- 1Department of Speech Therapy, School of Paramedical and Rehabilitation Sciences, Mashhad University of Medical Sciences, Mashhad, Iran
- 2Department of Communication Sciences and Disorders, College of Health and Human Services, Bowling Green State University, Bowling Green, OH, United States
- 3Department of Neurology, Mashhad University of Medical Sciences, Mashhad, Iran
- 4Department of Biostatistics, School of Health, Mashhad University of Medical Sciences, Mashhad, Iran
Swallowing is essential for human health, and the cerebellum is crucial for motor movement regulation. Cerebellar strokes may cause dysphagia, but their exact effects remain unexplored in swallowing function. Therefore, the aim of this study was to analyze the precise clinical characteristics of the oral and pharyngeal phases of swallowing after cerebellar stroke and to critically discuss the cerebellum’s contribution to swallowing. The study involved 34 participants with cerebellar strokes, gathered through convenience sampling. Neurologists diagnosed isolated strokes, and a speech and language pathologist examined swallowing ability using the Mann Assessment of Swallowing Ability. The study found that 52.9% of people experienced dysphagia after a cerebellar stroke. Dysphagia was significantly associated with a higher risk of aspiration. Age was also significantly correlated with dysphagia. No significant correlation was found between swallowing ability and sex. In conclusion, this study suggests isolated cerebellar stroke can adversely affect the motor and non-motor aspects of swallowing and cause severe dysphagia and aspiration risk. Thus, early diagnosis and timely management of dysphagia following a cerebellar stroke can help prevent serious consequences.
1 Introduction
Swallowing is a complex and basic mechanism allowing humans to eat and drink while preventing penetration or aspiration, which can result in pulmonary diseases (1). Swallowing, as Magendie described it first, is usually factored in as a process having three stages, including oral, pharyngeal, and esophageal (2, 3). This complex mechanism requires extreme coordination and arrangements among different body systems, such as the nervous system. Hence, different medical conditions of this system, such as stroke (4), multiple sclerosis (5), etc., can lead to swallowing disorders, also known as dysphagia.
The cerebellum, which makes up the majority of the hindbrain, is attached to the brainstem by three groups of cerebellar peduncles. The cerebellum has been acknowledged for its crucial involvement in the regulation of motor movement, postural maintenance, muscular tone control, balance, gait coordination, and volitional muscle activity. The cerebellar function appears to be only inhibitory (6). Indeed, the cerebellar cortex plays a crucial part in ensuring the accuracy, smoothness, and coordination of any muscular activity, even though it has no excitatory role and does not initiate movements (7, 8). The cerebellum also has many projections to the brainstem, such as nucleus tractus solitarius and nucleus ambiguous (9). The cerebellum is thought to affect different central pattern generators such as swallowing (10). Therefore, cerebellar damage can lead to tremors, incoordination, and inaccuracy in movements, especially in swallowing (7, 11).
Stroke is a widespread neurological deficit that affects 25% of people within their lifespan and is, respectively, the second and third significant cause of adults’ mortality and disability worldwide (12). A cerebellar infarct is a kind of stroke in which the posterior cranial fossa, in particular the cerebellum, gets involved. Cerebellar stroke has been reported to account for up to 4 % of all brain strokes (13, 14), and causes dysphagia in 11.45% of the affected population (15). Nevertheless, the effects of this infarct on the swallowing mechanism are still uncrystallized.
Although the accumulation of animal studies has revealed a prominent connection between swallowing and the cerebellum, for instance (16–21), the association between the cerebellum and swallowing has not been well explored among humans. Hence, several interventional studies have also been conducted within the last two decades to illuminate the cerebellum’s contribution to human swallowing. Accordingly, experimental studies have demonstrated that cerebellar transcranial magnetic stimulation can significantly evoke distinct pharyngeal neuromuscular responses as well as corticobulbar excitability [(e.g., 22–24)]. However, two recent studies reported the opposite view. An investigation of cerebellar transcranial direct current stimulation illustrated that it can adversely affect motor skill learning in the swallowing process by causing a deterioration in the timing of submental muscle activation during swallowing (25). Furthermore, another experiment also showed that cerebellar transcranial magnetic stimulation can suppress pharyngeal motor cortical activities and swallowing behavior (26). For more information on this topic, please refer to “The Role of the Cerebellum in Swallowing” by Sasegbon and Hamdy, published in 2021 (27). They provided a comprehensive review regarding this topic. In addition to these interventional studies, several observational investigations have been accomplished and demonstrated cerebellar bilateral activation during swallowing, for example (28–30). Further, a new exploration exhibited that the posterior lobe of the left cerebellum seems to be related to dysphagia severity among persons with isolated cerebellar lesions (31). Huang et al., through a retrospective cohort study in 2023, indicated that dysphagia affected almost 11% of people with cerebellar infracts. They found higher odds ratios of dysphagia among people with mixed strokes (a combination of ischemic and hemorrhagic strokes) and with older ages. They also discovered that the higher recovery rate of swallowing disorders was related to male gender, younger adults, hemorrhagic stroke, and right cerebellum (15).
These studies presented evidence that supports the idea that the cerebellum is likely to get involved in the swallowing mechanism as also previously discussed (32). Indeed, the cerebellum may even play a crucial role in swallowing (27). Nevertheless, the exact and elaborate contribution of the cerebellum and its activities to swallowing have been poorly addressed and understood in humans, analogous to the basal ganglia (33). In fact, it seems that these studies investigated swallowing as a simple three-phase mechanism including some straightforward subsets, while just the two first stages consist of almost 20 various sub-elements that work together to provide a flawless mechanism of swallowing (34). Therefore, this study aims to determine elaborate clinical characteristics of the oral and pharyngeal phases of swallowing after isolated cerebellar stroke and reflectively describe the cerebellum’s roles and involvements within swallowing, and address how these strokes may affect the swallowing process.
2 Materials and methods
All gathered participants received informed consent, including thorough explanations about the study and the use of their data for the exploration. They were also informed that they are free to leave the research whenever they do not tend to continue taking part.
2.1 Participants
The present study examined 34 participants (mean age = 51.76; SD = 17.06; range = 27–82), including 13 females (mean age = 52.31; SD = 18.35; range = 29–82) and 21 males (mean age = 51.43; SD = 16.66; range = 27–81), with cerebellar stroke. All invited participants received comprehensive information about the present study through informed consent to make their decision about their voluntary participation in this research. They were also gathered through convenience sampling over 6 months in the following manner.
