
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 01 December 2023
Sec. Stroke
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1277855
This article is part of the Research Topic Advances and controversies in ischemic stroke management: from prevention to diagnosis and acute treatment View all 95 articles
 Min Kyoung Kang1,2
Min Kyoung Kang1,2 Dongwhane Lee2
Dongwhane Lee2 Mi Sun Oh3
Mi Sun Oh3 Ji-Sung Lee4
Ji-Sung Lee4 Han-Yeong Jeong5
Han-Yeong Jeong5 Jung Hwan Shin5
Jung Hwan Shin5 Byung-Woo Yoon2
Byung-Woo Yoon2 Jong-Moo Park2*
Jong-Moo Park2*Aim: While the relationship between impaired kidney function and non-vitamin K antagonist oral anticoagulants (NOACs) is well established, there is limited research exploring the association between an elevated estimated glomerular filtration rate (eGFR) and the efficacy of NOACs, especially concerning the outcomes of acute ischemic stroke (AIS). This study aimed to examine the association between higher-than-normal eGFR and the severity of AIS during the use of NOACs using a nationwide multicenter stroke registry in Korea.
Material and methods: This study utilized data from the Korean Stroke Registry (KSR) database, examining information from 2,379 patients with AIS, who had atrial fibrillation (AF) and a history of utilizing NOACs prior to hospitalization due to incident stroke occurring between 2016 and 2021. Patients with a history involving two or more types of anticoagulants or one or more forms of antiplatelet agents were excluded. Baseline characteristics, medical history, medication usage, CHADS2-VASc score, and the anticoagulation and risk factors in atrial fibrillation (ATRIA) score were evaluated. Renal function was assessed using eGFR levels and calculated with the Cockcroft–Gault equation. The severity of stroke was measured by the National Institutes of Health Stroke Scale as an outcome. For sensitivity analysis, further evaluation was performed using eGFR levels according to the modification of diet in renal disease (MDRD) study equation.
Results: The mean age of subjects was 76.1 ± 8.9 years. The moderate-to-severe stroke severity group exhibited an elevation in creatinine levels. The eGFR of 60 to 89 mL/min/1.73 m2 group was associated with a decreased risk of moderate-to-severe stroke severity [hazard ratio (HR)] (0.77, 95% confidence interval (CI) [0.61, 0.98], p = 0.031) compared to the eGFR≥90 mL/min/1.73 m2 group. An increment of 10 units in eGFR was marginally associated with an increased risk of moderate-to-severe stroke severity (HR: 1.03, 95% CI [1.00, 1.07], p = 0.054).
Conclusion: The study revealed that individuals with eGFR ≥ 90 mL/min/1.73 m2 had an association linked to an increased risk of moderate-to-severe stroke severity. Our study suggests that patients taking NOACs with higher-than-normal eGFR levels may have an increased severity of AIS.
Renal function has been linked with a spectrum of cardiovascular diseases, including acute ischemic stroke (AIS), atrial fibrillation (AF), myocardial infarction, and heart failure. Chronic kidney disease, characterized predominantly by diminished estimated glomerular filtration rate (eGFR), is strongly associated with the risk of these cardiovascular diseases (1, 2). In addition to low eGFR, abnormally high eGFR has also been linked to various health conditions. While high eGFR is generally considered a normal physiological state, it can also indicate underlying adverse renal conditions, such as the onset of glomerular damage in hypertensive patients or diabetic nephropathy (3). High eGFR is also closely intertwined with hypertension, prediabetes, and obesity, all of which may contribute to cardiovascular events (4). In addition, recent studies have reported an association between high eGFR and the poor outcome of cardiovascular disease, including AIS (5, 6).
Non-vitamin K antagonist oral anticoagulants (NOACs) stand as the first-choice drugs for major conditions entailing a risk of thromboembolism in AF (7). It is widely known that patients with impaired kidney function have an increased risk of bleeding and necessitate dose adjustments while undergoing treatment with NOACs (8). However, there is controversy regarding the relationship between elevated eGFR and the efficacy of NOACs (9–12).
Limited studies have examined the relationship between high eGFR and the efficacy of NOACs, especially concerning outcomes of AIS. This study aimed to investigate the association between higher-than-normal eGFR and the risk of increased severity of AIS during the use of NOACs in a nationwide multicenter registry study in Korea.
The data for this study were derived from the Korean Stroke Registry (KSR) database. Established in 1999, the KSR has been operating as a prospective, multicenter, collaborative hospital-based stroke registry (13). To ensure the consistency and accuracy of data, dedicated auditors engaged in monthly reviews, address queries, and made corrections from researchers (14–16).
The KSR database comprehensively encompasses information on demographic characteristics, medical and medication history, stroke subtype, side of stroke involvement, lesion of stroke involvement, stroke outcomes, and treatment details of patients with AIS. The database entails measuring physical parameters such as height, weight, blood pressure, conducting laboratory tests, and surveying lifestyle habits (14–16).
Between January 2016 and December 2020, a total of 85,499 patients diagnosed with AIS (within 7 days of onset) were enrolled from 39 hospitals across Korea. From this registry, we selected the patients with AF and the documented history of administration of NOACs prior to the index stroke. We excluded the patients with a history involving the use of two or more types of anticoagulants, one or more forms of antiplatelet agents, and those with incomplete data at least one variable required to calculate eGFR. Ultimately, 2,379 patients were included in the analysis.
