- 1Department of Neurology and German Center for Vertigo and Balance Disorders, LMU University Hospital, LMU Munich, Munich, Germany
- 2Department of Pharmacology, University of Oxford, Oxford, United Kingdom
- 3Department of Medical Information Sciences, Biometry and Epidemiology, Ludwig Maximilians University, Munich, Germany
Background: Betahistine was registered in Europe in the 1970s and approved in more than 80 countries as a first-line treatment for Menière's disease. It has been administered to more than 150 million patients. However, according to a Cochrane systematic review of betahistine and recent meta-analyses, there is insufficient evidence to say whether betahistine has any effect in the currently approved dosages of up to 48 mg/d. A combination with the monoamine oxidase B (MAO-B) inhibitor, selegiline, may increase the bioavailability of betahistine to levels similar to the well-established combination of L-DOPA with carbidopa or benserazide in the treatment of Parkinson's disease. We investigated the effect of selegiline on betahistine pharmacokinetics and the safety of the combination in humans.
Methods: In an investigator-initiated prospective, non-randomized, single-sequence, two-period titration, open label single-center phase 1 study, 15 healthy volunteers received three single oral dosages of betahistine (24, 48, and 96 mg in this sequence with at least 2 days' washout period) without and with selegiline (5 mg/d with a loading period of 7 days). Betahistine serum concentrations were measured over a period of 240 min at eight time points (area under the curve, AUC0-240 min). This trial is registered with EudraCT (2019-002610-39) and ClinicalTrials.gov.
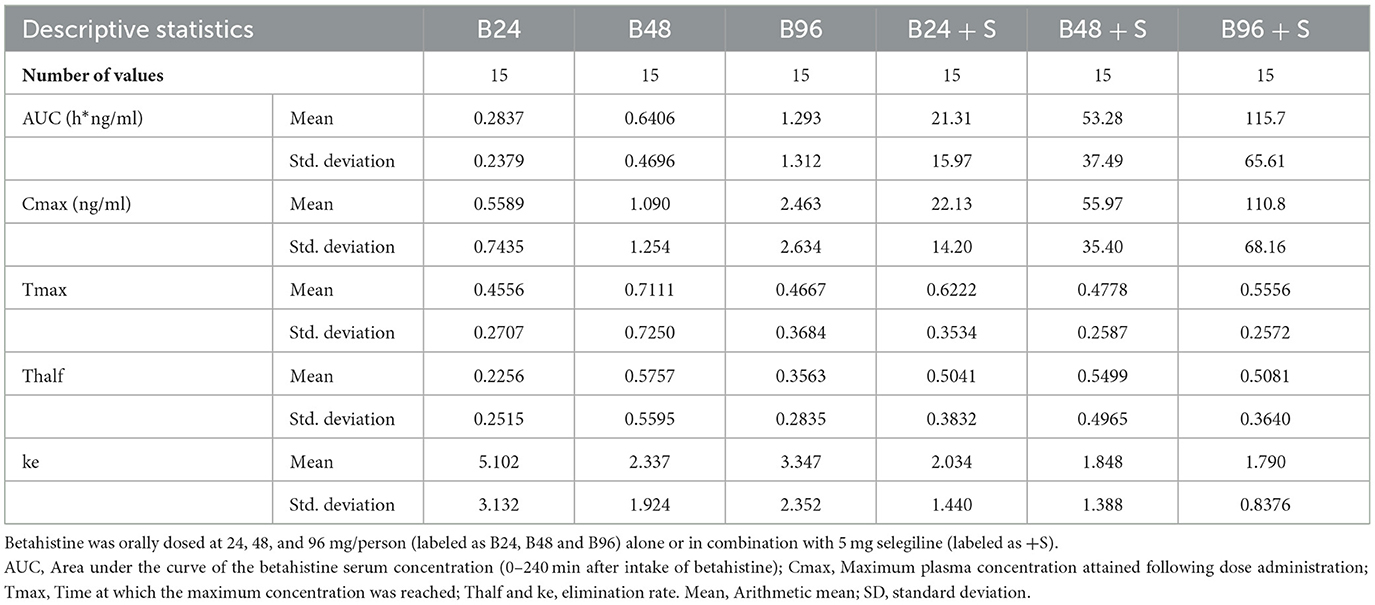
Findings: In all three single betahistine dosages, selegiline increased the betahistine bioavailability about 80- to 100-fold. For instance, the mean (±SD) of the area under curve for betahistine 48 mg alone was 0.64 (+/-0.47) h*ng/mL and for betahistine plus selegiline 53.28 (+/-37.49) h*ng/mL. The half-life time of around 30 min was largely unaffected, except for the 24 mg betahistine dosage. In total, 14 mild adverse events were documented.
Interpretation: This phase 1 trial shows that the MAO-B inhibitor selegiline increases betahistine bioavailability by a factor of about 80 to 100. No safety concerns were detected. Whether the increased bioavailability has an impact on the preventive treatment of Menière's disease, acute vestibular syndrome, or post-BPPV residual dizziness has to be evaluated in placebo-controlled trials.
Clinical trial registration: https://clinicaltrials.gov/study/NCT05938517?intr=betahistine%20and%20selegiline&rank=1, identifier: NCT05938517.
1. Introduction
Betahistine is a structural analog of histamine with weak histamine H1 receptor agonist and more potent histamine H3 receptor antagonist properties. Betahistine has been used for various indications, namely for the prophylactic treatment of Menière's disease for over 50 years and for the symptomatic treatment of vertigo. It is approved for Menière's disease in more than 80 countries and has been used in over 130 million patients with a favorable safety profile (1). A randomized, placebo-controlled, multi-center trial (conducted by our institution), however, demonstrated that its approved dosage (48 mg/day), and even 144 mg/day, were not superior to placebo (2); this finding is supported by a systematic review (3) and Cochrane analysis (4). Nevertheless, a survey in Italy published in 2023 showed that 28% of the patients still used betahistine as the main treatment (5). Further, it is the most frequently given drug for the treatment of vertigo according to a worldwide survey (6). Higher doses of up to 480 mg/d have shown benefit for severe cases in a small case series of Meniere's disease (7). This indicates that effectiveness seems to be a question of dosage and thereby serum concentration. For other indications, higher single dosages of betahistine were already studied, e.g., 200 mg per day in patients with attention deficit hyperactivity syndrome (8).
A basic pharmacokinetic problem of betahistine is that 99% of orally ingested betahistine is metabolized in the gastrointestinal tract and liver by MAO-B/A (9). This was also shown in a phase 1 trial in healthy volunteers (10). This first-pass effect can be reduced by MAO-B inhibitors, such as selegiline (11). Combination therapy like this is similar to the well-established combination of L-DOPA with carbidopa or benserazide in the treatment of Parkinson's disease.
In an animal model of unilateral vestibular neurectomized cats, oral treatment with betahistine in combination with selegiline led to significantly higher blood concentrations of betahistine and to a better posture function (12, 13). A study in guinea pigs demonstrated that cochlear microcirculation increased significantly after intravenous treatment with low-dose betahistine combined with selegiline compared to selegiline alone (14). An alternative approach to bypass the first-pass effect are intranasal (EudraCT 2018-002474-52) and transbuccal applications.
