- 1Department of Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- 3Department of Anesthesiology, Affiliated Sport Hospital of Chengdu Sport University, Chengdu, Sichuan, China
Background: The base deficit, international normalized ratio, and Glasgow Coma Scale (BIG) score was previously developed to predict the outcomes of pediatric trauma patients. We designed this study to explore and improve the prognostic value of the BIG score in adult patients with traumatic brain injury (TBI).
Methods: Adult patients diagnosed with TBI in a public critical care database were included in this observational study. The BIG score was calculated based on the Glasgow Coma Scale (GCS), the international normalized ratio (INR), and the base deficit. Logistic regression analysis was performed to confirm the association between the BIG score and the outcome of included patients. Receiver operating characteristic (ROC) curves were drawn to evaluate the prognostic value of the BIG score and novel constructed models.
Results: In total, 1,034 TBI patients were included in this study with a mortality of 22.8%. Non-survivors had higher BIG scores than survivors (p < 0.001). The results of multivariable logistic regression analysis showed that age (p < 0.001), pulse oxygen saturation (SpO2) (p = 0.032), glucose (p = 0.015), hemoglobin (p = 0.047), BIG score (p < 0.001), subarachnoid hemorrhage (p = 0.013), and intracerebral hematoma (p = 0.001) were associated with in-hospital mortality of included patients. The AUC (area under the ROC curves) of the BIG score was 0.669, which was not as high as in previous pediatric trauma cohorts. However, combining the BIG score with age increased the AUC to 0.764. The prognostic model composed of significant factors including BIG had the highest AUC of 0.786.
Conclusion: The age-adjusted BIG score is superior to the original BIG score in predicting mortality of adult TBI patients. The prognostic model incorporating the BIG score is beneficial for clinicians, aiding them in making early triage and treatment decisions in adult TBI patients.
Introduction
As the leading cause of mortality and disability in trauma patients, traumatic brain injury (TBI) brings damage to victims and their families' quality of life and economic burden to society. A recent study estimated that nearly 69 million individuals are diagnosed with TBI annually in the world (1). The high mortality of TBI patients makes early triage and clinical intervention extremely important. Many trauma scores have been developed to assess the injury severity of TBI and to predict the outcome of trauma patients, such as the revised trauma score (RTS), the injury severity score (ISS), and the comprehensive trauma revised injury severity score (TRISS) (2–5). Specific scoring systems aimed at predicting the outcome of TBI patients were also developed and validated, including the CRASH model and IMPACT model (6, 7). However, these scores composed of radiologic characteristics and other anatomical factors are too complex and time-consuming for clinicians making patient triage and treatment decisions in the early stage after initial brain injury.
The BIG (composed of base deficit, international normalized ratio, and Glasgow Coma Scale) score is a pediatric trauma score that was initially developed to assess children facing military and civilian traumatic injuries (8). It has been proven to accurately predict the mortality rate of pediatric trauma patients admitted to military trauma systems. Several subsequent studies externally confirmed the good performance of the BIG score in predicting the mortality of similar pediatric trauma patients (9–11). Moreover, one study confirmed that the BIG score in admission was associated with functional outcomes at hospital discharge in pediatric TBI patients (12). The BIG score performed better than other pediatric trauma scoring systems and was validated with similar accuracy in a separate pediatric population (8). A BIG score of <12 points suggests a mortality of <5%, whereas a cutoff of >26 points corresponds to a mortality of >50% (13). In addition, researchers also explored the prognostic value of the BIG score in non-specific adult trauma patients and found that the BIG score had a comparable predictive performance with TRISS and the probability of survival (PS09) score (14). Given that aging is a factor that affects the prognosis of trauma patients, we proposed to establish an age-adjusted BIG score to better predict the mortality of patients with trauma.
It was mentioned in all the above studies that the superiority of the BIG score was its availability and simplicity. Based on these findings, we designed this study to explore the prognostic value of the BIG score and compared it with other trauma triage scores in homogeneous adult TBI patients.
Materials and methods
Data source
This observational study was performed using data from the Multiparameter Intelligent Monitoring in Intensive Care Database III (MIMIC-III database), which was a large critical care database including patients admitted to ICUs (intensive care unit) of the Beth Israel Deaconess Medical Center between 2001 and 2012. This freely available database was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center and the Massachusetts Institute of Technology (MIT). All data of participants in this public database were deidentified and anonymized. We obtained access to utilize data from the MIMIC III database after passing the National Institutes of Health (NIH) web-based training course and the Protecting Human Research Participants examination. All needed data, including age, sex, vital signs, laboratory tests, diagnoses, length of hospital stay, records of operation, and blood transfusion of this study were extracted by us using Navicat Premium 12 (PremiumSoft, Hong Kong). The BIG score was calculated by base deficit + 2.5 × INR + (15-GCS). The computing methods of other trauma scoring systems, including RTS, new trauma score (NTS), and GCS, age, and systolic arterial pressure score (GAP), were referred to in previous studies (15, 16). The primary outcome of this study was in-hospital mortality.
