
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 12 October 2023
Sec. Stroke
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1271391
This article is part of the Research Topic Vascular Immunity and Ischemic Stroke View all 15 articles
A correction has been applied to this article in:
Corrigendum: Association between lactate/albumin ratio and 28-day all-cause mortality in ischemic stroke patients without reperfusion therapy: a retrospective analysis of the MIMIC-IV database
Objective: The lactate/albumin ratio (LAR) has been used as a novel prognostic indicator for aneurysmal subarachnoid hemorrhage, traumatic brain injury, sepsis, heart failure, and acute respiratory failure. However, its potential in predicting all-cause mortality in patients with ischemic stroke (IS) has not been evaluated. Therefore, this study aimed to elucidate the correlation between LAR and 28-day all-cause mortality in IS patients without reperfusion therapy.
Methods: This retrospective cohort study used data from the Medical Information Mart for Intensive Care (MIMIC-IV) (v2.0) database. It included 568 IS adult patients admitted to the intensive care unit (ICU). The correlation between LAR and ICU 28-day all-cause mortality rate was analyzed using multiple COX regression analysis and Kaplan–Meier survival analysis. Restricted cubic spline (RCS) curves were used to assess the relationship between LAR and 28-day mortality. In addition, a subgroup analysis was performed to investigate the impact of other influencing factors on outcomes. The primary outcome was the ability of LAR to predict 28-day mortality in IS patients.
Results: Among the 568 patients with IS, 370 survived (survival group) and 198 died (non-survival group) within 28 days of admission (mortality rate: 34.9%). A multivariate COX regression analysis indicated that LAR was an independent predictor of all-cause mortality within 28 days after admission for patients with IS (hazard ratio: 1.32; 95% confidence interval: 1.03–1.68; P = 0.025). We constructed a model that included LAR, age, race, sex, white blood cell count, Sequential Organ Failure Assessment (SOFA) score, and anion gap (AG) and established a prediction model with an area under the curve (AUC) value of 71.5% (95% confidence interval: 67.1%−75.8%). The optimal cutoff value of LAR that separated the survival group and the non-survival group based on the Youden index was 0.55. The Kaplan-Meier survival curves plotted using this critical value showed that patients with LAR ≥ 0.55 had a significantly higher 28-day all-cause mortality rate than patients with LAR < 0.55 (P = 0.0083).
Conclusion: LAR can serve as an independent predictor of all-cause mortality within 28 days after admission for patients with IS.
Ischemic stroke (IS) is a grave condition that affects the blood vessels in the brain and endangers the life of patients. It is the second most common cause of disability and death across the globe (1). Approximately 15% of IS patients die within 30 days (1). In recent years, the emergence of venous thrombolysis and endovascular thrombectomy has ushered us into a new era of IS treatment, wherein efficient reperfusion therapy is widely employed (2). However, numerous patients cannot be treated with reperfusion therapy in time, especially in developing countries. Therefore, there is an urgent need for simple and practical risk indicators that can inform the clinical management of IS patients without reperfusion therapy.
Lactate, a by-product of anaerobic metabolism, indicates the degree of tissue underperfusion and cellular oxygen deprivation (3). It can also forecast organ dysfunction and death in critically ill patients (4). Besides, it plays a crucial role in IS prognosis because it accumulates rapidly due to impaired diffusion of ischemic brain tissue, and its excess causes acidosis, which activates specific ion channels, leading to neurotoxic calcium accumulation and cytotoxic swelling (5). However, protein hydrolysis metabolism and metformin intake in patients with liver dysfunction or abnormalities can lead to abnormal lactate levels (6, 7). Therefore, relying solely on lactate levels for prediction may not guarantee reliable results.
Albumin is a vital protein that regulates blood osmotic pressure and influences the physiological function of the circulatory system. It also exhibits anti-inflammatory, antioxidant, and antithrombotic effects. However, serum albumin levels are influenced by kidney disease or nutritional status and therefore have limited value on their own in predicting IS outcomes (8, 9).
Some studies have explored the lactate/albumin ratio (LAR) as a potential predictor of acute pancreatitis, severe pneumonia, traumatic brain injury, and aneurysm subarachnoid hemorrhage (10–13). However, the link between LAR and mortality in IS patients remains unknown. Therefore, we obtained and analyzed data on IS patients admitted between 2008 and 2019 from the MIMIC-IV (v2.0) database. The current study aims to analyze the relationship between LAR and all-cause mortality within 28 days of admission in IS patients.
