 (95% confidence interval [CI], 0.749–0.926) in the development dataset and a C-index of 0.756 in the validation dataset (Figures 2A, B). The calibration curves for the development and validation dataset showed a high consistency between the predicted and observed outcomes (Figures 2C, D). The Hosmer-Lemeshow (H-L) goodness-of-fit test p-values were 0.920 and 0.752, respectively, indicating optimal calibration. The DCA demonstrated that our nomogram model also displayed a higher net benefit (Figure 3).
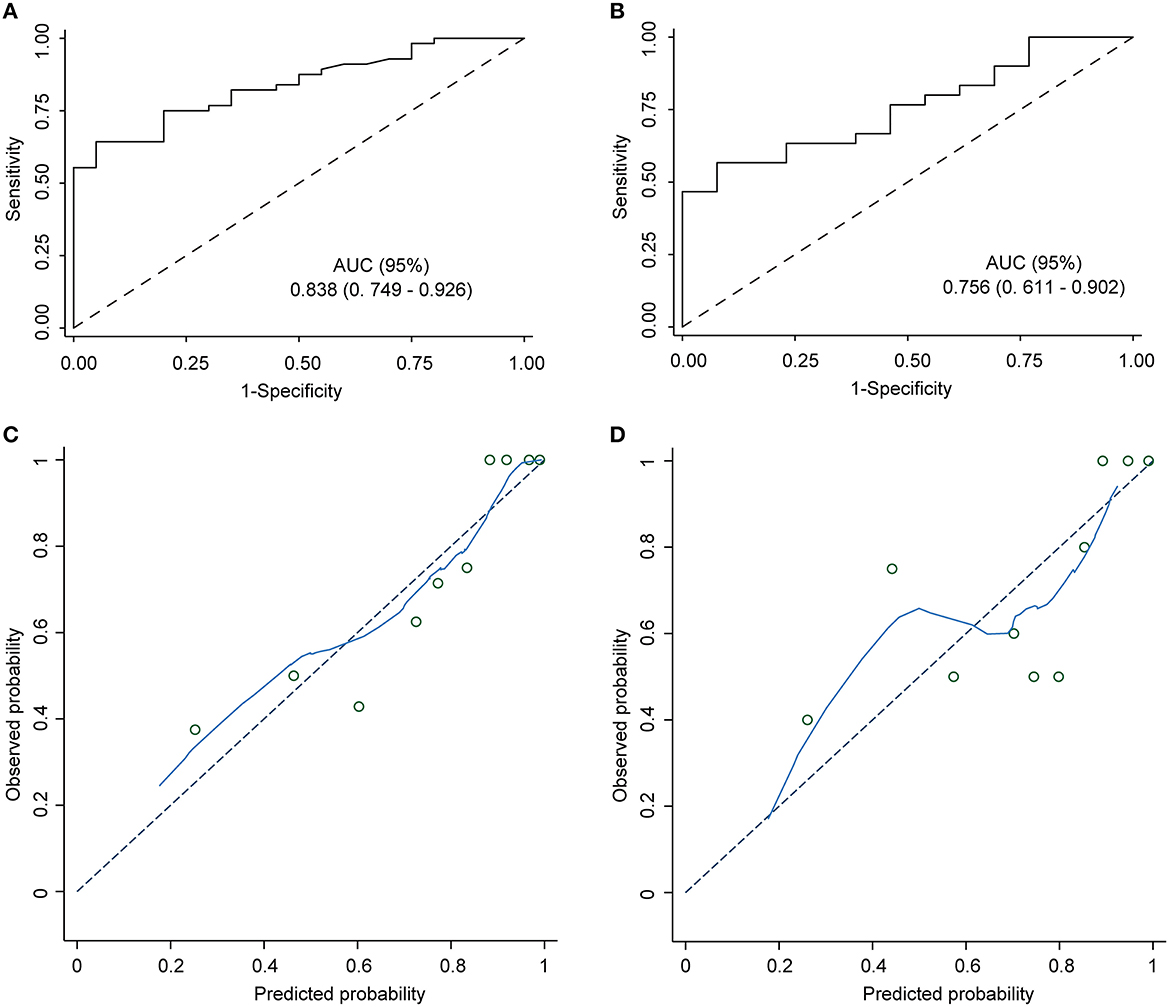
Figure 2. Nomogram model ROC and calibration curve. ROC curves of the predictive model in the development cohort and the validation cohort. Area under the ROC curve (A) shows the predictive ability of the model in the training cohort, and area under the ROC curve (B) validates the predictive ability of the model. ROC: receiver operating characteristic. The ROC curve plots the true positive rate (sensitivity) against the false positive rate (1 - specificity) at various classification thresholds. The diagonal dashed line represents the performance of a random guess, whereas the solid line represents the performance of the nomogram model. The closer the solid line is to the upper left corner of the plot, the better the model's discriminatory ability. (C) The nomogram calibration curve displays the predictive performance of the nomogram model in the training cohort. (D) The validation plot illustrates the distribution of the nomogram model's performance. The x-axis shows the predicted probability of the outcome, while the y-axis shows the actual probability of the outcome. The diagonal dashed line indicates perfect calibration, while the solid line represents the actual performance of the nomogram model.