Participants
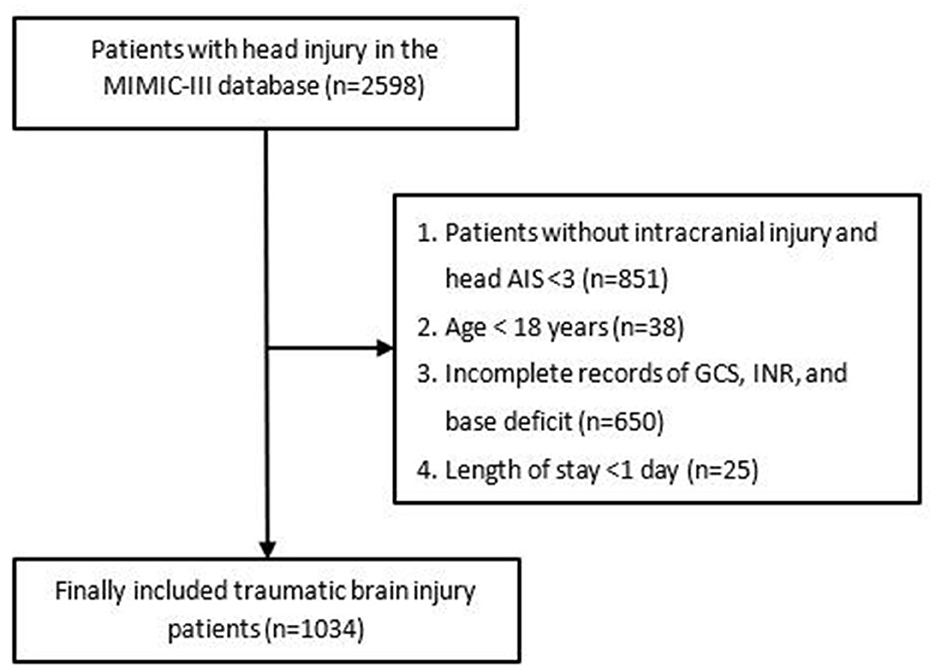
Patients with head injuries from the MIMIC-III database were enrolled for this study based on ICD-9 codes (800.00–801.99; 803.00–804.99; 850.0–854.19). However, patients were excluded from this study if they met any one of the following criteria: (1) diagnosed only with extracranial injury including scalp injury and skull fracture and head AIS <3; (2) age <18 years; (3) incomplete records of GCS, INR, and base deficit; (4) discharged within 24 h following admission (due to the hasty process, these patients did not receive standard medical treatment and examination, so they lacked many related variables). After exclusion, a total of 1,034 patients were included in the final cohort. The complete flowchart of participant inclusion is shown in Figure 1.
Statistical analysis
We utilized Kolmogorov–Smirnov tests to verify the normality of included variables. All continuous variables included in this study were expressed as median (interquartile range) and differences between groups of continuous variables were testified by the Mann–Whitney U-test because of their non-normal distribution. Categorical variables were expressed as numbers (percentage) and differences between groups of categorical variables were analyzed by the chi-square test. Univariable logistic regression was performed first and then, stepwise multivariable logistic regression with the entry method including significant variables in the univariable logistic regression was sequentially performed to explore the independent relationship between BIG score, other risk factors, and in-hospital mortality of included patients. The odds ratio (OR) and 95% confidence intervals (CI) of each factor were also shown. Then, multivariable logistic regression analysis was also performed to construct an age-adjusted BIG score and the multi-factor prognostic model. The nomogram of this multi-factor prognostic model was drawn for convenient clinical use. A calibration plot was drawn to evaluate the fit of the multi-factor prognostic model. Receiver operating characteristic (ROC) curves were drawn to evaluate the discriminatory ability to predict outcomes of included patients. The Youden index was used to identify cutoff values. We used the Z-test to compare the predictive values between factors and models.