A team of neurologists made diagnoses based on Computed Tomography Scan (CTS) and Magnetic Resonance Imaging (MRI) to determine only ones with isolated cerebellar strokes, and not any other neurological regions or conditions at that moment or previously. Then a speech and language pathologist (SLP), with 3 years of experience working on swallowing disorders, examined them and explored their records for no history of oropharyngolaryngeal pathologies, tracheostomy, dysphagia, perception problems, or any chronic medical conditions. Individuals were excluded from the study if they were identified with each of the records mentioned.
2.2 Swallowing assessment
The SLP applied the Mann Assessment of Swallowing Ability (MASA) manual to assess their swallowing function.
The MASA is an efficient, accurate, and reliable swallowing assessment tool, which consists of 24 items allowing professionals to assess swallowing characteristics by which dysphagia can be determined and scored (34). The tool is also capable of categorizing dysphagia into four classes of severity (no abnormality detected , mild 168–177, moderate 139–167, and severe ) (34). Furthermore, the manual can provide a cut-off score for the risk of aspiration (no abnormality detected , mild 149–169, moderate 141–148, and severe ) (34).
The MASA can be performed over time to check the swallowing function. The sensitivity and specificity of the MASA in diagnosing dysphagia among people with stroke have been reported as 71 and 72%, respectively (34). Moreover, the MASA’s sensitivity and specificity to predict aspiration have been valued as 93 and 55%, respectively (34).
2.3 Statistical analysis
In the present study, the Statistical Package for the Social Sciences (SPSS®) was used to analyze the data. The Kolmogorov–Smirnov test was used to calculate the normality of age and the MASA–score distribution to apply appropriate statistical methods.
A t-test was calculated to examine differences across parametric data. Spearman’s rank correlation coefficient was calculated to examine the correlation (ρ) between non-parametric data. Moreover, the Mann–Whitney U test was applied to analyze differences across non-parametric data. Furthermore, this test was used to examine any probable differences among different categories.
The significance level was considered as ≤0.05.
3 Results
According to the Kolmogorov–Smirnov test, the age distribution was normal (p = 0.59) among the participants, but the MASA-score distribution was not normal (p < 0.00).
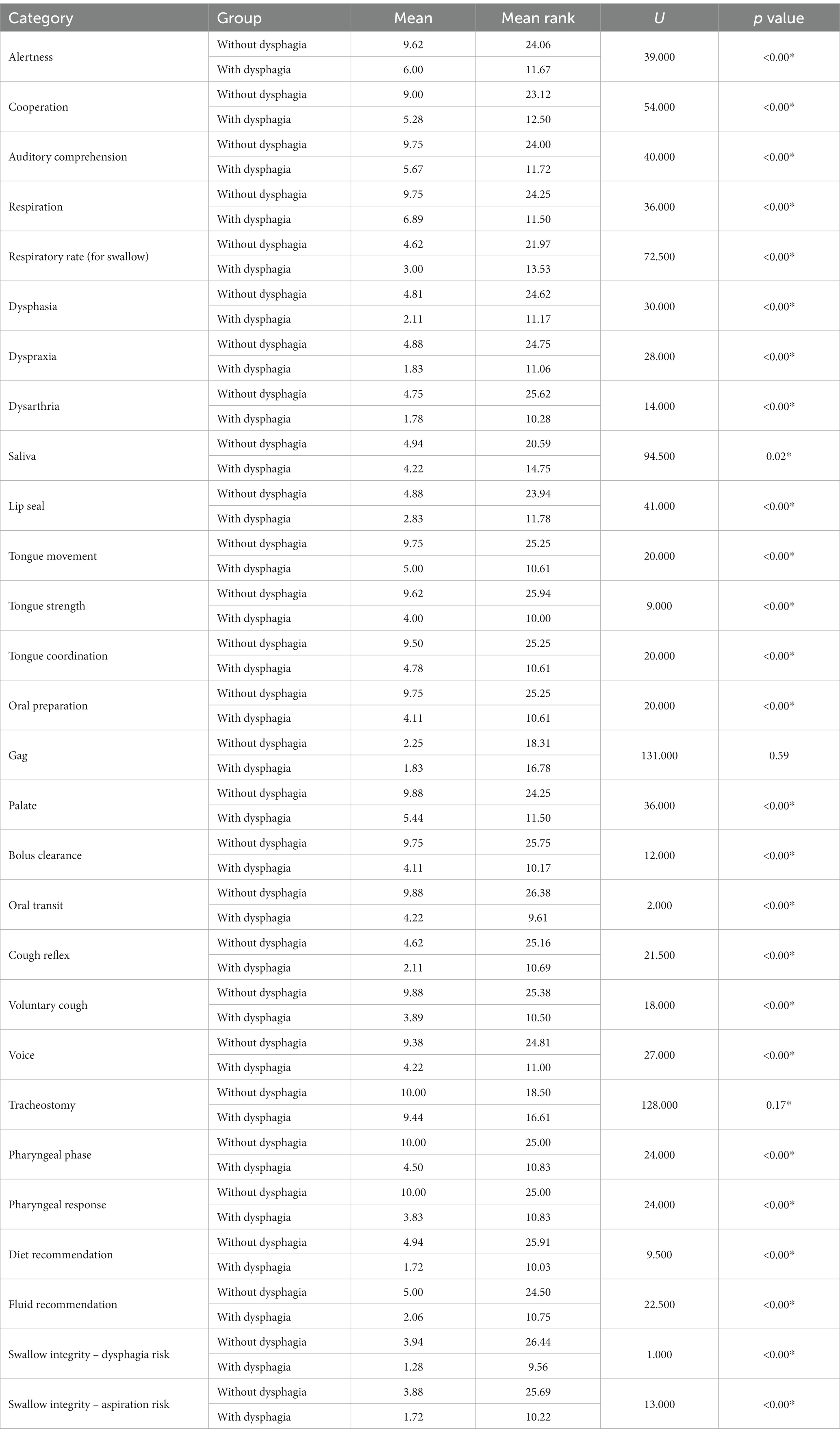
Out of 34 examined participants, 16 individuals (proportion = 47.1%; mean age = 44.94; SD = 15.36; range = 29–72), including 6 females and 10 males, were identified without dysphagia, and 18 participants (proportion = 52.9%; mean age = 57.83; SD = 16.55; range = 27–82), including 7 females and 11 males, were diagnosed to have dysphagia according to the MASA scores. According to the Mann Whitney U test, swallowing ability was significantly different (p < 0.00) between these two groups. This difference was also significant across all MASA categories, except the gag reflex and tracheostomy categories (Table 1). Figure 1 portrays these categories and compares the differences. Indeed, the gag reflex diminished in 91.2% of all participants. Also, 94.1% of all participants had no tracheostomy tube at the time of the assessment.