We obtained our data from the MIMIC-IV (v2.0), a large-scale, open-source database created and maintained by the MIT Computational Physiology Laboratory (https://physionet.org/content/mimiciv/2.0/). This database contains the records of all the patients hospitalized at the Beth Israel Deaconess Medical Center (BIDMC) between 2008 and 2019. It provides comprehensive data, such as length of stay, laboratory results, medication administration, vital signs, etc., for each patient. The data was anonymized by replacing personal information with random codes to protect patient privacy, so we did not require patient consent or ethical approval. The MIMIC-IV (v2.0) database is available for download from the PhysioNet online platform (https://physionet.org/). To access the database, the second author of this study, Chen HongZhuang, completed the Collaborative Institutional Training Initiative (CITI) course and passed the exams on “Conflict of Interest” and “Data or Sample Only Research” (ID: 52748910). The research team was then authorized to use the database and extract data.
We selected patients from the MIMIC-IV database using the following criteria: (1) age above 18 years, and (2) IS diagnosis based on ICD-9 codes 433, 434, 436, 437.0, and 437.1 or ICD-10 codes I63 and I65 (Figure 1). We excluded patients who underwent reperfusion therapy and those without lactate or albumin measurements. If patients had multiple ICU admissions, we only used clinical data from the first ICU admission. Ultimately, 568 patients were included in this study.
We selected LAR as the primary variable of interest. We used the first blood lactate and serum albumin levels measured after admission to reduce the influence of subsequent treatments on these values. Potential confounders, such as demographics (age, sex, and race), vital signs (heart rate, systolic blood pressure, and diastolic blood pressure), comorbidities (myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes, paraplegia, renal disease, AIDS, and hemorrhage), laboratory tests (red blood cell, white blood cell, and red blood cell distribution width, platelet, hemoglobin, and lymphocyte percentage, hematocrit, serum glucose level, anion gap, prothrombin time, and international normalized ratio), and sequential organ failure assessment (SOFA) scores were also extracted. Data extraction was performed using PostgreSQL (v13.7.1) and Navicate Premium (version 15) with structured query language. All the code for computing demographic features, laboratory tests, comorbidities, and severity scores were obtained from the GitHub website (GitHub - MIT-LCP/mimic-iv: Deprecated. For the latest MIMIC-IV code see: https://github.com/MIT-LCP/mimic-code).
This study classified the patients into two groups: those who survived in the hospital for 28 days (survival group, n = 370) and those who died in the hospital within 28 days (non-survival group, n = 198). The primary outcome of interest was all-cause mortality within 28 days of admission.
Variables with more than 15% missing values, such as blood cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, and C-reactive protein, were omitted to reduce bias. For variables with <15% missing values (lymphocyte, monocyte, and neutrophil counts), multiple imputation was applied to choose the best possible data set to impute the missing values (14).
The Kolmogorov-Smirnov test evaluated the normality of continuous variables. Continuous variables were reported as mean ± SD for normal distributions, median (IQR) for skewed distributions, and frequencies (%) for categorical variables. In the baseline characteristics analysis, continuous variables were compared using T-test or one-way ANOVA, while categorical variables were compared using Pearson's χ2 test and Fisher's test. A univariate COX regression analysis identified potential risk factors and a multivariate COX regression analysis determined the independent risk factors for in-hospital mortality with p-values below 0.05. A receiver operating characteristic (ROC) analysis assessed the model's predictive performance for 28-day in-hospital mortality by calculating the area under the curve (AUC), sensitivity, and specificity of the models. The Youden index was utilized to determine the optimal cutoff value.
Restricted cubic spline (RCS) analysis was employed to depict the non-linear relationship between LAR and 28-day all-cause mortality in IS subjects.
Kaplan–Meier curves were used to observe the relationship between LAR and mortality rate in IS patients. Subgroup analysis examined the effect of LAR on different characteristics, such as age, sex, race, SOFA scores, white blood cell (WBC) count, anion gap, ventilation status, and cerebral hemorrhage, and their p-values for interactions were also tested. All analyses were performed with free statistical software version 1.6 and R 4.1.3 (R Foundation for Statistical Computing, Vienna, Austria). P < 0.05 from two-tailed tests indicated statistical significance.
Table 1 shows the baseline characteristics of the survival and non-survival groups. Of the 568 patients who met the inclusion criteria, 309 (55.4%) were men and the median age was 67.8 (52.1, 83.5) years. The 28-day mortality rate was 34.9%. Non-survivors were older (P < 0.01) and had lower Glasgow Coma Scale scores, lower albumin levels (P < 0.05), higher SOFA scores, higher LAR [0.9 (0, 1.8) vs. 0.7 (0.1, 1.3), P = 0.002], and higher lactate levels (P < 0.05) than survivors. The other covariates were not significantly different between the groups (P > 0.05).
Unadjusted LAR showed a significant association with all-cause mortality within 28 days of admission [hazard ratio (HR), 1.45; 95% confidence interval (CI), 1.15–1.83; P = 0.002] according to the results of the univariate COX regression analysis (Table 2). In the multivariate COX regression analysis (Table 3), LAR remained significantly associated with higher in-hospital 28-day all-cause mortality after adjusting for potential confounding factors such as age, sex, and race (HR, 1.55; 95% CI, 1.23–1.96; P < 0.002) in Model 1. Moreover, in Model 2, which included additional adjustments for WBC count, anion gap, and SOFA score, LAR remained an independent predictor of increased mortality risk (HR, 1.32; 95% CI, 1.03–1.68; P = 0.025).