Based on these findings, the primary objective of this phase 1 trial in healthy subjects was to demonstrate that the serum concentration of betahistine is higher in a combination treatment with selegiline compared to betahistine monotherapy. The primary endpoint was the area under the curve (AUC0-240 min) of the serum concentrations of betahistine after combination treatment with betahistine and 5 mg/d selegiline compared to betahistine monotherapy, giving single dosages of 24, 48, and 96 mg betahistine in ascending dosage. The secondary objectives were the assessment of safety of the combination treatment and the pharmacokinetics of betahistine in different dosages in serum, namely the half-life time and the pharmacokinetic simulation of multiple dosing.
2. Methods
2.1. Study design
This study was an investigator-initiated (IIT), prospective, non-randomized, single sequence, two-period titration, open label single-center phase 1 study in healthy volunteers. It was conducted at the Department of Neurology, LMU University Hospital, LMU Munich, Munich, Germany with recruitment between 2 June 2021 and 9 September 2021.
2.2. Ethics approval and consent to participate
The study was performed in compliance with the requirements of the “Bundesinstitut für Arzneimittel und Medizinprodukte” (BfArM), Fachregistratur Klinische Pruefung, Kurt-Georg-Kiesinger-Allee 3, 53175 Bonn and Regierung von Oberbayern, Sachgebiet 55.2, 80534 Munich. It gained full regulatory approval from the Bundesinstitut für Arzneimittel und Medizinprodukte on 05.06.2020. The study was approved by the local ethics committees in Munich (19-992) on 25.05.2020. In the EudraCT register, the study was issued with the EudraCT number 2019-002610-39, registered on 17 June 2019 (first patient in 2 June 2021), named PK-BeST. As phase 1 studies are not public in the EudraCT register, the study was also registered in ClinicalTrials.gov.
2.3. Participants
Subjects were recruited via an announcement on the clinic homepage/intranet and were adult (age ≥18 years and ≤ 70 years) healthy volunteers (males and non-pregnant, non-breastfeeding women with adequate contraception) as determined by screening and the principal investigator's judgement. Participants were not allowed to take medication due to an illness and had normal blood pressure, heart rate, and ECG values.
Exclusion criteria were conditions in which repeated serum draws or injections pose more than minimal risk for the subjects, such as hemophilia, a positive test for alcohol or drugs, known contraindications for betahistine, such as bronchial asthma, and known contraindications for selegiline, e.g., hypersensitivity.
Each subject gave their written informed consent before any study-related procedures were performed.
2.4. Procedures
Study visits took place in an outpatient setting. The first step was to check the inclusion criteria at screening.
Subjects received single dosages of betahistine orally (24 mg, 48 mg, and 96 mg) with an at least 48-h wash-out period between the different dosages. In the second period, subjects were pre-treated with selegiline orally (5 mg/day) for 1 week and treated continuously with selegiline (5 mg/day) in combination with the single dosages of betahistine (24 mg, 48 mg, and 96 mg) with at least 2 days between the different dosages.
After absorption, betahistine is rapidly and almost completely metabolized into 2-PAA (which has no pharmacological activity in humans). After oral administration of betahistine, the plasma (and urinary) concentration of 2-PAA reaches its maximum 1 h after intake and declines with a half-life of about 3.5 h. A wash-out-time of 48 h, i.e., 15 times the half-life time, is sufficient and was feasible.
Vital signs and adverse events were checked for a minimum of 4 h after the intake of betahistine in the center. Serum samples to test serum concentrations of betahistine were obtained before and 10, 30, 60, 90, 120, 180, and 240 min after betahistine intake.
Safety parameters included assessment of adverse events, ECG, vital signs, laboratory measurements including kidney and liver function, full blood count, and pregnancy and drug screening test. Safety monitoring was conducted during every visit and assessment of vital parameters and adverse events was conducted 10, 30, 60, 90, 120, 180, and 240 min after betahistine intake. The clinical trial was conducted in a hospital of maximum care with the possibility to consult a specialist for possible symptoms of intoxication, specifically an anesthesiologist, cardiologist, and neurologist 24 h/7 days a week.
2.5. Randomization and masking
This was a phase 1 open label study. All subjects received the same treatment and no blinding process was involved. Due to safety reasons, ascending dosages were required by the federal institute so that no randomization was possible.
2.6. Outcomes
The primary endpoint was the area under the curve (AUC0-240 min) of the serum concentrations of betahistine after combination treatment with betahistine and 5 mg/d selegiline compared to betahistine monotherapy, giving single dosages of betahistine of 24, 48, and 96 mg in ascending dosage. Secondary endpoints were the half-life of betahistine in serum after intake of different dosages of betahistine and the occurrence of adverse effects due to combination treatment with betahistine and selegiline.
2.7. Simulations of multiple dosing of the three dosages examined
Computer simulations of multiple dosing with the three different dosages of betahistine and dosing regimens (three times per day and four times per day) were performed post-hoc to evaluate a possible accumulation over time. The simulations of multiple dosing regimens were conducted using a one-compartment model with PKTool, a free pharmacokinetic prediction tool developed with funding from the Bill & Melinda Gates Foundation (*https://www.mmv.org/research-development/computationalchemistry). As certain PK parameters necessary for simulating multiple dosing are unknown from empirical studies (e.g., Vd and F), estimates were obtained by fitting values to a one-compartment model using the single dose data with PKsolver (14).
2.8. Determination of sample size
The sample size chosen for this study was not based on statistical considerations. The number of subjects per dosage was chosen based on historical experience with safety and tolerance trials. The sample size falls within the range of those used in other studies of this nature. It was planned to enroll a total number of 15 subjects between 18 and 70 years of age in this trial at one trial site.
2.9. Statistical analysis
2.9.1. Preliminary analysis
Our preliminary analysis includes descriptive statistics of demographics, general physical examination, relevant medical history, and concomitant medication and neurological examinations. Numerical variables (e.g., age, BMI, serum pressure, and heart rate) are summarized in terms of mean (SD), median (minimum-maximum), and median [1st Qu., 3rd Qu.]. Categorical variables (e.g., gender and neurological abnormalities) are summarized in terms of frequency counts and percentages.
2.9.2. Preparatory graphical analysis
For our study, extensive graphical analyses preceded every population model analysis. Concentration-time data was plotted by individuals (one plot for each individual). Plots of the summarized data are also presented.
For pharmacokinetic parameters (AUC, half-life, elimination rate, Tmaxax, and Cmaxax), summary statistics are presented in a table followed by scatter plots on a linear y-axis and a log y-axis and bar charts. This is followed by the normality tests for linear and log transformed data.
2.9.3. Analysis of primary outcome
This trial aims to demonstrate that serum concentration of betahistine is higher due to combination treatment with selegiline compared to betahistine. The superiority of the combination treatment with selegiline compared to betahistine alone is based on the area under the curve (AUC0-240 min) of the serum concentrations of betahistine after combination treatment with betahistine and 5 mg/d selegiline compared to betahistine monotherapy, giving single dosages of betahistine of 24, 48, and 96 mg in ascending order. The efficacy of the experimental intervention was determined by AUC0-240 min of betahistine before and 10, 30, 60, 90, 120, 180, and 240 min after treatment in the two treatment arms.
All data were plotted as grouped (average of all participants) and as individual data. The graphs of each individual's serum betahistine concentration over time was used to detect possible errant data points. The data from individual plots were then used to calculate the pharmacokinetic parameters (AUC, half-life, elimination rate, Tmaxax, and Cmaxax). The values from each individual were then used to calculate a mean and standard error of the mean.