A P-value <0.05 was considered to be statistically significant. We used SPSS 22.0 Windows software (SPSS, Inc, Chicago, IL) and R (version 3.6.1; R Foundation) for all statistical analyses and for drawing the figures.
Results
Baseline comparison of included TBI patients based on in-hospital outcomes
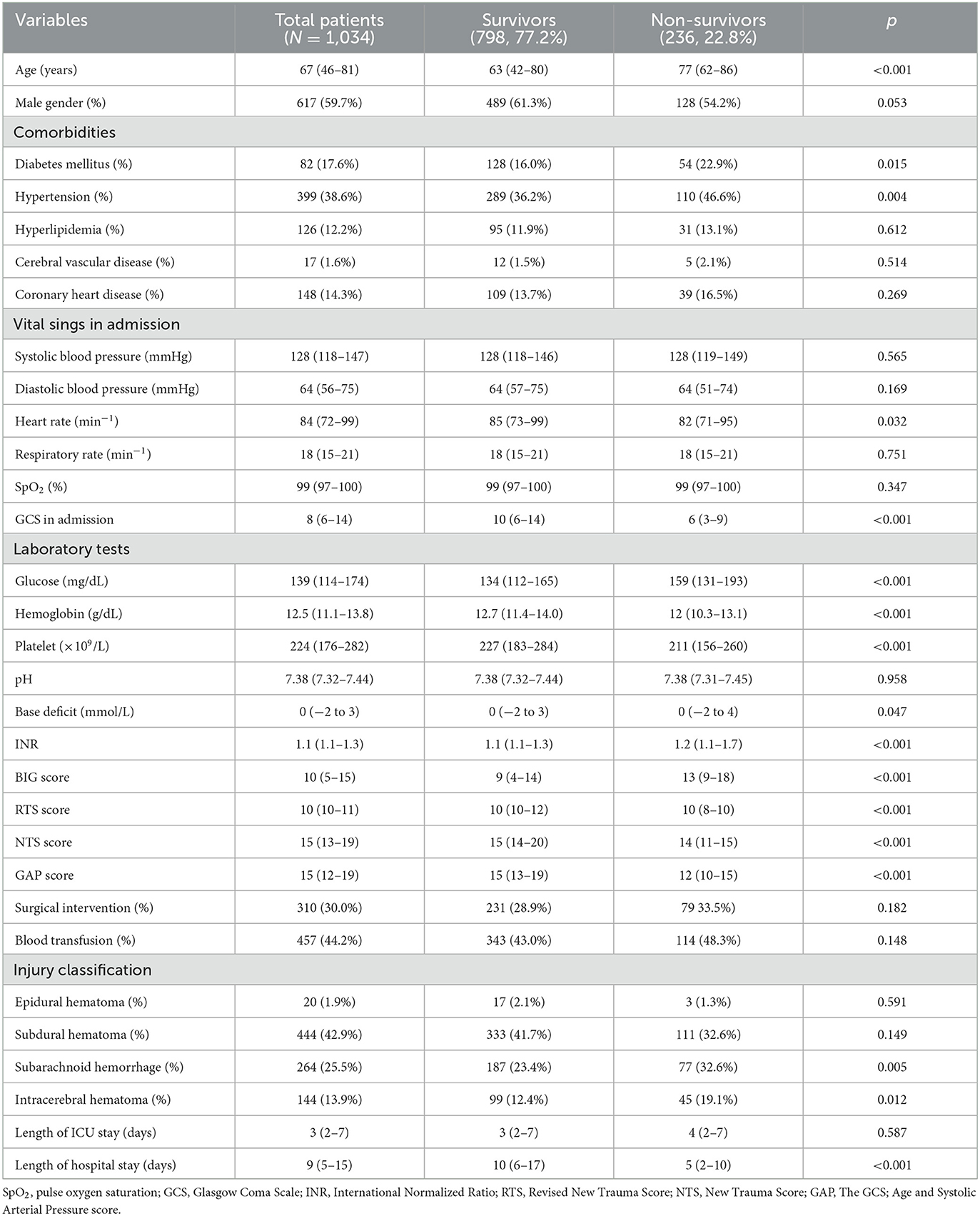
A total of 1,034 patients were included in this study with 798 survivors and 236 non-survivors (Table 1). The mortality rate and male ratio of included patients were 22.8 and 59.7%, respectively. The incidence of diabetes mellitus (p = 0.015) and hypertension (p = 0.004) were both higher among non-survivors. Vital signs including systolic blood pressure, diastolic blood pressure, and respiratory rate were not different between survivors and non-survivors. However, non-survivors had significantly lower heart rate (p = 0.032). Pulse oxygen saturation (SpO2) did not differ between those two groups, whereas the GCS score of non-survivors was lower than that of survivors with statistical significance (p < 0.001). Results of laboratory tests showed that non-survivors had a higher level of blood glucose (p < 0.001), base deficit (p = 0.047), and INR (p < 0.001), while the level of hemoglobin (p < 0.001) and platelet (p < 0.001) were significantly lower in non-survivors. Furthermore, the BIG score of non-survivors was significantly higher than that of survivors (p < 0.001), whereas survivors had a higher score of RTS (p < 0.001), NTS (p < 0.001), and GAP (p < 0.001). Results of injury types presented that non-survivors were more frequently diagnosed with subarachnoid hemorrhage (p = 0.005) and intracerebral hematoma (p = 0.012).
Univariate and multivariable logistic regression analysis of risk factors for in-hospital mortality of included TBI patients