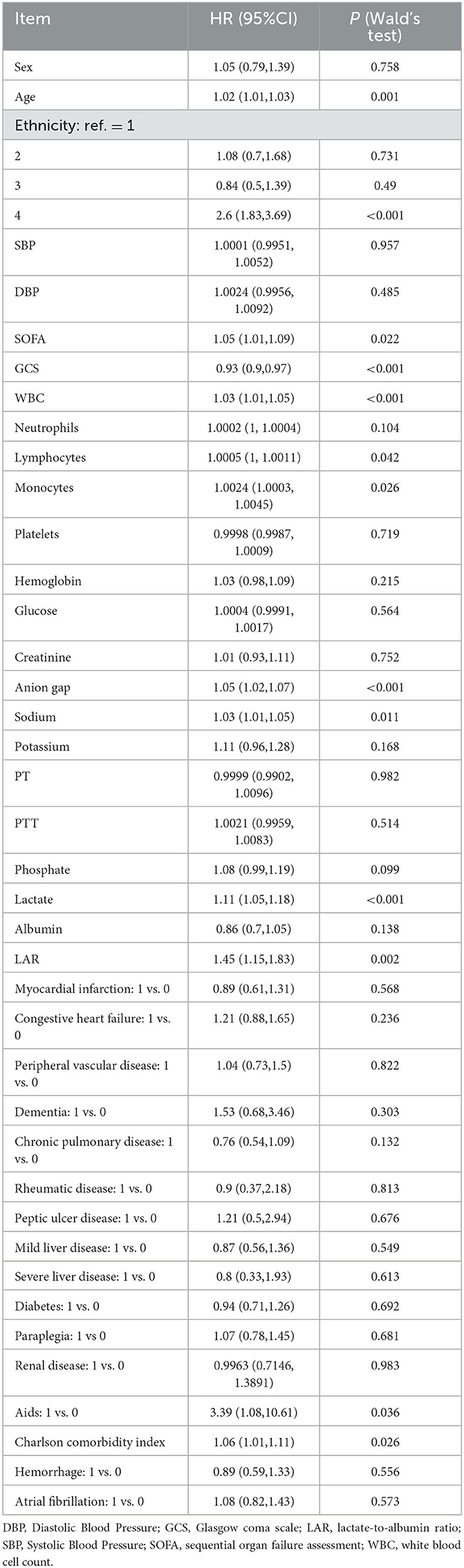
Table 2. Univariate Cox regression models evaluating the association between LAR and 28-day all-cause mortality with IS.
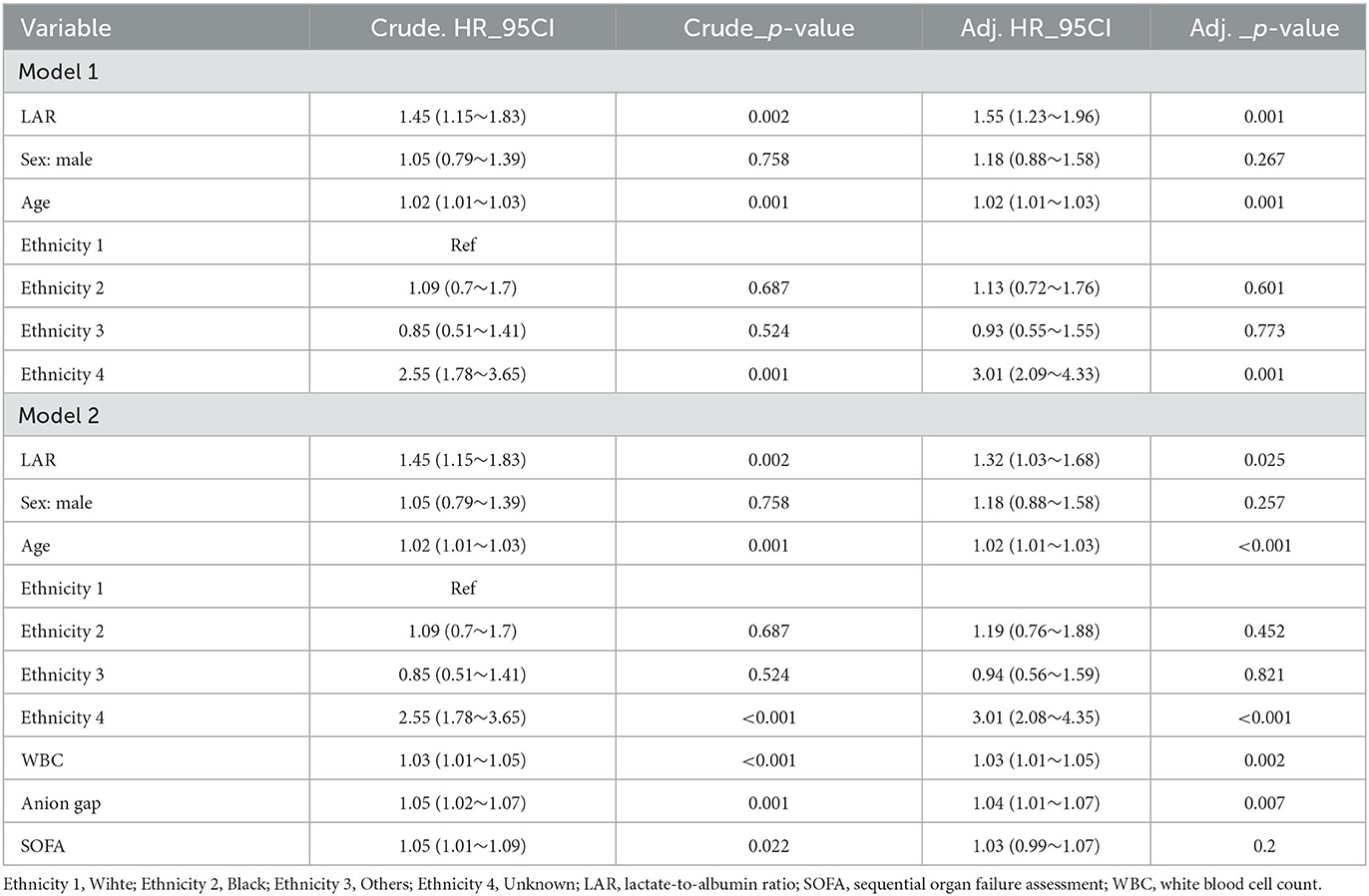
Table 3. Multivariable Cox regression models evaluating the association between LAR and 28-day all-cause mortality with IS.
Figure 2 displays the ROC curves of Model 2 plotted for predicting all-cause mortality within 28 days after admission of IS patients, and the AUC of the model was 71.5% (95% CI: 67.1%−75.8%). Additionally, Model 2 had a sensitivity of 75.13% and a specificity of 58.81%. Based on the Youden index, we selected the optimal threshold value to divide the IS patients into a high LAR group (LAR ≥ 0.55, n = 283) and a low LAR group (LAR < 0.55, n = 285). An RCS analysis was employed to assess the non-linear relationship between LAR and 28-day