Baseline characteristics were comprehensively assessed, including age, sex, body weight, body mass index, and comorbidities such as hypertension, diabetes mellitus, hyperlipidemia, congestive heart failure, peripheral artery disease, coronary heart disease, transient ischemia attack (TIA), stroke, and active cancer. The assessment was based on the diagnosis and medication record at admission for the index stroke. The side of the stroke involvement was divided into left, right, or multiple, and with regard to the lesion, it was divided into anterior circulation, posterior circulation, or multiple circulation. Smoking status was dichotomized as non-smoker/former smoker or current smoker. The medication history within 7 days preceding the index stroke was collected, including types of NOACs, anti-hypertensive drugs, antidiabetic drugs, and anti-hyperlipidemia agents. The dosages of NOACs or other agents were not recorded as it was determined based on the judgment of the medical professionals. Stroke subtype, the modified Rankin Scale (mRS) prior to admission, initial the National Institutes of Health Stroke Scale (NIHSS) score, and mRS at discharge were recorded in the KSR database (17, 18).
Laboratory assessments in the KSR database were based on the initial blood test results conducted in the emergency department for patients presenting with stroke symptoms, encompassing BUN (blood urea nitrogen), creatinine, leukocyte count, hemoglobin, hematocrit, platelet count, prothrombin time, CRP (C-reactive protein), LDL (low-density lipoprotein), HbA1c (glycated hemoglobin), and D-dimer. The estimated glomerular filtration rate (eGFR) was calculated using the serum creatinine level and Cockcroft–Gault equations (19).
The Cockcroft–Gault equation estimating creatinine clearance (Ccr) was calculated as eGFR (mL/min/1.73 m2):
We calculated the CHADS2-VASc score and the anticoagulation and risk factors in atrial fibrillation (ATRIA) bleeding score in order to assess the risk of occurrence of stroke and the bleeding risk in each patient (20, 21). For sensitivity analysis, further evaluation was performed using eGFR levels according to the modification of diet in renal disease (MDRD) study equation (22).
The modification of diet in renal disease (MDRD) study equation was calculated as eGFR (mL/min/1.73 m2):
The primary outcome was the initial stroke severity at admission, measured by the NIHSS score, with a range from 0 to 42 (23). A mild initial stroke severity was defined as an NIHSS score of 0 to 7 (24). To perform sensitivity analysis, we conducted additional assessments using the mRS scale at discharge. We categorized moderate-to-severe stroke severity as mRS scores ≥3, and death was defined as an mRS score of 6 upon discharge (25).
The study presented data in two formats: mean ± standard deviation or as number and percentage. The chi-square test, Fisher's exact test, Cochran–Mantel–Haenszel shift test, Student's t-test, and Wilcoxon rank sum test were employed to compare baseline characteristics between the two severity groups. The relationship between eGFR and initial stroke severity was examined by categorizing patients into ranges of eGFR (< 30, 30–59, 60–89, and ≥90 mL/min/1.73 m2), with the range of >90 mL/min/1.73 m2 serving as the reference group (26).
Multivariable logistic regression models were applied to investigate the factors that affected moderate-to-severe initial stroke severity. These analyses were adjusted for potential confounding variables including age, sex, hypertension, diabetes mellitus, hyperlipidemia, congestive heart failure, coronary heart disease, peripheral arterial disease, previous TIA or stroke, active cancer, current smoking status, history of taking NOACs, stroke subtype, laboratory data, the CHADS2-VASC score, and ATRIA score to identify the independent contributing power of kidney function for the use of NOACs. Potential determinants based on the results of univariate analysis (P < 0.1) and variables that were already known to be related to NOAC therapy were selected for multivariate analysis. Odds ratios (OR) with 95% CI were reported.
Subgroup analyses were conducted based on a stroke subtype or previous stroke history. For sensitivity analysis, we conducted additional assessments which included evaluating eGFR levels using the MDRD study equation and using mRS scores ≥3 or death at discharge as additional indicators of stroke severity.
Statistical analyses were conducted using SAS software (version 9.2, SAS Institute, Cary, NC, USA), with a p < 0.05 considered statistically significant.
The Institutional Review Board of Uijeongbu Eulji Medical Center approved this study and provided a consent waiver (Institutional Review Board approval number: 2022–07–004), as the KSR permitted restricted access to anonymized data for research purposes.
The average age of the patients with AIS was 76.1 ± 8.9 years, and male subjects accounted for 50.0% of the cohort. The prevalence of hypertension, diabetes mellitus, and hyperlipidemia was 85.1, 35.4, and 57.4%, respectively. In this study, we observed that the moderate-to-severe stroke severity group tended to be older, have lower body weight and BMI, and a lesser use of anti-hypertensive drugs and anti-diabetes drugs. Additionally, we found a lower prevalence of male, hyperlipidemia, and a previous TIA history among individuals in the moderate-to-severe stroke severity group. The side of the lesion did not show statistically significant differences, but when considering the location of the lesion, the moderate-to-severe stroke severity of stroke was higher in cases of anterior circulation infarction. Elevated BUN, decreased creatinine levels, higher leukocyte counts, CRP levels, and D-dimer levels were observed as well as lower hemoglobin, prothrombin time, and HbA1c levels compared to the mild severity group. Notably, there was no significant difference in eGFR levels upon comparison by means between the two groups, while lower creatinine levels were evident in the moderate-to-severe stroke severity group. The CHADS2-VASC score and ATRIA score, which determined stroke occurrence or complication risk in patients with atrial fibrillation, were higher in the moderate-to-severe stroke severity group (Table 1).