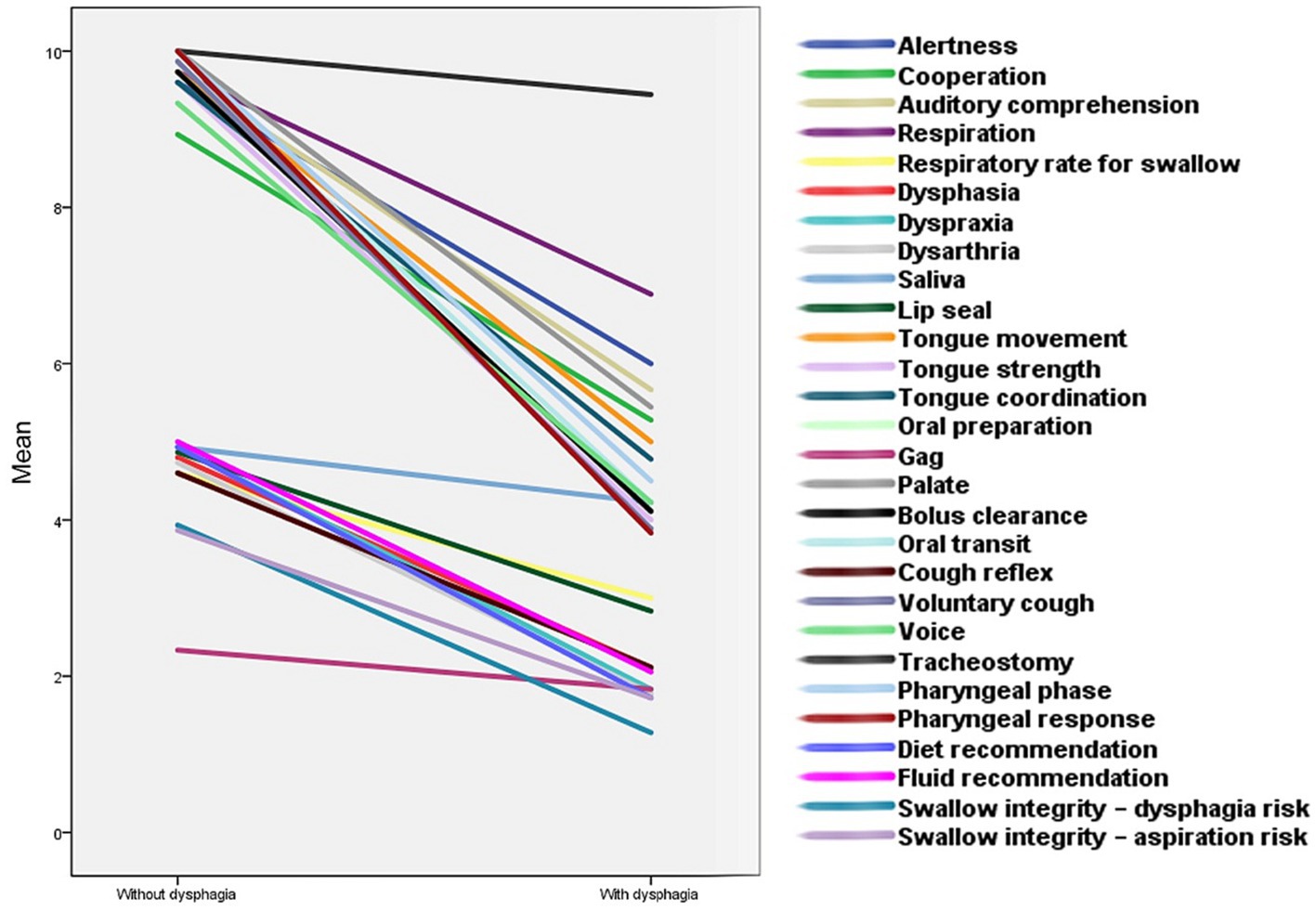
Figure 1. The comparison of swallowing aspects between people with and without dysphagia after cerebellar stroke. This diagram depicts swallowing characteristics between the two groups of subjects following cerebellar stroke. Almost every component of swallowing became considerably compromised among individuals with dysphagia. The slope of the lines indicates how much these factors were influenced.
16 individuals with dysphagia were identified to be at risk of aspiration (proportion = 47%; mean age = 57.12; SD = 16.27; range = 27–82). However, participants without dysphagia showed no risk of aspiration (Figure 2). Further, a significant correlation was also found between aspiration and the presence of dysphagia (ρ = 0.88; p < 0.00).