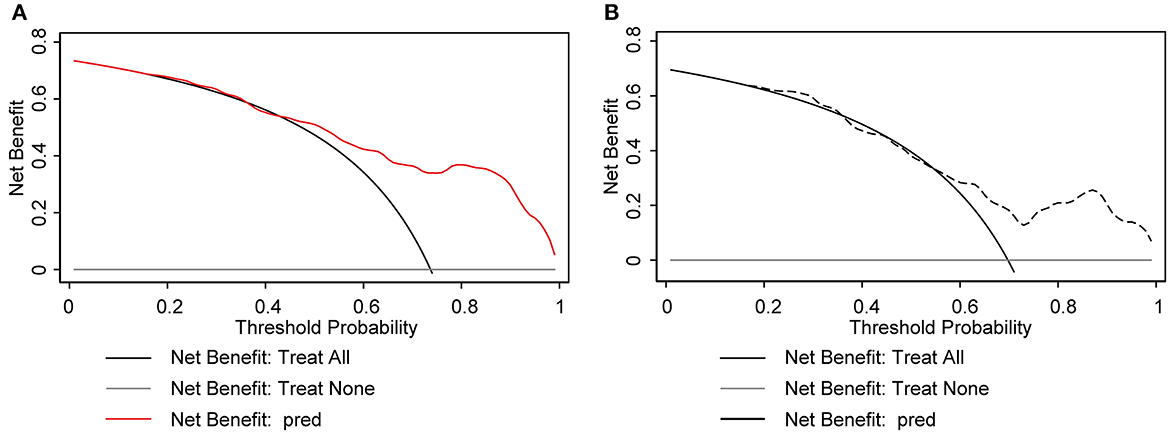
Figure 3. Decision curve analysis (DCA) plot for the nomogram model. The x-axis represents the threshold probability and the y-axis represents the net benefit. The gray solid line represents the net benefit of the “no-treatment” strategy, while the black solid line represents the net benefit of the “treat-all” strategy. The solid red line and black dotted line represents the net benefit of the nomogram model. The DCA plot in the development cohort (A) and the validation cohort (B) demonstrates that the nomogram model has a superior net benefit compared to the “treat-all” and “no-treatment” strategies across the range of threshold probabilities, indicating its potential usefulness as a clinical decision-making tool.
Discussion
As per the ILAE's standardized definition, DRE is characterized by the failure to achieve sustained seizure freedom following appropriate trials of two tolerated, suitably selected, and used ASMs, either in monotherapy or in combination therapy (4). Studies have reported DRE incidence rates ranging from 15 to 34% (19, 20). Recent studies suggest that adjunctive perampanel therapy can effectively reduce seizure frequency in patients with DRE (6, 21, 22). Nevertheless, not all patients with DRE benefit from perampanel adjunctive therapy. Some studies have reported instances of no significant improvement in seizure frequency and even adverse psychiatric effects due to perampanel (6, 23). Identifying DRE patients with a high probability of benefiting from perampanel adjunctive therapy and administering it early in their disease course may result in improved quality of life and early benefits. Therefore, identifying patients with DRE who are most likely to benefit from perampanel adjunctive therapy is crucial.