With respect to renal function, 21.0% of the patients with mild stroke severity and 25.1% of the patients with moderate-to-severe stroke severity fell into the eGFR≥90 mL/min/1.73 m2 group (reference), exhibiting higher-than-normal eGFR levels. After being adjusted for comorbidities and laboratory results, an eGFR of 60–89 mL/min/1.73 m2 (HR: 0.77, 95% CI [0.61, 0.98], p = 0.031) and eGFR of < 30 mL/min/1.73 m2 (HR: 0.42, 95% CI [0.22, 0.77], p = 0.005) were associated with a decreased risk of moderate-to-severe stroke severity, whereas an eGFR of 30–59 mL/min/1.73 m2 (HR: 0.91, 95% CI [0.69, 1.18], p = 0.466) was not associated with a decreased risk of moderate-to-severe stroke severity (Table 2). The hazard ratio plot demonstrated a bimodal decline in the hazard ratio for stroke severity as GFR decreased (Figure 1).
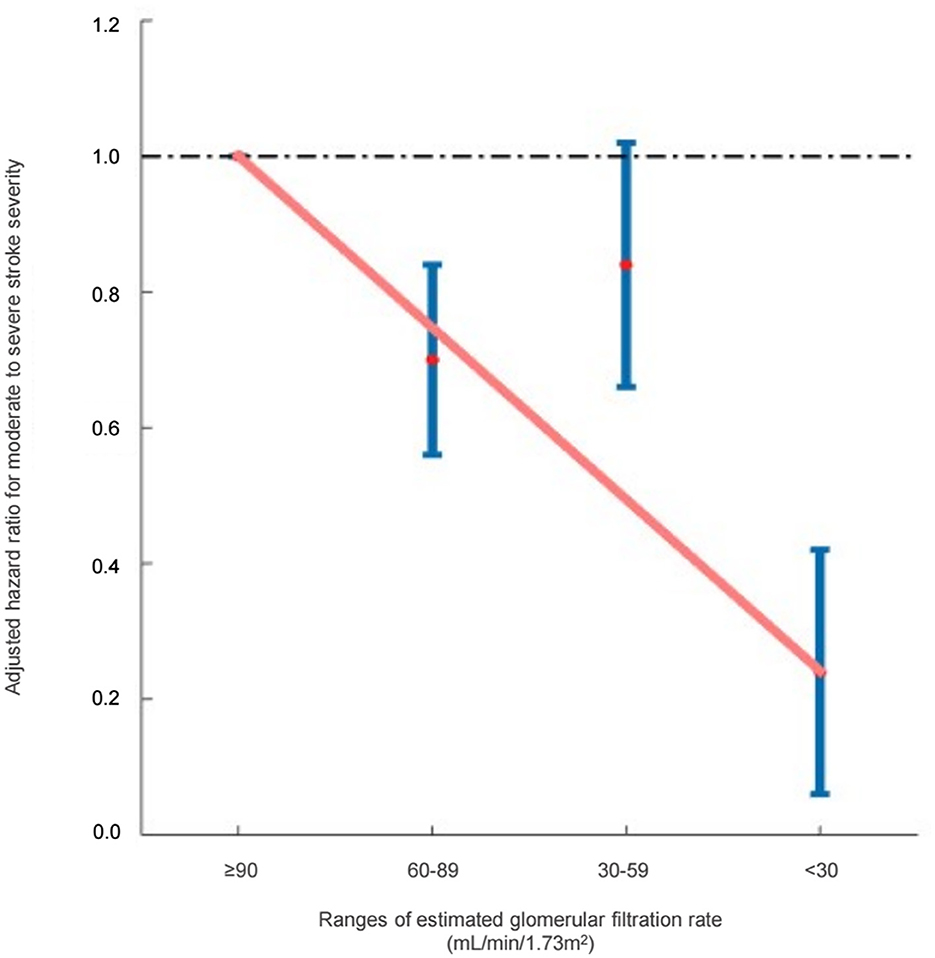
Figure 1. Hazard ratios for moderate-to-severe stroke severity based on the ranges of estimated glomerular filtration rate (Cockcroft–Gault equation).
In the multivariate analysis, a 10 unit increase in eGFR (Cockroft-Gault) was marginally associated with an increased risk of moderate-to-severe stroke severity (HR: 1.03, 95% CI [1.00, 1.07], p = 0.054) (Table 3). In addition, higher age, female, and absence of hyperlipidemia were significantly associated with stroke severity. When it comes to the stroke lesion, the side of the stroke did not have a significant correlation. Posterior circulation infarction was negatively associated moderate-to-severe strokes compared to anterior circulation infarction. When considering stroke subtypes, cases of small-vessel occlusion and strokes with an undetermined etiology exhibited a negative association with moderate-to-severe strokes in comparison to those attributed to large artery atherosclerosis.