The aforementioned pharmacokinetic parameters were plotted as individual points on scatter plots to see if the data was normally distributed and to evaluate the effect of treatment (dose ± selegiline).
For statistical analysis, as all pharmacokinetic parameters seemed to be not normally distributed, the data were log transformed. There was no evidence that the data were not log normal. All statistical tests were performed on the Log10 data.
Analysis of the primary endpoint was performed using repeated measures ANOVA on the Log10 transformed AUC0-240 min values. A repeated measure ANOVA was used with each participant used as a blocking factor (its own control to reduce signal to noise). To determine differences between means, rather than comparing all possible means, pre-planned comparisons (“post hoc tests”) were performed to determine the effect of selegiline on pharmacokinetics.
2.10. Role of the funding source
This study was funded by Cures within Reach (CwR), Chicago, USA. This trial was not industry co-sponsored. The funder of the trial had no role in the development of the study design, data collection, data analyses, data interpretation, or the writing of this report. All authors had full access to all data in the study and all authors had final responsibility for the decision to submit for publication.
3. Results
3.1. Participants
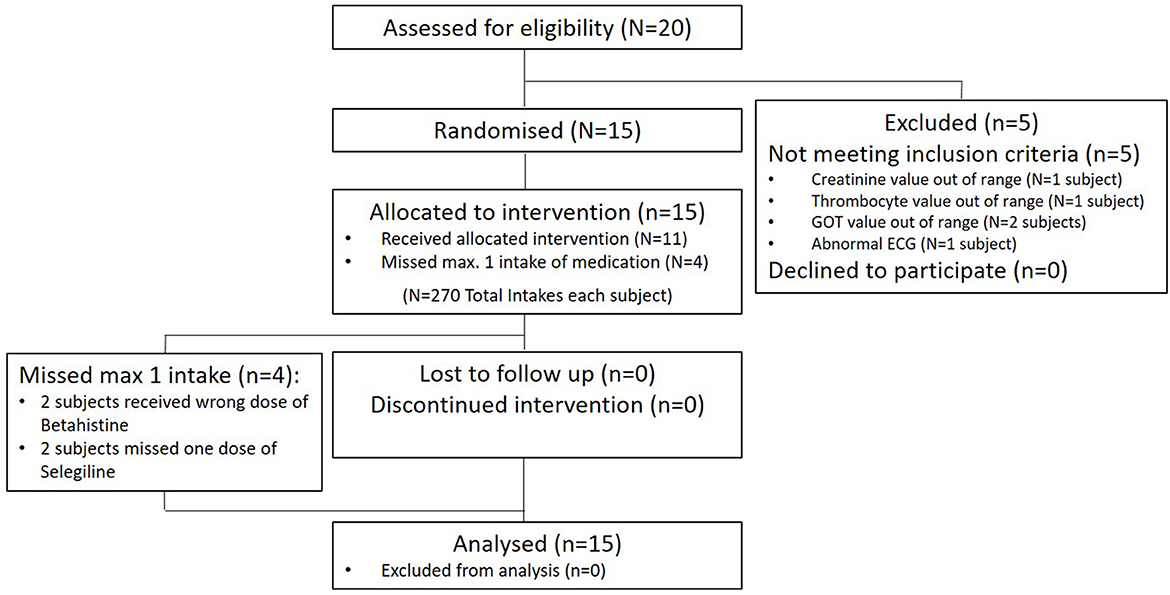
Twenty healthy volunteers were screened for this study between June 2, 2021 and September 9, 2021. Five subjects failed to enter the study for different reasons, mostly laboratory findings or abnormal ECG values. Fifteen participants were enrolled. Of these, all completed the trial and were included in the analysis. For details, see Figure 1. There were no dropouts over the course of the whole trial.
3.2. Descriptive data
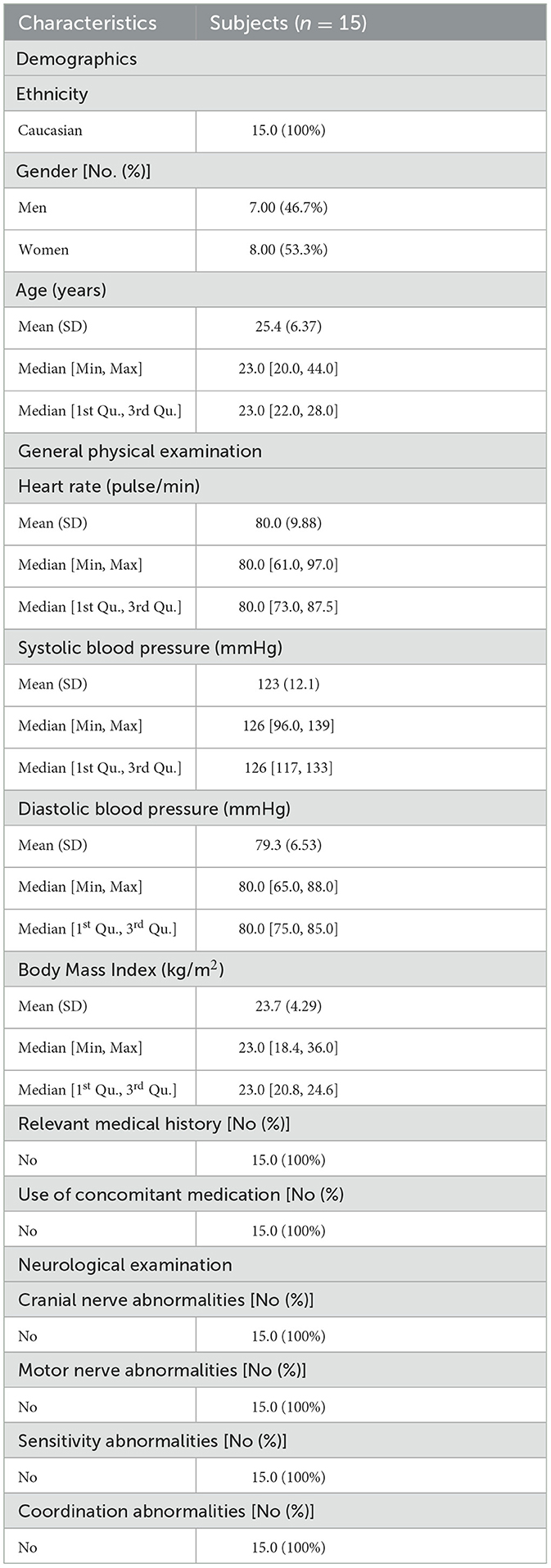
The baseline characteristics of the intention-to-treat sample include demographics, a general physical examination, relevant medical history, and concomitant medication and neurological examinations and are summarized in Table 1.
3.3. Results of the primary endpoint
The mean AUC of betahistine seems to increase with the increased dose of administered betahistine and, more prominently, with the administration of a combination treatment of betahistine and selegiline.
Our analysis showed that the primary endpoint (mean AUC of betahistine) increased in all three betahistine dosages:
- 77-fold (95% CI: 42.3 to 141.5) by the combination of betahistine 24 mg plus selegiline 5 mg/d,
- 86-fold (95% CI: 52.9 to 138.9) by the combination of 48 mg betahistine plus selegiline 5 mg/d, and
- 108-fold (95% CI: 61 to 190.9) by the combination of 96 mg betahistine plus selegiline 5 mg/d compared to betahistine monotherapy (Figure 2).