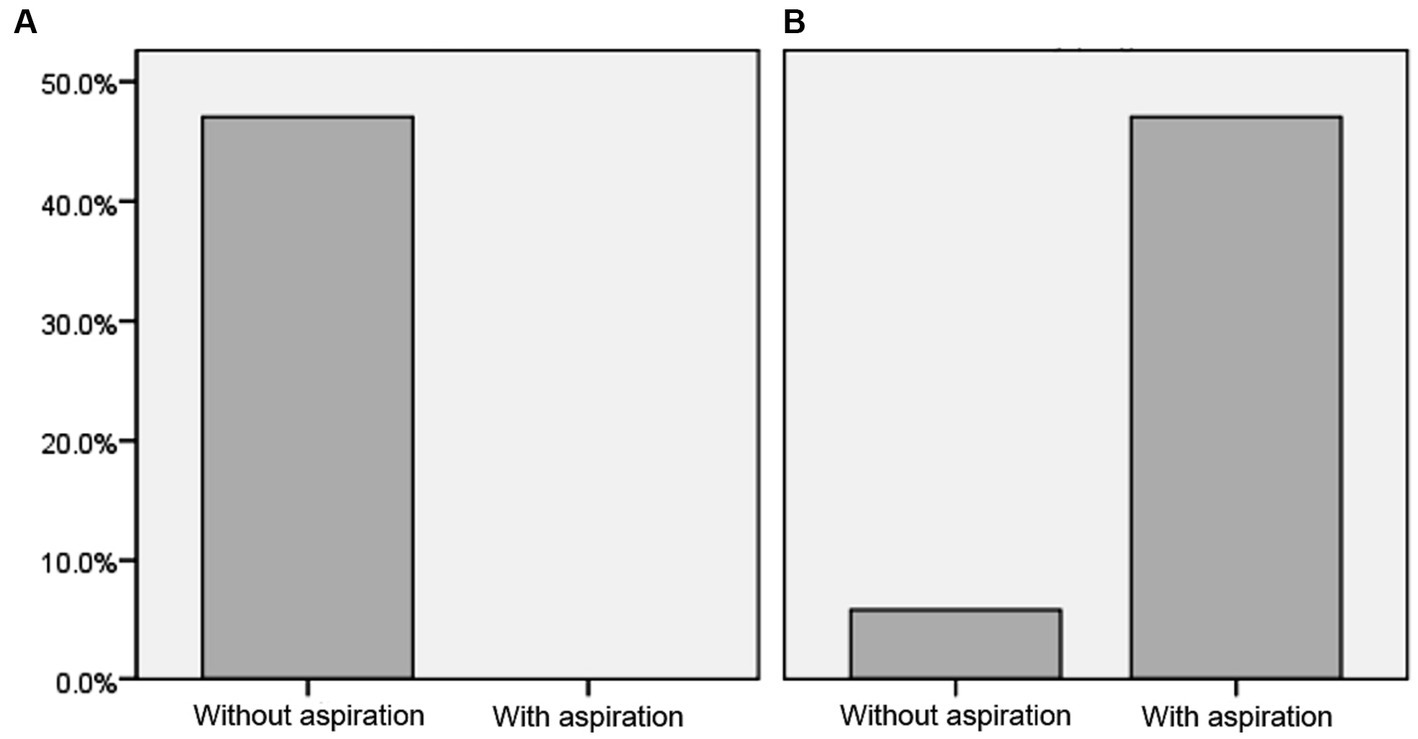
Figure 2. The risk of aspiration following cerebellar stroke among people with and without dysphagia. This figure illustrates the aspiration risk after cerebellar stroke. (A,B) show people without and with dysphagia, respectively.
Age and the presence of dysphagia were also significantly correlated (ρ = 0.39; p = 0.02), and individuals with dysphagia were significantly older than people without dysphagia (df = 32; p = 0.02; CI = 95%).
No significant correlation was found between the MASA-score and sex (p = 0.60). Furthermore, no significant difference (p = 0.95) was discovered regarding the presence of dysphagia between males and females.
Table 2; Figure 2 demonstrate further details about swallowing skills and the risk of aspiration among the two groups.
Table 2. Swallowing ability and aspiration risk in individuals with and without dysphagia after cerebellar stroke.
4 Discussion
An in-depth analysis of the clinical characteristics of the oral and pharyngeal phases of swallowing was conducted in patients with cerebellar strokes to understand how cerebellar strokes impact swallowing. The findings showed that cerebellar strokes can significantly affect the oral and pharyngeal phases of swallowing, leading to various issues such as dysphagia and aspiration.
This study indicated that almost half of the individuals who experienced a cerebellar stroke encountered dysphagia afterward. However, this is inconsistent with the results of the previous retrospective cohort study conducted in 2023, which demonstrated that dysphagia affected around 11% of individuals after cerebellar strokes (15). In the present study, people with dysphagia often underwent severe dysphagia, including prominent impairments in both oral and pharyngeal phases. Contrarily, Huang et al. could not provide detailed reports on the severity of dysphagia and the most affected phases of swallowing because they run their study through a retrospective chart review of the medical records and information reported after a swallowing bedside examination, videofuoroscopic swallowing study, fberoptic endoscopic evaluation of swallowing, etc. This might have affected the accuracy of dysphagia diagnosis after cerebellar strokes through their study. The present study suggests that the cerebellum may control both the motor and non-motor components of the swallowing process, such as tongue movements, alertness, etc. This supports the limited evidence revealing cerebellar activities during volitional swallowing among people with no medical conditions (28–30). Interestingly, an animal study has shown that the cerebellum modulates the excitability of the swallowing reflex via direct connections to the nucleus tractus solitarius (35), which has been known for its fundamental role in the oral and pharyngeal phases of swallowing (36). Although several studies have previously indicated a potential correlation between dysphagia and diseases related to the cerebellum, (e.g., 37–40), ones that investigated the risk of dysphagia after isolated cerebellar strokes have reported inconsistent results with these findings (41, 42). The reason behind this can be easily understood by looking at the way these studies evaluated the process of swallowing. Indeed, the two first phases of swallowing contain various parts that need to be considered together to assess swallowing (34), but it is unclear how exactly these studies assessed swallowing since they did not provide any specific information about their procedure. Some investigations, however, utilized specific assessment methods, but they did not include an adequate number of individuals with isolated cerebellar strokes (43–45). Thus, it is not surprising that there is still uncertainty about how cerebellar strokes lead to swallowing disorders, given the limited conclusive evidence in this regard.
According to the study, individuals with dysphagia were also significantly older than those who did not have the condition. This is also in line with previous studies, which have revealed that dysphagia is common among older adults, impacting around 10 to 33% of them (46). Furthermore, Huang et al. indicated that older individuals were more likely to encounter dysphagia after cerebellar strokes (15). Evidence has demonstrated that aging can change the swallowing mechanism (47). For example, aging can affect the oral phase of swallowing by causing a reduced oral sensation, poor dentition, hyposalivation, and xerostomia, which may also make it harder to sense how thick or thin the food or liquid is in the mouth (47). Aging also causes less strength in both the masticatory muscles and the lingual muscles (48). As well as the oral phase, the laryngopharyngeal response, musculature, pressure, timing, and coordination may become weakened as people get older (49, 50). Aging can also lead to various changes in the cerebellum, which affect its size, structure, and function (51, 52). These, for example, cause a decrease in size, particularly in the anterior and posterior cerebellum, and changes in neuron density, synapse connectivity, and white matter (51). Remarkably, aging decreases the volume of the nucleus tractus solitarius too (53), which has been found to be the key that the cerebellum uses to regulate the excitability of the swallowing reflex (35). Taking it all together, the findings suggest that aging can be a key factor that determines whether people with cerebellar strokes encounter dysphagia.