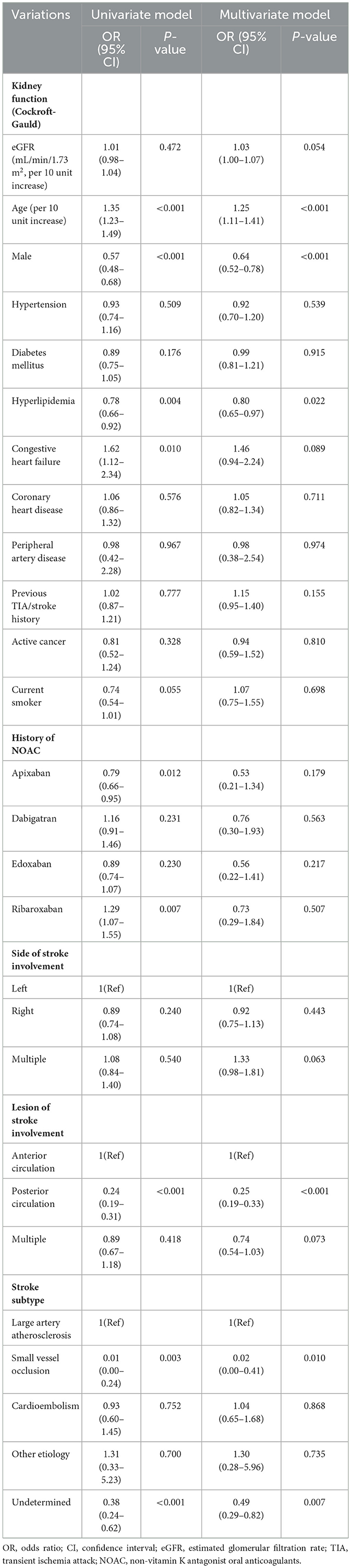
Table 3. Predictors for moderate to severe stroke severity in patients with non-vitamin K antagonist oral anticoagulants.
The scatter plot demonstrated an incline in the NIHSS score which represents stroke severity, as GFR increased (Figure 2).
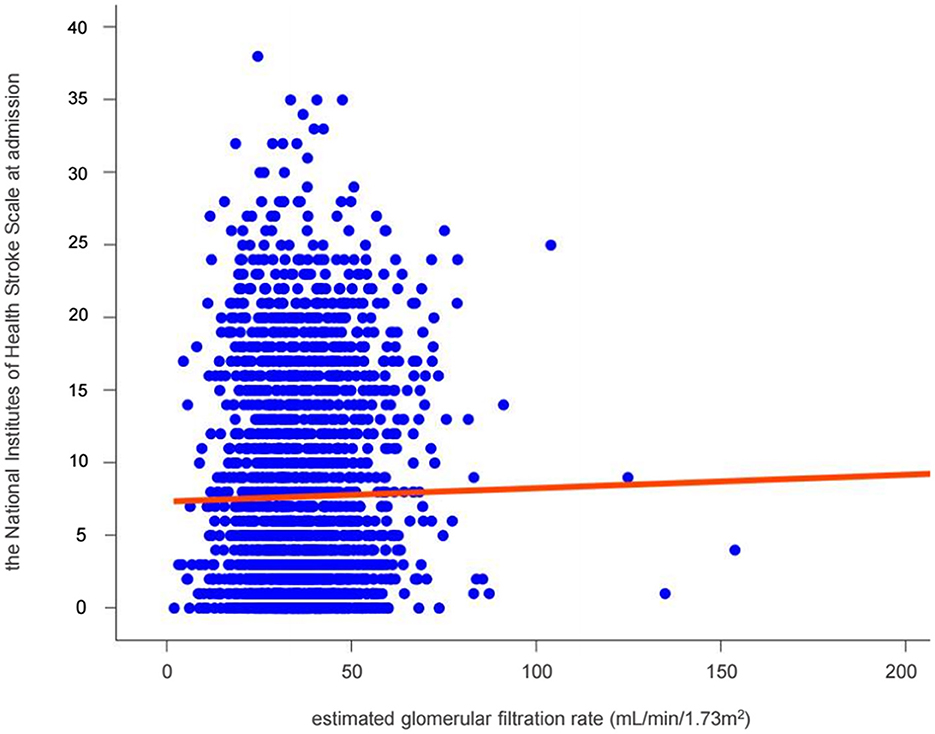
Figure 2. Relationship between renal function and stroke severity in patients with non-vitamin K antagonist oral anticoagulants. The solid red line represents the multivariate-adjusted linear regressions.
Subgroup analysis revealed no significant interactions between eGFR levels and stroke severity based on the stroke subtype. However, there was significant interaction based on previous TIA or stroke history (Table 4). In the presence of previous TIA or stroke history, a 10 unit increase in eGFR was significantly associated with an increased risk of moderate-to-severe stroke severity (HR: 1.07, 95% CI [1.01, 1.13], p = 0.013).