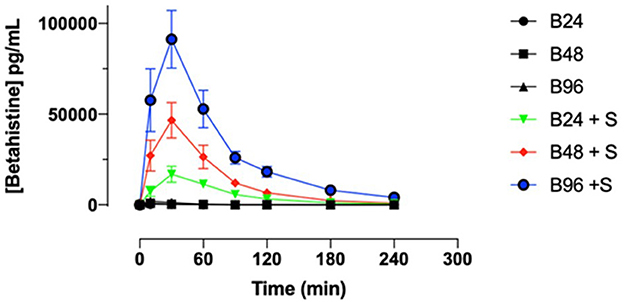
Figure 2. Selegiline increases the oral bioavailability of betahistine in humans as demonstrated by plots of serum concentrations of betahistine over time. Betahistine was orally dosed at 24, 48, and 96 mg/person (labeled on graph as B24, B48, and B96) alone or in combination with 5 mg selegiline (labeled on graph as B24 + S, B48 + S, and B96 + S). The total number of participants was 15 and each received all treatments.
For instance, the mean (+/-SD) of the area under curve for betahistine 48 mg alone was 0.64 (+/-0.47) h*ng/mL and for betahistine plus selegiline was 53.28 (+/-37.49) h*ng/ml. All the above results were statistically significant with a P < 0.0001, as shown in Table 2.
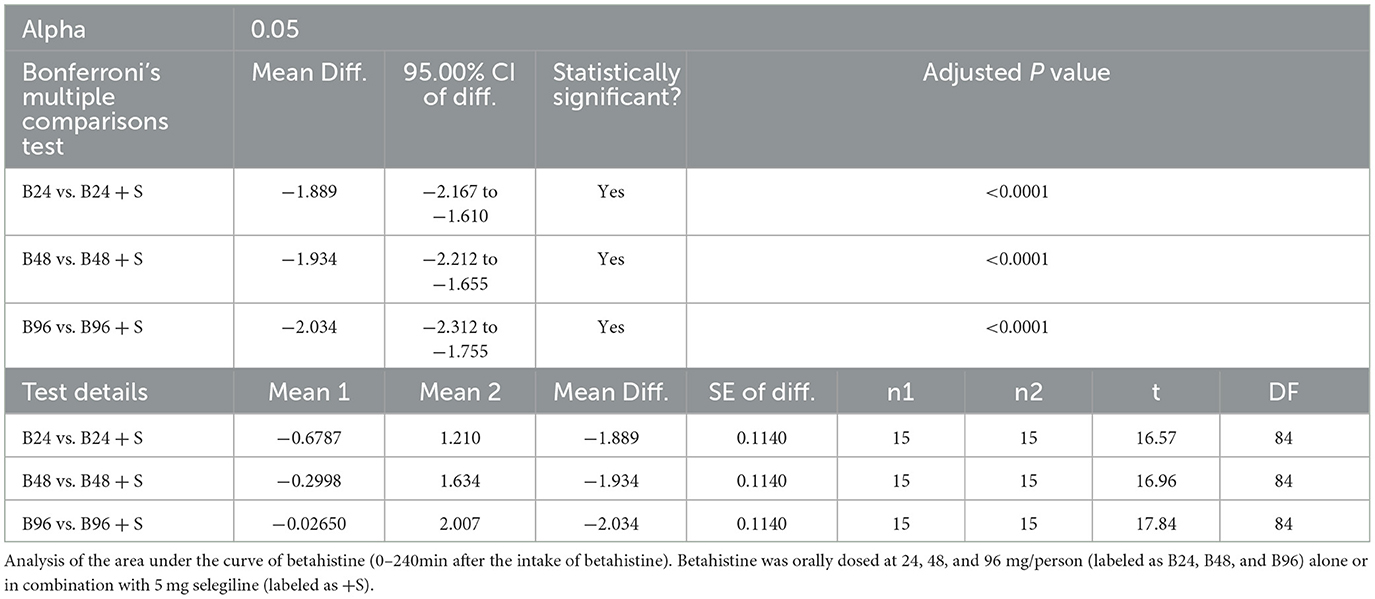
Table 2. Primary analysis for the ITT sample, mean AUC0−240min of betahistine following the administration of different betahistine doses with and without selegiline using repeated one-way ANOVA on the Log10 data.
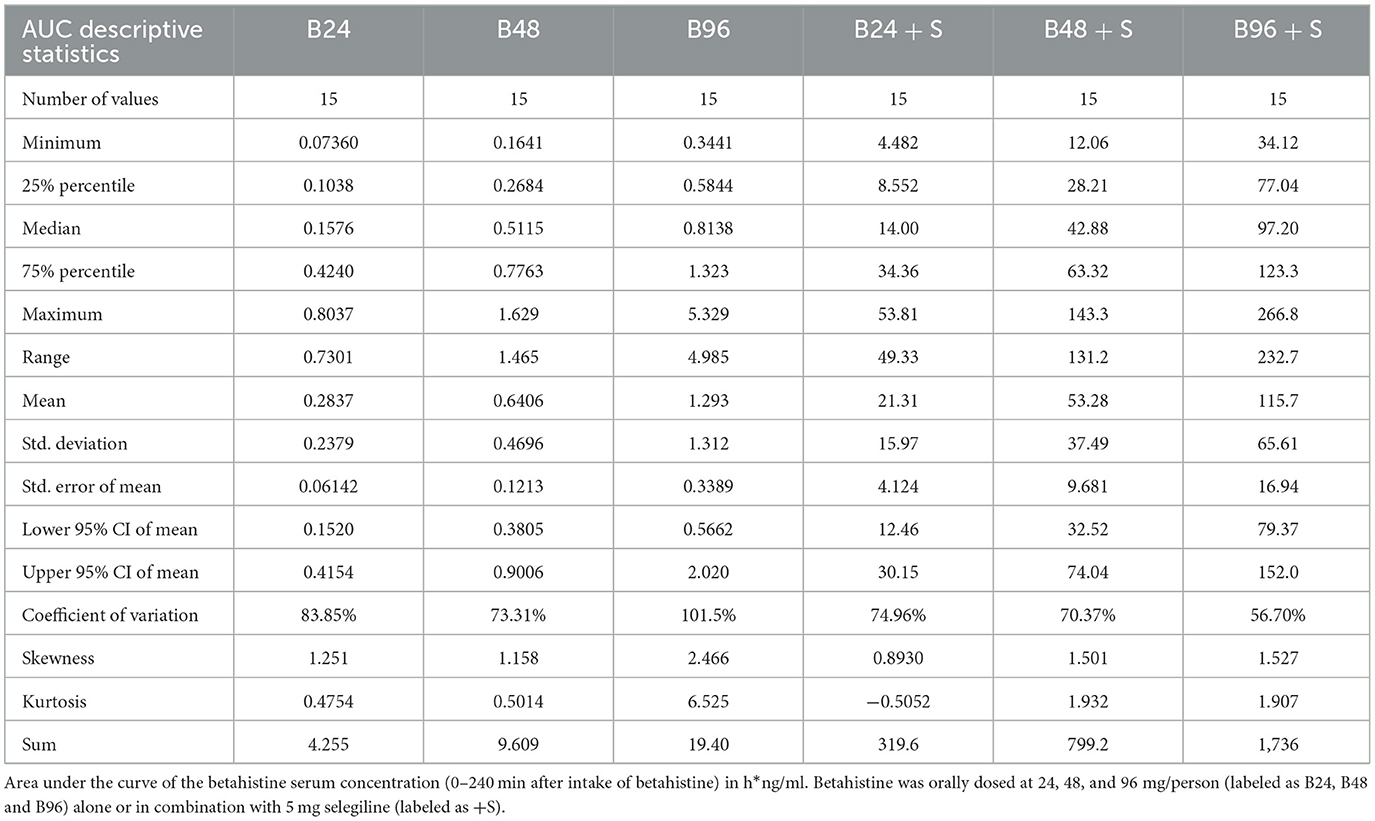
The summary statistics are shown in Table 3 and the details of the betahistine AUC0-240min in h*ng/ml are presented in Table 4. Figure 3 shows the scatter plots of the normal data and Figure 4 of log-normal AUC data following the administration of different betahistine doses with and without selegiline.
