
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 13 October 2023
Sec. Neuro-Otology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1272210
Background and Aim: Hearing loss in old age is associated with cognitive decline and with depression. Our study aimed to investigate the relationship between hearing loss, cognitive decline, and secondary depressive symptoms in a sample of younger and older cochlear implant candidates with profound to severe hearing loss.
Methods: This study is part of a larger cohort study designated to provide information on baseline data before CI. Sixty-one cochlear implant candidates with hearing loss from adulthood onwards (>18 years) were enrolled in this study. All had symmetrical sensorineural hearing loss in both ears (four-frequency hearing threshold difference of no more than 20 dB, PTA). Individuals with primary affective disorders, psychosis, below-average intelligence, poor German language skills, visual impairment, and a medical diagnosis with potential impact on cognition (e.g., neurodegenerative diseases,) were excluded. Four-frequency hearing thresholds (dB, PTA, better ear) were collected. Using the Abbreviated Profile of Hearing Aid Benefit, we assessed subjective hearing in noise. Clinical and subclinical depressive symptoms were assessed with the Beck Depression Inventory (BDI II). Cognitive status was assessed with a neurocognitive test battery.
Results: Our findings revealed a significant negative association between subjective hearing in noise (APHAB subscale “Background Noise”) and BDII. However, we did not observe any link between hearing thresholds, depression, and cognition. Additionally, no differences emerged between younger (25–54 years) and older subjects (55–75 years). Unexpectedly, further unplanned analyses unveiled correlations between subjective hearing in quiet environments (APHAB) and cognitive performance [phonemic fluency (Regensburg Word Fluency), cognitive flexibility (TMTB), and nonverbal episodic memory (Nonverbal Learning Test), as well as subjective hearing of aversive/loud sounds (APHAB)], cognitive performance [semantic word fluency (RWT), and inhibition (Go/Nogo) and depression]. Duration of hearing loss and speech recognition at quiet (Freiburg Monosyllables) were not related to depression and cognitive performance.
Conclusion: Impact of hearing loss on mood and cognition appears to be independent, suggesting a relationship with distinct aspects of hearing loss. These results underscore the importance of considering not only conventional audiometric measures like hearing thresholds but also variables related to hearing abilities during verbal communication in everyday life, both in quiet and noisy settings.
The impact of hearing loss is generally underestimated. The Global Burden of Disease study (1) reveals that hearing loss ranks as the third highest cause of disability globally in terms of years of life with disability, making it the foremost sensory impairment. Moreover, in the United States, hearing loss is the third most prevalent chronic physical condition, surpassing the occurrence of diabetes and cancer (2). Notably, it predominantly affects people aged 55 and older (1). In the United States, age-related lifetime hearing loss is estimated to impose an economic burden of approximately $297,000 per affected individual on society (3). Moreover, hearing loss is associated with cognitive decline (4–15).
Aging (16–18) is also associated with cognitive decline and increasing rates of dementia (19). and sensorineural hearing loss, especially if left untreated, appears to further accelerate cognitive decline. Significant associations were found between hearing thresholds (dB PTA) and cognitive performance [Retrospective: (6, 7, 9–14)]; Prospective: (5, 15, 20); Systematic review: (7, 8).1 Starting with a subclinical hearing loss < 25 dB, audiometric hearing loss (pure tone average) was associated with cognitive decline [Subclinical hearing loss (10), mild hearing loss (11, 12), moderate and severe hearing loss (5, 21)]. Further findings suggest that with increasing age, there is a progressively greater cognitive decline from older adults with hearing loss (14, 21). However, Croll et al. did not find that hearing loss is accelerating cognitive decline in non demented older adults (15).
Recently published studies have shed light on the significance of both peripheral hearing and central auditory components for cognitive performance. Among older adults, individuals with speech recognition scores in noise below the median exhibited poorer cognitive performance and experienced accelerated cognitive decline over an average follow-up period of 3.5 years compared to those with scores above the median (20). Furthermore, speech recognition in noise in healthy, mostly elderly Chinese was associated with both cognitive performance and parahippocampal cortex (medial temporal lobe) thickness. The parahippocampal cortex was identified as a partial mediator between hearing in noise and cognition, suggesting its potential role between the two (22).
Several articles discuss possible mechanism linking adult hearing loss in older adults and cognitive decline (3, 23–30). Causal mechanisms as well as shared (common) mechanisms have been proposed. An example of a hypothesis addressing common mechanisms (“common cause”) is that age-related functional and structural changes in the brain (spontaneous neural activity and functional brain connectivity, changes in brain areas)2 account for changes in auditory processes and cognitive processes in old age (24, 29). An example of a causal hypothesis is that hearing loss is the cause of social isolation that mediates cognitive decline [(e.g., 24, 29, 34)]. Rutherford et al., Uchida et al., Slade et al., and Sharma et al. discussed the possible contribution of depression as a consequence of hearing loss to cognitive decline in older adults (25, 27, 28, 30).3
To date, there has been a lack of research investigating the potential mediating role of depression between hearing loss and cognitive performance.
In addition to hearing loss, depression itself appears to independently contribute to cognitive decline. Multiple studies have consistently demonstrated a link between depressive problems and cognitive decline (35–41). Moreover, not only the severity but also the age of onset of depressive symptoms has been found to have a negative impact on cognitive performance (35, 39, 40, 42). Notably, individuals with bipolar disorder tend to experience more cognitive difficulties than those with major depression (43). Executive functions such as attention, working memory, and cognitive flexibility are affected, as well as episodic memory and cognitive processing speed. Depression is also associated with changes in the brain including the limbic system (hippocampus, amygdala, cingulate gyrus, hypothalamus, insula), the prefrontal cortex, and the cerebellum (44–47).
Numerous recent retrospective (6, 48–57) and prospective (58) studies indicate a connection between reduced hearing and depression. Bilateral audiometric hearing loss was found to be associated with depression [Golub et al. ≥ 25 dB HL better ear, Golub et al. beginning with subclinical HL of >15 dB, Shukla et al. ≥ 25 dB HL, better ear; Chern et al. ≥ 25 dB HL, left ear and right ear (53–56)]. Additionally, it seems to increase the risk for depression, also after multivariate comparison (58). However, an older study found no association between audiometric hearing loss and depression (59).
It is important to note that the assessment of depression in various studies may vary, leading to potential confusion. Most authors (6, 48, 51, 53–57) employed validated depression questionnaires, such as the Epidemiological Studies Depression Scale-10 (60). Brewster et al. and Tsimpida et al. utilized a continuum of depressive symptoms, covering both clinical and subclinical manifestations (51, 58). On the other hand, Li et al., Golub et al., Brewster et al., Chern et al., and Shukla focused solely on clinical symptoms (48, 52–56), while Tseng et al. and Kim relied on psychiatric diagnoses of depressive disorders (49, 58).
In addition, several studies inform about associations between hearing loss, depression, and brain changes in middle-aged and older adults. Tang et al. reported that middle-aged adults with acquired sensorineural hearing loss had higher levels of depression and anxiety and lower functional connectivity between the amygdala and auditory cortex, striatum, multimodal processing areas, and fronto-parietal control areas compared with normal-hearing peers (61). Aoki et al. (62) found that moderate/severe hearing loss was associated with higher depression scores in older adults, hippocampal atrophy and a higher cortisol/dehydroepiandrosterone sulfate ratio, which indicates higher stress levels (62). Brewster et al. examined older people with untreated hearing loss and major depression according to DSM 5 (Diagnostic and Statistical Manual) (63, 64). Significant associations were found between lower speech recognition and cortical thinning in the primary and secondary auditory cortex and lower integrity of the superior longitudinal fasciculus.
There is also evidence that hearing and depression are related to each other via the neurotransmitter serotonin. Impaired serotonergic function in the auditory cortex has been found in normal-hearing adults with major depression (65). Studies in rodents show that hearing damage is associated with changes in the serotonergic system within the auditory system, but also in the hypothalamus, striatum, and frontal cortex” (66–68).
Our clinical experience and research by GBD 19 Hearing Loss Collaborators (1) confirm that hearing loss is associated with depression. Many adults with hearing loss struggle to follow conversations, especially in noisy environments or with multiple people speaking, leading to feelings of depression.
To date, only a limited number of studies have explored the potential link between depression resulting from hearing loss and cognitive decline in adults with hearing loss. One study (69) found that the cognitive decline in older adults with hearing loss, compared to normal hearing, was partially associated with the severity of depressive symptoms. Another study (70) observed that older adults with hearing loss and clinical depressive symptoms experienced greater cognitive decline over a 10-year period compared to those without depression. However, the question of whether there is a direct relationship between depression as a result of hearing loss and cognitive performance, as hypothesized in the models of Rutherford et al., Uchida et al., Sharma et al. (see above), has not yet been investigated (25, 27, 30).
Taken together, the studies published to date support the hypotheses that there are associations between adult hearing loss (peripheral and central hearing) and depression and cognitive performance, and that depression mediates between hearing loss and cognitive performance. However, some limitations of these studies should also be considered. Brewster et al. (51) and Tsimpida et al. (57) used exclusively self-reports single questions to assess hearing loss, for example “Can you hear well enough to carry on a conversation in a crowded room?” (51). Hearing loss is usually defined as a four-frequency PTA of >25 dB HL on the better ear4 [see the website of US National Institute of Deafness and other Communication Disorders (71)]. Study results indicate that the exclusive use of self-report single questions is not sufficient to detect such a defined hearing loss (72–75). None of the studies, with the exception of Tseng et al. distinguished between primary and secondary depression, i.e., depression as a presumed consequence of hearing loss (58). In addition, several studies used verbal tasks, which may have confounded the results5 (10, 12, 13, 15, 20, 70). In addition, only some of the studies excluded individuals with suspected cognitive impairment or dementia (9, 12, 13, 15, 69, 76). Very few authors (13, 15, 58, 69) provide information on the time of onset of hearing loss (e.g., congenital hearing loss, hearing loss in adulthood). Finally, it is important to remember that hearing loss can affect not only older adults but also younger adults (1). To date, however, the majority of studies have been conducted in older adults, with an average age greater than 65 years [studies conducted with younger adults with a mean age less than 65 years include (5, 9, 48, 49, 58)]. It is not known, whether cognitive decline also occurs in younger adults with hearing loss and whether hearing loss is associated with depression.
The primary objective of our study was to unravel the interrelationships between hearing ability, depressive status (including subclinical and clinical symptoms as potential consequences of hearing loss), cognitive status, and age groups (younger and older adults) in individuals with bilateral sensory onset hearing loss starting in adulthood. Specifically, we investigated whether the severity of depressive symptoms acts as a mediator of cognitive performance. Additionally, we examined potential variations between the younger and older groups regarding secondary depression, cognitive status, and the mediating role of depressive symptoms.
a. There is a correlation between hearing ability (in quiet: hearing threshold dB PTA in the better ear, and in noise: Patient Report Outcome Measure) and
i. Severity (subclinical and clinical) of secondary depressive symptoms.
ii. Cognitive performance (episodic memory, executive functioning).
b. There is a correlation between severity of depressive symptoms and cognitive performance.
c. The severity of secondary depressive symptoms mediates between hearing ability and cognitive performance.
The following applies to a–c above: At least one connection between an auditory variable, depression and a cognitive variable can be established.
a. The main hypothesis applies to both the younger and the older group (Younger 25–54 years, older 55–74 years).
b. There are less depressive symptoms in the younger group.
c. The cognitive performance is higher in the younger group.
This study is part of a larger cohort study designated to provide information on baseline data before cochlear implantation. It was conducted at Medical University of Hannover, Germany (German Hearing Center) and ENT department of University Hospital Salzburg, Austria, from August 2019 to January 2023. The project was approved by local ethics committees of Hannover (protocol number: 8419_BO_S_2019) and Salzburg (protocol number: 415-E/22489/2-2019).
We recruited cochlear implant6 candidates as study participants because they have rather homogeneous levels of hearing loss (with a focus of profound and severe hearing loss). Sixty-one study participants with ages ranging from 25 to 75 years were recruited during outpatient and inpatient care at both centers. All participants with an indication for CI (cochlear implant) surgery in at least one ear according to the Association of the Scientific Medical Societies (AWMF) guidelines for cochlear implantation (77) who provided informed consent for surgery (first CI) were invited to participate in the study if they met the following inclusion criteria: There was symmetrical sensorineural hearing loss in both ears (hearing threshold difference of no more than 20 dB). Hearing loss begins from adulthood onwards (>18 years). The four-frequency PTA (0.5, 1, 2, 4 kHz) in the contralateral ear was at least 40 dB HL. Exclusion criteria comprised unilateral hearing loss, sudden sensorineural hearing loss less than 12 months before CI surgery, blindness, regular use of anticholinergic medication, presence and/or ongoing systemic treatment of malignant disease, an existing diagnosis of primary mood disorder (F30–F39, ICD 10), current psychotic illness (F20–F29) or affective disorder with psychotic symptoms (F 30. 3, F 31.3, F 32.3, F33.3), poor German language skills and very low nonverbal IQ. Primary mood disorders develop before hearing loss and have to be excluded because they may confound cognitive outcomes. As mentioned in the introduction, all mood disorders (primary and secondary as a result of hearing loss) are associated with cognitive decline. Nonverbal IQ was assessed using the Matrizen Test, part of the Wechsler Adult Intelligence Sale—Fourth Edition [WAIS-IV (78, 79)]. If the result of the WMT reached a percentage range of less than 5 percent, the patient was excluded from the study.
