
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 09 November 2023
Sec. Sleep Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1269945
Background: Obstructive sleep apnea (OSA) is an independent and modifiable risk factor in the initiation and maintenance of atrial fibrillation (AF). However, the effective of the continuous positive airway pressure (CPAP) on AF patients with OSA after ablation is elusive.
Methods: Cochrane Library, PubMed, Embase, and Web of Science were systematically searched up to February 1, 2023. Studies comprising the AF recurrence rate between the CPAP therapy group and non-CPAP therapy group for the AF patients with OSA were included. Meanwhile, trial sequential analysis (TSA) was conducted to adjust the lower statistical power and random error in this study. Subgroup analysis identified the potential determinants for the AF recurrence rate with CPAP therapy.
Results: A total of eight studies including 1,231 AF patients with OSA were eligible. Compared with non-CPAP treatment group, CPAP treatment group was statistically associated with a lower AF recurrence rate (risk ratio [RR], 0.58; p = 0.000). TSA indicated the firm evidence favoring CPAP group for AF recurrence risk. Three significant intervention-covariate interactions for AF recurrence was identified, including study design, non-paroxysmal AF (PAF) proportion, and CPAP treatment strategy.
Conclusion: Our study suggests that CPAP therapy might be an effective strategy on reducing AF recurrence post-ablation for AF patients with OSA. The CPAP treatment strategy and the non-PAF proportion might be the possible determinants on AF recurrence for AF patients with OSA after ablation.
Clinical trial registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023398588, identifier (CRD42023398588).
Atrial fibrillation (AF) is a commonly and clinically sustained arrhythmia, with an epidemiologically estimated prevalence of more than three million patients in the US, which could lead to stroke, heart failure, impaired quality of life, and increase of hospitalizations (1). Ablation therapy, such as radiofrequency (RF) or cryoballoon (Cryo) ablation, is an effective strategy for rhythm control for drug-refractory and symptomatic AF patients (2). Obstructive sleep apnea (OSA), a growingly common form of sleep-disordered breathing, is characterized by the repeated upper airway collapse during sleep, subsequently causing to sleep fragmentation and oxygen desaturation (3).
Accumulated studies demonstrated that OSA is an independent and modifiable risk factor for cardiovascular diseases, especially in the initiation and maintenance of AF, which was reportedly attributed to multiple abnormal pathophysiologies, including inflammation, oxidative stress, vascular endothelia dysfunction, and sympathetic overactivity (4–7). Moreover, OSA was also an key predictor for the AF prognosis post-ablation, suggesting that OSA could significantly influence the success rate of AF treatment (8). Therefore, the latest perspective for AF management recommended that the optimal management for OSA should be performed to reduce AF symptoms, AF progression, and AF recurrences (9).
CPAP has been considered to be an effective alternation to markly improve and correct the abnormal pathophysiologies underlying the OSA (10, 11). Growing evidence demonstrated that the CPAP therapy could significantly reduce the AF episodes in OSA patients (12, 13). However, the effective of the CPAP on AF patients with OSA after ablation is still controversial. The previous studies and meta-analysis have indicated that CPAP treatment on the AF patients with OSA could decline the risk of AF recurrence after ablation (14, 15). Whereas, a recent randomized controlled trial (RCT) showed the negative results (16).
Therefore, we perfomed this registered meta-analysis to clarify the role of CPAP on the AF patients with OSA after ablation, and identify the potential determinants for the AF recurrence rate between the CPAP therapy group and non-CPAP therapy group for the AF patients with OSA.
This study was performed following the preferred reporting items for reviews and the PRISMA guidelines. The official protocol was registered on the PROSPERO database (CRD42023398588).
Two reviewers (F. Li and C-J. He) independently and comprehensively searched for four databases, including PubMed, Embase, Web of Science, and Cochrane Library, from the establishment of the online databases up to 1 February 2023. The searching keywords mainly included “atrial fibrillation,” “AF,” “obstructive sleep apnea,” “OSA,” “continuous positive airway pressure,” “CPAP,” “ablation,” and “recurrence.” The detailed search strategy was presented in the Supplementary Text 1. Trails comprising the outcomes of AF ablation (mainly AF recurrence rate) between the CPAP therapy group and non-CPAP therapy group for the AF patients with OSA were included. A manual search was also conducted to screen the potential publications not being identified previously. Moreover, the relevant corresponding authors were contacted to acquire the missing data related outcomes in their publications.