Results of univariate logistic regression analysis indicated that age (p < 0.001), diabetes mellitus (p = 0.016), hypertension (p = 0.004), glucose (p < 0.001), BIG score (p < 0.001), occurrence of subarachnoid hemorrhage (p = 0.005), and intracerebral hematoma (p = 0.010) were positively associated with poor outcomes of included patients (Table 2), whereas heart rate (p = 0.026), SpO2 (p = 0.015), hemoglobin (p < 0.001), and platelet (p = 0.046) were inversely related with poor in-hospital outcomes. All variables were then included in multivariable analysis. After adjusting confounded factors, age (p < 0.001), SpO2 (p = 0.032), glucose (p = 0.015), hemoglobin (p = 0.047), BIG score (p < 0.001), subarachnoid hemorrhage (p = 0.013), and intracerebral hematoma (p = 0.001) were still correlated with in-hospital mortality of included TBI patients.
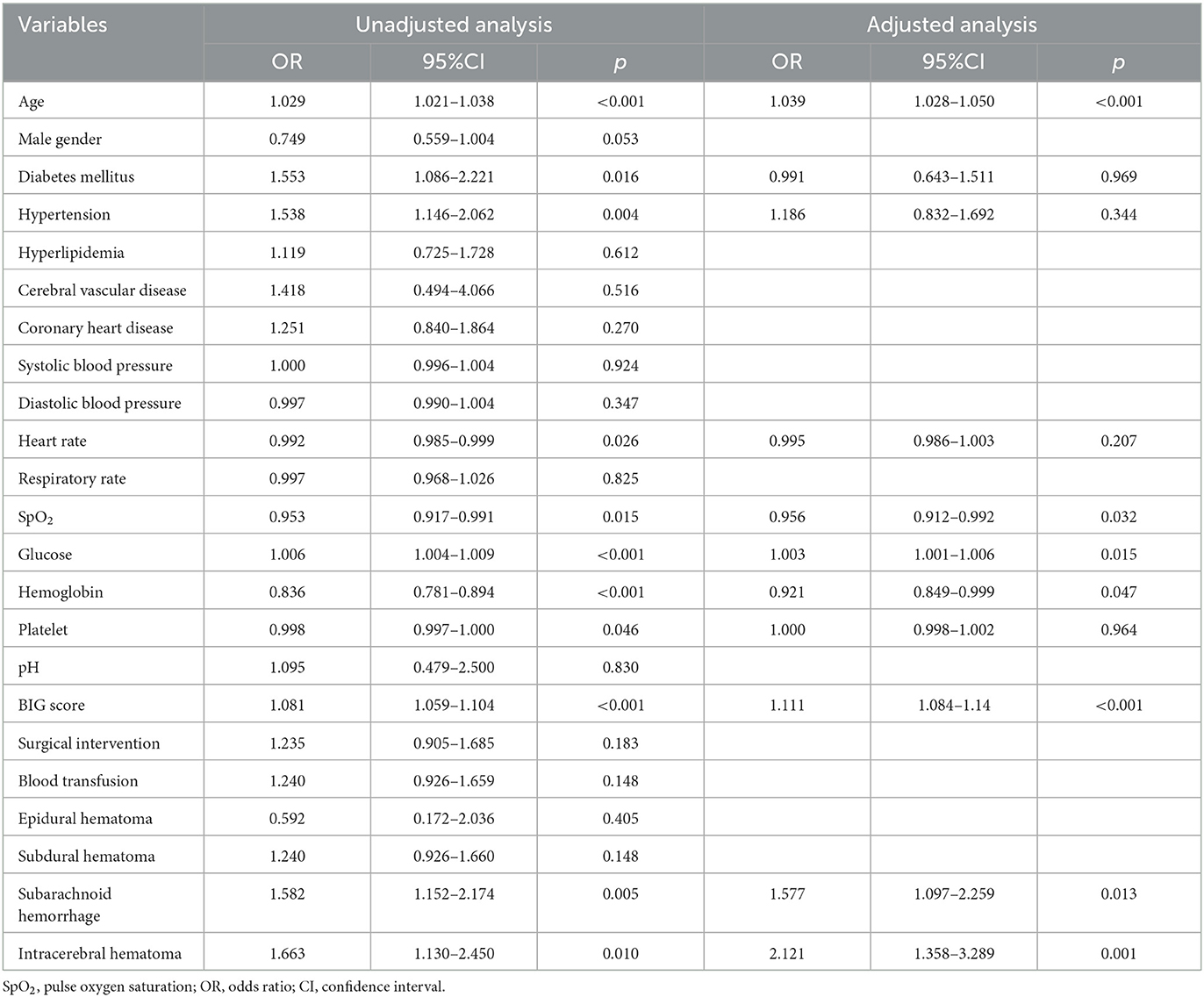
Table 2. Univariate and multivariable analysis of risk factors for in-hospital mortality in included TBI patients.
Construction of the age-adjusted BIG score and multi-factor prognostic model
Multivariable logistic regression was utilized to construct an age-adjusted BIG score. Utilizing regression coefficients of age and BIG score, we calculated the age-adjusted BIG score by 0.38 × age + BIG score for convenient application. Then, a multi-factor prognostic model was also constructed by multivariable logistic regression using the abovementioned seven significant factors, which were age, SpO2, glucose, hemoglobin, BIG score, subarachnoid hemorrhage, and intracerebral hematoma. The nomogram of this multi-factor prognostic model was drawn to evaluate its accuracy (Figure 2A). The calibration plot showed good consistency between the actual probability and predicted probability of in-hospital mortality (Figure 2B).
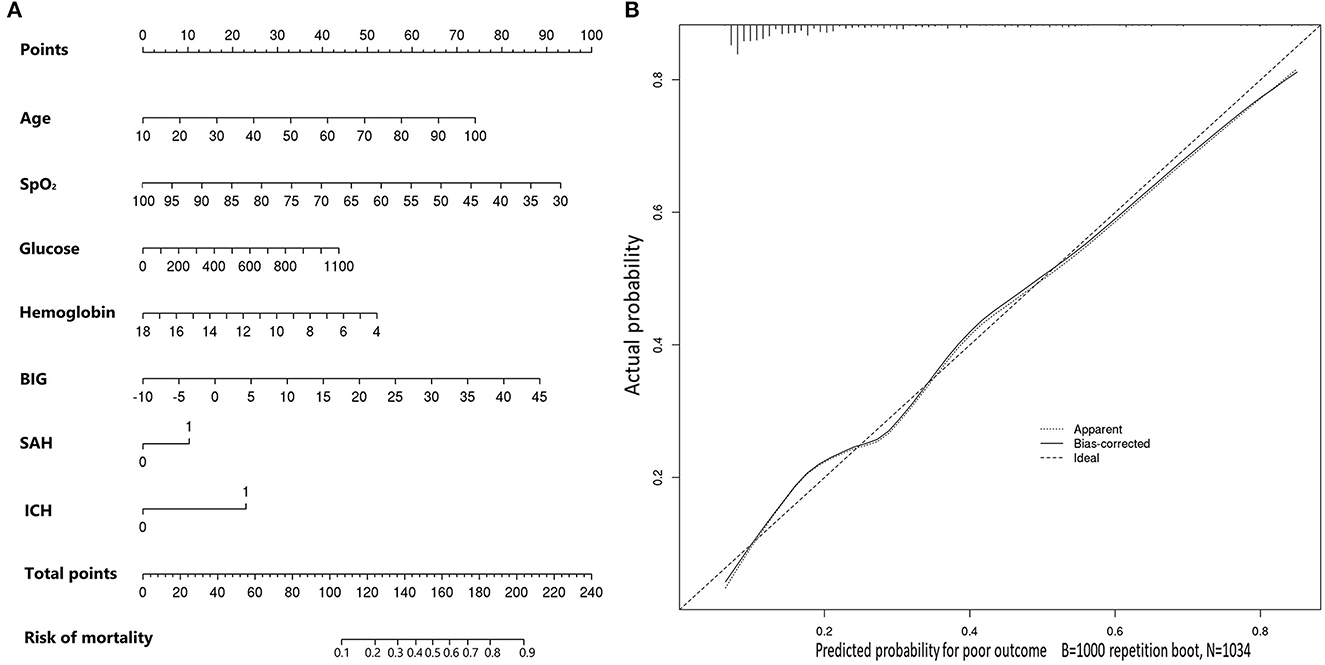
Figure 2. (A) Nomogram of the constructed prognostic model for predicting in-hospital mortality in included TBI patients. (B) Calibration plot of the constructed prognostic model for predicting in-hospital mortality in included TBI patients.
