- 1Melbourne School of Psychological Sciences, The University of Melbourne, Parkville, VIC, Australia
- 2Department of Psychology, The Alfred Hospital, Melbourne, VIC, Australia
- 3Developmental Imaging, Murdoch Children's Research Institute, Melbourne, VIC, Australia
- 4General Electric Healthcare, Melbourne, VIC, Australia
- 5Monash Alfred Psychiatry Research Centre, Melbourne, VIC, Australia
- 6Department of Paediatrics, The University of Melbourne, Parkville, VIC, Australia
- 7Neuroscience Research, Murdoch Children's Research Institute, Melbourne, VIC, Australia
- 8Department of Neurosurgery, Neuroscience Advanced Clinical Imaging Service (NACIS), The Royal Children's Hospital, Melbourne, VIC, Australia
Introduction: Recent developments in neuroimaging techniques enable increasingly sensitive consideration of the cognitive impact of damage to white matter tract (WMT) microstructural organisation after mild traumatic brain injury (mTBI).
Objective: This study investigated the relationship between WMT microstructural properties and cognitive performance.
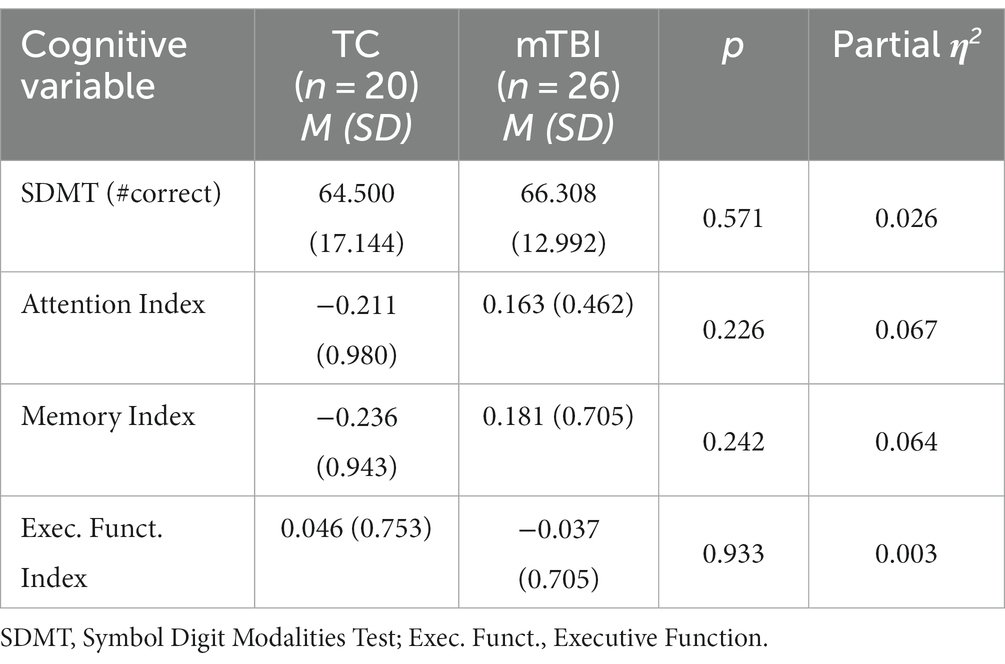
Participants, setting and design: Using an observational design, a group of 26 premorbidly healthy adults with mTBI and a group of 20 premorbidly healthy trauma control (TC) participants who were well-matched on age, sex, premorbid functioning and a range of physical, psychological and trauma-related variables, were recruited following hospital admission for traumatic injury.
Main measures: All participants underwent comprehensive unblinded neuropsychological examination and structural neuroimaging as outpatients 6–10 weeks after injury. Neuropsychological examination included measures of speed of processing, attention, memory, executive function, affective state, pain, fatigue and self-reported outcome. The WMT microstructural properties were estimated using both diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI) modelling techniques. Tract properties were compared between the corpus callosum, inferior longitudinal fasciculus, uncinate fasciculus, anterior corona radiata and three segmented sections of the superior longitudinal fasciculus.
Results: For the TC group, in all investigated tracts, with the exception of the uncinate fasciculus, two DTI metrics (fractional anisotropy and apparent diffusion coefficient) and one NODDI metric (intra-cellular volume fraction) revealed expected predictive linear relationships between extent of WMT microstructural organisation and processing speed, memory and executive function. The mTBI group showed a strikingly different pattern relative to the TC group, with no relationships evident between WMT microstructural organisation and cognition on most tracts.
Conclusion: These findings indicate that the predictive relationship that normally exists in adults between WMT microstructural organisation and cognition, is significantly disrupted 6–10 weeks after mTBI and suggests that WMT microstructural organisation and cognitive function have disparate recovery trajectories.
Introduction
Mild traumatic brain injury (mTBI) that results in hospital treatment, occurs in at least 100–300/100,000 individuals per year (1). Cognition is routinely affected by mTBI in the acute period, most commonly in the domains of speed of information processing, attention, memory and executive function (1). Full resolution of objective cognitive difficulties occurs for the majority of individuals within 3 months of injury (2). During normal recovery, however, as well as for the 20% who do not recover within the typical timeframe (1), cognitive impairment is a substantial contributor to poor psychosocial outcome and disability after mTBI (3–5).
In healthy adult populations, objective cognitive performance has been shown to be associated with WMT microstructural organisation, as measured with diffusion-tensor imaging (DTI) metrics. Specifically, fractional anisotropy (FA), which quantifies degrees of diffusion directionality, is positively associated with general cognition (6, 7), speed of processing (6), attention (6), memory (8) and executive function (6); mean diffusivity (MD), which quantifies magnitude of diffusivity, is negatively associated with processing speed (6) and executive function (6). Within the mTBI population, a recent meta-analysis of hospitalised mixed-mechanism adults found that structural white matter tract (WMT) changes are also associated with cognitive changes (9). Specifically, WMT pathology is associated with dysfunction in attention, memory and executive function, with poorer performance in these cognitive domains significantly associated with reduced FA. Poorer performance in the domains of attention and memory are also associated with increased MD. Despite being commonly affected by mTBI, processing speed has not been included in sufficient DTI studies to enable a meta-analysis.
Although there is currently no consensus regarding whether specific tracts are associated with deficits in specific cognitive domains post mTBI, it has been established that WMT damage after mTBI typically occurs in long-range association and interhemispheric tracts (10). These include the corpus callosum (CC), superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus (ILF), uncinate fasciculus (UF) and anterior corona radiata (ACR) (10–13). In particular, DTI metrics most typically demonstrate reduced FA and increased apparent diffusivity coefficient (ADC; considered broadly synonymous to MD, as MD is the mean ADC of the diffusion tensor) in these tracts after mTBI; these have been interpreted as representative of diffuse axonal injury (10).
Changes in DTI metrics are multi-determined, being influenced by many white matter microstructural properties that are directly affected by mTBI-related pathological mechanisms, including dysmyelination, axonal loss, and/or reduced axonal diameters (14, 15). The coherence of axonal fibre arrangements, a microstructural factor potentially unrelated to the brain injury, also influences DTI metrics (15–17). Importantly, DTI cannot model multiple axonal fibre orientations (“crossing fibres”) with a single MRI voxel (18), which is problematic given they are present in over 90% of brain white matter regions (19).
Neurite orientation dispersion and density imaging (NODDI) (20) is a biophysical diffusion weighted imaging (DWI) model that provides more specific information about WMT microstructural properties than DTI. The NODDI model fits the DWI signal into three assumed tissue compartments: the intra-neurite, extra-neurite and cerebrospinal fluid compartments. Two useful quantitative metrics derived from NODDI are the intra-cellular volume fraction (ICVF), which is a measure of neurite density, and the orientation dispersion index (ODI), which quantifies the bending and fanning of neurites (20). The metric ODI has a strong negative correlation with FA, whereas ICVF has a weak positive correlation with FA (20, 21).
It has been shown that NODDI is sensitive to mTBI-related WMT changes (13, 22–25). Indeed, the current cohort of mTBI participants has been shown to have WMT microstructural disorganisation relative to the current TC sample, which is evident on both DTI and NODDI metrics (13). Few studies have investigated the relationship between NODDI metrics and measures of cognition, however. One study showed linear relationships between ICVF and measures of attention, memory and executive function (25), but the direction of these linear relationships was reversed when mTBI participants’ performances were compared with a non-mTBI trauma control (TC) group. Individuals with mTBI showed negative linear relationships with measures of attention, memory and executive function. The researchers did not assess processing speed and examined individuals approximately 2 weeks after injury, when injury-related physiological fluctuations are still present and substantial recovery is ongoing; this makes cognitive function more variable and limits reliability of examination (26, 27). The study also used tract based spatial statistics (TBSS), which relies on a whole brain analysis of WMTs that have been normalised prior to analysis. This approach may not be appropriate for identifying subtle changes in WMT structure (28). A second study found ODI was positively correlated with processing speed in a combined mTBI and healthy control group, but also used TBSS and examined individuals with mTBI even earlier - less than 7 days after injury (29).