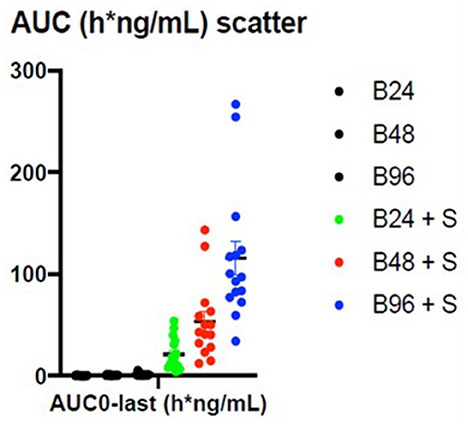
Figure 3. Scatter plots of the normal AUC0−240 min data in h*ng/ml following the administration of different betahistine doses with and without selegiline.
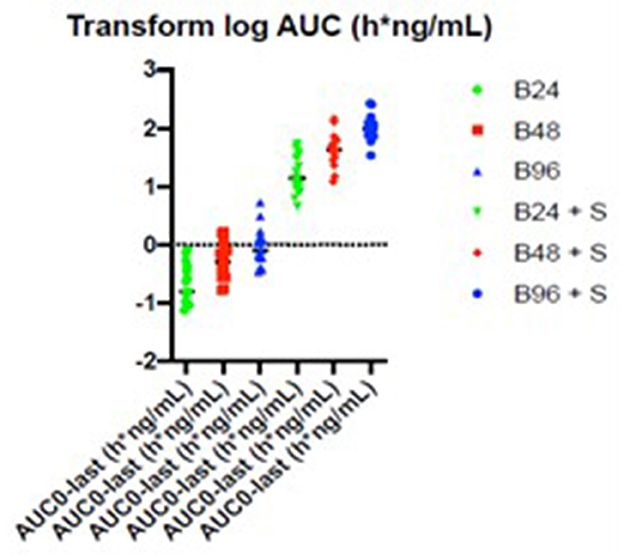
Figure 4. Scatter plots of the log-normal AUC0−240 min data in h*ng/ml following the administration of different betahistine doses with and without selegiline.
3.4. Results of the secondary endpoint
When examining the effect of the combination treatment of betahistine and selegiline vs. betahistine alone on the half-life of betahistine, a one-way repeated measure ANOVA and the post hoc tests (Bonferroni) showed a statistically significant increase in the half-life of betahistine following the combination treatment of betahistine 24 mg and selegiline 5 mg/d. The half-life of betahistine was 1.9 times higher, 95% CI (1 to 3.5) after a combination treatment of selegiline 5 mg/d with betahistine 24 mg. In contrast, there was no statistically significant difference in the half-life of betahistine between the combination treatment and the single treatment of betahistine 48 & 96 mg on the plasma half-life, as shown in Table 3 and the Supplementary Table S1.
When examining the effect of the combination treatment of selegiline and betahistine vs. betahistine alone on the Cmax of betahistine, a one-way repeated measure Anova and the post hoc tests (Bonferroni) showed a statistically significant 50-fold increase in Cmax of betahistine following the combination treatment of betahistine 24 mg and selegiline 5 mg/d.
There was no statistically significant difference between the combination treatment and the single treatment of betahistine 24, 48, and 96 mg on Tmax and elimination rate (ke) of betahistine.
3.5. Simulations of multiple dosing of the three dosages examined
Based on the above reported findings, simulations of multiple doses with different dosages of betahistine/selegiline and dosing regiments were performed. Figure 5 shows as an example the results for betahistine 48 mg eight times per day (348 mg daily dose) and 5 mg selegiline once daily. This analysis demonstrated that there was no accumulation over time. The other simulations are shown in the Supplementary Figures S1–S3.
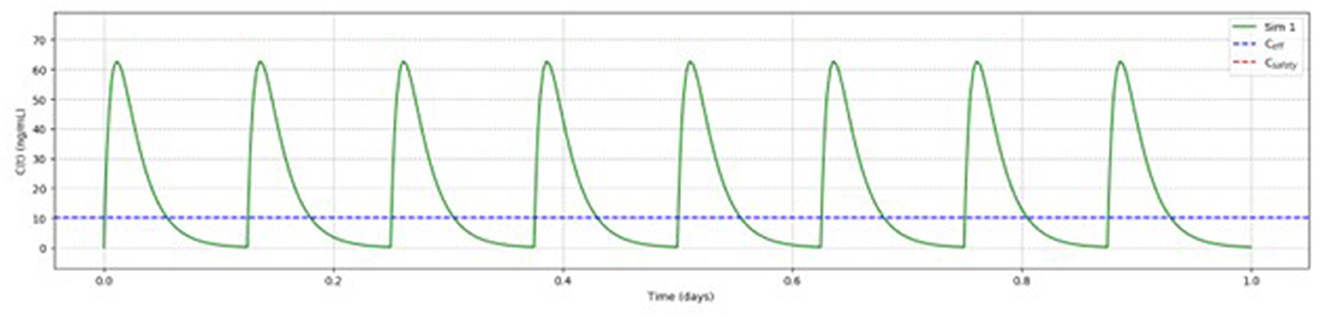
Figure 5. Pharmacokinetic modeling of 48 mg betahistine every 3 h (348 mg daily dose) and 5 mg selegiline once daily showed no accumulation of betahistine over time.