The flow chart in Figure 1 shows the selection of participants. Demographic, audiometric, and clinical data of n = 61 participants [n = 17 younger group (25–54 years), n = 44 older group (55–75 years)] are listed in Table 1. Apart from a significant difference in age, there were no differences in demographic variables between the younger group and the older group. All investigations were conducted prior to surgery.
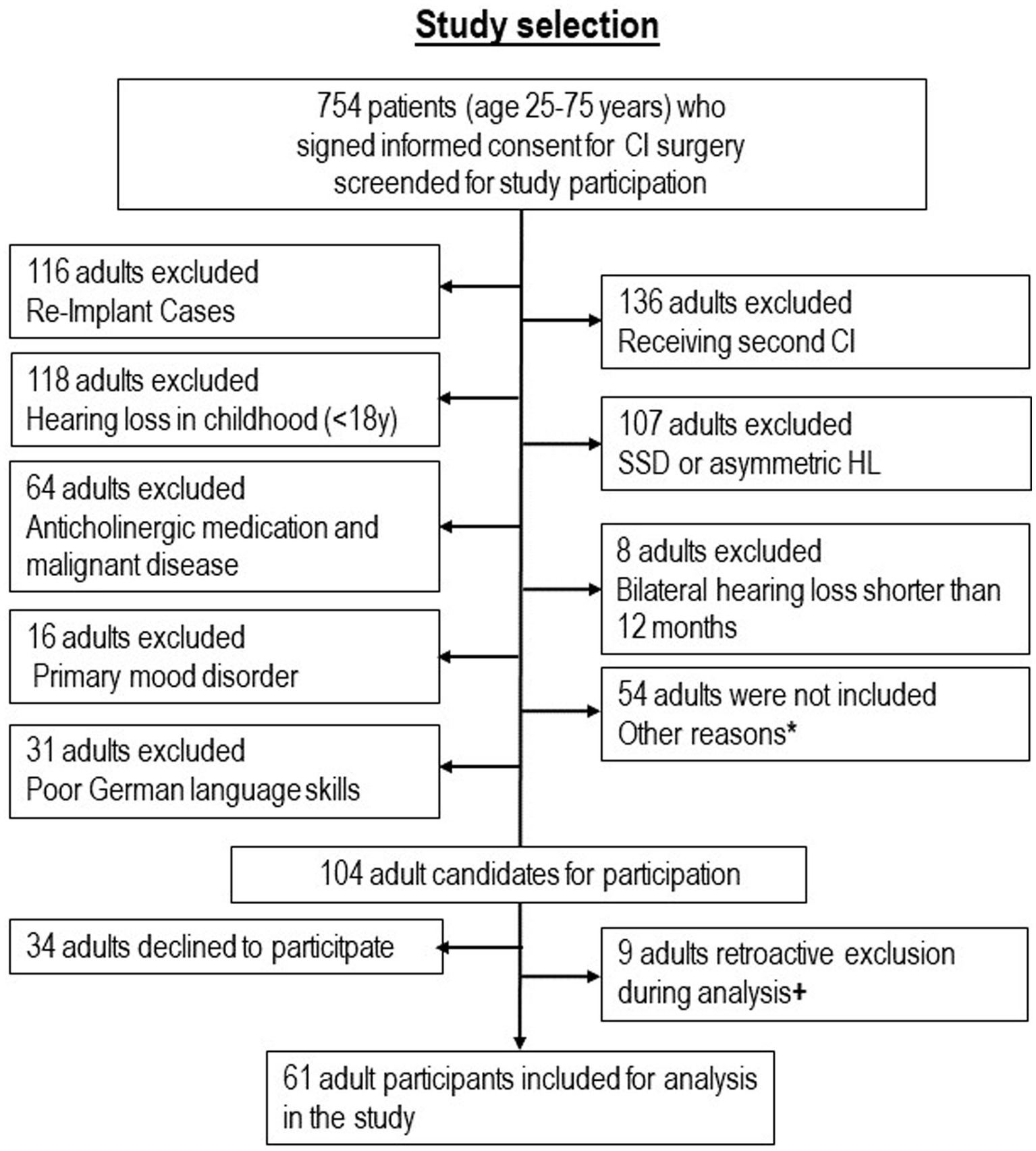
Figure 1. Selection of 61 study participants with hearing loss from adulthood. *Reasons for non-inclusion: 17 because of severe illness and replacement of one investigator, 6 because of illness and vacation of the other investigators, 25 because of logistical and organizational problems in one clinic, 6 for other reasons. + Reasons for retroactive exclusion: 3 because of hearing loss since childhood, 2 because of anticholinergic medication or malignant disease, 1 because of very low nonverbal IQ, 1 because of poor German language skills, 1 because adult was no CI candidate.
The primary outcomes of this study were possible associations between (a) hearing status and severity (subclinical and clinical) of secondary depressive symptoms in younger and older CI candidates, (b) depressive status and cognitive performance, and (c) hearing status and cognitive performance. The secondary outcomes were possible differences in these associations between the younger and older groups.
Hearing assessments were conducted by qualified audiologists in double-walled, soundproof booths, following International Electrotechnical Commission (IEC) regulations. Audiometric tests were performed using equipment that adheres to IEC 60645 standards and is calibrated based on ISO 389 1–9 guidelines. The audiometric tests included measurement of pure tone threshold, with higher thresholds [four-frequency PTA (0.5, 1, 2, 4 kHz) indicating lower hearing status].
Speech recognition was tested with the German Freiburg Numbers (recognition of 10 two-digit recorded numbers) and the Freiburg Monosyllables [recognition of 20 recorded monosyllabic words each per test series (80, 81)]. All speech recognition tests were performed with hearing aids the patients had received from their local acoustician in free-field measurement at 65 dB SPL. Consequently, these patients wore many different HighPower hearing aids. These were not fitted by us nor was any programming changed. The percentage of correctly perceived speech stimuli was documented.
We refrained from a speech recognition test in noise because clinical experience shows that many CI candidates fail a speech test in noise, with and without hearing aids (0% of speech stimuli are correctly recognized), which is due to their severe and profound hearing loss.
As a substitute, we used the “background noise” subscale (BN) of the German version of the APHAB [Abbreviated Profile of Hearing Aid Benefit (82–84)]. This patient-reported outcome measure allows assessment of hearing function in everyday situations. It consists of 24 questions with response options A–G. To analyze the outcomes the answer options A–G are assigned the numbers 1–7. The number 1 stands for pronounced difficulties or restrictions in hearing. The number 7 stands for no difficulties or good subjective hearing. There are four subscales: “Background Noise,” which assesses subjective hearing in noisy situations, “Ease of Communication,” which assesses subjective hearing when communicating in quiet situations, “Reverberation,” which assesses subjective hearing in reverberant situations, and “Aversiveness,” which assesses aversion and discomfort when hearing aversive environmental sounds. Studies show significant correlations between the APHAB total scale, the subscales EC and RV, and the PTA (85–88).
Although we only needed the results of the BN subscale, the participants were asked to answer all 24 questions of the APHAB.
The Beck’s Depression Inventory II (89–91), German version. Kühner et al. (91) consists of four questions on 21 depressive symptoms each. A score of 13 or higher (“clinical severity”) raises suspicion of a mood disorder. We documented the clinical severity and subclinical severity of depressive symptoms (score of 13 and below).
We tested visual episodic memory and the executive functions working memory, attentional control, inhibition, cognitive flexibility, and phonemic and semantic verbal fluency. We did not examine verbal episodic memory because we could not find a validated test that measures only verbal memory (and not a combination of visual and verbal element), that is suitable for younger and older people, that is also suitable for people with severe and profound hearing loss, and that also has a German version.
We used the short form of the Non Verbal Learning Test (NVLT), a German test of visual episodic memory based on Kimura’s Recurring Figures Test, which is also available in computer form (92–95). We used the computer form. On the screen, the subject is presented with a series of geometrically shaped or irregularly shaped figures in succession (120 in the short form). For each figure, a decision must be made whether it has been seen before during the test or whether it is being presented for the first time. During the test, 8 of the items shown are repeated a total of five times. Both the number of correct positive answers and the number of false positive answers are evaluated. The difference between correct and false positive answers is documented.
An n-back test was used to test attention and working memory. This test goes back to Kirchner et al. (96) and tests (executive functions) control of information flow “active maintenance,” Cohen et al. (97) and the ability to continuously update information. We used the computer version of TAP (98). In this task, participants are presented with numbers on the screen in rapid succession. The task is to determine with a mouse click whether the currently presented number is identical to the previously presented number (n = 1), to the previous number (n = 2), or even to a previous number (n = 3). Number of omissions and the number of errors are documented.
To test focused attention and inhibition the Go/NoGo Test was used, developed by Maquire et al. (99). Again, we used the computer version of the TAP (98). In this task, a response “triggered by an external stimulus” must be suppressed in favor “of an internally controlled behavioral response” (Testmanual TAP). In this task, either an x or a + appears on a dark screen. The test person should only press a button when an x is displayed and suppress the impulse to react when a + is displayed. Number of errors and reaction time are documented.
To test cognitive flexibility of executive function, the Trail Making Test B (TMTB) was used (100–102). In this paper-based test, numbers and letters are to be connected alternately in ascending order as fast as possible. Number of errors and reaction time are documented.
The Regensburg Word Test (103) was used to test phonemic and semantic word fluency [(e.g., 104)]. Participants are given the task of producing as many unique words as possible in a given time that begin with a specific letter (e.g., S; phonemic fluency), or belong to one of a specific category (e.g., fruit). The number of words produced correctly was documented.
All cognitive tests were presented visually. Written test instructions were provided for all tests. For communication during test administration (e.g., transitioning from one test to another), there was a template for communication that all investigators had to follow. In Salzburg, the tests were administered by clinical psychologists. In Hannover, the tests were administered by clinical staff who were experienced in communicating with individuals with hearing loss. Since the TAP tests may only be administered by psychologists (see German manual), they were trained by psychologists and then supervised.
To identify potential confounders such as comorbidities or other circumstances and to check the inclusion and exclusion criteria, all participants were examined by medical professionals before the audiological, cognitive and psychological investigations, which also included the general ENT status. Potential exclusion criteria were verified systematically with the help of a self-developed questionnaire, addressing predefined exclusion criteria as mentioned earlier. This questionnaire was filled out by each potential participant before the medical interview and subsequently controlled by medical staff during the examination.
Since cochlear implantation is not a life-saving procedure, patient volumes at the Hannover and Salzburg clinics declined by more than 50 percent during the COVID-19 pandemic compared to the years before the pandemic. Therefore, the study was extended by 1 year. In Salzburg and in Hannover, the assessments were performed in well-ventilated rooms and partition walls made of Plexiglas between the tester and the participant were used. In Salzburg, the NVLT (Non-verbal Learning Test) was performed on a PC (there was no extra laptop as in Hannover). Since no separation was possible in this test situation, FFP2 masks were worn by the investigators (please note that all test instructions were presented also visually). All participants were required to wear FFP2 masks. As soon as corona testing was available, all patients were tested for corona on admission to the ward.
Statistical analysis was carried out in IBM SPSS Statistics Version 27. Pearson correlations were used in order to assess (i) associations between hearing ability and cognitive functions, (ii) hearing ability and depression, and (iii) depression and cognitive functions. For mediation analyses were planned to use the mediate function in the mediation package of statistics software R. 4.2.3. In order to assess, whether the correlations addressed in (i)–(iii) differed significantly between the younger and older group, linear multiple regressions were used including age group as a moderator. For cognitive functions, age-adjusted T-values were used as performance estimates. p-values were FDR-corrected for multiple comparisons. All missing dada were documented.
Four-frequency hearing thresholds (PTA) in the better ear were significantly negatively related to subjective hearing ability in noise as assessed with the APHAB (r = −0.31, p = 0.02). Participants with objectively worse hearing, i.e., higher PTA, had significantly worse subjective hearing in noise, i.e., lower APHAB scores. Hearing thresholds were furthermore significantly related to subjective hearing in reverberant surroundings (r = −0.30, p = 0.02), but not to subjective hearing in quiet [“Ease of communication” (r = 0.08, p = 0.56) and also not with subjective hearing of aversive and loud sounds (“Aversiveness”; r = −0.13, p = 0.30)].
As for possible associations between hearing and depression, we found no significant association between four-frequency hearing thresholds (PTA) and BDI II (Beck’s Depression Inventory) results (r = 0.08, 95% CI = [−0.18, 0.33], pFDR = 0.54). Subjective hearing in noise as assessed with the Background Noise subscale (APHAB) was significantly negatively related to depressive symptoms as assessed with the BDI II (r = −0.30, 95% CI [−0.51, −0.052], pFDR = 0.04; see Figure 2): The better the subjective hearing in noise, the less severe are the depressive symptoms.

Figure 2. Association between subjective hearing ability in noise (Background Noise, Abbreviated Profile of Hearing Aid Benefit) with severity of depressive symptoms (Becks Depression Inventory II). APHAB: Higher scores indicate better subjective hearing. BDI II: Higher scores indicate more depression. Solid regression line: Total group. Orange dots: Younger group (n = 17), gray dots: Older group (n = 44).
As for possible associations between hearing and cognitive performance, neither objective hearing ability, as assessed with audiometric testing (PTA, the better ear), nor subjective hearing ability in background noise (BN) were significantly related to any cognitive variables (all │r│< 0.17, all p > 0.20).