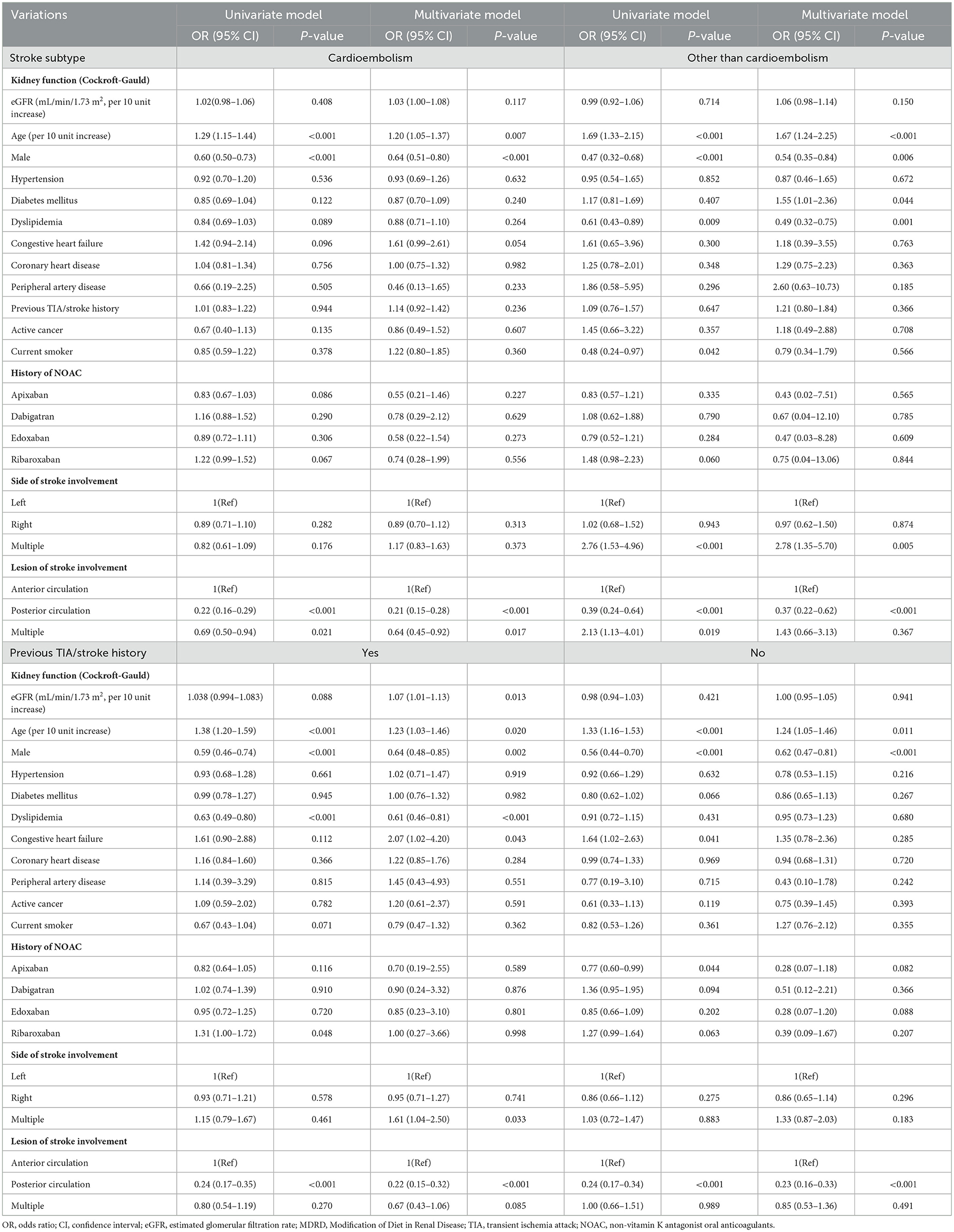
Table 4. Subgroup analysis of predictors for moderate to severe stroke severity in patients with non-vitamin K antagonist oral anticoagulants.
Sensitivity analysis consistently showed that an eGFR of 60–89 mL/min/1.73 m2 (HR: 0.75, 95% CI [0.61, 0.93], p = 0.007) and eGFR of <30 mL/min/1.73 m2 (HR: 0.37, 95% CI [0.20, 0.71], p = 0.003) were associated with a decreased risk of moderate-to-severe stroke severity, and the marginal association of eGFR levels and severity of stroke was also observed (HR: 1.03, 95% CI [1.00, 1.06], p = 0.050) even when eGFR levels were assessed using the MDRD method (Supplementary Tables 1, 2). In the sensitivity analysis, where an mRS score ≥3 was used as an indicator of neurological severity, it was found that eGFR of 60–89 mL/min/1.73 m2 (HR: 0.69, 95% CI [0.55, 0.87], p = 0.001) and eGFR of 30–59 mL/min/1.73 m2 (HR: 0.68, 95% CI [0.53, 0.88], p = 0.003) were associated with a decreased risk of moderate-to-severe stroke severity, while there was no association with in-hospital mortality (Supplementary Tables 3–1, 3–2). In the multivariate analysis, a 10 unit increase in eGFR was not associated with an increased risk of moderate-to-severe stroke severity defined as the mRS score of 3 or above (HR: 1.04, 95% CI [1.00, 1.07], p = 0.057) as well as for the patients with in-hospital mortality (Supplementary Tables 4–1, 4–2).