3.6. Safety results
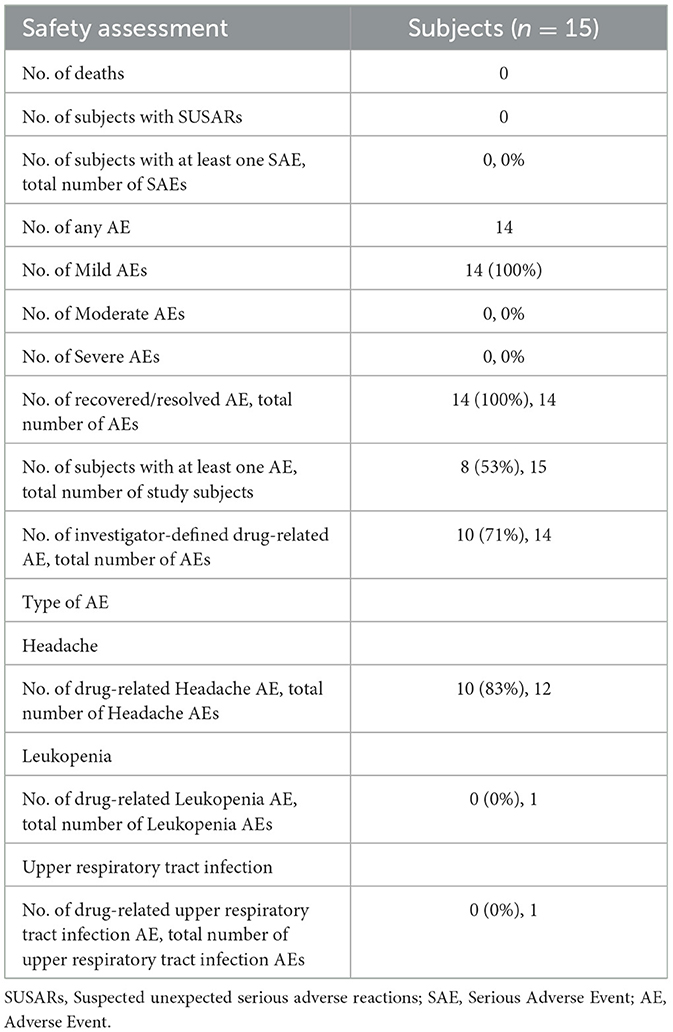
In total, 14 adverse events (AE) were documented throughout the course of the trial (Table 5). None of them were assessed as serious, hence no SAE and SUSAR were observed. All AEs were assessed as mild, and the outcomes of all AEs were classified as recovered or resolved. No concomitant medication was taken by the subjects.
In total, 12 adverse events reported were classified as headache (nervous system; MedDRA Code 10019211) and of these, 10 AEs were assessed as related to the combination treatment of betahistine and selegiline. One adverse event was classified as an upper respiratory infection (infections and infestations; MedDRA Code 10046300) and assessed as not related to the trial treatment, and one adverse event was classified as leukopenia (blood and lymphatic system disorders; MedDRA Code 10024384) and assessed as not related to the trial treatment. All adverse events that were assessed as related to the treatment were headaches. These headaches started 15 min to a few hours after the intake of betahistine after pre-treatment with selegiline and lasted a few hours without intake of concomitant medication. No AEs related to the therapy were observed due to therapy with betahistine without selegiline and due to concomitant treatment with 5 mg selegiline and 24 mg betahistine. Headaches related to the treatment were observed in six out of 15 subjects due to concomitant treatment with 5 mg selegiline and 48 mg betahistine and in six out of 15 subjects upon treatment with 5 mg selegiline and 96 mg betahistine.
4. Discussion
The major findings of this phase 1 pharmacokinetic study in 15 healthy volunteers are:
Firstly, the MAO-B inhibitor selegiline (5 mg orally per day) increases the bioavailability of orally administered betahistine (in dosages of 24, 48 and 96 mg) by a factor of between 77 and 108.
Secondly, the half-life of around 30 min was largely unaffected; simulations showed that there was no accumulation over time with multiple doses.
Thirdly, 14 AE were documented throughout the course of the trial. All of these were assessed as mild, and the outcomes of all AEs were classified as recovered or resolved. Twelve adverse events reported were classified as headache and, of these, 10 AEs were assessed as related to the combination treatment of betahistine and selegiline.
Based on these findings and pharmacokinetic analysis, which showed an increase in the bioavailability of betahistine when combined with selegiline, as well as a favorable safety profile, phase II studies are needed and justified to evaluate the clinical efficacy. This combination can be useful for the preventive therapy of Menière's disease (7), acute treatment of acute unilateral vestibulopathy (12, 13, 15), and post-BPPV residual dizziness (16, 17), in the latter two to improve central compensation.
In particular, for the preventive treatment of Menière's disease there is a high unmet medical need because
a) of its high prevalence: it is the second most frequent peripheral vestibular disorder (the reported life-time prevalence of Menière's disease is between 34 and 190 per 100,000) (16–18).
b) Betahistine has already been used for decades in dosages of up to 48 mg tid, although it is not superior to placebo (2); this finding is supported by a Cochrane review (4). It is also not approved by the FDA.
c) There is insufficient evidence for the effectiveness of many other measures that have been recommended, such as life-style changes, e.g., low salt diet (19), diuretics (4), pulsed low-pressure delivery (20), surgical interventions (21), intratympanic steroids (22), and intratympanic gentamicin (23). All in all, there is very limited evidence for the efficacy of any prophylactic therapy in MD that does not impair vestibular function.
Finally, in terms of possible mechanisms of action of betahistine in Menière's disease, it was demonstrated in pre-clinical studies that it dose-dependently increases cochlear blood flow (24) which is mediated via the H3-receptor (25); selegiline alone did not have an impact on cochlear blood flow (14). In another animal model, it was found that betahistine can even prevent endolymphatic hydrops (26), which may have an impact clinically in terms of even preventing bilateral Menière's disease.
Combining our pharmacokinetic data with published pharmacodynamic data enables us to refine the proposed mechanism of action of betahistine. Specifically, we can compare the Cmax values with the published affinities of the histamine receptors to evaluate target engagement. Based on radioactive binding experiments, H1, H3, and H2 receptors had Kd values for betahistine of 64 μM, 2 μM, and millimolar, respectively (27). In other words, betahistine had an affinity to H1 and H3 but not H2 and H4 receptors. Using the Hill equation to calculate occupancy and using Cmax (from data with selegiline) as the concentration of betahistine, it can be seen that H1 receptors are only occupied between 0.2 and 1%, whereas at the same Cmax values the H3 receptors are occupied between 6 and 26% (Table 6). In other words, the H3 receptor is occupied 25 times more than H1, and H1 is only 1% occupied at the highest dose of betahistine in the presence of selegiline. We conclude that betahistine is acting through pre-synaptic H3 receptors (autoreceptors), which when blocked release more histamine (and other neurotransmitters) and also increase histamine synthesis (28), which could then act on H1 receptors. In terms of Menière's disease, such indirect activation of the H1 receptor, which is found in a high density in the human endolymphatic membrane (29), could lead to an increased membrane permeability and reduction of endolymphatic pressure. However, it must be said that the theories regarding the etiology of Menière's disease are still not finally clarified and therefore it remains disputable what the effects of betahistine will be with regards to the course of the disease. In other vestibular diseases, namely central compensation of acute unilateral vestibulopathy and central compensation of post-BPPV residual dizziness, its benefit could be via the (indirect) activation of H1 receptor, as was demonstrated in an animal model (13, 30, 31).