Two reviewers (F. Li and C-J. He) comprehensively and independently reviewed full texts for screening the eligible studies, respectively. The eligible study would be confirmed if inclusion criteria were met: (1) RCT and observational studies, (2) studies comprising the AF recurrence rate between the CPAP therapy group and non-CPAP therapy group for the AF patients with OSA were included, and (3) studies with the most data of multiple publications for the same study. Studies review articles, editorials, letters, case reports, animal studies, and without original data were excluded. A third reviewer (H. Li) would discuss and resolve the potential disagreements on the eligibility.
Two researchers (C-J. He and F. Li), respectively, acquired the data from all eligible studies. Any controversies were consulted and resolved by a third one (R-X. Wang). First, we documented the eligible study characteristics, including publication year, the first author, study design, the sample size in the CPAP therapy group and non-CPAP therapy group, and follow-up time. Then, the study demographic and clinical characteristics, as well as the intervention-related indexes were also recorded.
A total of two appraisal tools were applied by two independent researchers (F. Li and C-J. He) to evaluate the quality of the eligible studies. For observational studies, the Newcastle-Ottawa Quality Assessment Scale (NOS), including three domains with nine points, was used. The study quality was divided into low quality (the total score < 6) and moderate-to-high quality (the total score ≥ 6). For randomized clinical trial included in this study, the Cochrane risk of bias assessment tool was used, providing a grade of risk of bias for the eligible study in multiple aspects of the study design (such as the selection bias, the performance bias, the detection bias, the attrition bias, and the reporting bias). Any potential controversies were resolved by a third one (C-H. Ding).
In this study, continuous variables were displayed as means ± standard deviations or median with interquartile range, and categorical variables were presented as frequencies or percentages. Relative risk (RR) and corresponding 95% confidence interval (CI) were calculated for the AF recurrence rate for all eligible studies. The Stata 16.0 was used for analyses. Statistically significant was defined as p < 0.05.
We utilized the I-squared (I2) and chi-squared test to quantify and evaluate the statistical heterogeneity of studies. If the I2 value was ≥50% and/or p < 0.05 for the chi-squared test, we defined that the between-study heterogeneity was substantial, and a random-effect model would be performed. Otherwise, a fixed-effect model was performed. Trial sequential analysis (TSA) is a useful method providing the required information size (RIS). We performed TSA with TSA viewer (version 0.9.5.10 Beta) to adjust the random error and lower statistical power potentially caused by the limited number of trials in the meta-analysis (17). We set the type I and II errors, respectively, to 5 and 20% (80% power). Sensitivity analysis was conducted by sequentially omitting one study at a time to evaluate the effect of a single study on the overall risk. Potential publication bias was also assessed using Egger’s test.
Additionally, subgroup analysis was conducted to screen the sources of heterogeneity, as well as the potential determinants for the AF recurrence rate between the CPAP therapy group and non-CPAP therapy group for the AF patients with OSA. Based on the study characteristics, previously reported factors and some potential factors, a total of eight subgroup factors were identified, including study design (single-center, observational study vs. RCT), CPAP group sample size (> 50 vs. ≤ 50), follow-up time (> 12 months vs. ≤ 12 months), CPAP treatment strategy (first ablation then CPAP vs. first CPAP then ablation), ablation strategy (PVI only vs. PVI plus), ablation energy (RF only vs. RF/Cryo), the non-PAF proportion (> 50% vs. ≤ 50%), and the mean age (≥60 years vs. <60 years).
Eight studies with 1,231 AF patients with OSA (628 in the CPAP therapy group and 603 in non-CPAP therapy group) were eligible, including seven observational studies (15, 18–23) and one RCT study (16). The selection flowchart was presented in Figure 1. The baseline characteristics and intervention-related indices of the eligible studies were displayed in Table 1.