Our observational investigation revealed a noteworthy discovery, wherein 72.3% of patients (n = 86) exhibited a positive response over the entire duration of observation, surpassing the typical range of efficacy reported in randomized controlled trials on perampanel, which typically register efficacy rates between 26 and 56% (24). A clinical practice conducted in Germany, based on 6 months of observation, indicated that 46% of patients responded to treatment, evincing a reduction of at least 50% in seizure frequency (25). Ishikawa et al.'s retrospective study demonstrated a response rate of 52.3% to perampanel therapy (26). The divergence in the efficacy results may be explained by the more treatment-resistant population in prior studies. Nevertheless, these findings underscore the efficacy of perampanel as an add-on therapy for patients with refractory epilepsy, which undoubtedly holds immense promise.
Variations in follow-up periods preclude comparisons of retention rates across distinct studies. However, our present study's retention rate of 81.60% in development dataset falls within the range of 44–89% reported by Lattanzi et al. (27), signifying the drug's good tolerability and efficacy. The effective dose of perampanel ranged from 2 to 12 mg/day. Notably, the final mean dose of perampanel in our study for all patients was 5.447 ± 2.18 mg/day in development dataset, which is lower than the mean doses reported in two other real-world studies (6.03 ± 2.43 mg/day and 6.3 ± 3 mg/day, respectively) (28, 29). This variation can be attributed to demographic and anthropometric differences, including geographical location, population distribution, race, and body weight. A Korean study further demonstrated that Asians require lower perampanel doses (30). Additionally, a comparative study of perampanel efficacy in Asians and non-Asians showed that Asians exhibit poor tolerability to high doses (10–12 mg/day) (31). A slow titration regimen (incrementally increasing the dose by 2 mg every 2–4 weeks) could be a potential solution to enhance retention rates, as reflected significantly in this study.
Our results demonstrate that several factors significantly influence the likelihood of perampanel therapy benefitting patients with DRE. These factors include sex, age, history of GTCS, and the number of ASMs taken. These factors were incorporated into a logistic regression model. Notably, the number of ASMs taken exhibited the highest predictive weight in estimating the benefit of perampanel therapy. Furthermore, patient age and sex also exhibited significant predictive potential in terms of the probability of treatment benefit, with older patients and male patients demonstrating a higher likelihood of benefit. The presence of GTCS had a predetermined value in predicting the development of DRE while also playing a critical role in predicting clinical outcomes; patients with a history of GTCS exhibited a superior clinical response to perampanel therapy.
Our analysis also indicates that patients who have previously taken fewer ASMs have a more favorable clinical response. These results parallel those observed in the context of lacosamide and lamotrigine in patients in the early stages of treatment (32). Accordingly, perampanel therapy exhibits a response pattern that aligns with that of other ASMs. Notably, in Vicente Villanueva's study on perampanel therapy for focal epilepsy, early perampanel adjunctive therapy was found to be more effective than extensive ASM use before perampanel initiation (25). Similar results were obtained in patients with idiopathic generalized epilepsy (33). Collectively, these findings suggest that perampanel is more effective as an early adjunctive therapy for patients with DRE. Our results represent a valuable contribution to the prediction of treatment efficacy with the addition of perampanel to the treatment of DRE. We postulate that the reason underlying these findings is that the success rate of seizure control after the first ASM failure declines with the introduction of subsequent ASMs.
Our study findings revealed that male patients exhibited a superior clinical response to perampanel administration. This result is in contrast with the findings reported by Vazquez et al. (34), who documented a better clinical response in females receiving perampanel. Nonetheless, no significant statistical disparity existed in the number of male and female patients who attained seizure freedom (34). Moreover, studies have established that sex does not influence the efficacy of perampanel, as evidenced by a prior sub-analysis of a phase III randomized clinical trial that found no sex-based disparity in the efficacy and tolerability of perampanel (35). Nevertheless, this discrepancy may stem from limitations in sample size, thus necessitating the conduct of larger-scale studies to validate whether sex is associated with the efficacy of perampanel adjunct therapy in patients with DRE.