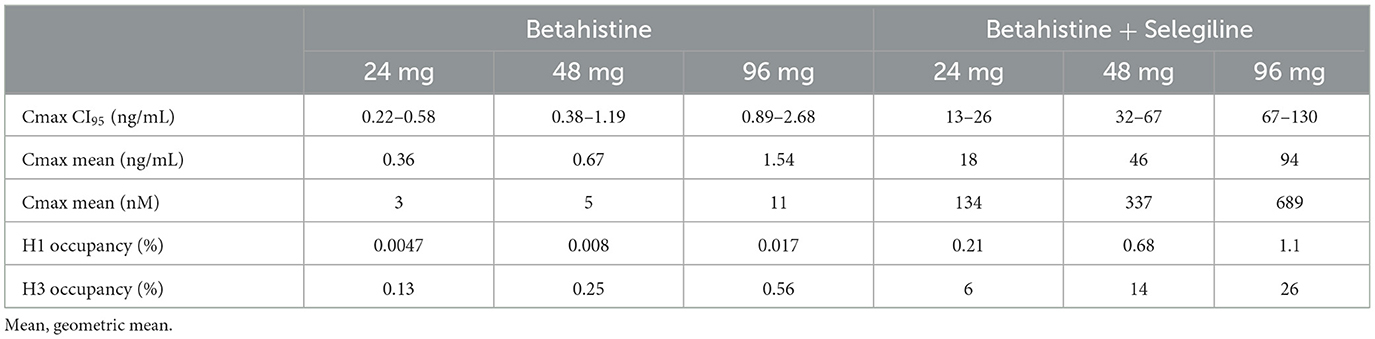
Table 6. Histamine receptor subtype occupancy during treatment with betahistine alone and betahistine combined with selegiline.
Finally, more broadly, these findings may also have an impact on the treatment of other diseases with betahistine. For instance, in high dosages it can improve cognition in schizophrenia (32).
4.1. Limitations
In the phase 1, single-center, open label study, only single dosages of betahistine and not the effects of repeated dosing was evaluated. However, the latter was studied by simulations. Only one dosage of selegiline was tested, which was half of the maximal dosage. As local authorities insist on ascending dosages for safety reasons, the study could not be randomized. For safety reasons, the sample size included only a small number of 15 healthy individuals.
Future phase 2 trials need to pay attention to contraindication of selegiline, a MAO-inhibitor, which can lead to serotonergic effects and cannot be combined with tricyclics (e.g., nortriptyline or amitriptyline) or SSRI/SNRI anti-anxiety antidepressants. This could limit the usability in patients with MD and migraine as well as depression. Both medications are well tolerated, and the side effect profile is well-defined. Side effects of betahistine include headache and gastric complaints; selegiline can lead to dizziness, headache, bradycardia, and a mild elevation of liver enzymes, so monitoring is necessary.
With regards to previous studies and analyses, which showed no effect of betahistine in MD in a dosage of up to 48 mg tid in comparison to placebo, and the uncertainties regarding the pathophysiology of Menière's disease and possible mode of action of betahistine in different diseases, it is uncertain if an increased bioavailability leads to a clinical effect. Therefore, placebo-controlled trials with a combination therapy in Menière's disease are needed. The same is true for acute vestibular syndrome or post-BPPV residual dizziness.
4.2. Conclusion
This phase 1 trial shows that the MAO-B inhibitor selegiline increases betahistine bioavailability with no safety concerns. Whether the increased bioavailability has an impact on the preventive treatment of Menière's disease, acute vestibular syndrome, or residual dizziness after BPPV has to be evaluated in placebo-controlled trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Local Ethics Committees in Munich (19-992). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MS: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing—original draft. GC: Data curation, Formal analysis, Methodology, Validation, Visualization, Writing—original draft. IN: Investigation, Project administration, Resources, Writing—review and editing. UM: Supervision, Writing—review and editing. AA: Data curation, Formal analysis, Validation, Writing—review and editing. AG: Investigation, Writing—review and editing. NG: Investigation, Methodology, Project administration, Validation, Visualization, Writing—original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by Cures within Reach (CwR), and the Knight family, Chicago, U.S.A.
Conflict of interest
The study received funding from Cures within Reach, and the Knight family, Chicago, U.S.A. The foundation and the Knight family had no role in the design, management, data collection, analyses, or interpretation of the data or in writing the manuscript or the decision to submit for publication. MS is Joint Chief Editor of the Journal of Neurology, Editor in Chief of Frontiers of Neuro-otology, and Section Editor of F1000, is investor and share-holder of IntraBio; IntraBio is the owner of the patent for the combined use of betahistine and selegiline, he has received speaker's honoraria from Abbott, Auris Medical, Biogen, Eisai, Grünenthal, GSK, Henning Pharma, Interacoustics, J&J, MSD, NeuroUpdate, Otometrics, Pierre-Fabre, TEVA, UCB, and Viatris, receives support for clinical studies from Decibel, U.S.A., Cures within Reach, U.S.A. and Heel, Germany, distributes “Mglasses” and “Positional vertigo App”, and acts as a consultant for Abbott, AurisMedical, Bulbitec, Heel, IntraBio, Sensorion and Vertify. GC is a founding scientist and consultant for IntraBio. NG acts as a reviewer for IntraBio and as Review Editor on the Editorial Board of Neuro-Otology (specialty section of Frontiers in Neurology).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1271640/full#supplementary-material
References
1. Jeck-Thole S, Wagner W. Betahistine: a retrospective synopsis of safety data. Drug Saf. (2006) 29:1049–59. doi: 10.2165/00002018-200629110-00004
2. Adrion C, Fischer CS, Wagner J, Gurkov R, Mansmann U, Strupp M. Efficacy and safety of betahistine treatment in patients with Meniere's disease: primary results of a long term, multicentre, double blind, randomised, placebo controlled, dose defining trial (BEMED trial). BMJ. (2016) 352:h6816. doi: 10.1136/bmj.h6816
3. Van Esch B, van der Zaag-Loonen H, Bruintjes T, van Benthem PP. Betahistine in Meniere's disease or syndrome: a systematic review. Audiol Neurootol. (2022) 27:1–33. doi: 10.1159/000515821
4. Webster KE, Galbraith K, Harrington-Benton NA, Judd O, Kaski D, Maarsingh OR, et al. Systemic pharmacological interventions for Ménière's disease. Cochrane Database Syst Rev. (2023) 2:Cd015171. doi: 10.1002/14651858.CD015171.pub2
5. Mammarella F, Loperfido A, Keeling EG, Bellocchi G, Marsili L. Ménière's disease: insights from an Italian Nationwide survey. Audiol Res. (2023) 13:160–8. doi: 10.3390/audiolres13020016
6. Agus S, Benecke H, Thum C, Strupp M. Clinical and demographic features of vertigo: findings from the REVERT registry. Front Neurol. (2013) 4:48. doi: 10.3389/fneur.2013.00048
7. Lezius F, Adrion C, Mansmann U, Jahn K, Strupp M. High-dosage betahistine dihydrochloride between 288 and 480 mg/day in patients with severe Meniere's disease: a case series. Eur Arch Otorhinolaryngol. (2011) 268:1237–40. doi: 10.1007/s00405-011-1647-2
8. Moorthy G, Sallee F, Gabbita P, Zemlan F, Sallans L, Desai PB. Safety, tolerability and pharmacokinetics of 2-pyridylacetic acid, a major metabolite of betahistine, in a phase 1 dose escalation study in subjects with ADHD. Biopharm Drug Dispos. (2015) 36:429–39. doi: 10.1002/bdd.1955
9. Sternson LA, Tobia AJ, Walsh GM, Sternson AW. The metabolism of betahistine in the rat. Drug Metab Dispos. (1974) 2:123–8.