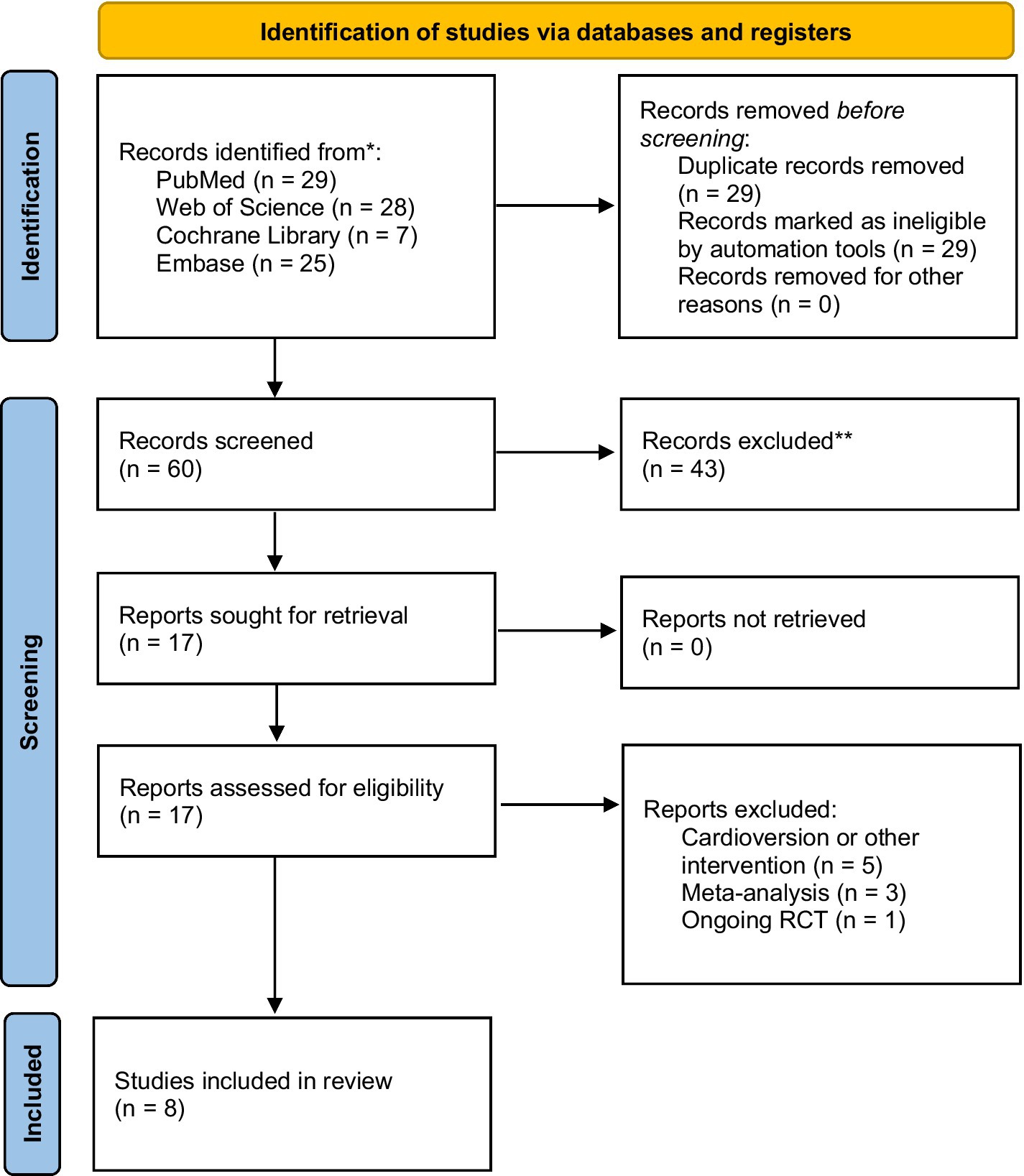
Figure 1. The flowchart for selecting the eligible studies (According to the PRISMA 2020 flow diagram).