The aim of the current study was to investigate the relationship between cognitive performance and DTI- and NODDI-derived WMT microstructural metrics of individual tracts, in the post-acute period (>6 weeks) after mTBI. It was hypothesised that both the mTBI and TC groups would demonstrate positive linear associations between FA and cognitive performance in all cognitive domains, and both groups would demonstrate a negative linear association between ADC and processing speed, attention and memory. In contrast, it was hypothesised that the mTBI group would demonstrate negative linear associations between ICVF and attention, memory and executive function, whereas the TC group would demonstrate linear associations in the opposite direction on these variables. Finally, it was hypothesised that the TC group would demonstrate negative linear relationships between ODI and all cognitive domains, whereas the mTBI group would demonstrate positive linear associations between ODI and processing speed.
Method
Participants
Participants comprised individuals, excluding professional athletes and war veterans, who had suffered any traumatic injury (systemic and/or head) between September 2015 and April 2018, and been consecutively admitted to The Alfred hospital, Melbourne, Australia, in the preceding 6–12 weeks. Detailed description of the recruitment process and the recruitment decision tree have been reported previously (30, 31). All admitted trauma patients were approached for recruitment consideration. The mTBI group comprised 26 premorbidly healthy adults (22 male) aged 18–60 years (Mean = 34.81, SD = 13.76) whose traumatic injury included a head strike and fulfilled criteria for a mTBI event as defined by the World Health Organisation criteria (32), which can be summarised as (i) 1 or more of: confusion or disorientation, loss of consciousness for 30 min or less, post-traumatic amnesia less than 24 h, and/or other transient neurological abnormalities not requiring surgery; (ii) Glasgow Coma Scale score of 13–15 after 30 min or later upon presentation for healthcare. Exclusion criteria were individuals with: any previous neurological history; any history of heavy alcohol consumption (>5 standard drinks/day), intravenous or regular Class A drug use; any current Class A drug use; history of any significant psychiatric disorder and any current/recent diagnosis or treatment of depression and/or anxiety and/or post-traumatic stress disorder; current TBI (at time of hospital admission) as a result of physical assault/attack; and lack of conversational English fluency. The TC participants comprised 20 premorbidly healthy adults (18 male) aged 18–60 years (Mean = 38.75, SD = 12.59) whose traumatic injury had not included a head strike and who did not report any symptoms of mTBI; this group had the same exclusion criteria as the mTBI group, with the addition of having no previous head injury. No ethnic group differences existed. All participants provided informed consent and the project was approved by The Alfred hospital Human Research Ethics Committee.
Measures
Premorbid cognitive functioning
The Wechsler Test of Adult Reading (WTAR) (33) is a word reading task, from which accurate estimates of premorbid intellectual functioning (PreIQ) can be derived in individuals with mTBI (34).
Processing speed
The Symbol Digit Modality Test – (SDMT) is a measure of processing speed that is sensitive to cognitive impairment after mTBI (35). It requires individuals to provide the correct number that corresponds to a given symbol, according to a reference key at the top of the page. On this version of the SDMT, the final score was number of correct items within 2 min.
PS/attention index
The Trail Making Test Parts A and B (TMTA and B) (36) measure processing speed and high level attention, respectively. These tests have high reliability and validity for mTBI populations (37, 38). The Victoria Stroop Dots trial (Stroop Dots) is a measure of processing speed (39). The Digit Span subtest from the Wechsler Adult Intelligence Scale – 4th is a valid, reliable and widely-used (40). Digit Span Total (DSp) is a global measure of attention; raw scores rather than aged scaled scores were used to enable comparative analyses with other cognitive measures.
Memory index
The Rey Auditory Verbal Learning Test (RAVLT) (41) is a reliable and valid measure of verbal memory (42). The total number of items learned on the 5 list learning trials (Total) assessed acquisition and the number of items recalled independently after a 25-min delay (Delay) assessed recall. The Rey-Osterrieth Complex Figure Test Delay (RCFT Delay) score (43) is a widely used measure of visual memory function that has good test–retest reliability (0.89) (44) and has been used with mTBI populations previously (45, 46). Ability to independently draw the stimulus figure after a 10-min delay was used as the measure of visual memory (RCFT Delay). All RCFT figures were scored by a single researcher, and intra-rater reliability was high (0.94).
Executive function index
The ratio of TMTB/TMTA was used as a measure of mental flexibility. The interference ratio of colour words/dots from the Victoria Stroop test (Stroop Int) is a measure of inhibitory control. Both of these measures have been shown to be sensitive to executive dysfunction after mTBI (37, 38, 47, 48).
Pain
The Short-Form McGill Pain Questionnaire (MPQ) (49) was used to measure pain. The MPQ requires respondents to endorse whether they have experienced different types (descriptions) of pain, and to what level, during the past week. It has excellent reliability and validity and has been used with mTBI populations (50).
Fatigue
The Multidimensional Fatigue Inventory (MFI) (51) was used to measure fatigue. The MFI is a 20-item questionnaire that measures five different types of fatigue and sums these together to provide a general measure of fatigue. It has good internal consistency (Cronbach’s alpha >0.076) and has previously been used in the TBI population (52, 53).
Quality of life
The RAND 36-item Short Form Survey (SF-36) (54) was used as a measure of general health related quality of life. This quality of life measure has been shown to be both reliable and valid in TBI populations (55).
Post-concussion symptoms
The Rivermead Post Concussion Symptoms Questionnaire (RPQ) is a widely used measure of post-concussion symptomatology. It assesses physical (10 items), psychological (3 items) and cognitive (3 items) symptoms experienced during the past 24 h (56). It has been shown to be elevated after mTBI and also other conditions (57–59).
Psychological distress index
Three widely used, valid and reliable questionnaires of psychological distress were used: The Inventory of Depressive Symptomatology (IDS) measures severity of overall depression (60). The Beck Anxiety Inventory (BAI) measures anxiety symptomatology (61). The PTSD Checklist for the DSM-5 (PCL-5) (62) measures the 20 symptoms of PTSD defined by DSM-5 (63). A single Psychological Distress Index (PDI) was created from standardised performances on the IDS, BAI and PCL-5. Specifically, responses on each measure were converted to a standardised 4-point scale and then summed together, resulting in a single variable comprising equivalent numbers of items measuring depression (IDS) and anxiety-based symptomatology (BAI and PCL-5).
Principal component analysis (PCA) was conducted on the cognitive measures to reduce the number of measures and identify a coherent group of cognitive indices in a statistically recommended manner (64). This analysis was conducted using a larger sample of mTBI and TC participants (n = 87), which had been recruited in an identical manner but had not undergone neuroimaging. As the SDMT was found to correlate moderately with most variables, it was removed and analysed separately. From the remaining variables, a three-component solution was supported, which explained 70.93% of the total variance. These components were interpreted as measuring Processing Speed/Attention, Memory and Executive Function. The variable to sample size ratio was 9:1, which compensates for a smaller sample size (n = 87) (65). The structure matrix of the PCA is presented in Supplementary Table S1.
Raw scores from test performances were converted to z-scores also using the aforementioned larger sample of mTBI and TC participants (n = 87). Index scores were then determined for each individual by averaging the z-scores for the tests comprising each index.
Measure of effort
The Digit Span (DSp) subtest from the Wechsler Adult Intelligence Scale, 4th Edition (WAIS-IV) (40) was used as a measure of effort (66). Participants were identified as having problematic effort on testing if they failed on the subscales of Longest Digits Forward (Fail = 4 or less) and Longest Digits Backward (Fail = 2 or less) (66).
Procedure
Following recruitment on the ward, within 1–4 days of injury, participants returned to the hospital for neuropsychological examination and MRI scans, conducted on the same day, 6–10 weeks after injury. Neuropsychological measures were conducted in the following sequence for all participants: SDMT, WTAR, Stroop, RAVLT, DSp, RCFT, TMT, RPQ, SF-36, MFI, IDS, BAI, PCL-5 and MPQ.