mortality in IS subjects (Figure 3). The Kaplan–Meier survival curves (Figure 4) show that the mortality rate of the high LAR group was significantly higher than that of the low LAR group (P = 0.0083).
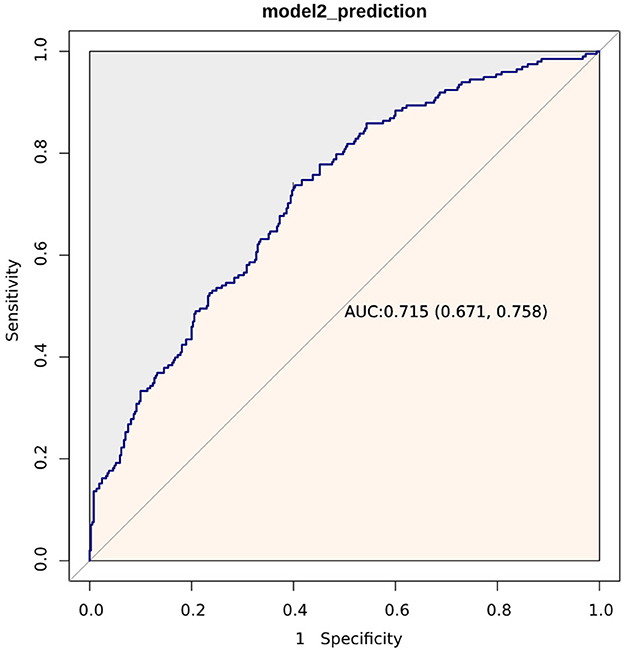
Figure 2. ROC curves of Model 2 for predicting 28-day mortality. Model 2 includes WBC count, anion gap, SOFA score, and LAR.
Figure 5 shows that the correlation between LAR and all-cause mortality within 28 days of admission of IS patients was stable across subgroups. The forest plot from the stratified analysis was performed for age, sex, race, SOFA score, WBC count, anion gap, ventilation status, and hemorrhagic transformation of cerebral infarction and showed that LAR had no significant interaction with each subgroup (interaction P: 0.073–0.735). These results prove that LAR was an independent prognostic factor.