The ablation energy was RF in two studies (16, 21) while that was RF/Cryo in the remaining six studies (15, 18–20, 22, 23). The ablation strategy of PVI plus was performed in six studies (18–23) while the PVI only was performed in the remaining two studies (15, 16). A total of seven eligible studies reported the CPAP treatment strategy (including first ablation then CPAP (15, 18) and first CPAP then ablation (16, 19, 20, 22, 23)) while one study (21) did not. Except for the Jongnarangsin et al. study (23) (only 7 months follow-up), the remaining seven studies (15, 16, 18–22) have a more than 12 months follow-up. Moreover, the non-PAF proportion was available only in five eligible studies (15, 16, 18, 19, 22), and the remaining three studies did not reported the proportion of AF type (20, 21, 23). All non-RCT studies showed a moderate-to-high quality (Supplementary Table S1). The RCT had a relatively high quality (Supplementary Table S2).
A total of eight studies (15, 16, 18–23), including 805 AF patients with OSA underwent ablation in our meta-analysis, reported the AF recurrence between CPAP group and non-CPAP group. Compared with non-CPAP treatment group, CPAP treatment group was statistically associated with a lower AF recurrence rate (RR, 0.58; 95% CI, 0.49–0.69; p = 0.000; I2 = 42.80%; Figure 2) using a fixed-effect model. Meanwhile, we conducted sensitivity analysis and found that no significant change, ranging from 0.53 (95% CI, 0.44–0.64) to 0.63 (95% CI, 0.52–0.76), in the overall combined proportion, indicated that the combined proportion and heterogeneity could not be dominated via a single study (Supplementary Figure S1). Additionally, publication bias was not displayed with Egger’s test (p = 0.939).
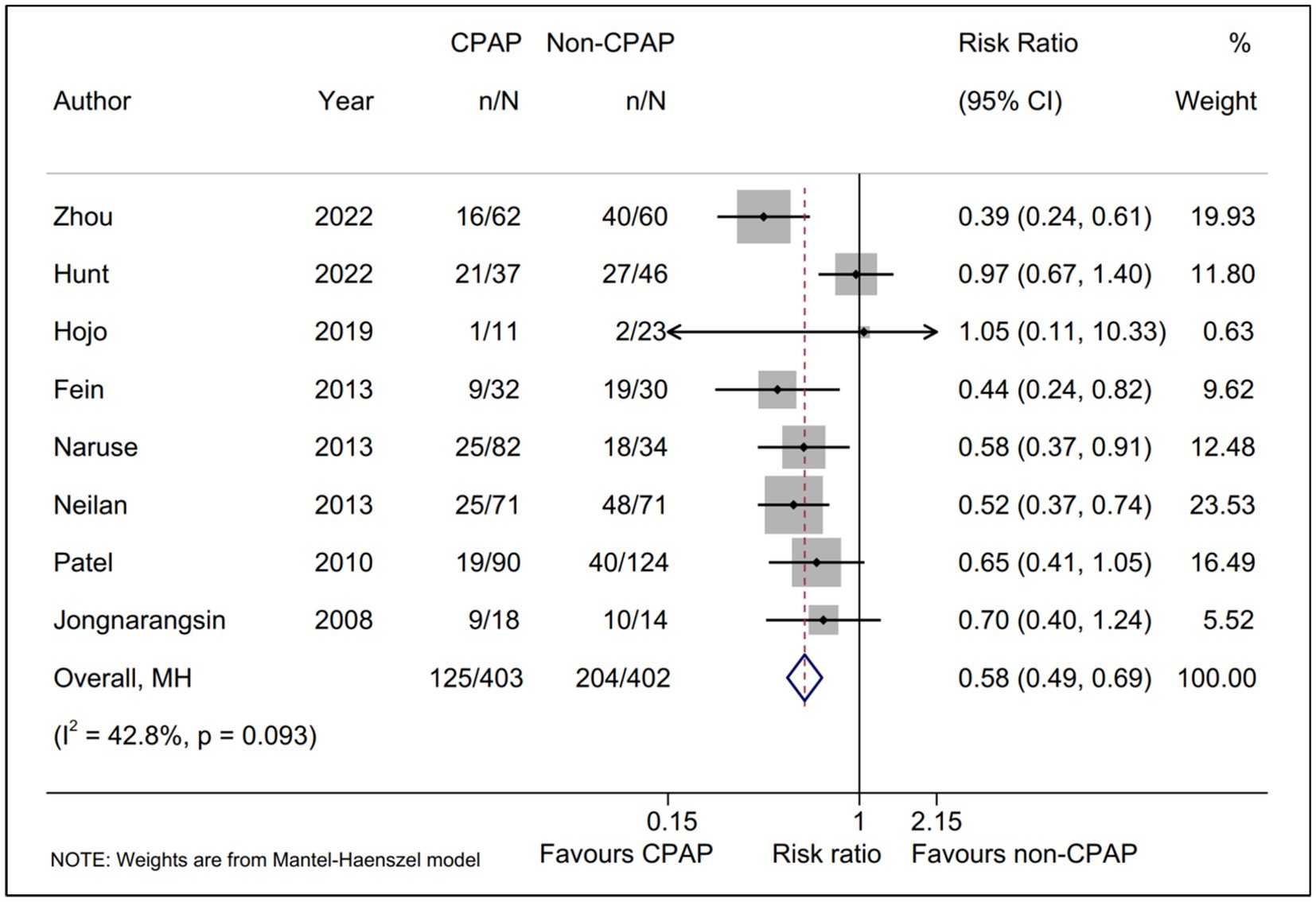