MRI data acquisition
MRI data (PRISMA3T Siemens, Erlangen, Germany) was acquired at the Baker IDI Heart and Diabetes Institute, Melbourne, Australia. High resolution volumetric T1-weighted images were acquired using the magnetisation prepared rapid gradient-echo (MPRAGE) sequence (240 × 256 mm acquisition matrix; FOV = 256 mm; 176 contiguous slices; 1mm3 isotropic voxel size; TR/TE = 2300/2.96 ms). The DWI data was acquired with multiband accelerated EPI sequences for multishell acquisition (128 × 128 mm acquisition matrix; FOV =256 mm; 75 contiguous slices; 64 b = 1,000 s/mm2 volumes, 64 b = 3,000 s/mm2 volumes, and 4 b = 0 s/mm2 volumes; 2mm3 isotropic resolution; TR/TE = 4800/88 ms; MB factor = 3). Additional volumes of reversed phase encoded b0 images were obtained to correct for susceptibility-induced geometric distortion (67).
MRI data processing
All DWI data and tractography reconstructions were processed using the MRtrix3 software package (version 0.3.16; Brain Research Institute, Melbourne, Australia1) with FSL functions (FMRIB’s Software Library2) incorporated in MRtrix3 command line usage. The NODDI model fitting and parametric maps were estimated using the NODDI Matlab Toolbox (Version 1.0.1). The DWI data was pre-processed to correct for thermal noise (68), gibbs-ringing artifacts (69), eddy current and motion artifacts (67, 70), susceptibility-induced geometric distortions (67), and β1 bias field inhomogeneities (71). Local WM fibre-orientation distributions were estimated based on multi-shell multi-tissue Constrained Spherical Deconvolution (msmt-CSD) (72). Tractography, representing the CC, and the six pairs of long-ranged association WMTs or WMT segmented components, were reconstructed from each participant, using a probabilistic tracking algorithm (73), retained 2,500 streamlines per WMT, a FOD cutoff = 0.1 and other default tracking parameters, and manually placed regions of interest (ROI) in areas with known anatomical priors based on modifications made from previous published methods (74). The DTI and NODDI model fittings and associated parametric maps were estimated based on the subset of b = 1,000 s/mm2 data and the multishell DWI data, respectively. Tract-wise averaged DTI and NODDI parametric estimations were then computed from binarised WMT masks derived from each tractography reconstruction.
Figure 1 illustrates the reconstructed tractography images from a representative study participant.
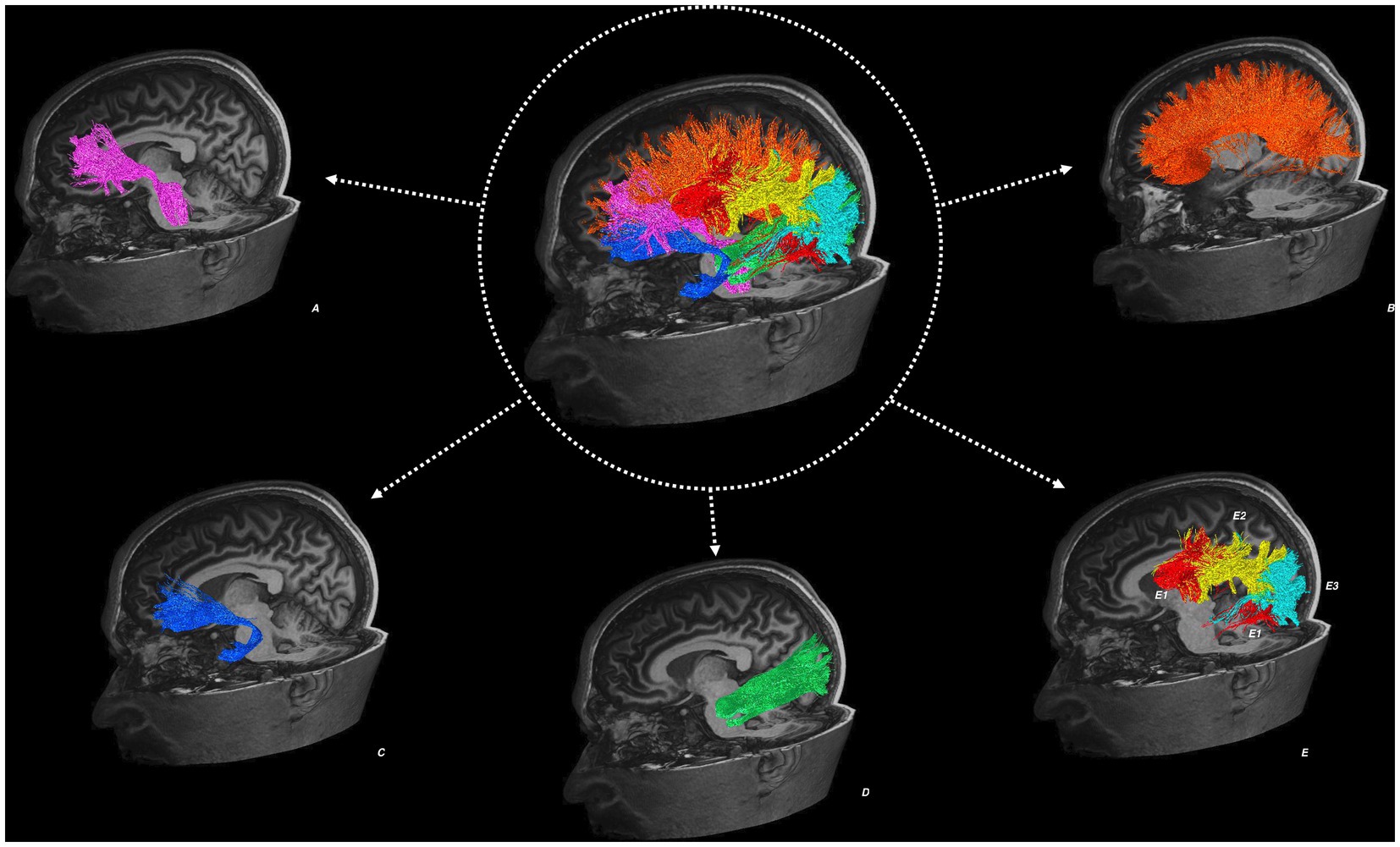
Figure 1. Tractography reconstructions from an example study participant. Showing the left anterior corona radiata (A), corpus callosum (B), uncinate fasciculus (C), inferior longitudinal fasciculus (D), superior longitudinal fasciculus (E) in three segmented components: the direct segment (E1), anterior indirect segment (E2), and posterior indirect segment (E3).
Statistical analysis
Statistical analyses were undertaken using Statistical Package for Social Sciences (SPSS version 26) software. Most demographic variables had a non-normal distribution, so Mann Whitney U tests were conducted to compare groups on these variables; the MPQ, MFI, SF-36 and days between injury and assessment were normally distributed enabling t-test analyses. Multivariate analyses of covariance (MANCOVA) were used to examine group differences on the cognitive variables, controlling for days post injury. Multiple regression analysis was conducted to identify group differences in the relationship between independent and cognitive variables. For significant interactions, additional regression analyses were undertaken separately for each group to examine whether DTI (FA, ADC) and NODDI (ICVF, ODI) metrics, from each WMT of interest, significantly predicted performance on cognitive testing. As research has demonstrated significant associations between DTI metrics and both age and predicted FSIQ (7, 75), these variables were included in the model as covariates. All analyses were re-run including sex as a co-variate. As this had no impact on the findings, we excluded this co-variate from the reported findings as increasing co-variates reduces the power of analyses. Consistent with modern statistical practise (76) in the context of using multiple univariate neuroimaging analyses, results were interpreted by identifying consistent overall patterns of findings and considering magnitudes of difference through inspection of confidence intervals, rather than focussing on specific p-values. A consistent overall pattern of results, which have confidence intervals that are not close to including 0.00, as we have in this paper, is considered to indicate a real effect (76).
Results
The demographic details and injury characteristics for each group are presented in Table 1. No individual in either group failed the measure of effort. The between group comparisons of the DWI data have been previously published, with between group differences evident on DTI and NODDI metrics on all white matter tracts (13).
The groups were well matched on all demographic variables and reported similar levels of post-concussion symptoms and quality of life, but the mTBI group had an average of 2–3 weeks of additional recovery time between injury and examination relative to the TC group. As between group comparisons were not carried out for the primary analyses of interest (regression analyses), this group difference was not included as a co-variate.
Analysis of the cognitive variables revealed no group differences on either the SDMT or cognitive indices (p > 0.225), as shown in Table 2. Effect sizes were small (0.01) to moderate (0.07) for all comparisons. T-test analyses indicated that the mTBI (p = 0.001) and TC (p = 0.002) groups were significantly slower on the SDMT than a previously reported healthy control sample ( = 13.61) (77).