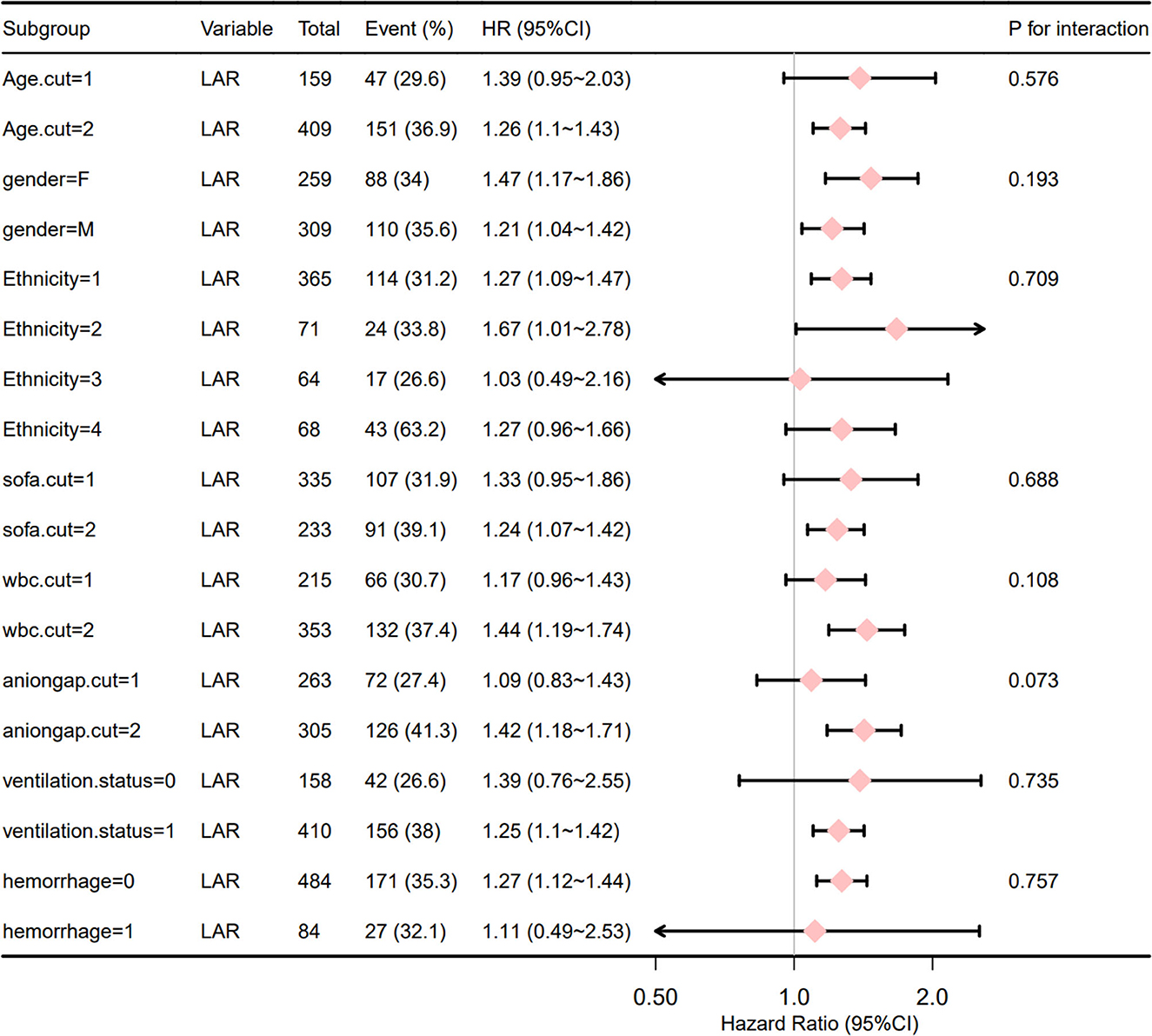
Figure 5. Forest plot for the subgroup analysis of the relationship between hospital mortality and LAR. Age.cut1, age < 67.8; Age.cut2, age > 67.8; Ethnicity1, White; Ethnicity2, Black; Ethnicity3, Other; Ethnicity4, Unknown; Sofa.cut1, sofa < 7; Sofa.cut2, sofa > 7; Wbc.cut1, WBC count < 13.1; Wbc.cut2, WBC count ≥ 13.1; aniogap.cut1, aniogap < 16.4; aniogap.cut2, aniogap > 16.4; for ventilation.status and hemorrhage, 0 means that the value does not exist and 1 means that it exists.
This is the first study examining the role of LAR in IS patients. The results of this retrospective study demonstrated that the LAR was an independent factor for all-cause mortality in IS patients without reperfusion therapy within 28 days of hospital admission. Our study included 568 patients from the MIMIC-IV (2.0) database. We conducted COX regression analysis to determine the independent predictive factors for 28-day mortality before and after adjusting for confounding factors. We found that LAR was consistently identified as an independent predictor of 28-day mortality. Additionally, we identified an optimal cutoff point (0.55), allowing us to construct a Kaplan–Meier curve and demonstrate that LAR effectively differentiated patients who died within 28 days. Furthermore, we adjusted for all confounding factors and created a forest plot, which showed that LAR remained a stable indicator unaffected by other variables. Therefore, LAR is reliable for predicting the 28-day mortality of IS patients and can be used as a novel clinical biomarker.