Our study outcomes suggest that patients of advanced age and those with a history of GTCS exhibit a superior clinical response to perampanel. GTCS history has long been regarded as an independent risk factor for DRE and likely validates the rationale behind perampanel as an adjunct treatment for this patient group. Similarly, previous studies revealed that epilepsy patients aged ≥65 years showed a superior clinical response to perampanel (24), concurring with our study findings. Notably, all our patients had DRE, and increased patient age correlated positively with the probability of perceiving benefits from adding perampanel treatment. Further studies are necessary to decipher the mechanisms underlying this association.
The salient aspect of our study was the development of a clinical prediction model to forecast the probability of DRE patients experiencing benefits from perampanel adjunct therapy, characterized by adequate discrimination and calibration. However, limitations such as inaccurate self-reporting of seizure frequency and inadequate attention to adverse reactions, albeit alongside extensive research on perampanel adjunct treatment tolerability, underscore the need for caution in generalizing the results. Thus, it is imperative to conduct long-term real-world studies in the future.
Conclusion
Our study established the efficacy of perampanel in minimizing seizure frequency in patients with DRE and devised a clinical prediction model to predict the probability of DRE patients benefitting from perampanel adjunct therapy. Sex, age, history of GTCS, and the number of ASMs remain essential predictive factors for this beneficial effect. Deploying predictive tools facilitates the identification of patients with DRE who are likely to benefit from adding perampanel adjunct therapy. Consequently, the early administration of perampanel has immense potential for enhancing treatment outcomes in such patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Fujian Medical University Union Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
CZ: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing—original draft. JL: Data curation, Formal analysis, Methodology, Software, Investigation, Writing—review & editing. DW: Data curation, Formal analysis, Investigation, Methodology, Software, Writing—review & editing. LW: Data curation, Formal analysis, Investigation, Methodology, Writing—review & editing. YZ: Formal analysis, Investigation, Methodology, Writing—review & editing, Conceptualization, Resources, Supervision, Validation. HH: Conceptualization, Formal analysis, Investigation, Resources, Supervision, Writing—review & editing, Funding acquisition, Project administration. WL: Conceptualization, Funding acquisition, Investigation, Project administration, Writing—review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Excellent Young Scholars Cultivation Project of Fujian Medical University Union Hospital (2022XH037); National key clinical specialty (Grant No. 21281003); Special support project for clinical research of young and middle-aged doctors in Lingnan Neurology; and the Joint Funds for the Innovation of Science and Technology, Fujian Province (Grant No. 2018Y9014).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Milligan TA. Epilepsy: a clinical overview. Am J Med. (2021) 134:840–7. doi: 10.1016/j.amjmed.2021.01.038
2. Ding D, Zhou D, Sander JW, Wang W, Li S, Hong Z. Epilepsy in China: major progress in the past two decades. Lancet Neurol. (2021) 20:316–26. doi: 10.1016/S1474-4422(21)00023-5
3. Kanner AM, Bicchi MM. Antiseizure medications for adults with epilepsy: a review. Jama. (2022) 327:1269–81. doi: 10.1001/jama.2022.3880
4. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. (2017) 58:512–21. doi: 10.1111/epi.13709
5. Sills GJ, Rogawski MA. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology. (2020) 168:107966. doi: 10.1016/j.neuropharm.2020.107966
6. Yamamoto T, Gil-Nagel A, Wheless JW, Kim JH, Wechsler RT. Perampanel monotherapy for the treatment of epilepsy: Clinical trial and real-world evidence. Epilepsy Behav. (2022) 136:108885. doi: 10.1016/j.yebeh.2022.108885
7. Hanada T. Ionotropic glutamate receptors in epilepsy: a review focusing on AMPA and NMDA receptors. Biomolecules. (2020) 10:464. doi: 10.3390/biom10030464
8. Hanada T. The discovery and development of perampanel for the treatment of epilepsy. Expert Opin Drug Discov. (2014) 9:449–58. doi: 10.1517/17460441.2014.891580
9. Schulze-Bonhage A. Perampanel for epilepsy with partial-onset seizures: a pharmacokinetic and pharmacodynamic evaluation. Expert Opin Drug Metab Toxicol. (2015) 11:1329–37. doi: 10.1517/17425255.2015.1061504
10. Ledingham DR, Patsalos PN. Perampanel: what is its place in the management of partial onset epilepsy? Neurol Ther. (2013) 2:13–24. doi: 10.1007/s40120-013-0012-3
11. Rossi J, Cavallieri F, Bassi MC, Biagini G, Rizzi R, Russo M, et al. Efficacy and tolerability of perampanel in brain tumor-related epilepsy: a systematic review. Biomedicines. (2023) 11:651. doi: 10.3390/biomedicines11030651
12. Potschka H, Trinka E. Perampanel: does it have broad-spectrum potential? Epilepsia. (2019) 60:22–36. doi: 10.1111/epi.14456
13. Rohracher A, Kalss G, Leitinger M, Granbichler C, Deak I, Dobesberger J, et al. Two-year real-world experience with perampanel in patients with refractory focal epilepsy: Austrian data. Ther Adv Neurol Disord. (2016) 9:445–53. doi: 10.1177/1756285616661115
14. Zaccara G, Giovannelli F, Cincotta M, Verrotti A, Grillo E. The adverse event profile of perampanel: meta-analysis of randomized controlled trials. Eur J Neurol. (2013) 20:1204–11. doi: 10.1111/ene.12170
15. Huang X, Zhang X, Wang X, Rong X, Li Y, Li H, et al. A nomogram to predict symptomatic epilepsy in patients with radiation-induced brain necrosis. Neurology. (2020) 95:e1392–403. doi: 10.1212/WNL.0000000000010190
16. Yu T, Liu X, Sun L, Lv R, Wu J, Wang Q. Risk factors for Drug-resistant Epilepsy (DRE) and a nomogram model to predict DRE development in post-traumatic epilepsy patients. CNS Neurosci Ther. (2022) 28:1557–67. doi: 10.1111/cns.13897
17. Allotey J, Fernandez-Felix BM, Zamora J, Moss N, Bagary M, Kelso A, et al. Predicting seizures in pregnant women with epilepsy: development and external validation of a prognostic model. PLoS Med. (2019) 16:e1002802. doi: 10.1371/journal.pmed.1002802
18. Wang X, Zhong J, Lei T, Chen D, Wang H, Zhu L, et al. An artificial neural network prediction model for posttraumatic epilepsy: retrospective cohort study. J Med Internet Res. (2021) 23:e25090. doi: 10.2196/25090
19. Beghi E. The epidemiology of epilepsy. Neuroepidemiology. (2020) 54:185–91. doi: 10.1159/000503831
20. Engel T, Smith J, Alves M. Targeting neuroinflammation via purinergic p2 receptors for disease modification in drug-refractory. Epilepsy J Inflamm Res. (2021) 14:3367–92. doi: 10.2147/JIR.S287740
21. Lim SN, Wu T, Tseng WE, Chiang HI, Cheng MY, Lin WR, et al. Efficacy and safety of perampanel in refractory and super-refractory status epilepticus: cohort study of 81 patients and literature review. J Neurol. (2021) 268:3744–57. doi: 10.1007/s00415-021-10506-9
22. Plosker GL. Perampanel: as adjunctive therapy in patients with partial-onset seizures. CNS Drugs. (2012) 26:1085–96. doi: 10.1007/s40263-012-0021-2
23. Rugg-Gunn F. Adverse effects and safety profile of perampanel: a review of pooled data. Epilepsia. (2014) 55:13–5. doi: 10.1111/epi.12504