After controlling for age and predicted FSIQ, a series of planned significant regression analyses revealed the groups repeatedly differed with respect to the relationship between the independent variables and the measure of cognition. Those models that were not significant revealed no relationship between the independent variables and cognitive measures for either group. Detailed results of the significant regression models, and subsequent regression analyses that identified a statistically independent relationship between an imaging metric and a cognitive variable, are presented in the Supplementary Tables S2–S6. Table 3 presents an overview, for each WMT, of those models that were significant overall (p < 0.05), and also revealed a significant independent relationship between one or more DWI metrics and the cognitive variable.
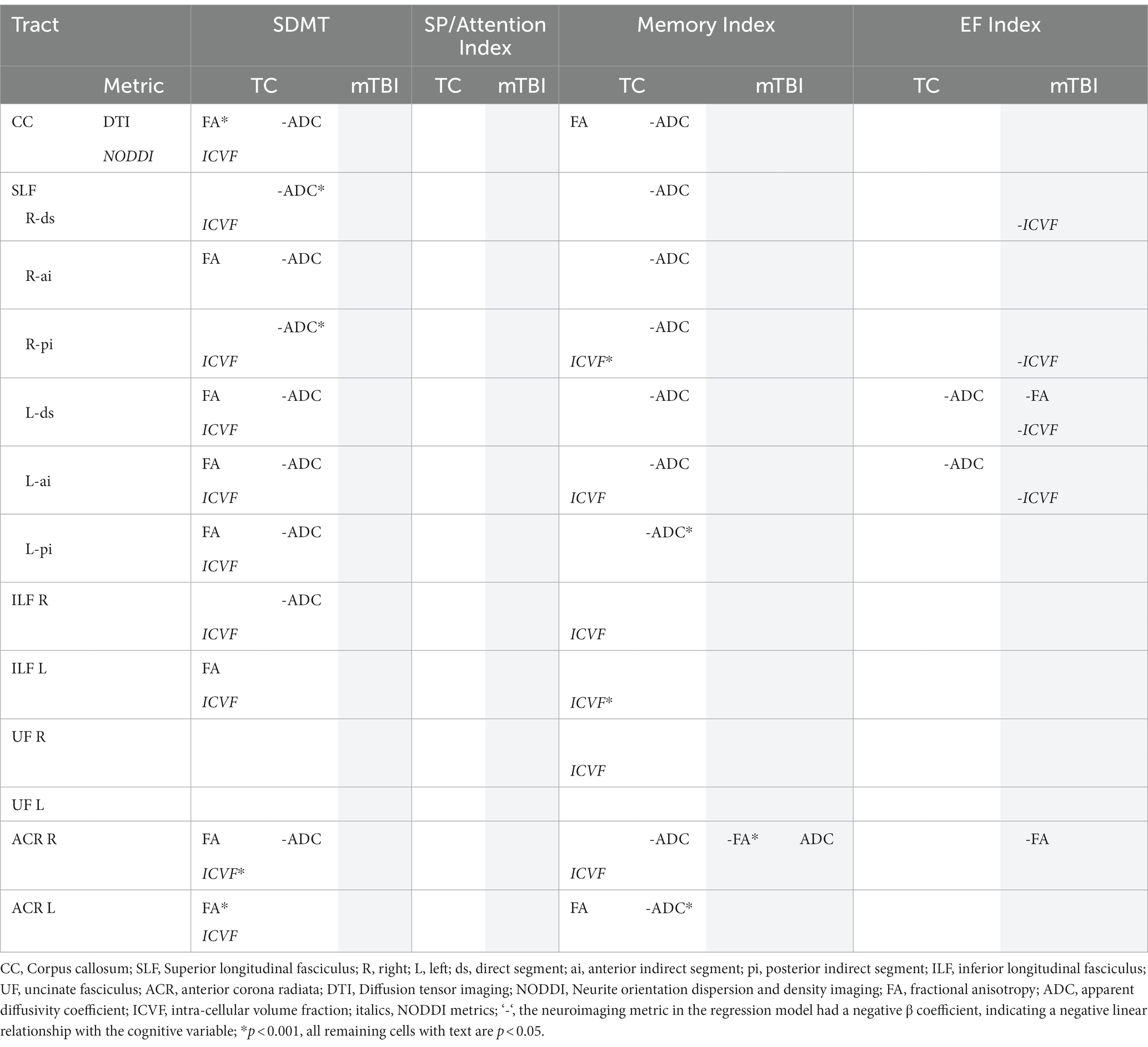
Table 3. Regression models with significant independent neuroimaging predictors by cognitive variables for each group.
Overall, the results revealed a consistent pattern of neuroimaging metrics significantly predicting cognitive function across a range of WMTs for the TC group. In contrast, almost no significant predictive relationships were evident in the mTBI group. In the few instances where neuroimaging metrics did significantly predict cognition in the mTBI group, either no significant relationship was evident in the TC group, or a significant predictive relationship, in the opposite direction, was evident in the TC group (ADC and Memory Index in right ACR). As an exemplar illustration of this broad pattern of significant findings, Figure 2 shows the relationship between DWI metrics and SDMT performance for the CC.
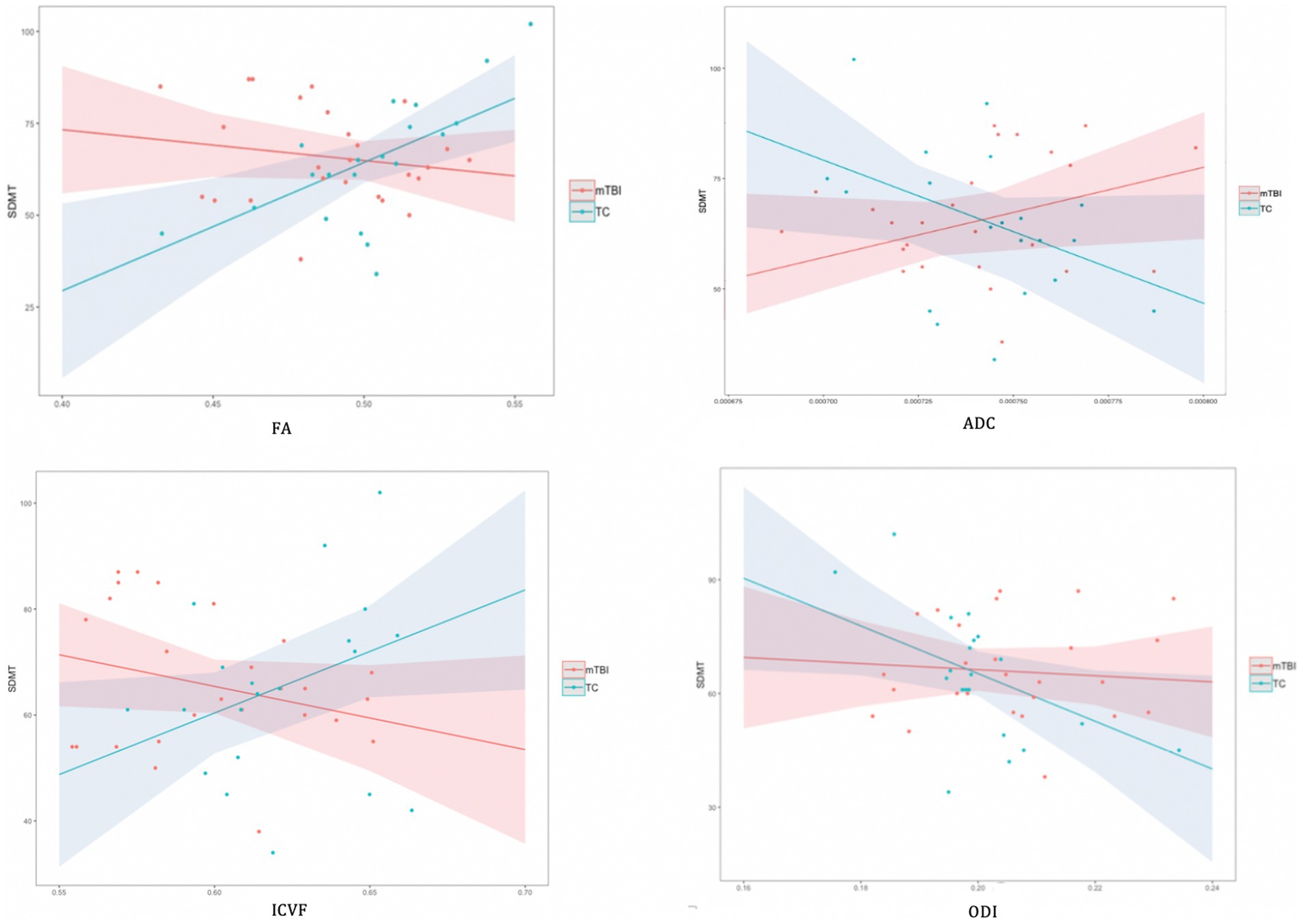
Figure 2. Associations between DWI metrics and SDMT performance for corpus callosum by group. In illustration of the broad pattern of findings across all significant regression analyses, the TC group showed a positive linear relationship between FA, ICVF and the cognitive variable, whereas a negative association was evident for the ADC. For the mTBI group, there were typically no significant associations between DWI metrics and cognition. There were no significant linear relationships between ODI and any cognitive variable in either group.