Lactate is an important indicator of tissue oxygenation, blood perfusion, and metabolism in the body. Hypoxia-induced acidosis in brain tissue is a sensitive indicator of brain injury (5). Lactate is a biomarker of ischemia produced by anaerobic glycolysis (15). Sakal et al. found that hyperlactatemia was correlated with increased mortality at 1, 3, and 12 months in IS patients (16, 17). However, interpreting serum lactate levels is indeed complex. For example, patients with liver disease may have abnormal lactate metabolism. Under hypoxic conditions, lactate production may also increase. Some drugs, such as salbutamol and metformin, can also elevate lactate levels. In addition, some critically ill patients may have lower lactate levels in venous blood, which reduces the reliability of lactate levels alone in predicting patient outcomes (18).
Serum albumin is associated with the outcome of IS (19). Serum albumin extravasation into the ischemic brain may provide neuroprotection by limiting metal-catalyzed oxidative stress (20). Gao et al. found that a decline in serum albumin levels after 90 days of acute large vessel occlusive stroke was independently associated with poor prognosis (21). Dziedzic et al. and Babu et al. suggested that higher serum albumin levels in acute stroke patients could reduce the risk of adverse outcomes (22, 23).
A meta-analysis (24) including 13,618 patients with acute IS or transient ischemic attack concluded that low serum albumin levels could predict adverse functional outcomes and mortality in patients with these diseases. However, different albumin detection methods in different studies may have biased the results. In addition, serum albumin levels are influenced by underlying diseases, nutritional status, and inflammation, which may limit its prognostic value as a single measurement. In the present study, we took the ratio of blood lactate to serum albumin, reducing the influence of a single factor on the regulatory mechanism by causing inverse changes through two different mechanisms, thus more accurately predicting the outcome for IS patients.
Recently, researchers explored the predictive value of LAR in the prognosis of neurosurgical diseases. For example, Wang et al.'s cohort study (12) on the mortality of patients with moderate to severe traumatic brain injury showed that non-survivors had higher LAR than survivors (1.09 vs. 0.53, P < 0.001), which was close to our results. Zhang et al. (13) established a prediction model for in-hospital mortality of patients with spontaneous subarachnoid hemorrhage. Independent predictors included age, LAR, anion gap, and Acute Physiology Score III, which was similar to our prediction model. Their results showed that LAR was closely related to increased in-hospital mortality of patients with spontaneous subarachnoid hemorrhage. However, studies using LAR to predict the outcome of ischemic stroke patients have yet to be reported.
Our results also confirmed previous research findings on the association between WBC count at admission and the prognosis of patients with ischemic stroke. Zheng et al. demonstrated that elevated WBC counts are correlated with stroke severity and adverse major and minor outcomes within a 3-month period (25). Furlan et al. reported that with each increase of 1 × 10(-9)/l in WBC count, there is a proportional rise in stroke severity, degree of disability at discharge, and 30-day mortality (26).
In addition to WBC count, our study also investigated the association between the anion gap and the prognosis of patients with ischemic stroke. Consistent with prior studies, Wang et al. observed a significant association between elevated AG values and increased all-cause mortality rates at 1 year, 4 years, and overall in patients with ischemic stroke who received rtPA treatment (27). Furthermore, Liu et al. demonstrated that high AG is an independent risk factor for all-cause mortality at 30 days, 60 days, and 180 days in patients with ischemic stroke (28). These collective study findings suggest that AG has the potential to serve as a biomarker for predicting the prognosis of patients with ischemic stroke.
Patients with IS admitted to the ICU have a higher mortality rate than other patients with IS. This may explain why the mortality rate of the patients included in our study was higher than the overall mortality rate of IS patients. In a study involving 370,386 ICU patients [including 7,046 (1.9%) stroke patients, with 4,072 having IS and 2,974 having intracerebral hemorrhage] (29), the short-term mortality rate of stroke patients admitted to the ICU was higher, with a 30-day mortality rate of 31% for IS patients, which is similar to our study.
In our study, LAR could be used as an independent predictor of 28-day all-cause mortality in IS patients without reperfusion therapy; it yielded a more accurate prognosis than blood lactate or serum albumin alone. This will provide medical workers with a better tool for clinic planning for poor patient outcomes. Further validation of LAR as a readily available and objective biomarker is still needed in large-scale multicenter prospective studies.
Our study has some limitations. First, it is a single-center retrospective cohort study, which cannot elucidate the relationship between LAR and IS as prospective studies do, to the extent that our findings need more persuasive power. Second, the drugs and hospital medical care, which may affect the LAR of patients with IS, were not recorded, which might bias our results. Lastly, although potential confounding factors such as myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, liver disease, and hemorrhage were not significantly present in our results, they should be considered and potentially excluded in future prospective studies.