10. Chen XY, Zhong DF, Duan JL, Yan BX, LC-MS-MS. analysis of 2-pyridylacetic acid, a major metabolite of betahistine: application to a pharmacokinetic study in healthy volunteers. Xenobiotica. (2003) 33:1261–71. doi: 10.1080/716689336
11. Myllyla VV, Sotaniemi KA, Vuorinen JA, Heinonen EH. Selegiline as initial treatment in de novo parkinsonian patients. Neurology. (1992) 42:339–43. doi: 10.1212/WNL.42.2.339
12. Tighilet B, Leonard J, Watabe I, Bernard-Demanze L, Lacour M. Betahistine treatment in a cat model of vestibular pathology: pharmacokinetic and pharmacodynamic approaches. Front Neurol. (2018) 9:431. doi: 10.3389/fneur.2018.00431
13. Antons M, Lindner M, Eilles E, Günther L, Delker A, Branner C, et al. Dose- and application route-dependent effects of betahistine on behavioral recovery and neuroplasticity after acute unilateral labyrinthectomy in rats. Front Neurol. (2023) 14:1175481. doi: 10.3389/fneur.2023.1175481
14. Kloos B, Bertlich M, Spiegel JL, Freytag S, Lauer SK, Canis M, et al. Low dose betahistine in combination with selegiline increases cochlear blood flow in Guinea Pigs. Ann Otol Rhinol Laryngol. (2022) 2022:34894221098803. doi: 10.1177/00034894221098803
15. Lacour M. Betahistine treatment in managing vertigo and improving vestibular compensation: clarification. J Vestib Res. (2013) 23:139–51. doi: 10.3233/VES-130496
16. Sayin I, Koç RH, Temirbekov D, Gunes S, Cirak M, Yazici ZM. Betahistine add-on therapy for treatment of subjects with posterior benign paroxysmal positional vertigo: a randomized controlled trial. Braz J Otorhinolaryngol. (2022) 88:421–6. doi: 10.1016/j.bjorl.2020.07.011
17. Li W, Sun J, Zhao Z, Xu J, Wang H, Ding R, et al. Efficacy of Epley's maneuver plus betahistine in the management of PC-BPPV: a systematic review and meta-analysis. Medicine (Baltimore). (2023) 102:e33421. doi: 10.1097/MD.0000000000033421
18. Harris JP, Alexander TH. Current-day prevalence of Meniere's syndrome. Audiol Neurootol. (2010) 15:318–22. doi: 10.1159/000286213
19. Webster KE, George B, Lee A, Galbraith K, Harrington-Benton NA, Judd O, et al. Lifestyle and dietary interventions for Ménière's disease. Cochrane Database Syst Rev. (2023) 2:Cd015244. doi: 10.1002/14651858.CD015244.pub2
20. Webster KE, George B, Galbraith K, Harrington-Benton NA, Judd O, Kaski D, et al. Positive pressure therapy for Ménière's disease. Cochrane Database Syst Rev. (2023) 2:Cd015248. doi: 10.1002/14651858.CD015248.pub2
21. Lee A, Webster KE, George B, Harrington-Benton NA, Judd O, Kaski D, et al. Surgical interventions for Ménière's disease. Cochrane Database Syst Rev. (2023) 2:Cd015249. doi: 10.1002/14651858.CD015249.pub2
22. Webster KE, Lee A, Galbraith K, Harrington-Benton NA, Judd O, Kaski D, et al. Intratympanic corticosteroids for Ménière's disease. Cochrane Database Syst Rev. (2023) 2:Cd015245. doi: 10.1002/14651858.CD015245.pub2
23. Webster KE, Galbraith K, Lee A, Harrington-Benton NA, Judd O, Kaski D, et al. Intratympanic gentamicin for Ménière's disease. Cochrane Database Syst Rev. (2023) 2:Cd015246. doi: 10.1002/14651858.CD015246.pub2
24. Ihler F, Bertlich M, Sharaf K, Strieth S, Strupp M, Canis M. Betahistine exerts a dose-dependent effect on cochlear stria vascularis blood flow in guinea pigs in vivo. PLoS ONE. (2012) 7:e39086. doi: 10.1371/journal.pone.0039086
25. Bertlich M, Ihler F, Freytag S, Weiss BG, Strupp M, Canis M. Histaminergic H3-Heteroreceptors as a potential mediator of betahistine-induced increase in cochlear blood flow. Audiol Neurootol. (2015) 20:283–93. doi: 10.1159/000368293
26. Pålbrink AK, In 't Zandt R, Magnusson M, Degerman E. Betahistine prevents development of endolymphatic hydrops in a mouse model of insulin resistance and diabetes. Acta Otolaryngol. (2023) 143:127–33. doi: 10.1080/00016489.2023.2171116
27. Fossati A, Barone D, Benvenuti C. Binding affinity profile of betahistine and its metabolites for central histamine receptors of rodents. Pharmacol Res. (2001) 43:389–92. doi: 10.1006/phrs.2000.0795
28. Nieto-Alamilla G, Márquez-Gómez R, García-Gálvez AM, Morales-Figueroa GE, Arias-Montaño JA. The histamine H3 receptor: structure, pharmacology, and function. Mol Pharmacol. (2016) 90:649–73. doi: 10.1124/mol.116.104752
29. Møller MN, Kirkeby S, Vikeså J, Nielsen FC, Caye-Thomasen P. Expression of histamine receptors in the human endolymphatic sac: the molecular rationale for betahistine use in Menieres disease. Eur Arch Otorhinolaryngol. (2016) 273:1705–10. doi: 10.1007/s00405-015-3731-5
30. Chen ZP, Zhang XY, Peng SY, Yang ZQ, Wang YB, Zhang YX, et al. Histamine H1 receptor contributes to vestibular compensation. J Neurosci. (2019) 39:420–33. doi: 10.1523/JNEUROSCI.1350-18.2018
31. Lang J, Ishikawa K, Hatakeyama K, Wong WH, Yin M, Saito T, et al. 3D body segment oscillation and gait analysis for vestibular disorders. Auris Nasus Larynx. (2013) 40:18–24. doi: 10.1016/j.anl.2011.11.007
Keywords: betahistine, selegiline, MAO-B inhibitor, phase 1 trial, Menière, vertigo
Citation: Strupp M, Churchill GC, Naumann I, Mansmann U, Al Tawil A, Golentsova A and Goldschagg N (2023) Examination of betahistine bioavailability in combination with the monoamine oxidase B inhibitor, selegiline, in humans—a non-randomized, single-sequence, two-period titration, open label single-center phase 1 study (PK-BeST). Front. Neurol. 14:1271640. doi: 10.3389/fneur.2023.1271640
Received: 02 August 2023; Accepted: 04 September 2023;
Published: 18 October 2023.
Edited by:
Shinichi Iwasaki, Nagoya City University, JapanReviewed by:
Tjasse Bruintjes, Gelre Hospitals, NetherlandsBabette Van Esch, Leiden University Medical Center (LUMC), Netherlands
Copyright © 2023 Strupp, Churchill, Naumann, Mansmann, Al Tawil, Golentsova and Goldschagg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Strupp, TWljaGFlbC5TdHJ1cHBAbWVkLnVuaS1tdWVuY2hlbi5kZQ==
 Michael Strupp
Michael Strupp Grant C. Churchill
Grant C. Churchill Ivonne Naumann1
Ivonne Naumann1 Amani Al Tawil
Amani Al Tawil Anastasia Golentsova
Anastasia Golentsova Nicolina Goldschagg
Nicolina Goldschagg