For the TC group, the majority of significant results revealed a predictive relationship between imaging metrics and the SDMT and Memory Index variables. Substantially more independent predictive relationships were evident between DTI metrics and cognitive variables than were evident for NODDI metrics. FA and/or ADC independently predicted performance on the SDMT and the Memory Index in most WMTs. All significant associations between cognitive variables and FA were positive and all significant associations with ADC were negative. Of the NODDI metrics, ODI did not independently predict cognitive performance on any WMT. ICVF independently predicted cognitive performance on most WMTs, however. All significant associations between cognitive variables and ICVF were positive. The EF Index was only associated with ADC, and this occurred only for the direct segment and the anterior indirect segment of the left SLF. There were no associations between any neuroimaging metric and the SP/Attention Index.
For the mTBI group, only the SLF and the right ACR revealed a small number of significant independent predictive relationships between FA, ADC and/or ICVF and cognitive performances. In the opposite pattern to the TC group, both FA and ICVF were negatively associated with cognition and ADC was positively associated with cognition. The right ACR revealed an independent predictive relationship between DTI metrics and the Memory Index, and a small number of WMTs revealed a predictive relationship between DTI and NODDI metrics and the EF Index. There were no associations between any neuroimaging metrics and the SDMT or the SP/Attention Index.
Discussion
Broadly consistent with the hypotheses, our findings showed a robust relationship between both DTI (FA/ADC) and NODDI (ICVF) metrics, and cognitive domains in the TC group. Specifically, the FA and ICVF metrics significantly, independently and positively predicted processing speed ability (SDMT) in the majority of WMTs; they also independently and positively predicted Memory Index performance, although these relationships were evident on a minority of WMTs. In addition, significant negative predictive relationships were found between ADC and both processing speed and the Memory Index, on the majority of WMTs. In contrast to hypotheses, for the mTBI group very few significant predictive relationships were evident between any DTI or NODDI metric and any cognitive measure on any WMT; the few significant predictive relationships that were observed were in the opposite direction to that predicted and were mostly evident for the EF Index. Also in contrast to hypotheses, neither group demonstrated any significant predictive relationships between the NODDI metric ODI, and any cognitive measure on any WMT.
These results strongly indicate that this premorbidly healthy, hospitalised TC group were performing in a manner that was commensurate with healthy performance (6, 7, 20, 21). The relationship between DWI metrics and cognition in this premorbidly healthy hospitalised mTBI group consistently contrasted that seen in the TC group, however. Wherever the TC group demonstrated a significant predictive linear relationship between DTI and/or NODDI metrics and a cognitive index, the mTBI group showed no significant predictive relationship or, in one instance, a significant relationship in the opposite direction. Further, in a small number of analyses, where no significant relationship was found for the TC group, the mTBI group did in fact reveal a significant predictive relationship.
This consistent finding of group difference in the pattern of significant relationships between DTI measures and cognition suggests that customary predictive relationships, which exist between WMT microstructure and cognitive function in premorbidly healthy hospitalised TC adults, are disrupted in premorbidly healthy hospitalised mTBI adults, 6–10 weeks after injury. Given the rigorous sample matching and the removal of influencing factors in the analyses, it is likely that this relationship disruption was specifically due to the mTBI.
The finding of contrasting group profiles between the NODDI metric, ICVF, and cognition is consistent with the single study that has looked at this previously after mTBI (25). Specifically, in the opposite pattern to the TC group, better cognitive performance by individuals with mTBI was associated with lower ICVF or lower axonal packing density (25). It was suggested this might be due to a higher ratio of astrocytes, as these have been shown to aid neuroprotection by contributing to post-traumatic tissue repair and synaptic remodelling following TBI (78).
The lack of any significant relationships between the NODDI metric, ODI, and cognition indirectly contrasts previous research, which has indicated that ODI is typically negatively correlated with FA (21) and is positively correlated with processing speed in a combined mTBI and healthy control sample (29). Given that no previous studies have reported a relationship between ODI and cognition in a pure mTBI sample, however, the present finding cannot be considered inconsistent with any previous research.
With respect to the DTI findings, a recent meta-analysis, which also examined hospitalised mixed-mechanism adults after mTBI, found a significant positive linear relationship between FA and attention, memory and executive function (9). In contrast, the present study identified no positive relationship between FA and cognitive function in the mTBI group on most WMTs, and even a negative relationship on two WMTs in the domains of memory and executive function. A possible mechanism underlying these negative relationships is that selective disruption of one WMT fibre population occurred in the presence of crossing fibres, leading to a subsequent increase in FA in the context of reduced cognitive performance (79).
The difference in DTI findings between the current and previous studies might be explained by the fact that the meta-analysis (9) collated data from different regions of interest to undertake analyses, whereas the current study looked at relationships for a substantial number of individual WMTs. In addition, most earlier studies undertook a more limited cognitive examination, included individuals that were either substantially earlier or later in the recovery process, or included individuals with moderate–severe TBI. These methodological discrepancies prevent meaningful inferential conclusions (32) regarding the discrepancy between past and present findings.
In the current study, both groups were slower than published healthy control data (77). Cognitive performances were commensurate between the groups, however, despite the relationship between cognition and WMT microstructural features (as measured by DWI metrics) being abnormal in the mTBI group. This appears to suggest that cognitive function had recovered in the mTBI group 6–10 weeks after injury to be consistent with cognitive abilities seen in general trauma patients, despite ongoing WMT structural disruptions or remodelling. It is also possible, however, that subtle group differences in cognition were not found because of the modest sample size. This is somewhat substantiated by the moderate between group effect size evident for some cognitive indices. Further research with larger sample sizes might clarify this issue.
It is noteworthy that the difference in brain-behaviour relationship between the groups occurred in the context of no group differences in post-concussion symptom reporting or quality of life. Given that symptom reporting is commonly elevated in trauma control samples (59), this does not mean that either group had recovered to premorbid levels of functioning. It does show, however, that these factors cannot explain the different pattern of relationships found between the mTBI and TC groups in this study. Future research is clearly warranted to investigate whether there is a relationship between the current pattern of findings and symptom resolution.
This study supported previous findings of CC, SLF, ILF and ACR involvement after mTBI (10–12), as the brain-behaviour relationships differed between the groups on all of these WMTs. It also indicated that the different SLF segments are comparably susceptible to an alteration in brain-behaviour relationship after mTBI. While the lack of group difference on the UF is noticeably discrepant from the remaining findings, it does not indicate that the UF was unaffected by mTBI pathology. Rather, 6–10 weeks post-injury there was no relationship between WMT structural pathology and cognition, irrespective of whether an individual suffered an mTBI or a traumatic injury without mTBI.
The present study provides an opportunity to contrast the relationship between cognition and various DWI metrics in a premorbidly healthy, hospitalised mTBI sample. Although FA and ICVF consistently demonstrated significant predictive relationships with cognition that were in the same direction, they were only concurrently significant in 30% of analyses; this is consistent with the notion that these metrics are positively correlated, but only weakly (20, 21). In contrast, whereas ODI has previously been shown to have a strong negative correlation with FA (20, 21), this relationship was not observed in the current study. Disentangling the underlying pathological mechanism for this observation is difficult, as it may relate to the complex effects of ongoing WMT microstructural damage and remodelling on the DWI signal and derived modelling metrics. Certainly, unexpected patterns of both DTI and NODDI metric changes have been reported in other mTBI studies (24, 25, 79). This finding does challenge the view, however, derived from a study using a sample of martial artists (23), that these metrics provide comparable information about underlying WMT microstructure after mTBI.
The lack of any significant predictive relationship between any DWI metric and the SP/Attention Index for either group was unexpected given previous findings of a relationship between attention and WMT microstructural organisation in healthy adults and individuals with mTBI (9, 80). The likely explanation for this was that the SP/Attention Index was not a pure measure of attention, as it included measures of attention, processing speed and mental flexibility. Although the index was created via a statistically robust method of creating a dimensionally coherent index (64), it resulted in an index that measured a construct other than pure attention. This prevents comparability to previous studies that utilised single measures of attention.