LAR can serve as an independent predictor of all-cause mortality within 28 days after admission for IS patients without reperfusion therapy.
The data analyzed in this study was obtained from the Medical Information Mart for Intensive Care IV (MIMIC-IV) database, the following licenses/restrictions apply: To access the files, users must be credentialed users, complete the required training (CITI Data or Specimens Only Research) and sign the data use agreement for the project. Requests to access these datasets should be directed to PhysioNet, https://physionet.org/, doi: 10.13026/6mm1-ek67.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
YZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review and editing. HS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review and editing. HC: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing—review and editing. WJ: Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing—review and editing. WC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review and editing. JM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This work received no funding.
We gratefully appreciate all of the participants and staff for their contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AUC, area under the curve; AG, anion gap; CI, confidence interval; DBP, Diastolic Blood Pressure; HR, hazard ratio; IS, ischemic stroke; ICU, intensive care unit; LAR, lactate/albumin ratio; MIMIC-IV, Medical Information Mart for Intensive Care; RCS, Restricted cubic spline; SBP, Systolic Blood Pressure; SOFA, Sequential Organ Failure Assessment; WBC count, white blood cell count.
1. Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. Lancet. (2014) 383:245–55. doi: 10.1016/S0140-6736(13)61953-4
2. Fisher M, Savitz SI. Pharmacological brain cytoprotection in acute ischaemic stroke — renewed hope in the reperfusion era. Nat Rev Neurol. (2022) 18:193–202. doi: 10.1038/s41582-021-00605-6
3. James JH, Luchette FA, McCarter FD, Fischer JE. Lactate is an unreliable indicator of tissue hypoxia in injury or sepsis. Lancet. (1999) 354:505–8. doi: 10.1016/S0140-6736(98)91132-1
4. Doenyas-Barak K, Beberashvili I, Marcus R, Efrati S. Lactic acidosis and severe septic shock in metformin users: a cohort study. Critical Care. (2016) 20:10. doi: 10.1186/s13054-015-1180-6
5. M.Tóth O, Menyhárt Á, Frank R, Hantosi D, Farkas E, Bari F. Tissue acidosis associated with ischemic stroke to guide neuroprotective drug delivery. Biology. (2020) 9:460. doi: 10.3390/biology9120460
6. Katopodis P, Pappas EM, Katopodis KP. Acid-base abnormalities and liver dysfunction. Ann Hepatol. (2022) 27:100675. doi: 10.1016/j.aohep.2022.100675
7. Park J, Hwang SY, Jo IJ, Jeon K, Suh GY, Lee TR, et al. Impact of metformin use on lactate kinetics in patients with severe sepsis and septic shock. Shock. (2017) 47:582–7. doi: 10.1097/SHK.0000000000000782
8. Manolis AA, Manolis TA, Melita H, Mikhailidis DP, Manolis AS. Low serum albumin: a neglected predictor in patients with cardiovascular disease. Eur J Intern Med. (2022) 102:24–39. doi: 10.1016/j.ejim.2022.05.004
9. Gillum RF, Ingram DD, Makuc DM. Relation between serum albumin concentration and stroke incidence and death: the nhanes i epidemiologic follow-up study. Am J Epidemiol. (1994) 140:876–88. doi: 10.1093/oxfordjournals.aje.a117176
10. Liu Q, Zheng H-L, Wu M-M, Wang Q-Z, Yan S-J, Wang M, et al. Association between lactate-to-albumin ratio and 28-days all-cause mortality in patients with acute pancreatitis: a retrospective analysis of the mimic-iv database. Front Immunol. (2022) 13:1076121. doi: 10.3389/fimmu.2022.1076121
11. Xu C, Liu H, Zhang H, Zeng J, Li Q, Yi Y, et al. Predictive value of arterial blood lactate to serum albumin ratio for in-hospital mortality of patients with community-acquired pneumonia admitted to the intensive care unit. Postgrad Med. (2022) 135:273–82. doi: 10.1080/00325481.2022.2110769
12. Wang R, He M, Qu F, Zhang J, Xu J. Lactate albumin ratio is associated with mortality in patients with moderate to severe traumatic brain injury. Front Neurol. (2022) 13:662385. doi: 10.3389/fneur.2022.662385
13. Zhang G-G, Hao J-H, Yong Q, Nie Q-Q, Yuan G-Q, Zheng Z-Q, et al. Lactate-to-albumin ratio is associated with in-hospital mortality in patients with spontaneous subarachnoid hemorrhage and a nomogram model construction. Front Neurol. (2022) 13:1009253. doi: 10.3389/fneur.2022.1009253