Comparison of prognostic values between BIG score, age-adjusted BIG score, and the constructed prognostic model
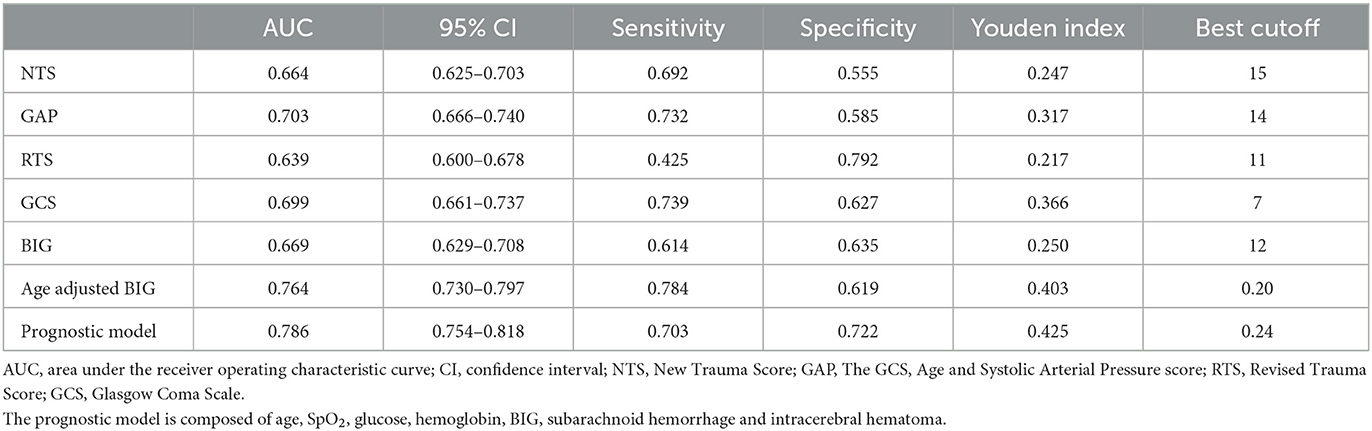
As shown in Table 3, the AUC value of single GCS and BIG scores were 0.699 and 0.669, respectively (Figure 3). The age-adjusted BIG score had higher AUC (AUC = 0.764) than GCS (Z = 15.795, p < 0.001) and BIG (Z = 5.352, p < 0.001). The constructed prognostic model composed of seven factors (age, SpO2, glucose, hemoglobin, BIG, subarachnoid hemorrhage, and intracerebral hematoma) had the highest AUC (AUC = 0.786).
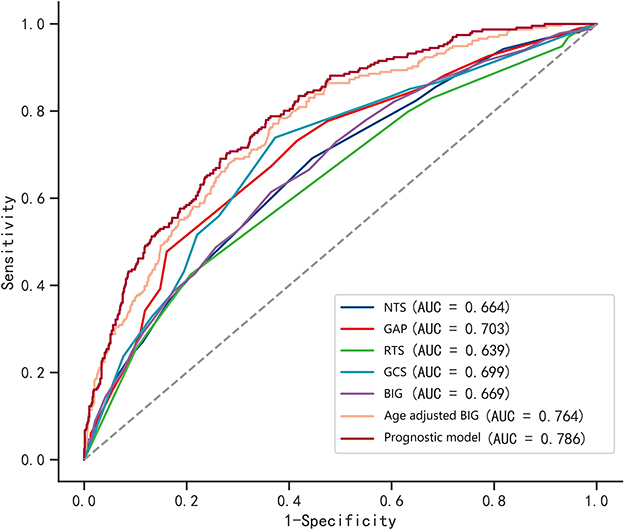
Figure 3. ROC curves of the BIG score, age-adjusted BIG score, other trauma triage scores, and constructed prognostic model. The constructed prognostic model is composed of age, SpO2, glucose, hemoglobin, BIG, subarachnoid hemorrhage, and intracerebral hematoma.
Discussion
In our study, the BIG score was not a valuable risk stratification tool for adult TBI patients. However, the age-adjusted BIG score performed well in predicting outcomes with an AUC of 0.764. The prognostic value of the age-adjusted BIG score was superior to the readily available physiological scoring system RTS. Combining four indicators of mortality (age, base deficit, international normalized ratio, and Glasgow Coma Scale) in trauma patients, the age-adjusted BIG score could comprehensively reflect the injury severity and possible progression of adult traumatic patients.
As an indicator of shock, base deficit was confirmed to be associated with injury severity and mortality in pediatric and adult trauma patients (17–20). Researchers also concluded that base deficit was a useful predictor of coagulation decompensation and shock-related complications after trauma (21, 22). One study found that an increased base deficit was associated with prolonged partial thromboplastin and prothrombin times and low protein C levels in trauma patients (23). A Evaluating level of base deficit could help physicians decide the requirements of early transfusion to avoid the development of hypoperfusion (21, 22). Maintaining appropriate blood pressure and cerebral perfusion was necessary to alleviate secondary brain injury in TBI patients. A previous study including pediatric TBI patients illustrated that patients with poor outcomes had a higher base deficit level than those with good outcomes (12). Our results were similar to the findings of a previous study that indicated that non-survivors had a higher base deficit level than survivors. Another study showed that the base deficit on admission was statistically negatively correlated with GCS and RTS in adult TBI patients (24). However, the level of base deficit did not significantly differ between survivors and non-survivors in this study. This contradictory and unconvincing result might be attributable to the small sample size of this study. Although statistically non-significant after adjusting confounders, the base deficit was significantly related to in-hospital mortality in the univariate logistic regression analysis of our study.