Furthermore, severity of depressive symptoms was not related to any cognitive variables (Clinical and subclinical cases (n = 61), all │r│< 0.23, all p > 0.07; clinical cases only, i.e., those that are at or above the cut-off value of 13 (n = 21), all │r│ < 0.24, all p > 0.29).
Given that no association between depressive symptoms and cognitive variables was observed, and that different aspects of hearing ability related to depressive symptoms and cognitive variables, no mediation analysis was performed.
The associations between subjective hearing in noise (BN) and severity of depressive symptoms (BDI II) were more pronounced in the younger group (r = −0.44, p = 0.07), than in the older group (r = −0.27, p = 0.08), though the difference in the associations between groups were not significant (β = 0.34, t = 1.34, p = 0.19, compare Figure 2). Associations between BN and cognitive performance did not differ significantly between the older and the younger group (all |β| < 0.57, all |t| < 1.87, all p > 0.07; compare Table 2). Associations between the BDI II results and cognitive variables were non-significant in both the younger and older group (all │r│ < 0.38, all p > 0.06).
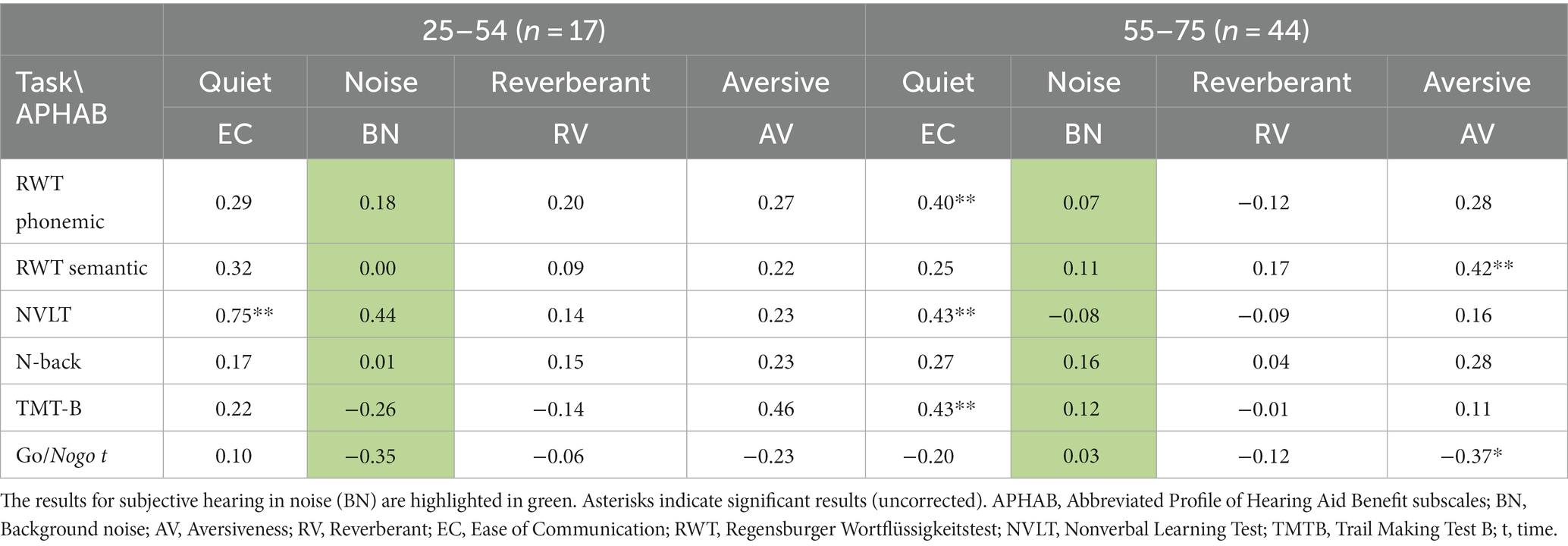
Table 2. Comparison between younger group and older group of CI-candidates, Pearson correlations between subjective hearing ability as assessed with the APHAB and cognitive variables, separately by group.
We also examined possible differences between the younger and older groups in BDI-II scores and cognitive performance. There was no significant difference between the two groups in the severity of depressive symptoms (BDI II, see Table 3). As for cognitive performance, the TMT-B performance was significantly reduced in the older age group compared to the younger age group. However, after correction for multiple comparisons the difference was no longer significant (compare Table 3). No significant difference was found between the younger and older groups on the other cognitive tests (Table 3).
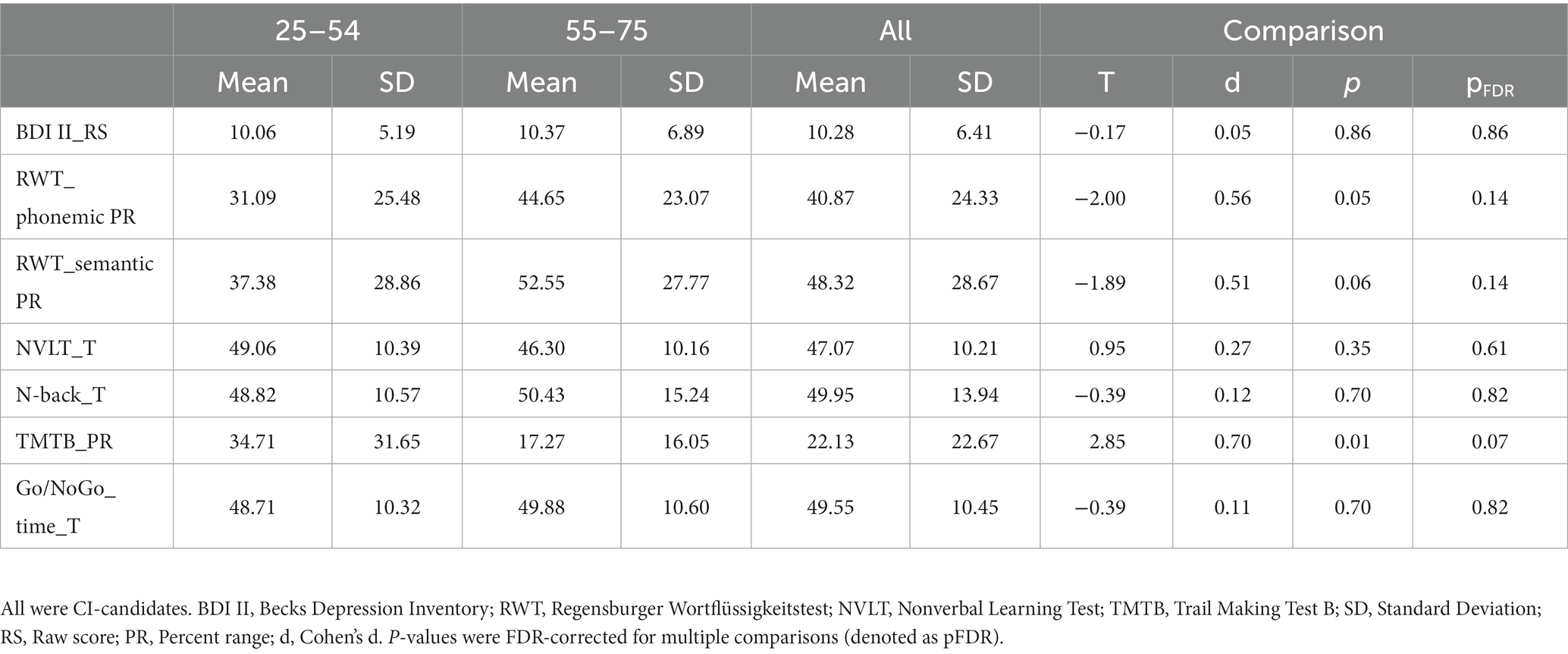
Table 3. Cognitive performance in younger adults (n = 17) and older adults (n = 44) with hearing loss.
In exploratory analyses, we looked for possible associations between additional hearing variables, depression (BDI II), and cognitive variables. Additional hearing variables included the remaining three subscales of the APHAB Ease of Communication (EC), Reverberant (RV) and Aversiveness (AV) as well as speech recognition in quiet (Freiburg Monosyllables) and duration of hearing loss (Table 1).
As for additional hearing variables and depression, APHAP subjective hearing in quiet (EC), or in reverberant surroundings (RV) were not associated with depressive symptoms (both │r│ < 0.17, both p > 0.19), while subjective hearing of aversive and loud sounds (AV) was also significantly negatively related to depressive symptoms (r = −0.31, p = 0.02). The duration of hearing loss and speech recognition were not significantly related to depression (duration: r = 0.03, p = 0.85; monosyllables: (all │r│ < 0.09, all p > 0.48) compare Figure 3).
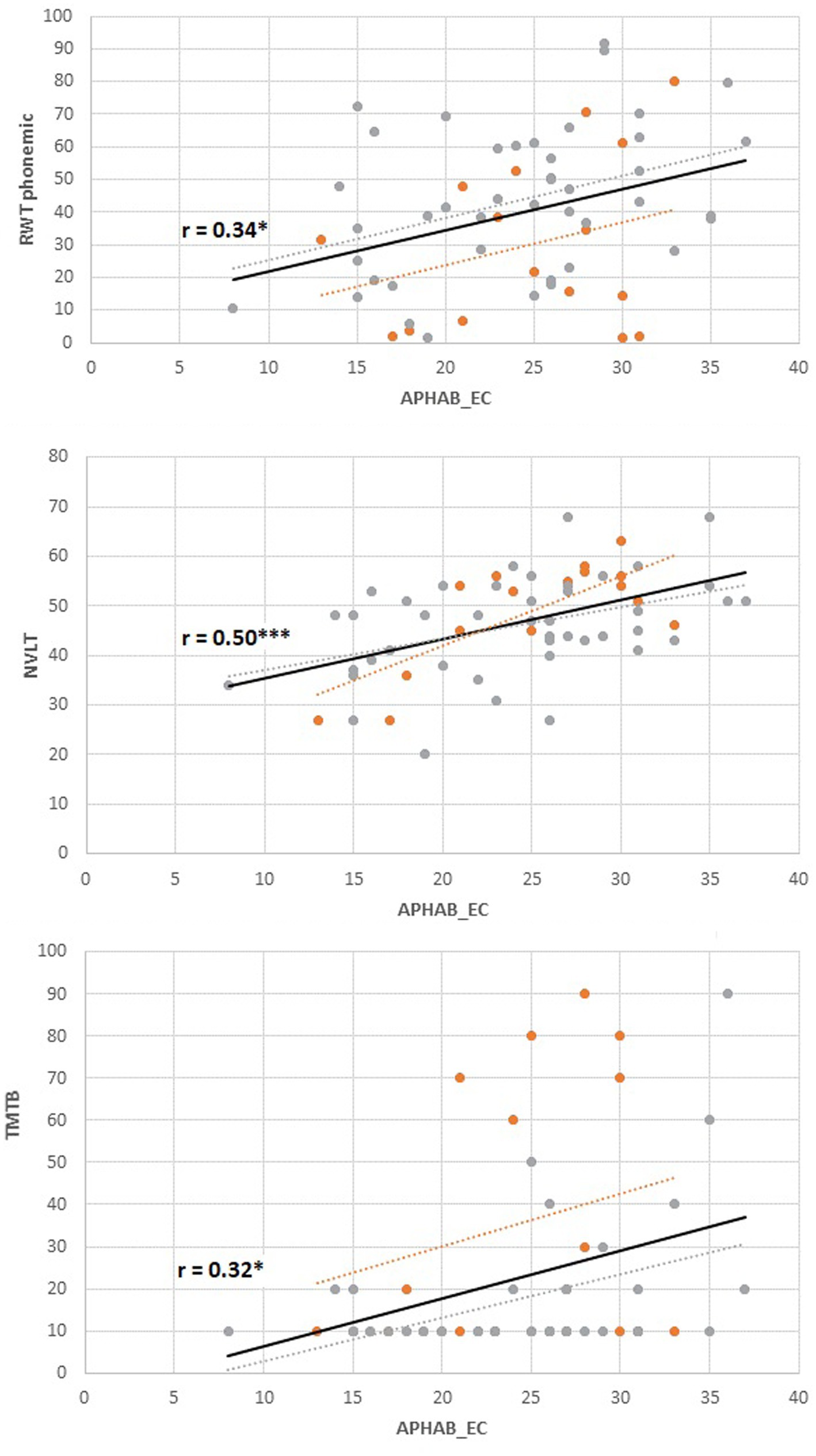
Figure 3. Associations between subjective hearing ability in quiet (Ease of Communication, APHAB) and cognitive performance. Orange dots: younger group (n = 17), gray dots; older group (n = 44). Solid regression line: Total group. RWT, Regensburger Wortflüssigkeit, phonemic fluency, percent range; NVLT, Non Verbal Learning Test, T-scores; TMT-B, Trail Making Test B, percent range; APHAB, Higher scores indicate better subjective hearing; Cognitive Tests, Higher scores indicate better results.