14. Austin PC, White IR, Lee DS, van Buuren S. Missing data in clinical research: A tutorial on multiple imputation. Can J Cardiol. (2021) 37:1322–31. doi: 10.1016/j.cjca.2020.11.010
15. Kraut JA, Madias NE. Lactic acidosis. N Engl J Med. (2014) 371:2309–19. doi: 10.1056/NEJMra1309483
16. Brouns R, Sheorajpanday R, Wauters A, De Surgeloose D, Mariën P, De Deyn PP. Evaluation of lactate as a marker of metabolic stress and cause of secondary damage in acute ischemic stroke or tia. Clinica Chimica Acta. (2008) 397:27–31. doi: 10.1016/j.cca.2008.07.016
17. Sakal C, Ak R, Taşçi A, Kirkpantur ED, Ünal Akoglu E, Cimilli Ozturk T. Admission blood lactate levels of patients diagnosed with cerebrovascular disease effects on short- and long-term mortality risk. Int J Clin Pract. (2021) 75:e14161. doi: 10.1111/ijcp.14161
18. Bou Chebl R, Geha M, Assaf M, Kattouf N, Haidar S, Abdeldaem K, et al. The prognostic value of the lactate/albumin ratio for predicting mortality in septic patients presenting to the emergency department: a prospective study. Ann Med. (2021) 53:2268–77. doi: 10.1080/07853890.2021.2009125
19. Wang A, Zhang Y, Xia G, Tian X, Zuo Y, Chen P, et al. Association of serum albumin to globulin ratio with outcomes in acute ischemic stroke. CNS Neurosc Ther. (2023) 29:1357–67. doi: 10.1111/cns.14108
20. Gum ET, Swanson RA, Alano C, Liu J, Hong S, Weinstein PR, et al. Human serum albumin and its n-terminal tetrapeptide (dahk) block oxidant-induced neuronal death. Stroke. (2004) 35:590–5. doi: 10.1161/01.STR.0000110790.05859.DA
21. Gao J, Zhao Y, Du M, Guo H, Wan T, Wu M, et al. Serum albumin levels and clinical outcomes among ischemic stroke patients treated with endovascular thrombectomy. Neuropsychiatr Dis Treat. (2021) 17:401–11. doi: 10.2147/NDT.S293771
22. Dziedzic T, Slowik A, Szczudlik A. Serum albumin level as a predictor of ischemic stroke outcome. Stroke. (2004) 35:e156–8. doi: 10.1161/01.STR.0000126609.18735.be
23. Babu MS, Kaul S, Dadheech S, Rajeshwar K, Jyothy A, Munshi A. Serum albumin levels in ischemic stroke and its subtypes: correlation with clinical outcome. Nutrition. (2013) 29:872–5. doi: 10.1016/j.nut.2012.12.015
24. Zhou H, Wang A, Meng X, Lin J, Jiang Y, Jing J, et al. Low serum albumin levels predict poor outcome in patients with acute ischaemic stroke or transient ischaemic attack. Stroke Vasc Neurol. (2021) 6:458–66. doi: 10.1136/svn-2020-000676
25. Zheng X, Zeng N, Wang A, Zhu Z, Zhong C, Xu T, et al. Prognostic value of white blood cell in acute ischemic stroke patients. Curr Neurovasc Res. (2018) 15:151–7. doi: 10.2174/1567202615666180626154857
26. Furlan JC, Vergouwen MDI, Fang J, Silver FL. White blood cell count is an independent predictor of outcomes after acute ischaemic stroke. Eur J Neurol. (2013) 21:215–22. doi: 10.1111/ene.12233
27. Wang H, Liu C, Xu H, Zhang Y, Gao P, Geng S, et al. The association between serum anion gap and all-cause mortality in cerebral infarction patients after treatment with rtpa: a retrospective analysis. Dis Markers. (2022) 2022:1–10. doi: 10.1155/2022/1931818
28. Liu X, Feng Y, Zhu X, Shi Y, Lin M, Song X, et al. Serum anion gap at admission predicts all-cause mortality in critically ill patients with cerebral infarction: evidence from the mimic-iii database. Biomarkers. (2020) 25:725–32. doi: 10.1080/1354750X.2020.1842497
Keywords: lactate/albumin ratio, ischemic stroke, all-cause mortality, 28-day, prognosis
Citation: Zhong Y, Sun H, Chen H, Jing W, Chen W and Ma J (2023) Association between lactate/albumin ratio and 28-day all-cause mortality in ischemic stroke patients without reperfusion therapy: a retrospective analysis of the MIMIC-IV database. Front. Neurol. 14:1271391. doi: 10.3389/fneur.2023.1271391
Received: 02 August 2023; Accepted: 19 September 2023;
Published: 12 October 2023.
Edited by:
Yanlin Zhang, Second Affiliated Hospital of Soochow University, ChinaReviewed by:
Shubham Misra, Yale University, United StatesCopyright © 2023 Zhong, Sun, Chen, Jing, Chen and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiqiang Chen, MTUzOTczNzA5NDhAMTYzLmNvbQ==; Junqiang Ma, NzM2MjQ3NzgxQHFxLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.