As may be expected from the previous discussion, the risk of aspiration (mostly severe) was accordingly significantly higher in individuals with dysphagia, while those without dysphagia showed no risk of aspiration, which supports the close association between dysphagia and aspiration pneumonia (54). Huang et al. could not also provide precise reports about aspiration risk and its severity, according to their study design. This might have also influenced the reports and made judgment biases through their study (15). Contrarily, a high incidence of aspiration has also been reported among people after stroke in the present study, which raises the risk of pneumonia (55). Furthermore, an investigation has pointed out that hospitalized older people are even more vulnerable since aspiration pneumonia is more likely to cause or develop mortality in older people (56). Therefore, early diagnosis and management of dysphagia are imperative to preserve the optimal quality of life for the affected individuals and reduce the risk of mortality.
The present study also indicated that the gag reflex faded away in almost all participants, with or without dysphagia, after an isolated cerebellar stroke. This may imply that there may be a possible link, somehow, between the gag reflex and the cerebellum. The gag reflex is thought to be an evolutionary reflex that developed to prevent individuals from choking and swallowing foreign objects. The gag reflex is mediated by both the glossopharyngeal (CN IX) and vagus (CN X) nerves, which pass to the medulla oblongata, which is also adjacent to the vomiting, salivary, and cardiac centers. Near this area, the reticular formation is also located, which fills the spaces among cranial nerves, olivary supplies, and fiber tracts. The reticular formation reciprocally carries information from the spinal cord, cranial nerve nuclei, cerebellum, and cerebrum. Some parts of this formation are tightly connected to the respiratory centers, including the dorsal and ventral respiratory groups (57). Surprisingly, the dorsal group is part of the nucleus tractus solitarius, which is the main area for termination and integration of sensory afferents from various vital body systems, such as the soft palate, epiglottis, and pharynx. They reciprocate various sensory information through the facial (CN VII), glossopharyngeal (CN IX), and vagus (CN X) nerves. The ventral respiratory group also contains a place called the preBötzinger complex, which is located in the ventral respiratory group that encompasses respiratory premotor interneurons that regulate inspiratory related muscles of the tongue and pharynx (58–61). Investigations have reported that this site gets involved in the reflexes provoked by the vestibular (CN VIII) and (superior) laryngeal (CN X) nerves, and in swallowing and vomiting (62, 63). Furthermore, the reticular formation also has projections to the cerebellum, which may imply the integrative role of these structures in pharyngeal movements (64–67). Interestingly, the cells forming spinal projections to the reticular formation are also placed mainly in the laminae of the abducens (CN VI), facial (CN VII), and vagus (CN X) nerves, where many last order premotor interneurons are also organized. Therefore, it seems that the cerebellum may indirectly receive tremendous information through a huge complex path called the spino-bulbar-reticular pathway (68), in order to regulate various motor adjustments with the help of these structures (69). Accordingly, a cerebellar stroke could possibly result in disruptions to the cerebellum’s function and also its connected structures, such as the reticular formation, by which the gag reflex faded away. This might be an explanation for why the gag reflex was found to be substantially poor among participants. However, further research is required to study these connections and their underlying mechanisms more precisely.
In conclusion, the findings of the present study support the notion that the cerebellum presumably plays a functional role in regulating the motor and non-motor aspects of swallowing. Accordingly, isolated cerebellar strokes can adversely affect both oral and pharyngeal phases of the swallowing process, particularly among older adults. Thus, dysphagia after a cerebellar stroke should be diagnosed early and timely managed, as it causes serious consequences resulting from aspiration.
5 Limitations and direction for the future study
Despite the present study offering promising evidence regarding dysphagia following cerebellar strokes, there are some limitations that should be factored in. First, we did not have access to the Videofluoroscopic Swallow Study in order to assess swallowing. This is currently the gold standard for diagnosing dysphagia (70). Second, this study was run through a master’s thesis; hence, effort was made to recruit as many participants as feasible within the time and resource constraints of the project. While a larger sample would be ideal, the researchers believe these findings still provide valuable preliminary insights that can inform future research. Therefore, the results of this study should be interpreted with caution. Third, we did not consider different conditions of stroke (ischemic, hemorrhagic, or mixed) in the present study. Thus, similar studies should be carried out taking these points into consideration in different regions of the world to study the effects of cerebellar strokes on the swallowing process in different populations. Although various medical conditions like swallowing disorders have been studied after cerebral strokes (e.g., (71, 72)), the role of the cerebellum in the swallowing process and the effects of its infracts on this process still need more illumination. Hence, further studies appear to be necessary for the expansion of sufficient practical information (73).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Mashhad University of Medical Sciences with the ethical code of IR.MUMS.REC.1398.286. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MH: Conceptualization, Writing – original draft, Data curation. SZ: Writing – original draft, Writing – review & editing, Methodology, Formal Analysis, Visualization. MF: Investigation, Writing – review & editing. SK: Formal analysis, Writing – review & editing. DS-R: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was financially supported by the Mashhad University of Medical Sciences (NO. 4001279), Mashhad, Iran.
Acknowledgments
The authors thank the participants and their families for their participation in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mandell, LA, and Niederman, MS. Aspiration pneumonia. N Engl J Med. (2019) 380:651–63. doi: 10.1056/NEJMra1714562
3. Panara, K, and Padalia, D. Physiology, swallowing. Treasure Island (FL): StatPearls Publishing (2019).
4. González-Fernández, M, Ottenstein, L, Atanelov, L, and Christian, AB. Dysphagia after stroke: an overview. Curr Phys Med Rehabil Rep. (2013) 1:187–96. doi: 10.1007/s40141-013-0017-y
5. Zainaee, S, Rahmani, S, and Ghaemi, H. Effective swallowing rehabilitation strategies in patients with multiple sclerosis suffering from dysphagia; a review of literature. J Rehab Sci Res. (2020) 7:106–13.