As for additional hearing variables and cognitive variables, APHAB subjective hearing in quiet (EC) was significantly positively associated with several cognitive variables (including phonemic fluency as assessed with the RWT (r = 0.34, pFDR = 0.03), nonverbal visual memory as assessed with the NVLT (r = 0.50, pFDR < 0.001), as well as the TMTB (r = 0.32, pFDR = 0.03), compare Figure 3). Non-significant results were observed for semantic fluency as assessed with the RWT (r = 0.24, pFDR = 0.09), the nback (r = 0.24, pFDR = 0.09) and Go/NoGo-Task (r = −0.14, pFDR = 0.31). APHAB Subjective hearing of aversive sounds (AV) was significantly associated with semantic fluency (r = 0.35, pFDR = 0.03) and GoNoGo time (r = −0.34, pFDR = 0.03), but non-significantly to phonemic fluency (r = 0.25, pFDR = 0.07), nonverbal memory (r = 0.17, pFDR = 0.17), n-back performance (r = 0.27, pFDR = 0.07) and TMTB performance (r = 0.22, pFDR = 0.12). APHAB subjective hearing in reverberant surroundings (RE) was not significantly related to any cognitive variables (all │r│ < 0.17, all p > 0.19). Although some correlations appear numerically larger in the older group, associations did not differ significantly between the older and the younger group (all |β| < 0.45, all |t| < 1.75, all p > 0.08; compare Table 2). Duration of hearing loss was not related to any cognitive variable (all │r│< 0.21, all p > 0.10). None of the cognitive variables were related to speech test results in quiet (Freiburg Monosyllables; all │r│< 0.23, all p > 0.09).
The present study was designed to assess depressive symptoms as a potential mediator of cognitive impairing effects of hearing loss. In otherwise healthy adults with bilateral hearing loss, all of whom were candidates for cochlear implantation, we found no evidence of a mediating quality of secondary depressive symptoms between hearing loss and cognitive performance. We found a significant association between subjective hearing ability in noise (APHAB) and severity of depressive symptoms, but no significant associations with cognitive performance. Hearing threshold (PTA) was not associated with depression or cognitive performance (Figure 4). There was no association between depressive symptom severity and any cognitive performance. Unexpectedly, there were also no significant differences between the younger and older groups in either depressive symptom severity or cognitive performance.
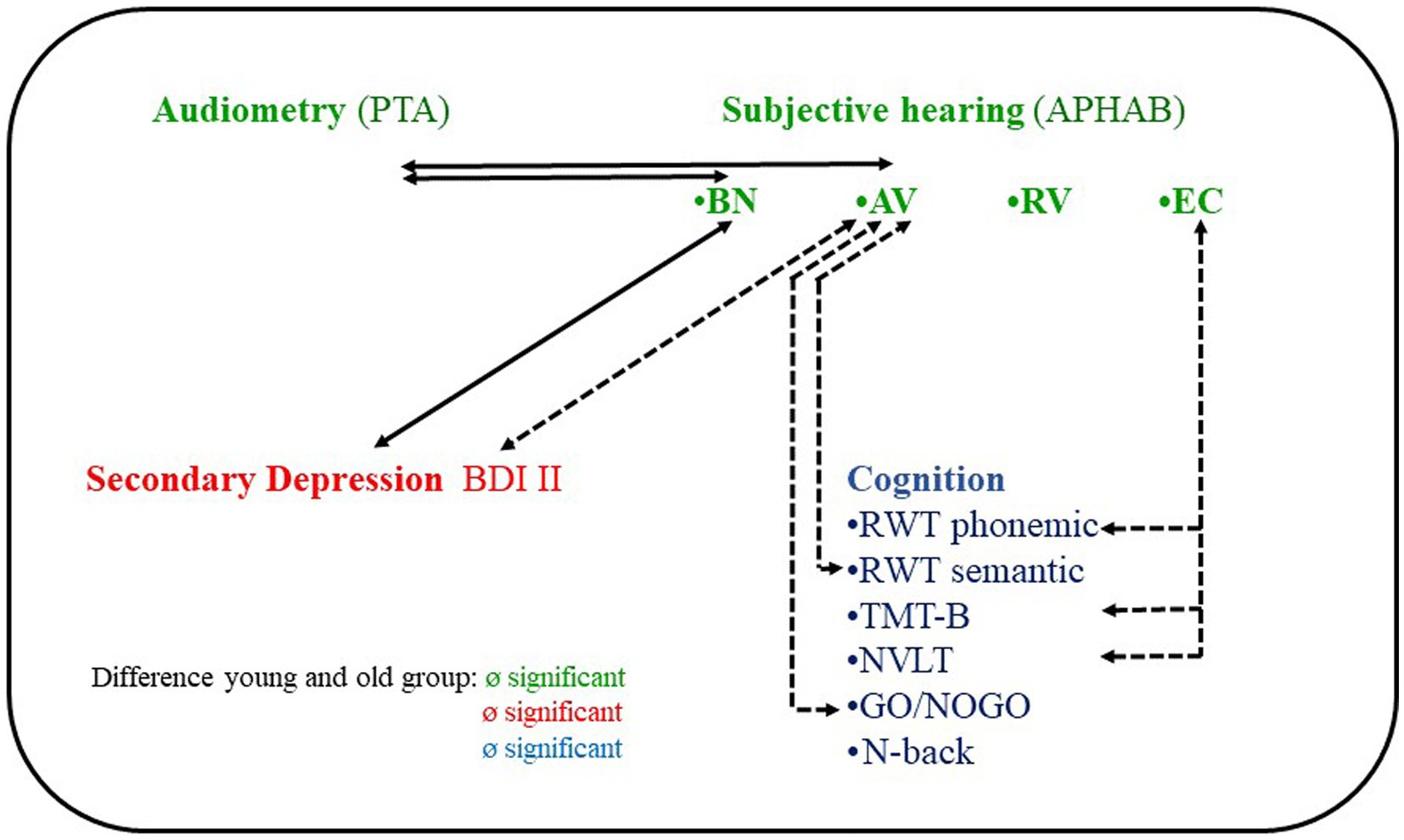
Figure 4. Correlations between hearing, depression and cognitive performance of 61 individuals with hearing loss and cochlear implant candidates. PTA, pure tone average; APHAB, Abbreviated Profile of Hearing Aid Benefit subscales; BN, Background noise; AV, Aversiveness; RV, Reverberant; EC, Ease of Communication; BDI, Becks Depression Inventory; RWT, Regensburger Wortfüssigkeitstest; TMT, Trail Making Test; NVLT, Non Verbal Learning Test.
To our knowledge, the present study was the first to examine the mediator quality of depression in both older people and younger people with hearing loss.
As expected, subjective APHAB hearing in noise was significantly related to the severity of secondary depressive symptoms, result of BDI II.
Speech understanding performance in noise depends on complex central processing and cognitive functions (105–110). Studies on adults without hearing loss suggest that depression may be associated with hearing in noise. For example, Xie et al. found that speech recognition in noise (talker masker) was associated with more errors and higher speech recognition accuracy in young normal hearing adults with major depressive disorder (111). The authors hypothesized that distractibility would be increased in individuals with depression due to speech interference in background noise. These findings were supported by behavioral neuroimaging studies that used both auditory and visual stimuli for distraction (112–115). In all studies, the authors found a higher distractibility in individuals with depressive disorders. Other studies addressed possible associations between speech recognition in noise—audiological test scores—and depressive symptom severity. Carvalho et al. found significant associations between speech perception performance in noise and depressive status in older hearing aid users (116). Heinze-Köhler et al. identified significant associations between short- and long-term speech perception and depressive status in middle-aged and older patients after cochlear implantation (117). Please note that all these studies, including the present study, are correlational studies. Therefore, the question of whether hearing disorders are the cause of depression or whether depression is the cause of hearing disorders cannot be answered.
Unexpectedly, peripheral hearing ability (PTA, the better ear) was not associated with the severity of depressive symptoms. Our results are consistent with those of Gobinath et al. (59) but not consistent with Li et al., Brewster et al., Golub et al., Golub et al., Shukla et al., Chern et al., and Tsimpida et al. (10, 48, 51–57, 118). It should be noted, however, that the nonconsistency refers to the significance of the results and not to the effect sizes. We obtained a small effect size, and this was also the case in these previous studies, with odd ratios ranging from 1.26 (52) to 1.64 (59). These previous studies, with the exception of Gobinath et al. used very large samples (ranging in size from n = 1,204 to 8,529) in which even small effect sizes became significant. With such a large sample, an │r│ of 0.08 as in the present study would most likely have become significant as well.
Furthermore, contrary to our expectations, we did not observe any significant correlation between four frequency hearing thresholds and cognitive performance. Instead, beyond the planned analyses, significant correlations were found between subjective hearing at quiet (APHAB “Ease of communication”) and cognitive performance (Figure 4, for details on the specific cognitive functions, see the discussion of our exploratory analyses below). We attribute this disparity to the fact that the APHAB “Ease of communication” captures different hearing aspects than an audiometric procedure. Hearing thresholds are determined through controlled tests in laboratory-like conditions, evaluating pure tone hearing and hearing with hearing aids without hearing aids (evaluating air conduction, bone conduction, loudness and frequency, best hearing ear)7 whereas subjective hearing in everyday situations is determined by a questionnaire. On the other hand, the APHAB subscale “Ease of communication” assesses hearing abilities in real-life verbal communication situations through a questionnaire-based approach, offering a more comprehensive representation of hearing in everyday settings. Hearing in verbal communication situations is assumed to be more cognitively demanding than hearing in audiometric tests of hearing thresholds, we believe it is not only reasonable but also clinically relevant to incorporate hearing variables that closely relate to verbal communication in daily life when investigating potential links between hearing loss and cognitive decline.
Another potentially interesting auditory variable in this context is aversion or misperception when listening to certain environmental sounds (APHAB AV subscale) (82). Beyond the planned analyses, we found significant associations between the APHAB AV and cognitive performance, which is a novel finding (Figure 4). AV differs from the EC and RV subscales in that it is not correlated with audiometric data [hearing thresholds, speech recognition (85–88)]. It also differs from EC, BN, and RV in that it does not correlate with the SSQ [Speech, Spatial, and Qualities of Hearing Scale (84)]. Possible relationships with other hearing variables for example hyperacusis8 and noise sensitivity9 has not yet been investigated.
We found no significant association between subclinical and clinical severity of depressive symptoms and cognitive performance. The results also do not support the models of Rutherford et al., Uchida et al., and Sharma et al., which hypothesize a direct relationship between depression and cognition of individuals with a hearing loss, see introduction (25, 27, 30). In addition, our findings on secondary depression in people with hearing loss are not consistent with the findings on primary depression in people without hearing loss, see introduction. Depression can be described as a result of genetic predisposition and environmental factors [(e.g., 124–126)]. We hypothesize that individuals with secondary depression caused by the critical life event of hearing loss represent a specific group that is distinct from other groups with depression.
There is a well-established consensus that both hearing loss and cognitive decline tend to increase with age. Notably, hearing loss accelerates cognitive decline in older adults, a phenomenon that also has been observed in individuals with normal hearing (14, 21). Given this relationship, one might anticipate that a younger group with hearing loss would outperform an older group with hearing loss on cognitive tests, even after accounting for age differences. However, our study’s findings did not support this hypothesis. To investigate this, we employed age norm scores, such as T scores and percentile ranks, and surprisingly, no significant difference was observed between the younger and older groups with hearing loss. These results challenge the assumption that cognitive decline solely affects older individuals with hearing loss. Instead, our findings lend support to the notion that cognitive decline can also manifest in younger individuals who experience hearing loss. Support for the validity of this hypothesis can be found in a study conducted by Kocabay et al. who compared a small group of younger adults with high-frequency hearing loss (mean age 39 years) with normal-hearing individuals (127). Moreover, it is usually assumed that older individuals are considered to be at increased risk for depression (see the website of US-National Institute on Aging). Unexpectedly, we found no significant differences between young and older groups in depressive status. Our results justify the hypothesis that depression scores are not only elevated in older people with hearing loss (69) but also in younger people.
In addition, there seems to be a widespread assumption that the longer the hearing loss, the more severe the depressive status. In our exploratory analyses, we found no significant association between the years of hearing loss and the severity of depressive symptoms. To our knowledge, there are no other studies on this topic yet. That depressive moods can also occur at any age in people with hearing loss, the use of depression questionnaires such as the BDI are recommended in clinical work.
In summary, our hypothesized model that depression mediates between hearing loss and cognition (see Figure 5A) was not supported by our results, neither in the overall group nor in the older or younger groups.