Figure 2. Forest plot of the AF recurrence between CPAP group and non-CPAP group. Comparison of the rate of AF recurrence between CPAP group and non-CPAP group. CPAP, continuous positive airway pressure; AF, atrial fibrillation.
Moreover, TSA was performed to evaluate whether there was a relatively adequate power for comparing the AF recurrence between CPAP group and non-CPAP group. The result suggested that the actual sample size (805) was more than the RIS (relative risk reduction = 30%; RIS = 629), and the cumulative Z curve crossed the conventional boundary, as well as the trial sequential alpha spending monitoring boundary. All TSA results revealed that there was the firm evidence favoring CPAP treatment group for the AF recurrence rate (Supplementary Figure S2).
A total of six subgroup factors for the AF recurrence was identified for the subgroup analysis, and the results were shown in Figure 3 and Supplementary Table S3. A statistically significant intervention-covariate interaction was identified in study design subgroup, including single-center, observational study (RR = 0.53; 95% CI, 0.44–0.64; p = 0.000) and RCT (RR = 0.97; 95% CI, 0.67–1.40; p = 0.859) with p = 0.005 for interaction. Meanwhile, a borderline statistically significant intervention-covariate interaction was screened in CPAP treatment strategy subgroup, including first ablation then CPAP (RR = 0.41; 95% CI, 0.26–0.64; p = 0.000) and first CPAP then ablation (RR = 0.67; 95% CI, 0.54–0.83; p = 0.000) with p = 0.049 for interaction. Additionally, the consistent trend with the pooled results was shown in the remaining four subgroup analysis, respectively. Interestingly, the potentially significant trend for intervention-covariate interaction were identified in sample size subgroup with p = 0.062.