Our study revealed that individuals with eGFR ≥ 90 mL/min/1.73 m2 demonstrated an association linked to an increased risk of moderate-or-severe stroke severity, which remains significant in individuals presenting with previous TIA or stroke history.
Numerous studies have investigated the relationship between eGFR and stroke. A multicenter-based prospective cohort-based study which was involving ~500,000 individuals, revealed that the hazard ratio for death at 1 year or severe stroke severity as defined by Barthel index <75, increased in decreased eGFR groups, with a proportional relationship between the extent of renal function impairment and a higher occurrence of death or severe sequelae (27). Contrarily, previous studies have reported “U” or “J”-shaped relationships between eGFR and stroke severity or mortality, suggesting that both low and high eGFR levels are associated with an increased mortality risk. A multicentre population-based stroke registry-based study conducted in Japan, involving ~1,400,000 individuals, revealed that the hazard ratio for all in-hospital death and at-discharge death/disability increased in eGFR < 45 mL/min/1.73 m2 and eGFR ≥ 90 mL/min/1.73 m2 groups (28).
When compared to normal eGFR levels, a relatively high eGFR was linked to an increased risk of cardiovascular diseases and mortality. In a prior study of the chronic kidney disease epidemiology collaboration dataset, participants with a high eGFR, defined as 95th percentiles of the age- and sex-specific eGFR quintile (HR 1.5, 95% CI [1.2–2.1]) exhibited a significantly heightened risk of cardiovascular events compared to those with normal eGFR (29). In a prospective cohort study comprising 16,958 participants without clinically evident vascular disease, an eGFR >90 mL/min/1.73 m2 was associated with a high risk for coronary heart disease and non-vascular mortality compared to the normal eGFR group (5). Furthermore, a retrospective study of roughly 43,500 individuals from a general population health screening cohort with a mean observation period of 12.4 years identified a correlation between high eGFR and increased risk of cardiovascular-associated mortality after adjustment for the age-, sex-, muscle mass-, and history of diabetes and/or hypertension (30). In summary, high eGFR levels may be associated with poor outcomes of cardiovascular events, including stroke, compared to normal eGFR levels.
Generally, although a high eGFR is frequently deemed favorable due to its indication of good kidney function, it can serve as a marker for underlying health conditions such as hypertension, diabetes, and obesity. As these conditions worsen, the risk of cardiovascular events and mortality increases. In our results, the mean eGFR did not differ between the mild and moderate/severe stroke severity groups before adjustment (Table 1). However, the association between high eGFR and increased risk of stroke was significant after adjusting cardiovascular risk factors. Furthermore, even when the criterion for moderate-to-severe stroke severity was changed to a discharge mRS score of 3 or above, a similar pattern was observed although a direct association with mortality was not evident (Supplementary Tables 3–1, 3–2). Since eGFR is the result of combining the effects of complex factors such as hypertension, diabetes, and BMI as well as renal hyperfiltration, the effect of renal function on stroke severity must be judged after controlling for confounding factors.
In our results, high eGFR exhibited a higher risk of moderate-to-severe stroke, as shown in the eGFR ≥ 90 mL /min/1.73 m2 group. High eGFR may be associated with dysfunction of the renin-angiotensin system, low-grade vascular or systemic inflammation, endothelial dysfunction, and increased arterial stiffness. These factors represent the primary mechanisms contributing to the development of cardiovascular/cerebrovascular disease and increased mortality risk (29, 31, 32). Another possible mechanism is that hyperfiltration may lead to enhanced removals, causing suboptimal plasma concentration of NOACs, first doubted by the ENGAGE AF-TIMI 48 study, which reported a higher ischemic stroke rate for edoxaban in patients with creatinine clearance > 95 mL/min (HR 1.45, 95% CI [0.90–2.35]). Similar findings were derived from subanalyses of apixaban and rivaroxaban trials (33, 34). However, pharmacokinetic studies have reported no significant alterations in hyperfiltrated patients with NOACs (35). Therefore, it seems more plausible to consider that poor outcomes of cardiovascular events in hyperfiltration patients could stem from the adverse effects of an early stage of chronic kidney disease as important and independent cardiovascular risk factors, rather than sole alteration of pharmacokinetics. In our subgroup analysis results, the fact that the effect of renal hyperfiltration was more evident in the group with a history of TIA or stroke, regardless of stroke side, lesion, or etiology including cardioembolism, could support the hypothesis that systemic involvement of renal hyperfiltration was the major component, rather than the change of concentration of NOACs induced by hyperfiltration. Other results of Mendelian analysis served as supplementary evidence. A previous Mendelian analysis study, conducted for myocardial infarction which was another type of cardiovascular disease, highlighted renal hyperfiltration as a causal risk factor for poor outcomes (36). Further investigation is necessary to substantiate the hypothesis.
Our research has limitations that should be acknowledged. First, there may have been a potential for ethnic selection bias, which could limit the applicability of our results to other populations. Additional research on diverse races and ethnicities is warranted (16). Second, we only assessed cross-sectional eGFR and did not perform cystatin C measurements, which were not included in the KSR dataset. Third, the dosages of NOACs or other agents were determined based on the medical professionals' discretion. Lastly, a retrospective nature of this study might impede the establishment of a causal relationship.