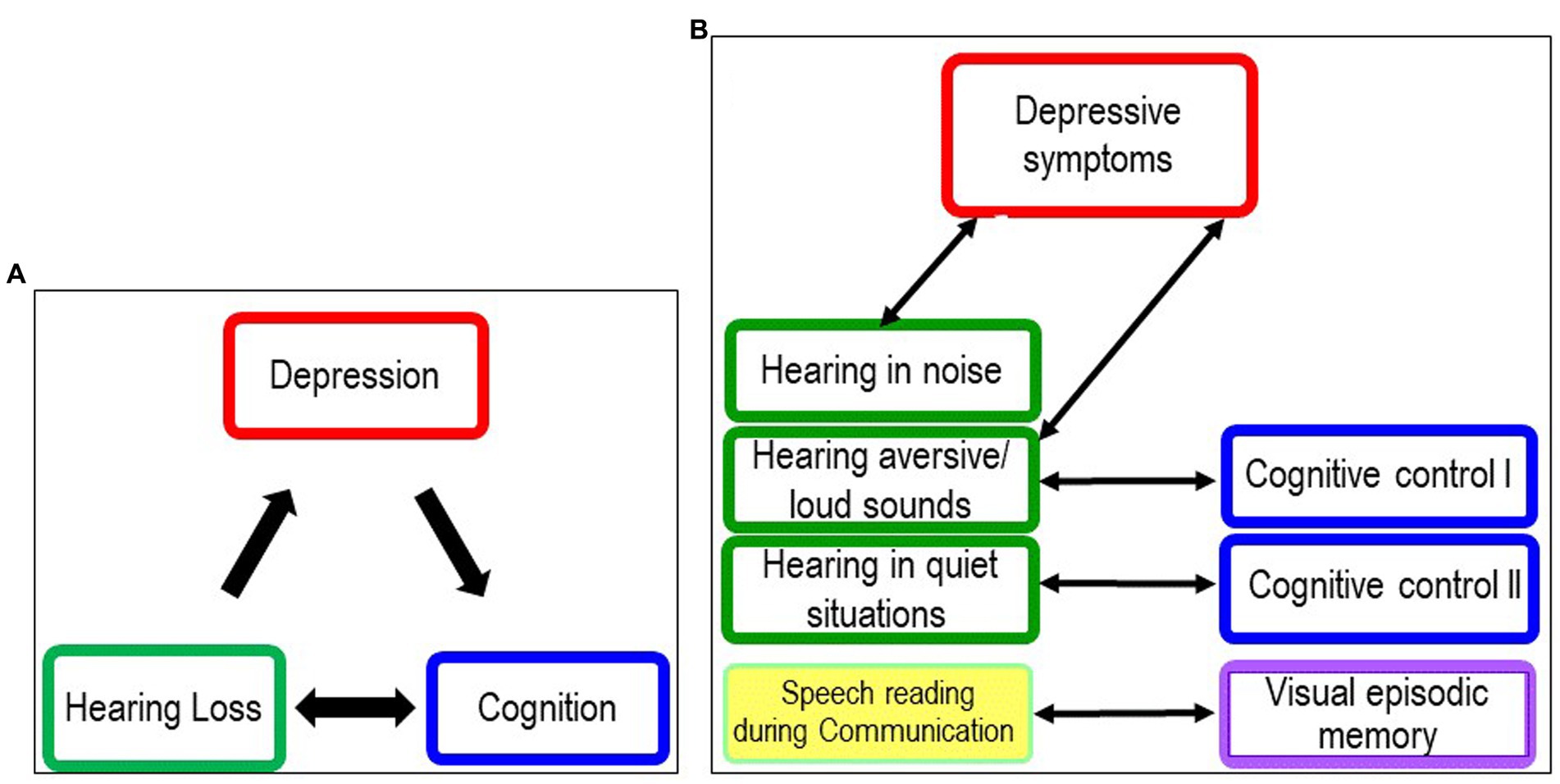
Figure 5. Models illustrating the relationships between hearing loss, depression and cognition. (A) Original model Original model at start of study. (B) New model following discussion of results of planned analyses and exploratory analyses.
Our results also seem to contrast with a recent study, finding that “depressive symptoms partially mediated the association between hearing loss and cognitive function [standardized regression B coefficient (β) = −0.114; 95% confidence interval (CI): (−0.158, −0.076)] (128). Despite this apparent inconsistency, we believe that there is basically no contradiction between the two studies. The sample of Cao’s studies was large enough (n = 8,094) to allow even small effects to become significant.10 Our studies would have needed a sample of (n = 120) to achieve an effect size of small to medium size and an even larger sample for small effect sizes.11 Therefore, the small effects found in Cao et al. (128) could not be detected in our study. Independent from this we have concerns about the validity of the measurement tools used in Cao et al. (128). The presence of hearing loss was only assessed using a single question to study participants (“Do you feel that you have difficulty hearing?”). As mentioned in the introduction, a self-report single question is insufficiently valid for assessing clinical hearing loss (72–75). Furthermore, single question data do not inform about the better and the worse ear or about unilateral deafness. No differentiation can be made between the difference between hearing in quiet and hearing in noise. Moreover, a single test, namely the MMSE (Mini Mental States Examination, Folstein et al.), was used to assess cognitive status (129). Because a substantial part of this test consists of verbal tasks, it is not a sufficiently valid instrument for assessing cognitive performance in individuals with hearing loss (see Introduction).
Because there were so many unexpected results, exploratory analyses were performed to find possible new paths for further investigation. We found that subjective hearing at quiet (APHAB subscale Ease of Communication) was significantly related to phonemic fluency (Regensburger Wortflüssigkstest), cognitive flexibility (Trail Making Test-B), and visual episodic memory (Non Verbal Learning Test). The better the hearing ability during communication in quiet situations, the higher the cognitive performance.
Fluency tests are among the tests of executive functioning or cognitive control. Studies show that these tests require not only controlled processing but also automatic processing (130–132). Previous studies showed that phonemic fluency is significantly associated with the hearing threshold of the better ear (PTA) of younger and older individuals with hearing loss (11) and that older individuals with age related hearing loss seem to have problems especially in the first more automatic part of the task (clustering) (133). The authors suggest that these problems are due to “impoverished auditory input in hearing loss,” which may “disrupt the automatic mapping of acoustic speech to representations in memory, leading to difficulties in implicit recall of such representations” (133).
Following this interpretation, we hypothesize that individuals with hearing loss in adulthood will have problems in automatically retrieving words from the lexicon.12 Furthermore, we hypothesize that impoverished auditory input is not only due to hearing loss, but also to social withdrawal and reduced verbal communication as a consequence of hearing loss.
Cognitive flexibility is defined as the ability to change “… perspectives or approaches to a problem, flexibly adjusting to new demands, rules, or priorities (as in switching between tasks)” (134).
Five of the six questions of the APHAB EC subscale refer to verbal interactions. Verbal turn taking requires a very fast switch between the speaker’s perspective and the listener’s perspective, even in a quiet situation. This is complicated by the additional task of planning a response while listening and at the same time anticipating the communication partner’s perspective (135). Additionally the listener “must predict …the end of the current speaker’s turn” to be able to begin with planning (135).
For people with hearing loss in particular this means that cognitive resources are tied up that are lacking elsewhere (136–140). For dialog situations, this likely means that they may be lacking for the cognitive flexibility tasks of perspective taking and task switching. We therefore hypothesize that cognitive load during verbal dialog (also in quiet) impairs the cognitive basis for dialog and thus dialog ability, for example by increasing reaction times when switching between listener and speaker. Furthermore, we expect that people with hearing loss will stop verbal communication when it becomes too demanding and too futile [despite effort, comprehension is no longer possible, see the model of Peele (138)], or avoid communication altogether. Studies in adults without hearing loss have shown that self-perceived stress is negatively related to cognitive control and flexibility (141).
We suspect that this correlation with NVLT is not related to hearing (we found no correlation between PTA and NVLT) but to speech reading. In our clinical experience, many patients with severe and profound hearing loss and cochlear implant candidates can read speech because they rely on visual perception (viseme) to follow a conversation. Studies show that a combination of visual and auditory speech information improves speech recognition [without hearing loss (142–144)]. Our hypothesis is that reading speech also trains visual memory. We also interpret the result as a possible indication that the APHAB subscale EC captures not only hearing during communication but also lip-reading ability.
This appears to be the first time that hearing variables other than traditional hearing thresholds and speech recognition scores have been used in a study addressing cognition in individuals with hearing loss—planned and exploratory. The exploratory part is, in our view, a strength of this study, indicating that it seems reasonable to leave the beaten path of ever-larger study samples and small effects with presumably little clinical relevance. Thus, we turned a supposed disadvantage -our hypotheses could not be confirmed in all respects into an advantage and generated a list of new possible hypotheses (see above).
There are also limitations. This study was conducted during COVID-19 times, which resulted in a decrease of more than 50% in patient volume at the Hannover and Salzburg clinics. As a further consequence, fewer study participants could be recruited than planned, so that the necessary sample size for a mediation analysis was clearly missed (n = 120 for a effect seize of an │r│ = 0.25). Extending the study period did not quite produce the expected effect because by the end of the study period, patient volume had not reached pre-Covid levels. A sample size of n = 61 may be sufficient to detect moderate to large effects in the associations between hearing and cognition, hearing and depression, and additionally depression and cognition. However, the subgroup of younger adults remained only small. The results of the younger group should therefore only be interpreted with caution.
Concerning potential selection bias, selection criteria were defined before participant enrollment and were controlled systematically by investigators. All cases of nonparticipation were documented, see flow diagram. However, it cannot be excluded that only the less anxious and less depressed patients entered the clinics during the COVID-19 pandemic. Studies report increased prevalence rates of depression and anxiety during the COVID-19 pandemic (145–147). Concerning potential performance bias, a monitor and evaluation plan was created before the investigation. All investigators of the study were monitored by the principal investigator and possible performance errors in the study performance were documented. To control potential bias during cognitive testing, see the procedure described in methods. The influence of the special precautions during the tests (methods) because of Covid is estimated to be low. In the statistics, the p’s of all outcomes in the planned analyses were controlled, as well as the p’s of the cognitive outcomes in the exploratory analyses. Note that the exploratory analyses were conducted to find new hypotheses, not to test hypotheses. Additionally, it cannot be excluded that the increased prevalence of depression and anxiety during COVID has minimally influenced the study outcomes of depression. Altogether, the risk of bias in selection, implementation, and statistics can be considered as still low.
The inclusion and exclusion criteria were quite strict in our study. Our results may not be valid for individuals with hearing loss and a suspected cognitive impairment or with multiple comorbidities. In addition, the results on hearing in noise may be valid only for those who are not remotely able (even with hearing aids) to recognize speech in background noise [four-frequency hearing threshold higher than 35 dB, better ear, best aided (1)]. Additionally cochlear implant candidates may be more engaged than individuals with similar hearing loss who have not considered cochlear implantation.
In summary, the results of the planned and exploratory analyses do not support our original model (see Figure 5A). We found no association between depressive symptoms and hearing loss in adulthood indicated by four-frequency hearing threshold (PTA) and cognitive performance in younger and older cochlear candidates. Instead, we found that subjective hearing limitation in noise was associated with higher depression scores, and subjective limitation in everyday communication was associated with lower cognitive test scores, independent of age (see Figure 5B). As discussed earlier, we hypothesize that the association between hearing in background noise and depression scores is also due to the increased distractibility of depressed patients and the stress caused by distracting information. The association between hearing in quiet and cognitive functions may be attributed to a lack of stimulation from social interactions and isolation when communication skills are affected.
For further studies, it may be useful to consider not only commonly studied auditory variables such as hearing thresholds, but also auditory variables that may play an important role in hearing in everyday life and verbal communication and that have been little studied in research on cognitive decline in individuals with hearing loss to date. This can be done with questionnaires such as the APHAB or other surveys and methods that are part of the ecological momentary assessment (EMA) (148). Another possibility is to study the neural correlates (EEG, MRI) of spoken language or turn-taking in conversations of persons with hearing loss [an example of a study in persons without hearing loss (149)].
Our study results indicate that depressive symptoms and cognitive impairment are less related to hearing loss per se, but more to different aspects of subjective limitations in everyday life due to hearing loss, regardless of age. Examples include distractibility and difficulty concentrating in noisy situations and communication problems in quiet situations that can quickly lead to frustration. These characteristics do not necessarily occur simultaneously in the same person and may affect patients differently depending on their occupation or other life situation.
Reporting of this observational study was done in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology statement (150). A checklist addressing each point may be found as Supplementary material.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Hannover (protocol number: 8419_BO_S_2019) and Salzburg (protocol number: 415-E/22489/2-2019). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MH: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LR: Investigation, Project administration, Writing – original draft, Writing – review & editing. LW: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. BP: Conceptualization, Formal Analysis, Methodology, Software, Validation, Writing – review & editing. SR: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. AI: Funding acquisition, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants of DFG (Deutsche Forschungsgemeinschaft) Nr. 675532, the International Hearing Foundation (www.internationale-hoerstiftung.de), and CochlearTM (IIR 1947).
The authors thank Isabella Gruber, Lars Larsen, Lucas Rainer, and Tabea Otte for cognitive examinations.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^Better ear: Deal et al., Loughrey et al., Golub et al., Jiang et al., Nicolas et al., and Shende et al. both ears Loughrey et al. and Samelli et al. (11) no information in the remaining studies (5, 7, 10, 13, 14, 20).
2. ^As a result of a systematic review and meta-analysis, gray matter and white matter appear to be affected “in almost every region /of the brain” in congenital and acquired hearing loss, with the frontal lobe being most affected (31). In individuals with acquired hearing loss, the insula is most affected (31). Age-Related Hearing Loss (ARHL) belongs to the group of acquired hearing losses. A meta-analysis of older adults with ARHL found that the temporal lobe was predominantly affected (32). Another review found that ARHL is “independently associated with accelerated atrophy of total and regional brain volume” (temporal lobes, particularly in the right hemisphere, entorhinal cortex, anterior cingulate cortex, superior frontal gyrus (associated with working memory), and parts of networks in default mode) and decreased white matter integrity (29). In addition, there are changes in spontaneous neural activity and cerebral functional connectivity of the brain in ARHL (resting-state or task negative networks and task-based or task positive networks). Increased neural activity in the middle frontal gyrus of individuals with presbycusis was found to be correlated with reduced cognitive flexibility (33).
3. ^For example, in Rutherford’s model, at the behavioral level, social isolation and loneliness caused by hearing loss in older adults lead to depressive disorder, which in turn is directly related to cognitive impairment. At the neurological level, prefrontal cortex functions are compromised by chronic overuse of the compensatory recruitment of the cognitive control network due to hearing loss. This impairment, combined with reduced or damaged auditory impulses that also damage the primary auditory cortex, negatively impacts the limbic system by affecting emotional responsiveness, emotional control and regulation, and emotional processing. This also leads to depressive disorder.