It was estimated that a quarter of patients with severe trauma would present an abnormal blood coagulation test on admission, which would be positively associated with poor outcomes for these patients (25, 26). As a reflection of coagulation function, INR was confirmed as an accurate predictor of mortality and organ failure in trauma patients (27, 28). Acute traumatic coagulopathy (ATC) was primarily caused by the endothelial activation of the protein C pathway, which was induced by tissue injury and hypoperfusion (29, 30). Other factors such as resuscitation-induced hemodilution, hypothermia, and acidosis would aggravate the ATC (26, 31). The prevalence of coagulopathy after TBI had been reported as ranging from 7 to 63%. The huge discrepancy might be attributable to the different definitions of coagulopathy and heterogeneous populations (32–35). Specifically, the TBI itself was independently correlated with the development of coagulopathy due to the excessive fibrinolysis caused by extensive tissue factor release from the injured brain (36, 37). Coagulopathy was acknowledged as an independent risk factor of mortality and neurological outcomes in TBI patients (38–41). Poor coagulation function could increase the potential of intracranial hemorrhage, extracranial hemorrhage, and secondary neuronal loss (26, 36). Some studies indicated that incorporating results of the coagulation test including INR could improve the value of the conventional TBI prognostic model (42, 43). Therefore, the BIG score incorporating INR could reflect the severity and possible progression of TBI patients more comprehensively.
GCS, which has been widely used for nearly five decades, is an indicator of brain injury severity and cerebral perfusion. However, the classic GCS could not be accurately evaluated in intubated, sedated, and intoxicated patients (44, 45). The comparison of the AUC value between the GCS score alone and our age-adjusted BIG score showed that incorporating base deficit, INR, and age into GCS could improve the prognostic value and stability of clinical use in TBI patients. The multivariable prognostic model we constructed was composed of seven factors, namely, age, SpO2, glucose, hemoglobin, BIG score, subarachnoid hemorrhage, and intracerebral hematoma. Although this model had a significantly higher AUC value than the age-adjusted BIG score, its evaluation was much more complex than the age-adjusted BIG score, which makes it more applicable in hospitalization but not in the emergency department. Instead, the simplicity and easy availability of the age-adjusted BIG score allow it to be quickly evaluated without the consideration of additional factors. This advantage is significant for physicians carrying out patient triage and providing intensive medical therapy for potentially high-risk TBI patients in the early stage after injury. Therefore, the age-adjusted BIG score has been specially applied by emergency department workers.
There were several limitations in this study. First, most of the included patients were those who received treatment in the ICU of a single medical center. Patients with mild TBI might not be included in this study. Nearly half of TBI patients in the database who did not meet inclusion criteria were excluded. Therefore, selection bias could not be avoided. The exact predictive value of the age-adjusted BIG score and the constructed prognostic model should be externally verified by a prospective study in other medical centers. Second, the predictive value of the age-adjusted BIG score was not specifically analyzed in subgroups of included TBI patients, such as patients with a penetrating injury or blunt injury. A previous study showed that the BIG score was more valuable in predicting outcomes of penetrating trauma patients than blunt trauma patients (14). Third, the level of base deficit and INR in admission could be influenced by pre-hospital intubation and resuscitation. Records of these two variables were not collected in the present study. Finally, in addition to RTS, other complex trauma scores such as ISS and TRISS were not evaluated in the present study. A study comparing the predictive value between age-adjusted BIG scores and these scores is worthwhile to conduct in the future. Despite these limitations, the readily available age-adjusted BIG score is more efficient than other complex scores in patient triage and treatment decisions in the early stage after brain injury.
Conclusion
As a pediatric trauma score, the BIG score is not applicable to adult TBI patients. However, the age-adjusted BIG score is a readily available and effective score that is beneficial for clinicians to triage adult TBI patients and evaluate possible progression in the early stage after injury. The prognostic model incorporating the BIG score has a better predictive value and could be used in TBI patients during hospitalization.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the West China Hospital of Sichuan University (NO: 2021-1684). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this observational study was performed using data from the Multiparameter Intelligent Monitoring in Intensive Care Database III (MIMIC-III database). All data of participants in this public database were deidentified and anonymized.