6. Purves, D, Augustine, G, Fitzpatrick, D, Katz, L, LaMantia, A, McNamara, J, et al. Circuits within the cerebellum. 2nd ed Sunderland (MA): Sinauer Associates (2001).
7. Roostaei, T, Nazeri, A, Sahraian, MA, and Minagar, A. The human cerebellum: a review of physiologic neuroanatomy. Neurol Clin. (2014) 32:859–69. doi: 10.1016/j.ncl.2014.07.013
8. Daskalakis, ZJ, Paradiso, GO, Christensen, BK, Fitzgerald, PB, Gunraj, C, and Chen, R. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol. (2004) 557:689–700. doi: 10.1113/jphysiol.2003.059808
9. Jean, A . Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. (2001) 81:929–69. doi: 10.1152/physrev.2001.81.2.929
10. Montgomery, JC . Roles for cerebellum and subsumption architecture in central pattern generation. J Comp Physiol A. (2023) 1-10. doi: 10.1007/s00359-023-01634-w
11. Witter, L, and De Zeeuw, CI. Regional functionality of the cerebellum. Curr Opin Neurobiol. (2015) 33:150–5. doi: 10.1016/j.conb.2015.03.017
12. Collaborators GLRoS . Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. doi: 10.1056/NEJMoa1804492
13. Tohgi, H, Takahashi, S, Chiba, K, and Hirata, Y. Cerebellar infarction. Clinical and neuroimaging analysis in 293 patients. The Tohoku cerebellar infarction study group. Stroke. (1993) 24:1697–701. doi: 10.1161/01.STR.24.11.1697
14. Kase, C, Norrving, B, Levine, S, Babikian, V, Chodosh, E, Wolf, P, et al. Cerebellar infarction. Clinical and anatomic observations in 66 cases. Stroke. (1993) 24:76–83. doi: 10.1161/01.STR.24.1.76
15. Huang, L, Wang, Y, Sun, J, Zhu, L, Liu, J, Wu, Y, et al. Incidence and risk factors for dysphagia following Cerebellar stroke: a retrospective cohort study. Cerebellum. (2023) 1–11. doi: 10.1007/s12311-023-01564-y
16. Mussen, AT . Symposium on the cerebellum: (4) experimental investigations on the cerebellum. Brain. (1927) 50:313–49. doi: 10.1093/brain/50.3-4.313
17. Mussen, AT . The cerebellum: the influence of the cortical reactions on the classification and the homology of the lobes and fissures in the cat, monkey and man. Arch Neurol Psychiatr. (1930) 24:913–20. doi: 10.1001/archneurpsyc.1930.02220170033004
18. Reis, DJ, Doba, N, and Nathan, MA. Predatory attack, grooming, and consummatory behaviors evoked by electrical stimulation of cat cerebellar nuclei. Science. (1973) 182:845–7. doi: 10.1126/science.182.4114.845
19. Ball, GG, Micco, DJ Jr, and Berntson, GG. Cerebellar stimulation in the rat: complex stimulation-bound oral behaviors and self-stimulation. Physiol Behav. (1974) 13:123–7. doi: 10.1016/0031-9384(74)90313-8
20. Colombel, C, Lalonde, R, and Caston, J. The effects of unilateral removal of the cerebellar hemispheres on motor functions and weight gain in rats. Brain Res. (2002) 950:231–8. doi: 10.1016/S0006-8993(02)03043-3
21. Zhu, JN, Li, HZ, Ding, Y, and Wang, JJ. Cerebellar modulation of feeding-related neurons in rat dorsomedial hypothalamic nucleus. J Neurosci Res. (2006) 84:1597–609. doi: 10.1002/jnr.21059
22. Sasegbon, A, Watanabe, M, Simons, A, Michou, E, Vasant, DH, Magara, J, et al. Cerebellar repetitive transcranial magnetic stimulation restores pharyngeal brain activity and swallowing behaviour after disruption by a cortical virtual lesion. J Physiol. (2019) 597:2533–46. doi: 10.1113/JP277545
23. Sasegbon, A, Smith, CJ, Bath, P, Rothwell, J, and Hamdy, S. The effects of unilateral and bilateral cerebellar rTMS on human pharyngeal motor cortical activity and swallowing behavior. Exp Brain Res. (2020) 238:1719–33. doi: 10.1007/s00221-020-05787-x
24. Zhong, L, Wen, X, Liu, Z, Li, F, Ma, X, Liu, H, et al. Effects of bilateral cerebellar repetitive transcranial magnetic stimulation in poststroke dysphagia: a randomized sham-controlled trial. NeuroRehabilitation. (2023) 52:227–34. doi: 10.3233/NRE-220268
25. Erfmann, KL, Macrae, PR, Jones, RD, Guiu Hernandez, E, and Huckabee, M-L. Effects of cerebellar transcranial direct current stimulation (tDCS) on motor skill learning in swallowing. Disabil Rehabil. (2022) 44:2276–84. doi: 10.1080/09638288.2020.1827303
26. Sasegbon, A, Niziolek, N, Zhang, M, Smith, CJ, Bath, PM, Rothwell, J, et al. The effects of midline cerebellar rTMS on human pharyngeal cortical activity in the intact swallowing motor system. Cerebellum. (2021) 20:101–15. doi: 10.1007/s12311-020-01191-x
27. Sasegbon, A, and Hamdy, S. The role of the cerebellum in swallowing. Dysphagia. (2023) 38:497–509. doi: 10.1007/s00455-021-10271-x
28. Suzuki, M, Asada, Y, Ito, J, Hayashi, K, Inoue, H, and Kitano, H. Activation of cerebellum and basal ganglia on volitional swallowing detected by functional magnetic resonance imaging. Dysphagia. (2003) 18:71–7. doi: 10.1007/s00455-002-0088-x
29. Malandraki, GA, Sutton, BP, Perlman, AL, Karampinos, DC, and Conway, C. Neural activation of swallowing and swallowing-related tasks in healthy young adults: an attempt to separate the components of deglutition. Hum Brain Mapp. (2009) 30:3209–26. doi: 10.1002/hbm.20743
30. Hamdy, S, Rothwell, JC, Brooks, DJ, Bailey, D, Aziz, Q, and Thompson, DG. Identification of the cerebral loci processing human swallowing with H2 15O PET activation. J Neurophysiol. (1999) 81:1917–26. doi: 10.1152/jn.1999.81.4.1917
31. Moon, HI, Jeong, YJ, and Suh, JH. Voxel-based lesion symptom mapping analysis for dysphagia in stroke patients with isolated cerebellar lesions. J Neural Transm. (2022) 129:65–74. doi: 10.1007/s00702-021-02438-5
32. Mahdipour, R, Hajipour, M, Shahin, M-A, and Zainaee, S. Comment on “prevalence and risk factors of dysphagia in patients with multiple sclerosis”. Mult Scler Relat Disord. (2022) 66:104017. doi: 10.1016/j.msard.2022.104017
33. Ghaemi, H, Sobhani-Rad, D, Arabi, A, Saifpanahi, S, and Ghayoumi, AZ. Role of basal ganglia in swallowing process: a systematic review. Iran Rehabil J. (2016) 14:239–45.