4. ^According to the latest WHO definition, hearing loss starts at a hearing threshold of >20 dB (PTA) https://www.who.int/health-topics/hearing-loss#tab=tab_1.
5. ^Cochlear implants (CIs) are electronic medical devices that bypass the hair cells in the cochlea and directly stimulate the auditory nerve (76, 77). They allow people with severe and profound hearing loss “to receive and process sounds and speech” (Medlineplus, National Library of Medicine https://medlineplus.gov/ency/article/007203.htm), where traditional hearing aids have been of little or no use.
6. ^“Sound field testing evaluates the composite best hearing ear, which means that the threshold recorded will be the quietest sound audible by either ear at each frequency presented. A normal sound-field audiogram, therefore, does not indicate normal hearing across all tested frequencies in both ears but rather that at each tested frequency, at least one of the ears could hear the tone normally” (78). There are two pathways: Air conduction and bone conduction.
7. ^Note that individuals with hearing loss are at a disadvantage in cognitive tasks that are only verbal, e.g., “I’m going to tell you three words to remember. Repeat them after I say all three words.”
8. ^“Hyperacusis can be defined as an intolerance to certain everyday sounds that are perceived as too loud or unpleasant and that cause the person significant stress and impairment in his or her daily activities” (119). Hyperacusis is common in patients with tinnitus and also occurs in individuals without hearing loss (120, 121). Although there are several models/theories that address the cause of hyperacusis (121, 122) the phenomenon hyperacusis is not yet fully understood. For example, the central auditory gain model assumes that hyperacusis is the result of an increased (dysfunctional) increase in neural gain that is due to deprived peripheral auditory input. Loudness discomfort level was found to be decreased in individuals with hyperacusis (121).
9. ^Noise sensitivity can be described as an “…increased reactivity to sounds that may include general discomfort (annoyance or feeling overwhelmed) due to a perceived noisy environment, regardless of its loudness.” Some authors (123) differed between noise sensitivity and hyperacusis, other authors did not (119, 120).
10. ^Studies with very large samples have also often been used to examine possible associations between hearing thresholds (PTA) and cognitive performance (5, 7, 8, 10, 11, 14, 15, 20). Small effect sizes were found in all these studies. Nevertheless, all p’s were significant. [Papers on smaller studies (n = 39–119)] looking at the relationship between hearing thresholds and cognition are from Jayakody et al., Nixon et al., Ren et al., and Shende et al. (6, 9, 12, 13).
11. ^https://davidakenny.shinyapps.io/MedPower/
12. ^A lexicon is the knowledge that a native speaker has about a language” Glossary of Linguistic Terms https://glossary.sil.org/term/lexicon.
1. Collaborators, GHL . Hearing loss prevalence and years lived with disability, 1990-2019: findings from the global burden of disease Study 2019. Lancet. (2021) 397:996–1009. doi: 10.1016/S0140-6736(21)00516-X
2. Blackwell, DL , Lucas, JW , and Clarke, TC . Summary health statistics for U.S. adults: national health interview survey, 2012. Vital Health Stat. (2014) 10:1–161.
3. Powell, DS , Oh, ES , Reed, NS , Lin, FR , and Deal, JA . Hearing loss and cognition: what we know and where we need to go. Front Aging Neurosci. (2021) 13:769405. doi: 10.3389/fnagi.2021.769405
4. Tay, T , Wang, JJ , Kifley, A , Lindley, R , Newall, P , and Mitchell, P . Sensory and cognitive association in older persons: findings from an older Australian population. Gerontology. (2006) 52:386–94. doi: 10.1159/000095129
5. Deal, JA , Betz, J , Yaffe, K , Harris, T , Purchase-Helzner, E , Satterfield, S, et al. Hearing impairment and incident dementia and cognitive decline in older adults: the Health ABC Study. J Gerontol A Biol Sci Med Sci. (2017) 72:703–9. doi: 10.1093/gerona/glw069
6. Jayakody, DMP , Friedland, PL , Eikelboom, RH , Martins, RN , and Sohrabi, HR . A novel study on association between untreated hearing loss and cognitive functions of older adults: baseline non-verbal cognitive assessment results. Clin Otolaryngol. (2018) 43:182–91. doi: 10.1111/coa.12937
7. Loughrey, DG , Kelly, ME , Kelley, GA , Brennan, S , and Lawlor, BA . Association of age-Related Hearing Loss with Cognitive Function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. (2018) 144:115–26. doi: 10.1001/jamaoto.2017.2513
8. Yuan, J , Sun, Y , Sang, S , Pham, JH , and Kong, WJ . The risk of cognitive impairment associated with hearing function in older adults: a pooled analysis of data from eleven studies. Sci Rep. (2018) 8:2137. doi: 10.1038/s41598-018-20496-w
9. Nixon, G , Sarant, JZ , Tomlin, D , and Dowell, R . The relationship between peripheral hearing loss and higher order listening function on cognition in older Australians. Int J Audiol. (2019) 58:933–44. doi: 10.1080/14992027.2019.1641752
10. Golub, JS , Brickman, AM , Ciarleglio, AJ , Schupf, N , and Luchsinger, JA . Association of Subclinical Hearing Loss with Cognitive Performance. JAMA Otolaryngol Head Neck Surg. (2020) 146:57–67. doi: 10.1001/jamaoto.2019.3375
11. Samelli, AG , Santos, IS , Deal, JA , Brunoni, AR , Padilha, FYOM , Matas, CG, et al. Hearing loss and cognitive function: baseline findings from the Brazilian longitudinal Study of adult Health: ELSA-brasil. Ear Hear. (2022) 43:1416–25. doi: 10.1097/AUD.0000000000001205
12. Ren, F , Luo, J , Ma, W , Xin, Q , Xu, L , Fan, Z, et al. Hearing loss and cognition among older adults in a Han Chinese cohort. Front Neurosci. (2019) 13:632. doi: 10.3389/fnins.2019.00632
13. Shende, SA , Nguyen, LT , Lydon, EA , Husain, FT , and Mudar, RA . Cognitive flexibility and inhibition in individuals with age-related hearing loss. Geriatrics. (2021) 6:22. doi: 10.3390/geriatrics6010022
14. Nicholas, SO , Koh, EJ , Wee, SL , Eikelboom, RH , Jayakody, DMP , Lin, F, et al. Peripheral hearing loss and its association with cognition among ethnic Chinese older adults. Dement Geriatr Cogn Disord. (2021) 50:394–400. doi: 10.1159/000519291
15. Croll, PH , Vinke, EJ , Armstrong, NM , Licher, S , Vernooij, MW , Baatenburg de Jong, RJ, et al. Hearing loss and cognitive decline in the general population: a prospective cohort study. J Neurol. (2021) 268:860–71. doi: 10.1007/s00415-020-10208-8
16. Salthouse, TA . Selective review of cognitive aging. J Int Neuropsychol Soc. (2010) 16:754–60. doi: 10.1017/S1355617710000706
17. Harada, CN , Natelson Love, MC , and Triebel, KL . Normal cognitive aging. Clin Geriatr Med. (2013) 29:737–52. doi: 10.1016/j.cger.2013.07.002
18. Zaninotto, P , Batty, GD , Allerhand, M , and Deary, IJ . Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English longitudinal Study of ageing. J Epidemiol Community Health. (2018) 72:685–94. doi: 10.1136/jech-2017-210116
19. Waller, M , Mishra, GD , and Dobson, AJ . Estimating the prevalence of dementia using multiple linked administrative health records and capture-recapture methodology. Emerg Themes Epidemiol. (2017) 14:3. doi: 10.1186/s12982-017-0057-3
20. Jiang, K , Armstrong, NM , Agrawal, Y , Gross, AL , Schrack, JA , Lin, FR, et al. Associations of audiometric hearing and speech-in-noise performance with cognitive decline among older adults: the Baltimore longitudinal Study of aging (BLSA). Front Neurol. (2022) 13:1029851. doi: 10.3389/fneur.2022.1029851
21. Deal, JA , Sharrett, AR , Albert, MS , Coresh, J , Mosley, TH , Knopman, D, et al. Hearing impairment and cognitive decline: a pilot study conducted within the atherosclerosis risk in communities neurocognitive study. Am J Epidemiol. (2015) 181:680–90. doi: 10.1093/aje/kwu333
22. Li, R , Miao, X , Han, B , and Li, J . Cortical thickness of the left parahippocampal cortex links central hearing and cognitive performance in aging. Ann N Y Acad Sci. (2023) 1522:117–25. doi: 10.1111/nyas.14971
23. Lin, FR , Yaffe, K , Xia, J , Xue, QL , Harris, TB , Purchase-Helzner, E, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. (2013) 173:293–9. doi: 10.1001/jamainternmed.2013.1868
24. Lin, FR , and Albert, M . Hearing loss and dementia—who is listening? Aging Ment Health. (2014) 18:671–3. doi: 10.1080/13607863.2014.915924
25. Rutherford, BR , Brewster, K , Golub, JS , Kim, AH , and Roose, SP . Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am J Psychiatry. (2018) 175:215–24. doi: 10.1176/appi.ajp.2017.17040423
26. Bowl, MR , and Dawson, SJ . Age-related hearing loss. New York, USA: Cold Spring Harbor Perspective Medicine (2019). 9 p.
27. Uchida, Y , Sugiura, S , Nishita, Y , Saji, N , Sone, M , and Ueda, H . Age-related hearing loss and cognitive decline—the potential mechanisms linking the two. Auris Nasus Larynx. (2019) 46:1–9. doi: 10.1016/j.anl.2018.08.010
28. Slade, K , Plack, CJ , and Nuttall, HE . The effects of age-related hearing loss on the brain and cognitive function. Trends Neurosci. (2020) 43:810–21. doi: 10.1016/j.tins.2020.07.005
29. Jafari, Z , Kolb, BE , and Mohajerani, MH . Age-related hearing loss and cognitive decline: MRI and cellular evidence. Ann N Y Acad Sci. (2021) 1500:17–33. doi: 10.1111/nyas.14617
30. Sharma, RK , Chern, A , and Golub, JS . Age-related hearing loss and the development of cognitive impairment and late-life depression: a scoping overview. Semin Hear. (2021) 42:10–25. doi: 10.1055/s-0041-1725997
31. Manno, FAM , Rodríguez-Cruces, R , Kumar, R , Ratnanather, JT , and Lau, C . Hearing loss impacts gray and white matter across the lifespan: systematic review, meta-analysis and meta-regression. NeuroImage. (2021) 231:117826. doi: 10.1016/j.neuroimage.2021.117826
32. Slade, K , Reilly, JH , Jablonska, K , Smith, E , Hayes, LD , Plack, CJ, et al. The impact of age-related hearing loss on structural neuroanatomy: a meta-analysis. Front Neurol. (2022) 13:950997. doi: 10.3389/fneur.2022.950997
33. Chen, YC , Chen, H , Jiang, L , Bo, F , Xu, JJ , Mao, CN, et al. Presbycusis disrupts spontaneous activity revealed by resting-state functional MRI. Front Behav Neurosci. (2018) 12:44. doi: 10.3389/fnbeh.2018.00044
34. Maharani, A , Pendleton, N , and Leroi, I . Hearing impairment, loneliness, social isolation, and cognitive function: longitudinal analysis using English longitudinal Study on ageing. Am J Geriatr Psychiatry. (2019) 27:1348–56. doi: 10.1016/j.jagp.2019.07.010
35. McDermott, LM , and Ebmeier, KP . A meta-analysis of depression severity and cognitive function. J Affect Disord. (2009) 119:1–8. doi: 10.1016/j.jad.2009.04.022
36. Bruder, G , Kayser, J , and Tenke, C . Event-related brain potentials in depression: clinical, cognitive and neurophysiologic implications In: SJ Luck and ES Kappenman, editors. Oxford handbook of event-related potential components. New York: Oxford University, Press (2012). 563–92.
37. Snyder, HR . Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. (2013) 139:81–132. doi: 10.1037/a0028727
38. Vu, NQ , and Aizenstein, HJ . Depression in the elderly: brain correlates, neuropsychological findings, and role of vascular lesion load. Curr Opin Neurol. (2013) 26:656–61. doi: 10.1097/WCO.0000000000000028
39. Roca, M , Vives, M , López-Navarro, E , García-Campayo, J , and Gili, M . Cognitive impairments and depression: a critical review. Actas Esp Psiquiatr. (2015) 43:187–93.