The primary limitation of the current study was a relatively modest sample size, which prevented investigation of interactions between WMTs and increased the likelihood of making Type II errors. To address this, we limited interpretation of our findings to overall patterns of consistent findings, as inferential conclusions are most robust when results are consistent across a range of measures (81). To reduce the likelihood of Type I error, consistent with modern statistical practise (76), rather than correcting for multiple comparisons, the findings were again interpreted with respect to overall patterns and uniformity of findings as well as considering magnitudes of difference, rather than focussing on specific p-values (76). Given the consistency of the pattern of significant findings in this study, there is strong evidence to support generalisability of these findings to premorbidly healthy civilian adults who are admitted to hospital with mTBI in the context of a traumatic injury.
Next, the presence of CSF partial voluming effect can confound both the DTI and NODDI metric estimates, by reducing contribution of anisotropic diffusion signal estimated from the white matter (82, 83). This is reflected by reduced FA value for the DTI model. The NODDI model has been reported to overestimate the CSF volume fraction (i.e., fiso) due to not accounting for compartment-specific T2 relaxation and its model parameters are usually estimated from data acquired with a single echo time (TE) (84–86). This can lead to erroneous NDI estimates in the white matter (86). Newly proposed methods, including acquiring data with multiple echo time (86), or rescaling/constrained the fiso modelling term (84, 85) are promising methods to adopt in future studies. Finally, tract-wise average diffusion metrics were used to represent WMT microstructural properties in this study. This has the potential of negating regional differences in microstructural properties along the WMT. To address this, alternative analyses based on obtaining diffusion metrics along the WMT profile can be adopted in future studies (87).
The clinical implications of these findings are significant. As outlined earlier, this research programme has previously reported that the present mTBI sample demonstrates WMT microstructural disorganisation relative to the TC group at this same time point post injury (13). In combination with these previously reported findings, the present study suggests that different profiles exist for cognitive vs. neurostructural resolution after mTBI. Although functional cognition appeared broadly recovered at a group level approximately 9 weeks after injury, the relationship between WMT microstructural organisation and functional cognition had significantly altered from that seen in healthy individuals. That is, functional cognition may have largely resolved not as a consequence of underlying WMT microstructural resolution, but despite underlying WMT microstructural disorganisation (13). This disconnection between cognitive function and WMT microstructure in recovery after mTBI raises the question of how cognition might be resolving and whether there are modifiable factors at play that could be appropriate targets for intervention.
In conclusion, this study provided strong evidence that, despite normal cognitive function at a group level, the expected predictive relationship between WMT microstructural organisation and cognitive function, which is evident in premorbidly healthy hospitalised trauma control participants, is significantly disrupted 6–10 weeks after mTBI in those who were premorbidly healthy and were hospitalised for injury. The atypical WMT structuro-functional relationship that was seen in the mTBI group was evident in the CC, the bilateral ILF and ACR, and in all SLF segments, but was essentially absent in the UF. The DTI metrics, FA and ADC, as well as the NODDI metric, ICVF, demonstrated this disruption in the domains of processing speed, memory and executive function, but the NODDI metric, ODI, did not demonstrate disruption in any domain. These findings provide robust evidence that the mTBI has caused an alteration in the way WMT microstructural organisation relates to cognitive function (i.e., brain-behaviour relationship), and that this alteration is evident in the post-acute period after mTBI. Future research is needed to understand if these relationships normalise over time or demonstrate lasting disruption.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Alfred Hospital Human Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JA: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LO: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – review & editing. JC: Formal analysis, Software, Writing – review & editing. JM: Conceptualization, Methodology, Writing – review & editing. MS: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – review & editing, Formal analysis. JY: Conceptualization, Methodology, Resources, Supervision, Validation, Writing – review & editing, Funding acquisition, Software.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by The University of Melbourne [2017 MRGSS]. Imaging analysis was conducted within the Developmental Imaging research group, Murdoch Children’s Research Institute at the Children’s MRI Centre, Royal Children’s Hospital, Melbourne, Victoria. This was supported by the Murdoch Children’s Research Institute, Royal Children’s Hospital, The University of Melbourne, Department of Paediatrics and the Victorian Government’s Operational Infrastructure Support Program. This work was also part funded by a grant from The University of Melbourne, and the Royal Children’s Hospital Foundation (RCH1000 and RCH2022-1402).
Acknowledgments
The authors would like to acknowledge the contribution of post-graduate students and research assistants: Georgia Bolt, Emily Cockle, Nicolette Ingram, Courtney Lewis, Katie Priestley, Joshua Nash, Aimee Savage, Nicola Singleton and Patrick Summerell for their assistance in collecting this data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1278908/full#supplementary-material
Footnotes
References
1. Carroll, LJ, Cassidy, JD, Cancelliere, C, Cote, P, Hincapie, CA, Kristman, VL, et al. Systematic review of the prognosis after mild traumatic brain injury in adults: cognitive, psychiatric, and mortality outcomes: results of the international collaboration on mild traumatic brain injury prognosis. Arch Phys Med Rehabil. (2014) 95:S152–73. doi: 10.1016/j.apmr.2013.08.300
2. Carroll, LJ, Cassidy, JD, Peloso, PM, Borg, J, von Holst, H, Holm, L, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. (2004) 36:84–105. doi: 10.1080/16501960410023859
3. Benedictus, MR, Spikman, JM, and van der Naalt, J. Cognitive and behavioral impairment in traumatic brain injury related to outcome and return to work. Arch Phys Med Rehabil. (2010) 91:1436–41. doi: 10.1016/j.apmr.2010.06.019
4. Caplain, S, Blancho, S, Marque, S, Montreuil, M, and Aghakhani, N. Early detection of poor outcome after mild traumatic brain injury: predictive factors using a multidimensional approach a pilot study. Front Neurol. (2017) 8:666. doi: 10.3389/fneur.2017.00666
5. Ponsford, J, Olver, J, Ponsford, M, and Nelms, R. Long-term adjustment of families following traumatic brain injury where comprehensive rehabilitation has been provided. Brain Inj. (2003) 17:453–68. doi: 10.1080/0269905031000070143
6. Cremers, LG, de Groot, M, Hofman, A, Krestin, GP, van der Lugt, A, Niessen, WJ, et al. Altered tract-specific white matter microstructure is related to poorer cognitive performance: the Rotterdam study. Neurobiol Aging. (2016) 39:108–17. doi: 10.1016/j.neurobiolaging.2015.11.021
7. Malpas, CB, Genc, S, Saling, MM, Velakoulis, D, Desmond, PM, and O'Brien, TJ. MRI correlates of general intelligence in neurotypical adults. J Clin Neurosci. (2016) 24:128–34. doi: 10.1016/j.jocn.2015.07.012
8. Niogi, SN, Mukherjee, P, Ghajar, J, Johnson, CE, Kolster, R, Lee, H, et al. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. (2008) 131:3209–21. doi: 10.1093/brain/awn247
9. Oehr, L, and Anderson, J. Diffusion-tensor imaging findings and cognitive function following hospitalized mixed-mechanism mild traumatic brain injury: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2017) 98:2308–19. doi: 10.1016/j.apmr.2017.03.019
10. Aoki, Y, Inokuchi, R, Gunshin, M, Yahagi, N, and Suwa, H. Diffusion tensor imaging studies of mild traumatic brain injury: a meta-analysis. J Neurol Neurosurg Psychiatry. (2012) 83:870–6. doi: 10.1136/jnnp-2012-302742
11. Bigler, ED. Neuroimaging biomarkers in mild traumatic brain injury (mTBI). Neuropsychol Rev. (2013) 23:169–209. doi: 10.1007/s11065-013-9237-2
12. Hulkower, MB, Poliak, DB, Rosenbaum, SB, Zimmerman, ME, and Lipton, ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol. (2013) 34:2064–74. doi: 10.3174/ajnr.A3395
13. Oehr, LE, Yang, JY, Chen, J, Maller, JJ, Seal, ML, and Anderson, JFI. Investigating white matter tract microstructural changes at 6–12 weeks following mild traumatic brain injury: a combined DTI and NODDI study. J Neurotrauma. (2020) 38:2255–63. doi: 10.1089/neu.2020.7310
14. Alexander, AL, Lee, JE, Lazar, M, and Field, AS. Diffusion tensor imaging of the brain. Neurotherapeutics. (2007) 4:316–29. doi: 10.1016/j.nurt.2007.05.011
15. Beaulieu, C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. (2002) 15:435–55. doi: 10.1002/nbm.782