34. Mann, G . Masa: the Mann assessment of swallowing ability. Australia: Singular/Thomson Learning (2002).
35. Reed, MD, English, M, English, C, Huff, A, Poliacek, I, Musselwhite, MN, et al. The role of the cerebellum in control of swallow: evidence of inspiratory activity during swallow. Lung. (2019) 197:235–40. doi: 10.1007/s00408-018-00192-2
36. Bieger, D, and Neuhuber, W. Neural circuits and mediators regulating swallowing in the brainstem. GI Motility. (2006). doi: 10.1038/gimo74
37. Vasant, DH, Sasegbon, A, Michou, E, Smith, C, and Hamdy, S. Rapid improvement in brain and swallowing behavior induced by cerebellar repetitive transcranial magnetic stimulation in poststroke dysphagia: a single patient case-controlled study. Neurogastroenterology Motility. (2019) 31:e13609. doi: 10.1111/nmo.13609
38. Almotairi, FS, Andersson, M, Andersson, O, Skoglund, T, and Tisell, M. Swallowing dysfunction in adult patients with Chiari I malformation. J Neurol Surg Part B. (2018) 79:606–13. doi: 10.1055/s-0038-1655758
39. Yu, T, Li, J, Wang, K, Ge, Y, Jiang, A, Duan, L, et al. Clinical characteristics of neurogenic dysphagia in adult patients with Chiari malformation type I. Beijing da xue xue bao Yi xue ban. (2017) 49:315–21.
40. Kumral, E, Kısabay, A, and Atac, C. Lesion patterns and etiology of ischemia in the anterior inferior cerebellar artery territory involvement: a clinical–diffusion weighted–MRI study. Eur J Neurol. (2006) 13:395–401. doi: 10.1111/j.1468-1331.2006.01255.x
41. Min, WK, Kim, YS, Kim, JY, Park, SP, and Suh, CK. Atherothrombotic cerebellar infarction: vascular lesion–MRI correlation of 31 cases. Stroke. (1999) 30:2376–81. doi: 10.1161/01.STR.30.11.2376
42. Izumi, M, Terao, S, Sobue, G, Koshimura, J, Takatsu, S, Yokoi, Y, et al. Clinical features of anterior inferior cerebellar artery territory infarcts--a study of ten patients. No to Shinkei. (1996) 49:152–6.
43. Dehaghani, SE, Yadegari, F, Asgari, A, Chitsaz, A, and Karami, M. Brain regions involved in swallowing: evidence from stroke patients in a cross-sectional study. J Res Med Sci. (2016) 21:45. doi: 10.4103/1735-1995.183997
44. Mo, SJ, Jeong, HJ, Han, YH, Hwang, K, and Choi, JK. Association of brain lesions and videofluoroscopic dysphagia scale parameters on patients with acute cerebral infarctions. Ann Rehabil Med. (2018) 42:560–8. doi: 10.5535/arm.2018.42.4.560
45. Fernández-Pombo, A, Seijo-Raposo, IM, López-Osorio, N, Cantón-Blanco, A, González-Rodríguez, M, Arias-Rivas, S, et al. Lesion location and other predictive factors of dysphagia and its complications in acute stroke. Clin Nutr ESPEN. (2019) 33:178–82. doi: 10.1016/j.clnesp.2019.05.019
46. Thiyagalingam, S, Kulinski, AE, Thorsteinsdottir, B, Shindelar, KL, and Takahashi, PY, editors. Dysphagia in older adults. Mayo clinic proceedings; (2021): Elsevier.
47. Walshe, M . Swallowing and ageing. Speech, Lang Hear. (2019) 22:2–8. doi: 10.1080/2050571X.2019.1567898
48. Hara, K, Tohara, H, Kenichiro, K, Yamaguchi, K, Ariya, C, Yoshimi, K, et al. Association between tongue muscle strength and masticatory muscle strength. J Oral Rehabil. (2019) 46:134–9. doi: 10.1111/joor.12737
49. Mulheren, RW, Azola, AM, Kwiatkowski, S, Karagiorgos, E, Humbert, I, Palmer, JB, et al. Swallowing changes in community-dwelling older adults. Dysphagia. (2018) 33:848–56. doi: 10.1007/s00455-018-9911-x
50. Namasivayam-MacDonald, AM, Barbon, CE, and Steele, CM. A review of swallow timing in the elderly. Physiol Behav. (2018) 184:12–26. doi: 10.1016/j.physbeh.2017.10.023
51. Gellersen, HM, Guell, X, and Sami, S. Differential vulnerability of the cerebellum in healthy ageing and Alzheimer’s disease. NeuroImage. (2021) 30:102605. doi: 10.1016/j.nicl.2021.102605
52. Woodruff-Pak, DS, Foy, MR, Akopian, GG, Lee, KH, Zach, J, Nguyen, KPT, et al. Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci. (2010) 107:1624–9. doi: 10.1073/pnas.0914207107
53. Daulatzai, MA . Dysfunctional nucleus Tractus Solitarius: its crucial role in promoting Neuropathogentic Cascade of Alzheimer’s dementia—a novel hypothesis. Neurochem Res. (2012) 37:846–68. doi: 10.1007/s11064-011-0680-2
54. Lo, W-L, Leu, H-B, Yang, M-C, Wang, D-H, and Hsu, M-L. Dysphagia and risk of aspiration pneumonia: a nonrandomized, pair-matched cohort study. J Dent Sci. (2019) 14:241–7. doi: 10.1016/j.jds.2019.01.005
55. Teasell, R, Foley, N, Martino, R, Richardson, M, Bhogal, S, and Speechley, M. Dysphagia and aspiration following stroke. Evidence-Based Rev Stroke Rehab, Chap. (2013):1–74.