40. John, A , Patel, U , Rusted, J , Richards, M , and Gaysina, D . Affective problems and decline in cognitive state in older adults: a systematic review and meta-analysis. Psychol Med. (2019) 49:353–65. doi: 10.1017/S0033291718001137
41. Zainal, NH , and Newman, MG . Elevated anxious and depressed mood relates to future executive dysfunction in older adults: a longitudinal network analysis of psychopathology and cognitive functioning. Clin Psychol Sci. (2023) 11:218–38. doi: 10.1177/21677026221114076
42. Koenig, AM , Bhalla, RK , and Butters, MA . Cognitive functioning and late-life depression. J Int Neuropsychol Soc. (2014) 20:461–7. doi: 10.1017/S1355617714000198
43. Lai, S , Zhong, S , Wang, Y , Zhang, Y , Xue, Y , Zhao, H, et al. The prevalence and characteristics of MCCB cognitive impairment in unmedicated patients with bipolar II depression and major depressive disorder. J Affect Disord. (2022) 310:369–76. doi: 10.1016/j.jad.2022.04.153
44. Vollmert, C , Tost, H , Brassen, S , Jatzko, A , and Braus, DF . Depression and modern neuroimaging. Fortschr Neurol Psychiatr. (2004) 72:435–45. doi: 10.1055/s-2004-818398
45. Zhao, H , Lai, S , Zhong, S , Zhang, Y , Yang, H , and Jia, Y . Variation in thyroid-stimulating hormone and cognitive disorders in unmedicated middle-aged patients with major depressive disorder: a proton magnetic resonance spectroscopy Study. Mediat Inflamm. (2022) 2022:1–12. doi: 10.1155/2022/1623478
46. Lu, X , Lai, S , Luo, A , Huang, X , Wang, Y , Zhang, Y, et al. Biochemical metabolism in the anterior cingulate cortex and cognitive function in major depressive disorder with or without insomnia syndrome. J Affect Disord. (2023) 335:256–63. doi: 10.1016/j.jad.2023.04.132
47. Wulff, L . Amygdala-Volumenveränderung bei Depression? Entwicklung und Anwendung eines Segmentierprotokolls für hochauflösende. Marburg, Germany: MRT Philipps-Universität Marburg (2018).
48. Li, CM , Zhang, X , Hoffman, HJ , Cotch, MF , Themann, CL , and Wilson, MR . Hearing impairment associated with depression in US adults, National Health and nutrition examination survey 2005-2010. JAMA Otolaryngol Head Neck Surg. (2014) 140:293–302. doi: 10.1001/jamaoto.2014.42
49. Kim, SY , Kim, HJ , Park, EK , Joe, J , Sim, S , and Choi, HG . Severe hearing impairment and risk of depression: a national cohort study. PLoS One. (2017) 12:e0179973. doi: 10.1371/journal.pone.0179973
50. Kim, HJ , Jeong, S , Roh, KJ , Oh, YH , and Suh, MJ . Association between hearing impairment and incident depression: a Nationwide follow-up Study. Laryngoscope. (2023). doi: 10.1002/lary.30654
51. Brewster, KK , Ciarleglio, A , Brown, PJ , Chen, C , Kim, HO , Roose, SP, et al. Age-related hearing loss and its association with depression in later life. Am J Geriatr Psychiatry. (2018) 26:788–96. doi: 10.1016/j.jagp.2018.04.003
52. Brewster, KK , Hu, MC , Zilcha-Mano, S , Stein, A , Brown, PJ , Wall, MM, et al. Age-related hearing loss, late-life depression, and risk for incident dementia in older adults. J Gerontol A Biol Sci Med Sci. (2021) 76:827–34. doi: 10.1093/gerona/glaa242
53. Golub, JS , Brewster, KK , Brickman, AM , Ciarleglio, AJ , Kim, AH , Luchsinger, JA, et al. Association of Audiometric age-Related Hearing Loss with Depressive Symptoms among Hispanic Individuals. JAMA Otolaryngol Head Neck Surg. (2019) 145:132–9. doi: 10.1001/jamaoto.2018.3270
54. Golub, JS , Brewster, KK , Brickman, AM , Ciarleglio, AJ , Kim, AH , Luchsinger, JA, et al. Subclinical hearing loss is associated with depressive symptoms. Am J Geriatr Psychiatry. (2020) 28:545–56. doi: 10.1016/j.jagp.2019.12.008
55. Shukla, A , Reed, NS , Armstrong, NM , Lin, FR , Deal, JA , and Goman, AM . Hearing loss, hearing aid use, and depressive symptoms in older adults-findings from the atherosclerosis risk in communities neurocognitive Study (ARIC-NCS). J Gerontol B Psychol Sci Soc Sci. (2021) 76:518–23. doi: 10.1093/geronb/gbz128
56. Chern, A , Irace, AL , and Golub, JS . The laterality of age-related hearing loss and depression. Otol Neurotol. (2022) 43:625–31. doi: 10.1097/MAO.0000000000003531
57. Tsimpida, D , Kontopantelis, E , Ashcroft, DM , and Panagioti, M . The dynamic relationship between hearing loss, quality of life, socioeconomic position and depression and the impact of hearing aids: answers from the English longitudinal Study of ageing (ELSA). Soc Psychiatry Psychiatr Epidemiol. (2022) 57:353–62. doi: 10.1007/s00127-021-02155-0
58. Tseng, CC , Hu, LY , Liu, ME , Yang, AC , Shen, CC , and Tsai, SJ . Risk of depressive disorders following sudden sensorineural hearing loss: a nationwide population-based retrospective cohort study. J Affect Disord. (2016) 197:94–9. doi: 10.1016/j.jad.2016.03.020
59. Gopinath, B , Wang, JJ , Schneider, J , Burlutsky, G , Snowdon, J , McMahon, CM, et al. Depressive symptoms in older adults with hearing impairments: the Blue Mountains Study. J Am Geriatr Soc. (2009) 57:1306–8. doi: 10.1111/j.1532-5415.2009.02317.x
60. Andresen, EM , Malmgren, JA , Carter, WB , and Patrick, DL . Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. (1994) 10:77–84. doi: 10.1016/S0749-3797(18)30622-6
61. Tang, TY , Luan, Y , Jiao, Y , Zhang, J , Ju, SH , and Teng, GJ . Disrupted amygdala connectivity is associated with elevated anxiety in sensorineural hearing loss. Front Neurosci. (2020) 14:616348. doi: 10.3389/fnins.2020.616348
62. Aoki, M , Okuda, H , Ishihara, H , Hayashi, H , Ohashi, T , Nishihori, T, et al. Hearing loss is associated with hippocampal atrophy and high cortisol/dehydroepiandrosterone sulphate ratio in older adults. Int J Audiol. (2021) 60:293–9. doi: 10.1080/14992027.2020.1831703
63. Brewster, K , Choi, CJ , He, X , Kim, AH , Golub, JS , Brown, PJ, et al. Hearing rehabilitative treatment for older adults with comorbid hearing loss and depression: effects on depressive symptoms and executive function. Am J Geriatr Psychiatry. (2022) 30:448–58. doi: 10.1016/j.jagp.2021.08.006
64. Falkai, P , and Wittchen, HU . Diagnostisches und statistisches Manual psychischer Störungen DSM-5. Göttingen: Hogrefe (2015).
65. Steinberg, LJ , Underwood, MD , Bakalian, MJ , Kassir, SA , Mann, JJ , and Arango, V . 5-HT1A receptor, 5-HT2A receptor and serotonin transporter binding in the human auditory cortex in depression. J Psychiatry Neurosci. (2019) 44:294–302. doi: 10.1503/jpn.180190
66. Kang, HH , Wang, CH , Chen, HC , Li, IH , Cheng, CY , Liu, RS, et al. Investigating the effects of noise-induced hearing loss on serotonin transporters in rat brain using 4-[18F]-ADAM/small animal PET. NeuroImage. (2013) 75:262–9. doi: 10.1016/j.neuroimage.2012.06.049
67. Li, IH , Shih, JH , Jhao, YT , Chen, HC , Chiu, CH , Chen, CF, et al. Regulation of noise-induced loss of serotonin transporters with resveratrol in a rat model using 4-[18F]-ADAM/small-animal positron emission tomography. Molecules. (2019) 24:1344. doi: 10.3390/molecules24071344
68. Keesom, SM , and Hurley, LM . Silence, solitude, and serotonin: neural mechanisms linking hearing loss and social isolation. Brain Sci. (2020) 10:367. doi: 10.3390/brainsci10060367
69. Huber, M , Roesch, S , Pletzer, B , Lukaschyk, J , Lesinski-Schiedat, A , and Illg, A . Cognition in older adults with severe to profound sensorineural hearing loss compared to peers with normal hearing for age. Int J Audiol. (2020) 59:254–62. doi: 10.1080/14992027.2019.1687947
70. Powell, DS , Brenowitz, WD , Yaffe, K , Armstrong, NM , Reed, NS , Lin, FR, et al. Examining the combined estimated effects of hearing loss and depressive symptoms on risk of cognitive decline and incident dementia. J Gerontol B Psychol Sci Soc Sci. (2022) 77:839–49. doi: 10.1093/geronb/gbab194
71. Disoriders NIoDaOC . What the numbers mean: An epidemiological perspective on hearing. (2011). Available at: www.nidcd.nih.gov/health/statistics/what-numbers-mean-epidemiological-perspective-hearing#measuring.
72. Kirk, KM , McGuire, A , Nasveld, PE , and Treloar, SA . Comparison of self-reported and audiometrically-measured hearing loss in the Australian defence force. Int J Audiol. (2012) 51:294–8. doi: 10.3109/14992027.2011.625981
73. Choi, JE , Moon, IJ , Baek, SY , Kim, SW , and Cho, YS . Discrepancies between self-reported hearing difficulty and hearing loss diagnosed by audiometry: prevalence and associated factors in a national survey. BMJ Open. (2019) 9:e022440. doi: 10.1136/bmjopen-2018-022440
74. Curti, SA , Taylor, EN , Su, D , and Spankovich, C . Prevalence of and characteristics associated with self-reported good hearing in a population with elevated audiometric thresholds. JAMA Otolaryngol Head Neck Surg. (2019) 145:626–33. doi: 10.1001/jamaoto.2019.1020
75. Heigl, K , Gerstner, D , Huß, J , Weilnhammer, V , Jenkac, C , Perez-Alvarez, C, et al. The validity of using a self-report single question as a means to detect hearing loss in an adolescent population. Int J Audiol. (2022) 1-8:1–8. doi: 10.1080/14992027.2022.2129852
76. Armstrong, NM , An, Y , Doshi, J , Erus, G , Ferrucci, L , Davatzikos, C, et al. Association of Midlife Hearing Impairment with Late-Life Temporal Lobe Volume Loss. JAMA Otolaryngol Head Neck Surg. (2019) 145:794–802. doi: 10.1001/jamaoto.2019.1610
77. Deutsche Gesellschaft für Hals-Nasen-Ohren-Heilkunde, Kopf-und Hals-Chirurgie eV . AWMF S2k-Leitlinie Cochlea-Implantat Versorgung Bonn2020. (2020) Available at: www.awmf.org/.
79. Petermann, F , and Lepach, AC . Wechsler Memory Scale – Fourth Edition(WMS-IV) Manual zur Durchführung und Auswertung. F rankfurt: Pearson Assessment & Information GmbH (2012).
80. Hesse, G , Janssen, T , Kinkel, M , Mrowinski, D , Müller-Deile, J , and Ptok, M . Praxis der Audiometrie. Stuttgart: Georg Thieme Verlag (2009).
82. Cox, RM , and Alexander, GC . The abbreviated profile of hearing aid benefit. Ear Hear. (1995) 16:176–86. doi: 10.1097/00003446-199504000-00005
83. Löhler, J , Moser, L , Heinrich, D , Hörmann, K , and Walther, LE . Results of clinical use of the German version of the APHAB. HNO. (2012) 60:626–36. doi: 10.1007/s00106-011-2466-x
84. Srinivasan, N , and O'Neill, S . Comparison of speech, spatial, and qualities of hearing scale (SSQ) and the abbreviated profile of hearing aid benefit (APHAB) questionnaires in a large cohort of self-reported Normal-hearing adult listeners. Audiol Res. (2023) 13:143–50. doi: 10.3390/audiolres13010014
85. Löhler, J , Akcicek, B , Wollenberg, B , Kappe, T , Schlattmann, P , and Schönweiler, R . The influence of frequency-dependent hearing loss to unaided APHAB scores. Eur Arch Otorhinolaryngol. (2016) 273:3587–93. doi: 10.1007/s00405-016-3966-9
86. Brännström, KJ , Andersson, K , Sandgren, O , and Whitling, S . Clinical application and psychometric properties of a Swedish translation of the abbreviated profile of hearing aid benefit. J Am Acad Audiol. (2020) 31:656–65. doi: 10.1055/s-0040-1718702
87. Dornhoffer, JR , Meyer, TA , Dubno, JR , and McRackan, TR . Assessment of hearing aid benefit using patient-reported outcomes and audiologic measures. Audiol Neurootol. (2020) 25:215–23. doi: 10.1159/000506666
88. Cox, RM , Alexander, GC , and Gray, GA . Audiometric correlates of the unaided APHAB. J Am Acad Audiol. (2003) 14:361–71. doi: 10.1055/s-0040-1715755
89. Wang, YP , and Gorenstein, C . Psychometric properties of the Beck depression inventory-II: a comprehensive review. Braz J Psychiatry. (2013) 35:416–31. doi: 10.1590/1516-4446-2012-1048
90. At, B , Ch, W , Mendelson, M , Mock, J , and Erbaugh, J . An inventory for measuring depression. Arch Gen Psychiatry. (1961) 4:561–71. doi: 10.1001/archpsyc.1961.01710120031004
91. Kühner, C , Bürger, C , Keller, F , and Hautzinger, M . Reliability and validity of the revised Beck depression inventory (BDI-II). Results from German samples. Nervenarzt. (2007) 78:651–6. doi: 10.1007/s00115-006-2098-7
92. Sturm, W , and Willlmes, K . WienerTestsystem. Nonverbaler Lerntest (NVLT). Mödling: Schuhfried GmbH (2018).
93. Kindsvater, S , and Sturm, W . Computer- vs papier-Bleistiftvorgabe: Äquivalenzstudie zum nonverbalen Lerntest (NVLT) [computerised vs paper-pencil testing: a study concerning the equivalence of these two test procedures for the nonverbal learning test (NVLT)]. Z Neuropsychol. (2003) 14:13–21. doi: 10.1024//1016-264X.14.1.13
95. Kimura, D . Right temporal-lobe damage. Perception of unfamiliar stimuli after damage. Arch Neurol. (1963) 8:264–71. doi: 10.1001/archneur.1963.00460030048004
96. Wk, K . Age differences in short-term retention of rapidly changing information. J Exp Psychol. (1958) 55:352–8. doi: 10.1037/h0043688
97. Cohen, JD , Perlstein, WM , Braver, TS , Nystrom, LE , Noll, DC , Jonides, J, et al. Temporal dynamics of brain activation during a working memory task. Nature. (1997) 386:604–8. doi: 10.1038/386604a0
98. Zimmermann, P , and Fimm, B . Testbatterie zur Aufmerksamkeitsprüfung (TAP) Version 2.3.1. Herzogenrath, Germany: Psytest (2019).