16. Tuch, DS, Reese, TG, Wiegell, MR, Makris, N, Belliveau, JW, and Wedeen, VJ. High angular resolution diffusion imaging reveals intravoxel white matter fiber heterogeneity. Magn Reson Med. (2002) 48:577–82. doi: 10.1002/mrm.10268
17. Wheeler-Kingshott, CA, and Cercignani, M. About axial and radial diffusivities. Magn Reson Med. (2009) 61:1255–60. doi: 10.1002/mrm.21965
18. Tournier, JD, Calamante, F, and Connelly, A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. NeuroImage. (2007) 35:1459–72. doi: 10.1016/j.neuroimage.2007.02.016
19. Jeurissen, B, Leemans, A, Tournier, JD, Jones, DK, and Sijbers, J. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp. (2013) 34:2747–66. doi: 10.1002/hbm.22099
20. Zhang, H, Schneider, T, Wheeler-Kingshott, CA, and Alexander, DC. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. (2012) 61:1000–16. doi: 10.1016/j.neuroimage.2012.03.072
21. Timmers, I, Roebroeck, A, Bastiani, M, Jansma, B, Rubio-Gozalbo, E, and Zhang, H. Assessing microstructural substrates of white matter abnormalities: a comparative study using DTI and NODDI. PLoS One. (2016) 11:e0167884. doi: 10.1371/journal.pone.0167884
22. Churchill, NW, Caverzasi, E, Graham, SJ, Hutchison, MG, and Schweizer, TA. White matter during concussion recovery: comparing diffusion tensor imaging (DTI) and neurite orientation dispersion and density imaging (NODDI). Hum Brain Mapp. (2018) 40:1908–18. doi: 10.1002/hbm.24500
23. Mayer, AR, Ling, JM, Dodd, AB, Meier, TB, Hanlon, FM, and Klimaj, SD. A prospective microstructure imaging study in mixed-martial artists using geometric measures and diffusion tensor imaging: methods and findings. Brain Imaging Behav. (2017) 11:698–711. doi: 10.1007/s11682-016-9546-1
24. Palacios, EM, Owen, JP, Yuh, EL, Wang, MB, Vassar, MJ, Ferguson, AR, et al. The evolution of white matter microstructural changes after mild traumatic brain injury: a longitudinal DTI and NODDI study. Sci Adv. (2020) 6:eaaz6892. doi: 10.1126/sciadv.aaz6892
25. Wu, YC, Mustafi, SM, Harezlak, J, Kodiweera, C, Flashman, LA, and McAllister, TW. Hybrid diffusion imaging in mild traumatic brain injury. J Neurotrauma. (2018) 35:2377–90. doi: 10.1089/neu.2017.5566
26. Bergsneider, M, Hovda, DA, McArthur, DL, Etchepare, M, Huang, SC, Sehati, N, et al. Metabolic recovery following human traumatic brain injury based on FDG-PET: time course and relationship to neurological disability. J Head Trauma Rehabil. (2001) 16:135–48. doi: 10.1097/00001199-200104000-00004
27. Iverson, GL. Outcome from mild traumatic brain injury. Curr Opin Psychiatry. (2005) 18:301–17. doi: 10.1097/01.yco.0000165601.29047.ae
28. Melonakos, ED, Shenton, ME, Rathi, Y, Terry, DP, Bouix, S, and Kubicki, M. Voxel-based morphometry (VBM) studies in schizophrenia-can white matter changes be reliably detected with VBM? Psychiatry Res. (2011) 193:65–70. doi: 10.1016/j.pscychresns.2011.01.009
29. Huang, S, Huang, C, Li, M, Zhang, H, and Liu, J. White matter abnormalities and cognitive deficit after mild traumatic brain injury: comparing DTI, DKI, and NODDI. Front Neurol. (2022) 13:803066. doi: 10.3389/fneur.2022.803066
30. Anderson, JFI, and Fitzgerald, P. Associations between coping style, illness perceptions and self-reported symptoms after mild traumatic brain injury in prospectively studied pre-morbidly healthy individuals. Neuropsychol Rehabil. (2020) 30:1115–28. doi: 10.1080/09602011.2018.1556706
31. Anderson, JFI, and Jordan, AS. An observational study of the association between sleep disturbance, fatigue and cognition in the post-acute period after mild traumatic brain injury in prospectively studied premorbidly healthy adults. Neuropsychol Rehabil. (2020) 31:1444–65. doi: 10.1080/09602011.2020.1781665
32. Carroll, LJ, Cassidy, JD, Holm, L, Kraus, J, and Coronado, VG, W. H. O. Collaborating Centre Task Force on Mild Traumatic Brain Injury. Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. (2004) 36:113–25. doi: 10.1080/16501960410023877
33. Wechsler, D. The Wechsler test of adult Reading. New York: The Psychological Corporation (2001).
34. Steward, KA, Novack, TA, Kennedy, R, Crowe, M, Marson, DC, and Triebel, KL. The Wechsler Test of Adult Reading as a measure of premorbid intelligence following traumatic brain injury. Arch Clin Neuropsychol. (2017) 32:98–103. doi: 10.1093/arclin/acw081
35. McCauley, SR, Wilde, EA, Barnes, A, Hanten, G, Hunter, JV, Levin, HS, et al. Patterns of early emotional and neuropsychological sequelae after mild traumatic brain injury. J Neurotrauma. (2014) 31:914–25. doi: 10.1089/neu.2012.2826
36. Partington, JE, and Leiter, RG. Partington’s pathway test. Psychol Serv Center Bull. (1949) 1:9–20.
37. Johansson, B, Berglund, P, and Rönnbäck, L. Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Inj. (2009) 23:1027–40. doi: 10.3109/02699050903421099
38. Spreen, O, and Strauss, E. A compendium of neuropsychological tests: Administration, norms, and commentary. 2nd ed. New York, NY, US: Oxford University Press (1998).
39. Stroop, JR. Studies of interference in serial verbal reactions. J Exp Psychol. (1935) 18:643–62. doi: 10.1037/h0054651
40. Wechsler. Wechsler Adult Intelligence Scale. 4th ed. New York: The Psychological Corporation (2008).
41. Schmidt, M. Rey auditory-verbal learning test. Los Angeles: Western Psychological Services (1996).
42. Helmes, E. Learning and memory In: GG Marnat, editor. Neuropsychological assessment in clinical practice: a guide to test interpretation and integration. New York, NY, US: John Wiley & Sons, Inc (2000). 293–334.
43. Rey, A. L'examen psychologie dans les cas d'encephalopathie traumatique. Arch Psychol. (1941) 28:286–340.
44. Shin, MS, Park, SY, Park, SR, Seol, SH, and Kwon, JS. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat Protoc. (2006) 1:892–9. doi: 10.1038/nprot.2006.115
45. French, LM, Lange, RT, and Brickell, T. Subjective cognitive complaints and neuropsychological test performance following military-related traumatic brain injury. J Rehabil Res Dev. (2014) 51:933–50. doi: 10.1682/JRRD.2013.10.0226
46. Spencer, RJ, Drag, LL, Walker, SJ, and Bieliauskas, LA. Self-reported cognitive symptoms following mild traumatic brain injury are poorly associated with neuropsychological performance in OIF/OEF veterans. J Rehabil Res Dev. (2010) 47:521–30. doi: 10.1682/JRRD.2009.11.0181
47. Landre, N, Poppe, CJ, Davis, N, Schmaus, B, and Hobbs, SE. Cognitive functioning and postconcussive symptoms in trauma patients with and without mild TBI. Arch Clin Neuropsychol. (2006) 21:255–73. doi: 10.1016/j.acn.2005.12.007
48. Smits, M, Dippel, DW, Houston, GC, Wielopolski, PA, Koudstaal, PJ, Hunink, MG, et al. Postconcussion syndrome after minor head injury: brain activation of working memory and attention. Hum Brain Mapp. (2009) 30:2789–803. doi: 10.1002/hbm.20709
49. Dworkin, RH, Turk, DC, Revicki, DA, Harding, G, Coyne, KS, Peirce-Sandner, S, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. (2009) 144:35–42. doi: 10.1016/j.pain.2009.02.007
50. Anderson, JFI. The association between pain type, cognition and complaint after mild traumatic brain injury in prospectively studied premorbidly healthy adults admitted to hospital. Neuropsychology. (2020) 34:53–62. doi: 10.1037/neu0000585
51. Smets, EM, Garssen, B, Bonke, B, and De Haes, JC. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. (1995) 39:315–25. doi: 10.1016/0022-3999(94)00125-O
52. Beaulieu-Bonneau, S, and Ouellet, MC. Fatigue in the first year after traumatic brain injury: course, relationship with injury severity, and correlates. Neuropsychol Rehabil. (2017) 27:983–1001. doi: 10.1080/09602011.2016.1162176
53. Ouellet, MC, and Morin, CM. Fatigue following traumatic brain injury: frequency, characteristics and associated factors. Rehabil Psychol. (2006) 51:140–9. doi: 10.1037/0090-5550.51.2.140
54. Hays, RD, Sherbourne, CD, and Mazel, RM. The RAND 36-item health survey 1.0. Health Econ. (1993) 2:217–27. doi: 10.1002/hec.4730020305
55. Findler, M, Cantor, J, Haddad, L, Gordon, W, and Ashman, T. The reliability and validity of the SF-36 health survey questionnaire for use with individuals with traumatic brain injury. Brain Inj. (2001) 15:715–23. doi: 10.1080/02699050010013941
56. King, NS, Crawford, S, Wenden, FJ, Moss, NE, and Wade, DT. The rivermead post concussion symptoms questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J Neurol. (1995) 242:587–92. doi: 10.1007/BF00868811
57. Cassidy, JD, Cancelliere, C, Carroll, LJ, Cote, P, Hincapie, CA, Holm, LW, et al. Systematic review of self-reported prognosis in adults after mild traumatic brain injury: results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. (2014) 95:S132–51. doi: 10.1016/j.apmr.2013.08.299
58. Ettenhofer, ML, and Barry, DM. A comparison of long-term postconcussive symptoms between university students with and without a history of mild traumatic brain injury or orthopedic injury. J Int Neuropsychol Soc. (2012) 18:451–60. doi: 10.1017/S1355617711001895
59. Laborey, M, Masson, F, Ribereau-Gayon, R, Zongo, D, Salmi, LR, and Lagarde, E. Specificity of postconcussion symptoms at 3 months after mild traumatic brain injury: results from a comparative cohort study. J Head Trauma Rehabil. (2014) 29:E28–36. doi: 10.1097/HTR.0b013e318280f896
60. Rush, AJ, Gullion, CM, Basco, MR, Jarrett, RB, and Trivedi, MH. The inventory of depressive symptomatology (IDS): psychometric properties. Psychol Med. (1996) 26:477–86. doi: 10.1017/S0033291700035558