Author contributions
XB: Conceptualization, Data curation, Methodology, Resources, Software, Validation, Writing—original draft, Writing—review & editing. RW: Conceptualization, Data curation, Methodology, Resources, Software, Validation, Writing—original draft, Writing—review & editing. CZ: Investigation, Methodology, Project administration, Writing—original draft. DW: Formal analysis, Methodology, Resources, Supervision, Validation, Writing—review & editing. LM: Data curation, Formal analysis, Methodology, Resources, Supervision, Visualization, Writing—review & editing. MH: Data curation, Formal analysis, Methodology, Supervision, Validation, Visualization, Writing—review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Natural Science Foundation of China (Grant Number 82201450).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. (2018) 130:1080–97. doi: 10.3171/2017.10.JNS17352
2. Champion HR, Sacco WJ, Carnazzo AJ, Copes W, Fouty WJ. Trauma score. Crit Care Med. (1981) 9:672–6. doi: 10.1097/00003246-198109000-00015
3. Baker SP, O'Neill B, Haddon W Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. (1974) 14:187–96. doi: 10.1097/00005373-197403000-00001
4. Boyd CR, Tolson MA, Copes WS. Evaluating trauma care: the TRISS method. Trauma Score Inj Severity Score. (1987) 27:370–8. doi: 10.1097/00005373-198704000-00005
5. Champion HR, Sacco WJ, Hannan DS, Lepper RL, Atzinger ES, Copes WS, et al. Assessment of injury severity: the triage index. Crit Care Med. (1980) 8:201–8. doi: 10.1097/00003246-198004000-00001
6. Perel P, Arango M, Clayton T, Edwards P, Komolafe E, Poccock S, et al. Predicting outcome after traumatic brain injury: practical prognostic models based on large cohort of international patients. BMJ. (2008) 336:425–9. doi: 10.1136/bmj.39461.643438.25
7. Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. (2008) 5:e165. doi: 10.1371/journal.pmed.0050165
8. Borgman MA, Maegele M, Wade CE, Blackbourne LH, Spinella PC. Pediatric trauma BIG score: predicting mortality in children after military and civilian trauma. Pediatrics. (2011) 127:e892–7. doi: 10.1542/peds.2010-2439
9. El-Gamasy MA, Elezz AA, Basuni AS, Elrazek ME. Pediatric trauma BIG score: predicting mortality in polytraumatized pediatric patients. Ind J Crit Care Med. (2016) 20:640–6. doi: 10.4103/0972-5229.194011
10. Muisyo T, Bernardo EO, Camazine M, Colvin R, Thomas KA, Borgman MA, et al. Mortality prediction in pediatric trauma. J Pediatr Surg. (2019) 54:1613–6. doi: 10.1016/j.jpedsurg.2018.08.045
11. Grandjean-Blanchet C, Emeriaud G, Beaudin M, Gravel J. Retrospective evaluation of the BIG score to predict mortality in pediatric blunt trauma. CJEM. (2018) 20:592–9. doi: 10.1017/cem.2017.379
12. Davis AL, Hochstadter E, Daya T, Kulkarni AV, Wales P, Stephens D, et al. The base deficit, international normalized ratio, and Glasgow Coma Scale (BIG) score, and functional outcome at hospital discharge in children with traumatic brain injury. Pediatr Crit Care Med. (2019) 20:970–9. doi: 10.1097/PCC.0000000000002050
13. Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, et al. Causes of death in U.S. special operations forces in the global war on terrorism: 2001-2004. Ann Surg. (2007) 245:986–91. doi: 10.1097/01.sla.0000259433.03754.98
14. Brockamp T, Maegele M, Gaarder C, Goslings JC, Cohen MJ, Lefering R, et al. Comparison of the predictive performance of the BIG, TRISS, and PS09 score in an adult trauma population derived from multiple international trauma registries. Crit Care. (2013) 17:R134. doi: 10.1186/cc12813
15. Jeong JH, Park YJ, Kim DH, Kim TY, Kang C, Lee SH, et al. The new trauma score (NTS): a modification of the revised trauma score for better trauma mortality prediction. BMC Surg. (2017) 17:77. doi: 10.1186/s12893-017-0272-4
16. Hasler RM, Mealing N, Rothen HU, Coslovsky M, Lecky F, Jüni P. Validation and reclassification of MGAP and GAP in hospital settings using data from the Trauma Audit and Research Network. J Trauma Acute Care Surg. (2014) 77:757–63. doi: 10.1097/TA.0000000000000452
17. Randolph LC, Takacs M, Davis KA. Resuscitation in the pediatric trauma population: admission base deficit remains an important prognostic indicator. J Trauma. (2002) 53:838–42. doi: 10.1097/00005373-200211000-00006
18. Hindy-François C, Meyer P, Blanot S, Marqué S, Sabourdin N, Carli P, et al. Admission base deficit as a long-term prognostic factor in severe pediatric trauma patients. J Trauma. (2009) 67:1272–7. doi: 10.1097/TA.0b013e31819db828
19. Kincaid EH, Chang MC, Letton RW, Chen JG, Meredith JW. Admission base deficit in pediatric trauma: a study using the National Trauma Data Bank. J Trauma. (2001) 51:332–5. doi: 10.1097/00005373-200108000-00018
20. Hodgman EI, Morse BC, Dente CJ, Mina MJ, Shaz BH, Nicholas JM, et al. Base deficit as a marker of survival after traumatic injury: consistent across changing patient populations and resuscitation paradigms. J Trauma Acute Care Surg. (2012) 72:844–51. doi: 10.1097/TA.0b013e31824ef9d2
21. Rixen D, Raum M, Bouillon B, Lefering R, Neugebauer E. Base deficit development and its prognostic significance in posttrauma critical illness: an analysis by the trauma registry of the Deutsche Gesellschaft für unfallchirurgie. Shock. (2001) 15:83–9. doi: 10.1097/00024382-200115020-00001
22. Davis JW, Parks SN, Kaups KL, Gladen HE, O'Donnell-Nicol S. Admission base deficit predicts transfusion requirements and risk of complications. J Trauma. (1996) 41:769–74. doi: 10.1097/00005373-199611000-00001
23. Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. (2007) 245:812–8. doi: 10.1097/01.sla.0000256862.79374.31