24. Lossius IM, Svendsen T, Sødal HF, Kjeldstadli K, Lossius MI, Nakken KO, et al. Effect and tolerability of perampanel in patients with drug-resistant epilepsy. Epilepsy Behav. (2021) 119:107965. doi: 10.1016/j.yebeh.2021.107965
25. Villanueva V, D'Souza W, Goji H, Kim DW, Liguori C, McMurray R, et al. PERMIT study: a global pooled analysis study of the effectiveness and tolerability of perampanel in routine clinical practice. J Neurol. (2022) 269:1957–77. doi: 10.1007/s00415-021-10751-y
26. Ishikawa N, Tateishi Y, Tani H, Kobayashi Y, Kobayashi M. Clinical profiles associated with serum perampanel concentrations in children with refractory epilepsy. Epilepsy Behav. (2019) 94:82–6. doi: 10.1016/j.yebeh.2019.02.004
27. Lattanzi S, Trinka E, Zaccara G, Striano P, Russo E, Giovane CD, et al. Third-generation antiseizure medications for adjunctive treatment of focal-onset seizures in adults: a systematic review and network meta-analysis. Drugs. (2022) 82:199–218. doi: 10.1007/s40265-021-01661-4
28. D'Souza W, Alsaadi T, Montoya J, Carreño M, Di Bonaventura C, Mohanraj R, et al. Perampanel for the treatment of patients with myoclonic seizures in clinical practice: evidence from the PERMIT study. Seizure. (2022) 100:56–66. doi: 10.1016/j.seizure.2022.06.008
29. Sagar P, Wawryk O, Vogrin S, Whitham E, Kiley M, Frasca J, et al. Efficacy and tolerability of adjuvant perampanel: an Australian multicenter real-world observational study in refractory focal and generalized epilepsy syndromes. Epilepsy Behav. (2021) 119:107935. doi: 10.1016/j.yebeh.2021.107935
30. Hwang SK, Lee YJ, Nam SO, Kim WS, Kim JS, Kim SJ, et al. Real-life effectiveness and tolerability of perampanel in pediatric patients aged 4 years or older with epilepsy: a Korean national multicenter study. J Clin Neurol. (2020) 16:53–9. doi: 10.3988/jcn.2020.16.1.53
32. Ben-Menachem E, Grebe HP, Terada K, Jensen L, Li T, De Backer M, et al. Long-term safety and efficacy of lacosamide and controlled-release carbamazepine monotherapy in patients with newly diagnosed epilepsy. Epilepsia. (2019) 60:2437–47. doi: 10.1111/epi.16381
33. French JA, Wechsler RT, Trinka E, Brandt C, O'Brien TJ, Patten A, et al. Long-term open-label perampanel: generalized tonic-clonic seizures in idiopathic generalized epilepsy. Epilepsia Open. (2022) 7:393–405. doi: 10.1002/epi4.12602
34. Jaramillo JA, María JC, Úbeda JM, López ÓV, Rivas ME, Díaz HP, et al. Effectiveness and safety of perampanel as early add-on treatment in patients with epilepsy and focal seizures in the routine clinical practice: Spain prospective study (PERADON). Epilepsy Behav. (2020) 102:106655. doi: 10.1016/j.yebeh.2019.106655
Keywords: epilepsy, perampanel, nomogram, predictive model, development and validation
Citation: Zhu C, Li J, Wei D, Wu L, Zhang Y, Huang H and Lin W (2023) A nomogram to predict the treatment benefit of perampanel in drug-resistant epilepsy patients. Front. Neurol. 14:1284171. doi: 10.3389/fneur.2023.1284171
Received: 30 August 2023; Accepted: 30 October 2023;
Published: 14 November 2023.
Edited by:
Fernando Cendes, State University of Campinas, BrazilReviewed by:
Jacopo Lanzone, Istituti Clinici Scientifici Maugeri IRCCS, ItalyGiovanni Battista Dell'Isola, University of Perugia, Italy
Copyright © 2023 Zhu, Li, Wei, Wu, Zhang, Huang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huapin Huang, aGgtcEAxNjMuY29t; Yuying Zhang, eXFsbXp5eUAxNjMuY29t; Wanhui Lin, d2FuaHVpbGluQGZqbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
‡These authors share senior authorship
§ORCID: Wanhui Lin orcid.org/0000-0002-9100-0449
 Chaofeng Zhu
Chaofeng Zhu Juan Li1†
Juan Li1† Dazhu Wei
Dazhu Wei Huapin Huang
Huapin Huang Wanhui Lin
Wanhui Lin