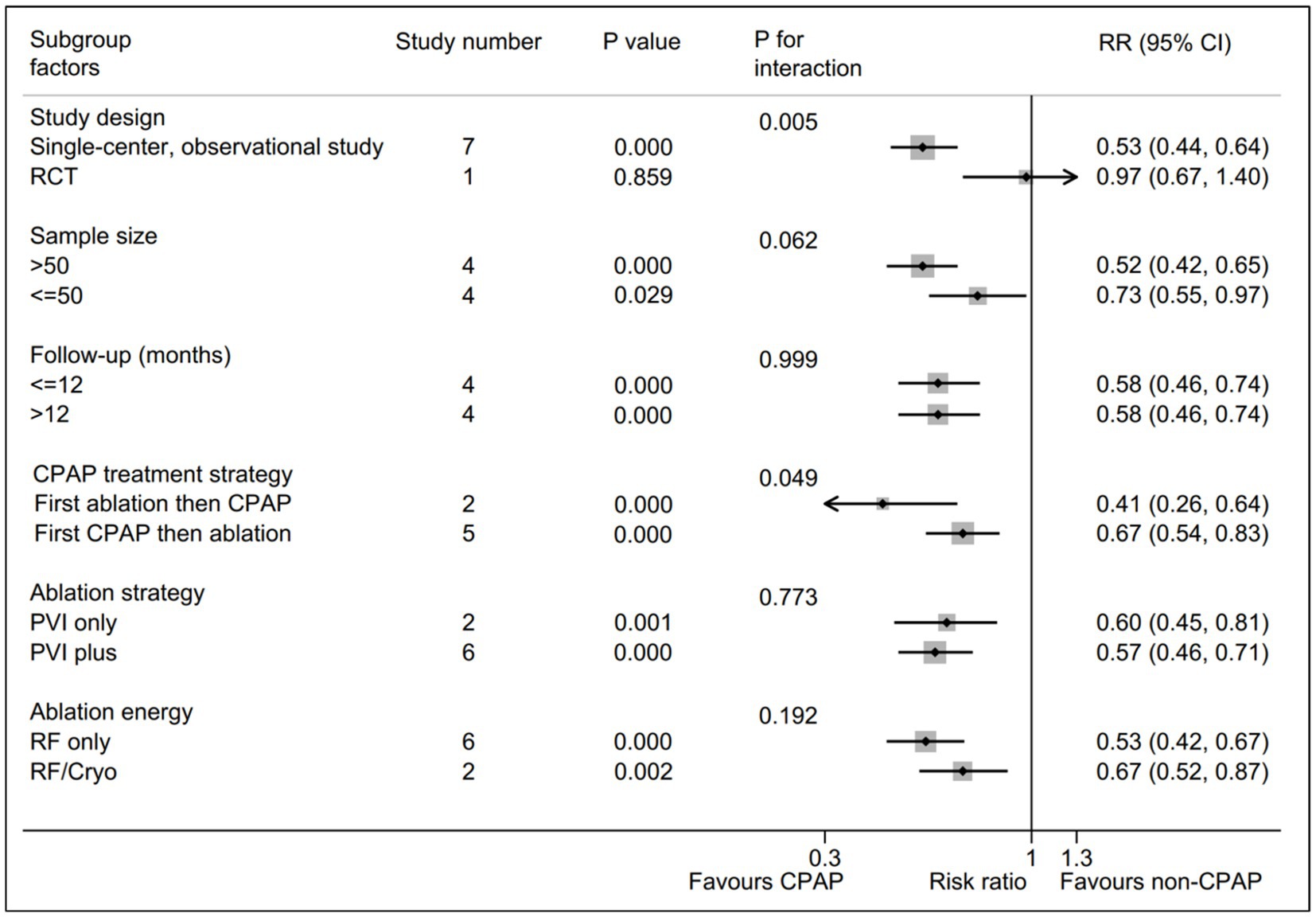
Figure 3. Forest plot of subgroup analysis for the AF recurrence between CPAP group and non-CPAP group. Subgroup analysis of the rate of AF recurrence between CPAP group and non-CPAP group. CPAP, continuous positive airway pressure; AF, atrial fibrillation.
Considering the limited data, we separately evaluated the non-PAF proportion subgroup (Figure 4). The pooled AF recurrence with the five eligible studies (15, 16, 18, 19, 22) using a random-effect model was consistent with the pooled result from all eligible studies (15, 16, 18–23). Interestingly, a statistically significant intervention-covariate interaction for AF recurrence was identified in non-PAF proportion subgroup, including >50% subgroup (RR = 0.49; 95% CI, 0.35–0.68; p = 0.000) and ≤ 50% subgroup (RR = 0.97; 95% CI, 0.67–1.40; p = 0.866) with p = 0.006 for interaction. Meanwhile, the five eligible studies (15, 16, 18, 19, 22) reported the age between CPAP and non-CPAP group, and we found that compared with non-CPAP group, the AF recurrence of CPAP group was significantly decreased in <60 years subgroup (RR = 0.57; 95% CI, 0.39–0.83; p = 0.003) and it was not in ≥60 years subgroup (Figure 5).