24. Shallwani H, Waqas M, Waheed S, Siddiqui M, Froz A, Bari ME. Does base deficit predict mortality in patients with severe traumatic brain injury? Int J Surg. (2015) 22:125–30. doi: 10.1016/j.ijsu.2015.05.054
25. MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. (2003) 55:39–44. doi: 10.1097/01.TA.0000075338.21177.EF
26. Whittaker B, Christiaans SC, Altice JL, Chen MK, Bartolucci AA, Morgan CJ, et al. Early coagulopathy is an independent predictor of mortality in children after severe trauma. Shock. (2013) 39:421–6. doi: 10.1097/SHK.0b013e31828e08cb
27. Verma A, Kole T. International normalized ratio as a predictor of mortality in trauma patients in India. World J Emerg Med. (2014) 5:192–5. doi: 10.5847/wjem.j.issn.1920-8642.2014.03.006
28. Peltan ID, Vande Vusse LK, Maier RV, Watkins TR. An international normalized ratio-based definition of acute traumatic coagulopathy is associated with mortality, venous thromboembolism, and multiple organ failure after injury. Crit Care Med. (2015) 43:1429–38. doi: 10.1097/CCM.0000000000000981
29. Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, Kluger Y, et al. The coagulopathy of trauma: a review of mechanisms. J Trauma. (2008) 65:748–54. doi: 10.1097/TA.0b013e3181877a9c
30. Cohen MJ, Call M, Nelson M, Calfee CS, Esmon CT, Brohi K, et al. Critical role of activated protein C in early coagulopathy and later organ failure, infection and death in trauma patients. Ann Surg. (2012) 255:379–85. doi: 10.1097/SLA.0b013e318235d9e6
31. Fries D, Innerhofer P, Schobersberger W. Time for changing coagulation management in trauma-related massive bleeding. Curr Opin Anaesthesiol. (2009) 22:267–74. doi: 10.1097/ACO.0b013e32832678d9
32. Joseph B, Aziz H, Zangbar B, Kulvatunyou N, Pandit V, O'Keeffe T, et al. Acquired coagulopathy of traumatic brain injury defined by routine laboratory tests: which laboratory values matter? J Trauma Acute Care Surg. (2014) 76:121–5. doi: 10.1097/TA.0b013e3182a9cc95
33. Laroche M, Kutcher ME, Huang MC, Cohen MJ, Manley GT. Coagulopathy after traumatic brain injury. Neurosurgery. (2012) 70:1334–45. doi: 10.1227/NEU.0b013e31824d179b
34. Maegele M, Schöchl H, Menovsky T, Maréchal H, Marklund N, Buki A, et al. Coagulopathy and haemorrhagic progression in traumatic brain injury: advances in mechanisms, diagnosis, and management. Lancet Neurol. (2017) 16:630–47. doi: 10.1016/S1474-4422(17)30197-7
35. Maegele M, Aversa J, Marsee MK, McCauley R, Chitta SH, Vyakaranam S, et al. Changes in coagulation following brain injury. Semin Thromb Hemost. (2020) 46:155–66. doi: 10.1055/s-0040-1702178
36. Takayama W, Endo A, Koguchi H, Murata K, Otomo Y. Age-related differences in the impact of coagulopathy in patients with isolated traumatic brain injury: an observational cohort study. J Trauma Acute Care Surg. (2020) 89:523–8. doi: 10.21203/rs.2.19876/v1
37. Samuels JM, Moore EE, Silliman CC, Banerjee A, Cohen MJ, Ghasabyan A, et al. Severe traumatic brain injury is associated with a unique coagulopathy phenotype. J Trauma Acute Care Surg. (2019) 86:686–93. doi: 10.1097/TA.0000000000002173
38. James V, Chong SL, Shetty SS, Ong GY. Early coagulopathy in children with isolated blunt head injury is associated with mortality and poor neurological outcomes. J Neurosurg Pediatr. (2020) 25:663–9. doi: 10.3171/2019.12.PEDS19531
39. van Gent GAN, van Essen TA, Bos MHA, Cannegieter SC, van Dijck J, Peul WC. Coagulopathy after hemorrhagic traumatic brain injury, an observational study of the incidence and prognosis. Acta Neurochir. (2020) 162:329–36. doi: 10.1007/s00701-019-04111-z
40. Talving P, Benfield R, Hadjizacharia P, Inaba K, Chan LS, Demetriades D. Coagulopathy in severe traumatic brain injury: a prospective study. J Trauma. (2009) 66:55–61. doi: 10.1097/TA.0b013e318190c3c0
41. Folkerson LE, Sloan D, Davis E, Kitagawa RS, Cotton BA, Holcomb JB, et al. Coagulopathy as a predictor of mortality after penetrating traumatic brain injury. Am J Emerg Med. (2018) 36:38–42. doi: 10.1016/j.ajem.2017.06.057
42. Yuan Q, Yu J, Wu X, Sun YR Li ZQ, Du ZY, et al. Prognostic value of coagulation tests for in-hospital mortality in patients with traumatic brain injury. Scand J Trauma Resusc Emerg Med. (2018) 26:3. doi: 10.1186/s13049-017-0471-0
43. Solla DJF, de Amorim RLO, Kolias AG, Hutchinson PJ, de Andrade AF, Teixeira MJ, et al. Incremental prognostic value of coagulopathy in addition to the crash score in traumatic brain injury patients. Neurocrit Care. (2020) 34:130–8. doi: 10.1007/s12028-020-00991-7
44. Ghaffarpasand F, Razmkon A, Dehghankhalili M. Glasgow Coma Scale score in pediatric patients with traumatic brain injury; limitations and reliability. Bull Emerg Trauma. (2013) 1:135–6.
Keywords: BIG score, traumatic brain injury, prognosis, trauma score, adult
Citation: Bai X, Wang R, Zhang C, Wen D, Ma L and He M (2023) The prognostic value of an age-adjusted BIG score in adult patients with traumatic brain injury. Front. Neurol. 14:1272994. doi: 10.3389/fneur.2023.1272994
Received: 05 August 2023; Accepted: 09 October 2023;
Published: 02 November 2023.
Edited by:
Jifeng Cai, Central South University, ChinaReviewed by:
Alessandro Orlando, Trauma Research LLC, United StatesTsen-Hsuan Lin, GlaxoSmithKline, United States
Copyright © 2023 Bai, Wang, Zhang, Wen, Ma and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Ma, YWxleDgwMzUwMzA1QHNjdS5lZHUuY24=; Min He, aGVtaW4xOTkxMDMwNkB3Y2hzY3UuY24=
†These authors share first authorship
 Xue Bai
Xue Bai Ruoran Wang
Ruoran Wang Cuomaoji Zhang3
Cuomaoji Zhang3 Dingke Wen
Dingke Wen Lu Ma
Lu Ma Min He
Min He