In summary, this study revealed that individuals with eGFR ≥ 90 mL/min/1.73 m2 demonstrated an association linked to an increased risk of moderate-to-severe stroke severity. Our study suggests that patients taking NOACs with higher-than-normal eGFR levels may have an increased severity of AIS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Institutional Review Board of Uijeongbu Eulji Medical Center approval number: 2022–07–004. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
MK: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. DL: Conceptualization, Supervision, Writing—original draft. MO: Conceptualization, Data curation, Formal analysis, Project administration, Writing— original draft. J-SL: Conceptualization, Data curation, Methodology, Validation, Writing—original draft. H-YJ: Data curation, Writing—original draft. JS: Methodology, Software, Validation, Writing—original draft. B-WY: Methodology, Project administration, Writing—original draft. J-MP: Conceptualization, Writing—original draft, Writing—review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study used the Korean Stroke Registry database supported by the Korean Stroke Society (KSR-2021-01).
The authors would like to thank the Korean Stroke Registry Open Proposal System for statistical analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1277855/full#supplementary-material
1. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, Jafar TH, Heerspink HJ, Mann JF, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. (2013) 382:339–52. doi: 10.1016/S0140-6736(13)60595-4
2. Song TJ, Kim J, Lee HS, Nam CM, Nam HS, Kim YD, et al. Distribution of cerebral microbleeds determines their association with impaired kidney function. J Clin Neurol. (2014) 10:222–8. doi: 10.3988/jcn.2014.10.3.222
3. Helal I, Fick-Brosnahan GM, Reed-Gitomer B, Schrier RW. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat Rev Nephrol. (2012) 8:293–300. doi: 10.1038/nrneph.2012.19
4. Kanbay M, Ertuglu LA, Afsar B, Ozdogan E, Kucuksumer ZS, Ortiz A, et al. Renal hyperfiltration defined by high estimated glomerular filtration rate: a risk factor for cardiovascular disease and mortality. Diabetes Obes Metab. (2019) 21:2368–83. doi: 10.1111/dom.13831
5. Di Angelantonio E, Chowdhury R, Sarwar N, Aspelund T, Danesh J, Gudnason V. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. BMJ. (2010) 341:c4986. doi: 10.1136/bmj.c4986
6. Tonelli M, Klarenbach SW, Lloyd AM, James MT, Bello AK, Manns BJ, et al. Higher estimated glomerular filtration rates may be associated with increased risk of adverse outcomes, especially with concomitant proteinuria. Kidney Int. (2011) 80:1306–14. doi: 10.1038/ki.2011.280
7. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. (2019) 140:e125–51. doi: 10.1161/CIR.0000000000000665
8. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non-vitamin K antagonist oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J Am Coll Cardiol. (2017) 69:2779–90. doi: 10.1016/j.jacc.2017.03.600
9. Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, et al. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation. (2016) 134:24–36. doi: 10.1161/CIRCULATIONAHA.116.022361
10. Fanikos J, Burnett AE, Mahan CE, Dobesh PP. Renal function considerations for stroke prevention in atrial fibrillation. Am J Med. (2017) 130:1015–23. doi: 10.1016/j.amjmed.2017.04.015
11. Yao X, Inselman JW, Ross JS, Izem R, Graham DJ, Martin DB, et al. Comparative effectiveness and safety of oral anticoagulants across kidney function in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. (2020) 13:e006515. doi: 10.1161/CIRCOUTCOMES.120.006515
12. Huqi A, Zoccali C, Giugliano RP, De Caterina R. The non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with high-normal renal function - A systematic review. Vascul Pharmacol. (2022) 147:107123. doi: 10.1016/j.vph.2022.107123
13. Lee BC, Roh JK, Korean Stroke R. International experience in stroke registries: Korean stroke registry. Am J Prev Med. (2006) 31:S243–5. doi: 10.1016/j.amepre.2006.08.019
14. Oh MS Yu KH, Roh JK, Lee BC, Korean Stroke Registry Study G. Gender differences in the mortality and outcome of stroke patients in Korea. Cerebrovasc Dis. (2009) 28:427–34. doi: 10.1159/000235986
15. Jung KH, Lee SH, Kim BJ Yu KH, Hong KS, Lee BC, et al. Secular trends in ischemic stroke characteristics in a rapidly developed country: results from the Korean stroke registry study (secular trends in Korean stroke). Circ Cardiovasc Qual Outcomes. (2012) 5:327–34. doi: 10.1161/CIRCOUTCOMES.111.963736
16. Jeong HY, Jung KH, Mo H, Lee CH, Kim TJ, Park JM, et al. Characteristics and management of stroke in Korea: 2014-2018 data from Korean stroke registry. Int J Stroke. (2020) 15:619–26. doi: 10.1177/1747493019884517
17. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