61. Beck, AT, and Steer, RA. Beck anxiety inventory manual. San Antonio, TX: Psychological Corporation (1993).
62. Weathers, FW, Litz, BT, Keane, TM, Palmieri, PA, Marx, BP, and Schnurr, PP. The PTSD checklist for DSM-5 (PCL-5). Retrieved from the National Center for PTSD. (2013). Available at: http://www.ptsd.va.gov
63. Ashbaugh, AR, Houle-Johnson, S, Herbert, C, El-Hage, W, and Brunet, A. Psychometric validation of the English and French versions of the posttraumatic stress disorder checklist for DSM-5 (PCL-5). PLoS One. (2016) 11:e0161645. doi: 10.1371/journal.pone.0161645
64. Tabachnick, BG, and Fidell, LS. Using multivariate statistics. 6th ed. Boston: Allyn and Bacon (2013).
65. Osborne, JW, and Costello, AB. Sample size and subject to item ratio in principal components analysis. Pract Assess Res Eval. (2004):9. doi: 10.7275/ktzq-jq66
66. Iverson, GL, and Tulsky, DS. Detecting malingering on the WAIS-III. Unusual digit span performance patterns in the normal population and in clinical groups. Arch Clin Neuropsychol. (2003) 18:1–9. doi: 10.1093/arclin/18.1.1
67. Andersson, JLR, Skare, S, and Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. (2003) 20:870–88. doi: 10.1016/S1053-8119(03)00336-7
68. Veraart, J, Fieremans, E, and Novikov, DS. Diffusion MRI noise mapping using random matrix theory. Magn Reson Med. (2016) 76:1582–93. doi: 10.1002/mrm.26059
69. Kellner, E, Dhital, B, Kiselev, VG, and Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med. (2016) 76:1574–81. doi: 10.1002/mrm.26054
70. Andersson, JLR, and Sotiropoulos, SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. (2016) 125:1063–78. doi: 10.1016/j.neuroimage.2015.10.019
71. Tustison, NJ, Avants, BB, Cook, PA, Zheng, Y, Egan, A, Yushkevich, PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. (2010) 29:1310–20. doi: 10.1109/TMI.2010.2046908
72. Jeurissen, B, Tournier, JD, Dhollander, T, Connelly, A, and Sijbers, J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. NeuroImage. (2014) 103:411–26. doi: 10.1016/j.neuroimage.2014.07.061
73. Tournier, JD, Calamante, F, and Connelly, A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. Proc Intl Soc Mag Reson Med. (2010) 18:1670.
74. Yang, JY, Beare, R, Seal, ML, Harvey, AS, Anderson, VA, and Maixner, WJ. A systematic evaluation of intraoperative white matter tract shift in pediatric epilepsy surgery using high-field MRI and probabilistic high angular resolution diffusion imaging tractography. J Neurosurg Pediatr. (2017) 19:592–605. doi: 10.3171/2016.11.PEDS16312
75. Hsu, JL, Leemans, A, Bai, CH, Lee, CH, Tsai, YF, Chiu, HC, et al. Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. NeuroImage. (2008) 39:566–77. doi: 10.1016/j.neuroimage.2007.09.017
76. Kirkwood, BR, and Sterne, JAC. Essential medical statistics. 2nd ed. Malden, MA: Blackwell Pub (2003).
77. Anderson, JFI, and Cockle, E. Investigating the effect of fatigue and psychological distress on information processing speed in the postacute period after mild traumatic brain injury in premorbidly healthy adults. Arch Clin Neuropsychol. (2021) 36:918–20. doi: 10.1093/arclin/acaa123
78. Burda, JE, Bernstein, AM, and Sofroniew, MV. Astrocyte roles in traumatic brain injury. Exp Neurol. (2016) 275:305–15. doi: 10.1016/j.expneurol.2015.03.020
79. Groeschel, S, Tournier, JD, Northam, GB, Baldeweg, T, Wyatt, J, Vollmer, B, et al. Identification and interpretation of microstructural abnormalities in motor pathways in adolescents born preterm. NeuroImage. (2014) 87:209–19. doi: 10.1016/j.neuroimage.2013.10.034
80. Charlton, RA, Barrick, TR, McIntyre, DJ, Shen, Y, O'Sullivan, M, Howe, FA, et al. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. (2006) 66:217–22. doi: 10.1212/01.wnl.0000194256.15247.83
81. Open Science Collaboration. PSYCHOLOGY. Estimating the reproducibility of psychological science. Science. (2015) 349:aac4716. doi: 10.1126/science.aac4716
82. Alexander, AL, Hasan, KM, Lazar, M, Tsuruda, JS, and Parker, DL. Analysis of partial volume effects in diffusion-tensor MRI. Magn Reson Med. (2001) 45:770–80. doi: 10.1002/mrm.1105
83. Hoy, AR, Koay, CG, Kecskemeti, SR, and Alexander, AL. Optimization of a free water elimination two-compartment model for diffusion tensor imaging. NeuroImage. (2014) 103:323–33. doi: 10.1016/j.neuroimage.2014.09.053
84. Alsameen, MH, Gong, Z, Qian, W, Kiely, M, Triebswetter, C, Bergeron, CM, et al. C-NODDI: a constrained NODDI model for axonal density and orientation determinations in cerebral white matter. Front Neurol. (2023) 14:1205426. doi: 10.3389/fneur.2023.1205426
85. Bouyagoub, S., Dowell, N.G., Hurley, S.A., Wood, T.C., and Cercignani, M.. "Overestimation of CSF fraction in NODDI: possible correction techniques and the effect on neurite density and orientation dispersion measures." 24th annual meeting of the international society for magnetic resonance in medicine, Singapore (2016).
86. Gong, T, Tong, Q, He, H, Sun, Y, Zhong, J, and Zhang, H. MTE-NODDI: multi-TE NODDI for disentangling non-T2-weighted signal fractions from compartment-specific T2 relaxation times. NeuroImage. (2020) 217:116906. doi: 10.1016/j.neuroimage.2020.116906
Keywords: mild traumatic brain injury, cognition, white matter tract, diffusion tensor imaging, neurite orientation dispersion and density imaging (NODDI)
Citation: Anderson JFI, Oehr LE, Chen J, Maller JJ, Seal ML and Yang JY-M (2023) The relationship between cognition and white matter tract damage after mild traumatic brain injury in a premorbidly healthy, hospitalised adult cohort during the post-acute period. Front. Neurol. 14:1278908. doi: 10.3389/fneur.2023.1278908
Edited by:
Ramon Diaz-Arrastia, University of Pennsylvania, United StatesReviewed by:
John Ollinger, Walter Reed National Military Medical Center, United StatesJeff Ware, University of Pennsylvania, United States
Copyright © 2023 Anderson, Oehr, Chen, Maller, Seal and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacqueline F. I. Anderson, amZhbmRlQHVuaW1lbGIuZWR1LmF1
 Jacqueline F. I. Anderson
Jacqueline F. I. Anderson Lucy E. Oehr1
Lucy E. Oehr1 Jian Chen
Jian Chen Joseph Yuan-Mou Yang
Joseph Yuan-Mou Yang