56. Shin, D, Lebovic, G, and Lin, RJ. In-hospital mortality for aspiration pneumonia in a tertiary teaching hospital: a retrospective cohort review from 2008 to 2018. J Otolaryngol-Head Neck Surg. (2023) 52:23. doi: 10.1186/s40463-022-00617-2
57. Sergievskii, M, and Kireeva, NY. On the mutual connections of the nuclei of the respiratory center. Byull Éksp Biol Med. (1980) 90:652–4.
58. Chamberlin, NL, Eikermann, M, Fassbender, P, White, DP, and Malhotra, A. Genioglossus premotoneurons and the negative pressure reflex in rats. J Physiol. (2007) 579:515–26. doi: 10.1113/jphysiol.2006.121889
59. Peever, J, Shen, L, and Duffin, J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience. (2002) 110:711–22. doi: 10.1016/S0306-4522(01)00594-2
60. Stanek, E IV, Cheng, S, Takatoh, J, Han, B-X, and Wang, F. Monosynaptic premotor circuit tracing reveals neural substrates for oro-motor coordination. elife. (2014) 3:e02511. doi: 10.7554/eLife.02511
61. Welzl, H, and Bureš, J. Lick-synchronized breathing in rats. Physiol Behav. (1977) 18:751–3. doi: 10.1016/0031-9384(77)90079-8
62. Nakazawa, K, Zheng, Y, Umezaki, T, and Miller, AD. Role of pre-Bötzinger complex respiratory neurons in vestibular and laryngeal reflexes and in swallowing and vomiting. Otolaryngol Head Neck Surg. (1997) 117:P146–7. doi: 10.1016/S0194-5998(97)80283-5
63. Fukuda, H, and Koga, T. Neuronal gagging activity patterns may be generated by neurons in the reticular area dorsomedial to the retrofacial nucleus in dogs. Exp Brain Res. (1997) 113:394–401. doi: 10.1007/PL00005593
64. Valle, MS, Garifoli, A, Maci, T, and Perciavalle, V. Reticulocerebellar projections to the anterior and posterior lobes of the rat cerebellum. Neurosci Lett. (2001) 314:41–4. doi: 10.1016/S0304-3940(01)02278-9
65. Tang, Z, and Zhang, S. The cerebellar projection from the reticular formation of the brain stem in the rabbit: an experimental study using HRP as a retrograde tracer. Anat Embryol. (1987) 175:521–6. doi: 10.1007/BF00309687
66. Wu, HS, Sugihara, I, and Shinoda, Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J Comp Neurol. (1999) 411:97–118. doi: 10.1002/(SICI)1096-9861(19990816)411:1<97::AID-CNE8>3.0.CO;2-O
67. Parenti, R, Cicirata, F, Pantò, MR, and Serapide, MF. The projections of the lateral reticular nucleus to the deep cerebellar nuclei. An experimental analysis in the rat. Eur J Neurosci. (1996) 8:2157–67. doi: 10.1111/j.1460-9568.1996.tb00737.x
68. Huma, Z, and Maxwell, DJ. The spino-bulbar-cerebellar pathway: organization and neurochemical properties of spinal cells that project to the lateral reticular nucleus in the rat. Front Neuroanat. (2015) 9:1. doi: 10.3389/fnana.2015.00001
69. Alstermark, B, and Ekerot, CF. The lateral reticular nucleus: a precerebellar Centre providing the cerebellum with overview and integration of motor functions at systems level. A new hypothesis. J Physiol. (2013) 591:5453–8. doi: 10.1113/jphysiol.2013.256669
70. Helliwell, K, Hughes, V, Bennion, C, and Manning-Stanley, A. The use of videofluoroscopy (VFS) and fibreoptic endoscopic evaluation of swallowing (FEES) in the investigation of oropharyngeal dysphagia in stroke patients: a narrative review. Radiography. (2023) 29:284–90. doi: 10.1016/j.radi.2022.12.007
71. Rexrode, KM, Madsen, TE, Yu, AY, Carcel, C, Lichtman, JH, and Miller, EC. The impact of sex and gender on stroke. Circ Res. (2022) 130:512–28. doi: 10.1161/CIRCRESAHA.121.319915
72. Saber-Moghadam, R, Zeinalzadeh, A, Momenzadeh, M, Farzadfar, MT, Ghaemi, H, and Sobhani-Rad, D. The relationship between memory, type, and severity of aphasia with confrontation naming in post-stroke patients with chronic aphasia. Iran Rehabil J. (2022) 20:561–8. doi: 10.32598/irj.20.4.1693.1
Keywords: cerebellum, cerebellar stroke, cerebellar infarct, dysphagia, swallowing disorders, deglutition disorders, swallowing, swallowing function
Citation: Hajipour M, Sobhani-Rad D, Zainaee S, Farzadfar MT and Khaniki SH (2023) Dysphagia following cerebellar stroke: analyzing the contribution of the cerebellum to swallowing function. Front. Neurol. 14:1276243. doi: 10.3389/fneur.2023.1276243
Edited by:
Emily Keshner, Temple University, United StatesReviewed by:
Mario Stampanoni Bassi, Mediterranean Neurological Institute Neuromed (IRCCS), ItalyHuiyu Liu, Yuebei People's Hospital, China
Copyright © 2023 Hajipour, Sobhani-Rad, Zainaee, Farzadfar and Khaniki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Davood Sobhani-Rad, c29iaGFuaWRAbXVtcy5hYy5pcg==
†ORCID: Masoume Hajipour, https://orcid.org/0009-0007-9140-5909
Shahryar Zainaee, https://orcid.org/0000-0003-2104-0151
Mohammad Taghi Farzadfar, https://orcid.org/0000-0002-4270-6621
Saeedeh Hajebi Khaniki, https://orcid.org/0000-0001-7041-3802
Davood Sobhani-Rad, https://orcid.org/0000-0003-1147-9865
 Masoume Hajipour1†
Masoume Hajipour1† Davood Sobhani-Rad
Davood Sobhani-Rad Shahryar Zainaee
Shahryar Zainaee