18. Rost NS, Bottle A, Lee JM, Randall M, Middleton S, Shaw L, et al. Stroke severity is a crucial predictor of outcome: an international prospective validation study. J Am Heart Assoc. (2016) 5:33. doi: 10.1161/JAHA.115.002433
19. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. (1976) 16:31–41. doi: 10.1159/000180580
20. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. (2010) 137:263–72. doi: 10.1378/chest.09-1584
21. Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, et al. A new risk scheme to predict warfarin-associated hemorrhage: the ATRIA (anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol. (2011) 58:395–401. doi: 10.1016/j.jacc.2011.03.031
22. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, et al. more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modif Diet Renal Dis Study Group Ann Intern Med. (1999) 130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002
23. Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. (1989) 20:864–70. doi: 10.1161/01.STR.20.7.864
24. Muchada M, Rubiera M, Rodriguez-Luna D, Pagola J, Flores A, Kallas J, et al. Baseline national institutes of health stroke scale-adjusted time window for intravenous tissue-type plasminogen activator in acute ischemic stroke. Stroke. (2014) 45:1059–63. doi: 10.1161/STROKEAHA.113.004307
25. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. (1988) 19:604–7. doi: 10.1161/01.STR.19.5.604
26. Potpara TS, Lenarczyk R, Larsen TB, Deharo JC, Chen J, Dagres N, et al. Management of atrial fibrillation in patients with chronic kidney disease in Europe results of the European heart rhythm association survey. Europace. (2015) 17:1862–7. doi: 10.1093/europace/euv416
27. Yahalom G, Schwartz R, Schwammenthal Y, Merzeliak O, Toashi M, Orion D, et al. Chronic kidney disease and clinical outcome in patients with acute stroke. Stroke. (2009) 40:1296–303. doi: 10.1161/STROKEAHA.108.520882
28. Widhi Nugroho A, Arima H, Miyazawa I, Fujii T, Miyamatsu N, Sugimoto Y, et al. The association between glomerular filtration rate estimated on admission and acute stroke outcome: the Shiga stroke registry. J Atheroscler Thromb. (2018) 25:570–9. doi: 10.5551/jat.42812
29. Reboldi G, Verdecchia P, Fiorucci G, Beilin LJ, Eguchi K, Imai Y, et al. Glomerular hyperfiltration is a predictor of adverse cardiovascular outcomes. Kidney Int. (2018) 93:195–203. doi: 10.1016/j.kint.2017.07.013
30. Park M, Yoon E, Lim YH, Kim H, Choi J, Yoon HJ. Renal hyperfiltration as a novel marker of all-cause mortality. J Am Soc Nephrol. (2015) 26:1426–33. doi: 10.1681/ASN.2014010115
31. Cherney DZ, Sochett EB, Lai V, Dekker MG, Slorach C, Scholey JW, et al. Renal hyperfiltration and arterial stiffness in humans with uncomplicated type 1 diabetes. Diabetes Care. (2010) 33:2068–70. doi: 10.2337/dc10-0767
32. Cherney DZ, Reich HN, Jiang S, Har R, Nasrallah R, Hebert RL, et al. Hyperfiltration and effect of nitric oxide inhibition on renal and endothelial function in humans with uncomplicated type 1 diabetes mellitus. Am J Physiol Regul Integr Comp Physiol. (2012) 303:R710–8. doi: 10.1152/ajpregu.00286.2012
33. Hijazi Z, Hohnloser SH, Andersson U, Alexander JH, Hanna M, Keltai M, et al. Efficacy and safety of Apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. (2016) 1:451–60. doi: 10.1001/jamacardio.2016.1170
34. Lindner SM, Fordyce CB, Hellkamp AS, Lokhnygina Y, Piccini JP, Breithardt G, et al. Treatment consistency across levels of baseline renal function with rivaroxaban or warfarin: a ROCKET AF (rivaroxaban once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation) analysis. Circulation. (2017) 135:1001–3. doi: 10.1161/CIRCULATIONAHA.116.024666
35. Corrochano M, Acosta-Isaac R, Plaza M, Munoz R, Mojal S, Moret C, et al. Impact of increased kidney function on clinical and biological outcomes in real-world patients treated with direct oral anticoagulants. PLoS ONE. (2022) 17:e0278693. doi: 10.1371/journal.pone.0278693
Keywords: acute ischemic stroke, non-vitamin K antagonist oral anticoagulant, high estimated glomerular filtration rate, nationwide multicenter study, glomerular hyperfiltration, high estimated glomerular filtration rate
Citation: Kang MK, Lee D, Oh MS, Lee J-S, Jeong H-Y, Shin JH, Yoon B-W and Park J-M (2023) Association of high-estimated glomerular filtration rate with the severity of ischemic stroke during non-vitamin K antagonist oral anticoagulants therapy: a nationwide cohort study. Front. Neurol. 14:1277855. doi: 10.3389/fneur.2023.1277855
Received: 15 August 2023; Accepted: 13 November 2023;
Published: 01 December 2023.
Edited by:
Matteo Foschi, Azienda Unità Sanitaria Locale (AUSL) della Romagna, ItalyReviewed by:
Andrea Surcinelli, Santa Maria delle Croci Hospital, ItalyCopyright © 2023 Kang, Lee, Oh, Lee, Jeong, Shin, Yoon and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jong-Moo Park, am1wYXJrQGV1bGppLmFjLmty
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.