99. Maguire, MJ , Brier, MR , Moore, PS , Ferree, TC , Ray, D , Mostofsky, S, et al. The influence of perceptual and semantic categorization on inhibitory processing as measured by the N2-P3 response. Brain Cogn. (2009) 71:196–203. doi: 10.1016/j.bandc.2009.08.018
100. Giovagnoli, AR , Del Pesce, M , Mascheroni, S , Simoncelli, M , Laiacona, M , and Capitani, E . Trail making test: normative values from 287 normal adult controls. Ital J Neurol Sci. (1996) 17:305–9. doi: 10.1007/BF01997792
101. Tombaugh, TN . Trail making test a and B: normative data stratified by age and education. Arch Clin Neuropsychol. (2004) 19:203–14. doi: 10.1016/S0887-6177(03)00039-8
102. Reitan, RM . Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. (1958) 8:271–6. doi: 10.2466/pms.1958.8.3.271
103. Aschenbrenner, S , Oliver, T , and Klaus, L . Regensburger Wortflüssigkeits-Test (RWT). Göttingen, Bern, Toronto, Seattle: Hogrefe Verlag für Psychologie (2000).
104. Baldo, JV , Schwartz, S , Wilkins, D , and Dronkers, NF . Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J Int Neuropsychol Soc. (2006) 12:896–900. doi: 10.1017/S1355617706061078
105. Pichora-Fuller, MK , Schneider, BA , and Daneman, M . How young and old adults listen to and remember speech in noise. J Acoust Soc Am. (1995) 97:593–608. doi: 10.1121/1.412282
106. Song, JH , Skoe, E , Banai, K , and Kraus, N . Perception of speech in noise: neural correlates. J Cogn Neurosci. (2011) 23:2268–79. doi: 10.1162/jocn.2010.21556
107. Souza, P , Arehart, K , Miller, CW , and Muralimanohar, RK . Effects of age on F0 discrimination and intonation perception in simulated electric and electroacoustic hearing. Ear Hear. (2011) 32:75–83. doi: 10.1097/AUD.0b013e3181eccfe9
108. Shojaei, E , Ashayeri, H , Jafari, Z , Zarrin Dast, MR , and Kamali, K . Effect of signal to noise ratio on the speech perception ability of older adults. Med J Islam Repub Iran. (2016) 30:342.
109. Wong, PC , Ettlinger, M , Sheppard, JP , Gunasekera, GM , and Dhar, S . Neuroanatomical characteristics and speech perception in noise in older adults. Ear Hear. (2010) 31:471–9. doi: 10.1097/AUD.0b013e3181d709c2
110. Halevi-Katz, DN , Yaakobi, E , and Putter-Katz, H . Exposure to music and noise-induced hearing loss (NIHL) among professional pop/rock/jazz musicians. Noise Health. (2015) 17:158–64. doi: 10.4103/1463-1741.155848
111. Xie, Z , Zinszer, BD , Riggs, M , Beevers, CG , and Chandrasekaran, B . Impact of depression on speech perception in noise. PLoS One. (2019) 14:e0220928. doi: 10.1371/journal.pone.0220928
112. Cornblatt, BA , Lenzenweger, MF , and Erlenmeyer-Kimling, L . The continuous performance test, identical pairs version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Res. (1989) 29:65–85. doi: 10.1016/0165-1781(89)90188-1
113. Lemelin, S , Baruch, P , Vincent, A , Everett, J , and Vincent, P . Distractibility and processing resource deficit in major depression. Evidence for two deficient attentional processing models. J Nerv Ment Dis. (1997) 185:542–8. doi: 10.1097/00005053-199709000-00002
114. Lepistö, T , Soininen, M , Ceponiene, R , Almqvist, F , Näätänen, R , and Aronen, ET . Auditory event-related potential indices of increased distractibility in children with major depression. Clin Neurophysiol. (2004) 115:620–7. doi: 10.1016/j.clinph.2003.10.020
115. Desseilles, M , Balteau, E , Sterpenich, V , Dang-Vu, TT , Darsaud, A , Vandewalle, G, et al. Abnormal neural filtering of irrelevant visual information in depression. J Neurosci. (2009) 29:1395–403. doi: 10.1523/JNEUROSCI.3341-08.2009
116. De Carvalho, LM , Gonsalez, EC , and Iorio, MC . Speech perception in noise in the elderly: interactions between cognitive performance, depressive symptoms, and education. Braz J Otorhinolaryngol. (2017) 83:195–200. doi: 10.1016/j.bjorl.2016.03.017
117. Heinze-Köhler, K , Lehmann, EK , and Hoppe, U . Depressive symptoms affect short- and long-term speech recognition outcome in cochlear implant users. Eur Arch Otorhinolaryngol. (2021) 278:345–51. doi: 10.1007/s00405-020-06096-3
118. Brewster, KK , and Rutherford, BR . Hearing loss, psychiatric symptoms, and cognitive decline: An increasingly important triad in older adults. Am J Geriatr Psychiatry. (2021) 29:554–6. doi: 10.1016/j.jagp.2020.10.015
119. Kula, FB , Cropley, M , and Aazh, H . Hyperacusis and Misophonia: a systematic review of psychometric measures. J Am Acad Audiol. (2023) 33. doi: 10.1055/a-1896-5032
120. Aazh, H , Moore, BC , Lammaing, K , and Cropley, M . Tinnitus and hyperacusis therapy in a UK National Health Service audiology department: Patients' evaluations of the effectiveness of treatments. Int J Audiol. (2016) 55:514–22. doi: 10.1080/14992027.2016.1178400
122. Knipper, M , Van Dijk, P , Nunes, I , Rüttiger, L , and Zimmermann, U . Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol. (2013) 111:17–33. doi: 10.1016/j.pneurobio.2013.08.002
123. Henry, JA , Theodoroff, SM , Edmonds, C , Martinez, I , Myers, PJ , Zaugg, TL, et al. Sound tolerance conditions (hyperacusis, Misophonia, noise sensitivity, and phonophobia): definitions and clinical management. Am J Audiol. (2022) 31:513–27. doi: 10.1044/2022_AJA-22-00035
124. Gronewold, J , Duman, EE , Engel, M , Engels, M , Siegrist, J , Erbel, R, et al. Association between life events and later depression in the population-based Heinz Nixdorf recall study-the role of sex and optimism. PLoS One. (2022) 17:e0271716. doi: 10.1371/journal.pone.0271716
125. School, HM . How genes and life events affect mood and depression: Harvard Health Publishing; (2022) Available at: https://www.health.harvard.edu/depression/how-genes-and-life-events-affect-mood-and-depression.
126. Coleman, JRI , Gaspar, HA , Bryois, J , and Breen, G, Consortium BDWGotPG, Consortium MDDWGotPG . The genetics of the mood disorder Spectrum: genome-wide association analyses of more than 185,000 cases and 439,000 controls. Biol Psychiatry. (2020) 88:169–84. doi: 10.1016/j.biopsych.2019.10.015
127. Kocabay, AP , Aslan, F , Yüce, D , and Turkyilmaz, D . Speech in noise: implications of age, hearing loss, and cognition. Folia Phoniatr Logop. (2022) 74:345–51. doi: 10.1159/000525580
128. Cao, X , Liu, Q , Liu, J , Yang, B , and Zhou, J . The impact of hearing loss on cognitive impairment: the mediating role of depressive symptoms and the moderating role of social relationships. Front Public Health. (2023) 11:1149769. doi: 10.3389/fpubh.2023.1149769
129. Folstein, MF , Folstein, SE , and McHugh, PR . "mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. (1975) 12:189–98. doi: 10.1016/0022-3956(75)90026-6
130. Friedman, NP , and Robbins, TW . The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology. (2022) 47:72–89. doi: 10.1038/s41386-021-01132-0
131. Shiffrin, RM , and Schneider, W . Automatic and controlled processing revisited. Psychol Rev. (1984) 91:269–76.
132. Fabio, RA , Caprì, T , and Romano, M . From controlled to automatic processes and Back again: the role of contextual features. Eur J Psychol. (2019) 15:773–88. doi: 10.5964/ejop.v15i4.1746
133. Loughrey, DG , Pakhomov, SVS , and Lawlor, BA . Altered verbal fluency processes in older adults with age-related hearing loss. Exp Gerontol. (2020) 130:110794. doi: 10.1016/j.exger.2019.110794
134. Diamond, A . Executive functions. Annu Rev Psychol. (2013) 64:135–68. doi: 10.1146/annurev-psych-113011-143750
135. Levinson, SC , and Torreira, F . Timing in turn-taking and its implications for processing models of language. Front Psychol. (2015) 6:731. doi: 10.3389/fpsyg.2015.00731
136. Campbell, J , and Sharma, A . Compensatory changes in cortical resource allocation in adults with hearing loss. Front Syst Neurosci. (2013) 7:71. doi: 10.3389/fnsys.2013.00071
137. Hornsby, BW , and Kipp, AM . Subjective ratings of fatigue and vigor in adults with hearing loss are driven by perceived hearing difficulties not degree of hearing loss. Ear Hear. (2016) 37:e1–e10. doi: 10.1097/AUD.0000000000000203
138. Peelle, JE . Listening effort: how the cognitive consequences of acoustic challenge are reflected in brain and behavior. Ear Hear. (2018) 39:204–14. doi: 10.1097/AUD.0000000000000494
139. Rosemann, S , and Thiel, CM . Neuroanatomical changes associated with age-related hearing loss and listening effort. Brain Struct Funct. (2020) 225:2689–700. doi: 10.1007/s00429-020-02148-w
140. Ayasse, ND , Hodson, AJ , and Wingfield, A . The principle of least effort and comprehension of spoken sentences by younger and older adults. Front Psychol. (2021) 12:629464. doi: 10.3389/fpsyg.2021.629464
141. Gabrys, RL , Tabri, N , Anisman, H , and Matheson, K . Cognitive control and flexibility in the context of stress and depressive symptoms: the cognitive control and flexibility questionnaire. Front Psychol. (2018) 9:2219. doi: 10.3389/fpsyg.2018.02219
142. Sanchez, K , Dias, JW , and Rosenblum, LD . Experience with a talker can transfer across modalities to facilitate lipreading. Atten Percept Psychophys. (2013) 75:1359–65. doi: 10.3758/s13414-013-0534-x
143. Bernstein, JGW , Venezia, JH , and Grant, KW . Auditory and auditory-visual frequency-band importance functions for consonant recognition. J Acoust Soc Am. (2020) 147:3712–27. doi: 10.1121/10.0001301
144. Bernstein, LE , Jordan, N , Auer, ET , and Eberhardt, SP . Lipreading: a review of its continuing importance for speech recognition with an acquired hearing loss and possibilities for effective training. Am J Audiol. (2022) 31:453–69. doi: 10.1044/2021_AJA-21-00112
145. Deng, J , Zhou, F , Hou, W , Silver, Z , Wong, CY , Chang, O, et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci. (2021) 1486:90–111. doi: 10.1111/nyas.14506
146. Collaborators, C-MD . Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. (2021) 398:1700–12. doi: 10.1016/S0140-6736(21)02143-7
147. WHO . Mental Health and COVID-19: early evidence of the pandemic’s impact. Geneva, Switzerland: WHO (2022).
148. Holube, I , von Gablenz, P , and Bitzer, J . Ecological momentary assessment in hearing research: current state, challenges, and future directions. Ear Hear. (2020) 41:79S–90S. doi: 10.1097/AUD.0000000000000934
149. Bögels, S . Neural correlates of turn-taking in the wild: response planning starts early in free interviews. Cognition. (2020) 203:104347. doi: 10.1016/j.cognition.2020.104347
Keywords: hearing loss from adulthood, secondary depression, cognition, younger and older adults, hearing restrictions during communication in quiet and in noise
Citation: Huber M, Reuter L, Weitgasser L, Pletzer B, Rösch S and Illg A (2023) Hearing loss, depression, and cognition in younger and older adult CI candidates. Front. Neurol. 14:1272210. doi: 10.3389/fneur.2023.1272210
Received: 03 August 2023; Accepted: 04 September 2023;
Published: 13 October 2023.
Edited by:
Andrés Soto-Varela, Complejo Hospitalario Universitario de Santiago, SpainReviewed by:
Emilio Domínguez-Durán, Hospital QuironSalud Infanta Luisa, SpainCopyright © 2023 Huber, Reuter, Weitgasser, Pletzer, Rösch and Illg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Huber, bS5odWJlckBzYWxrLmF0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.