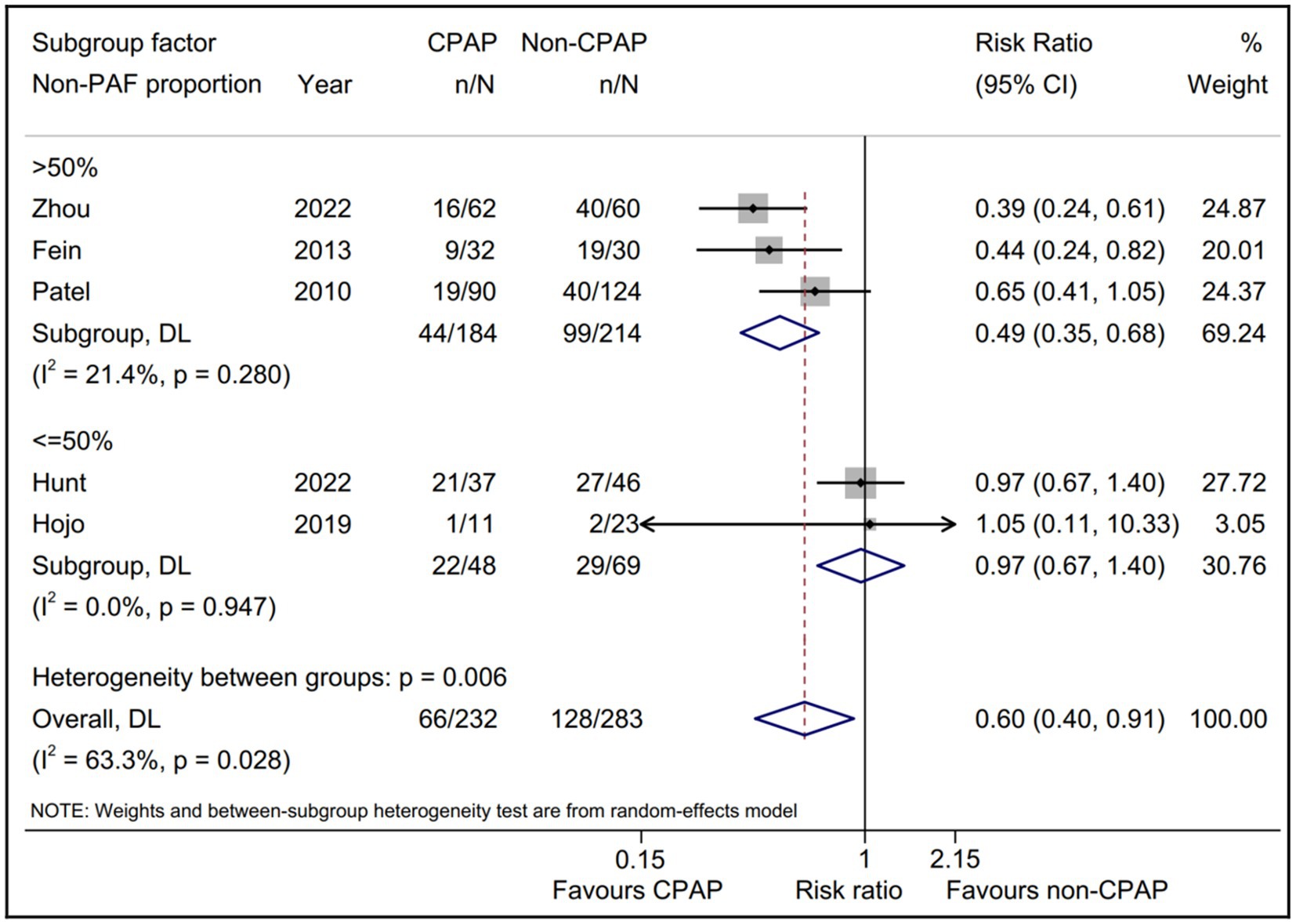
Figure 4. Forest plot of the non-PAF proportion subgroup analysis for the AF recurrence between CPAP group and non-CPAP group. CPAP, continuous positive airway pressure; AF, atrial fibrillation; PAF, paroxysmal atrial fibrillation.
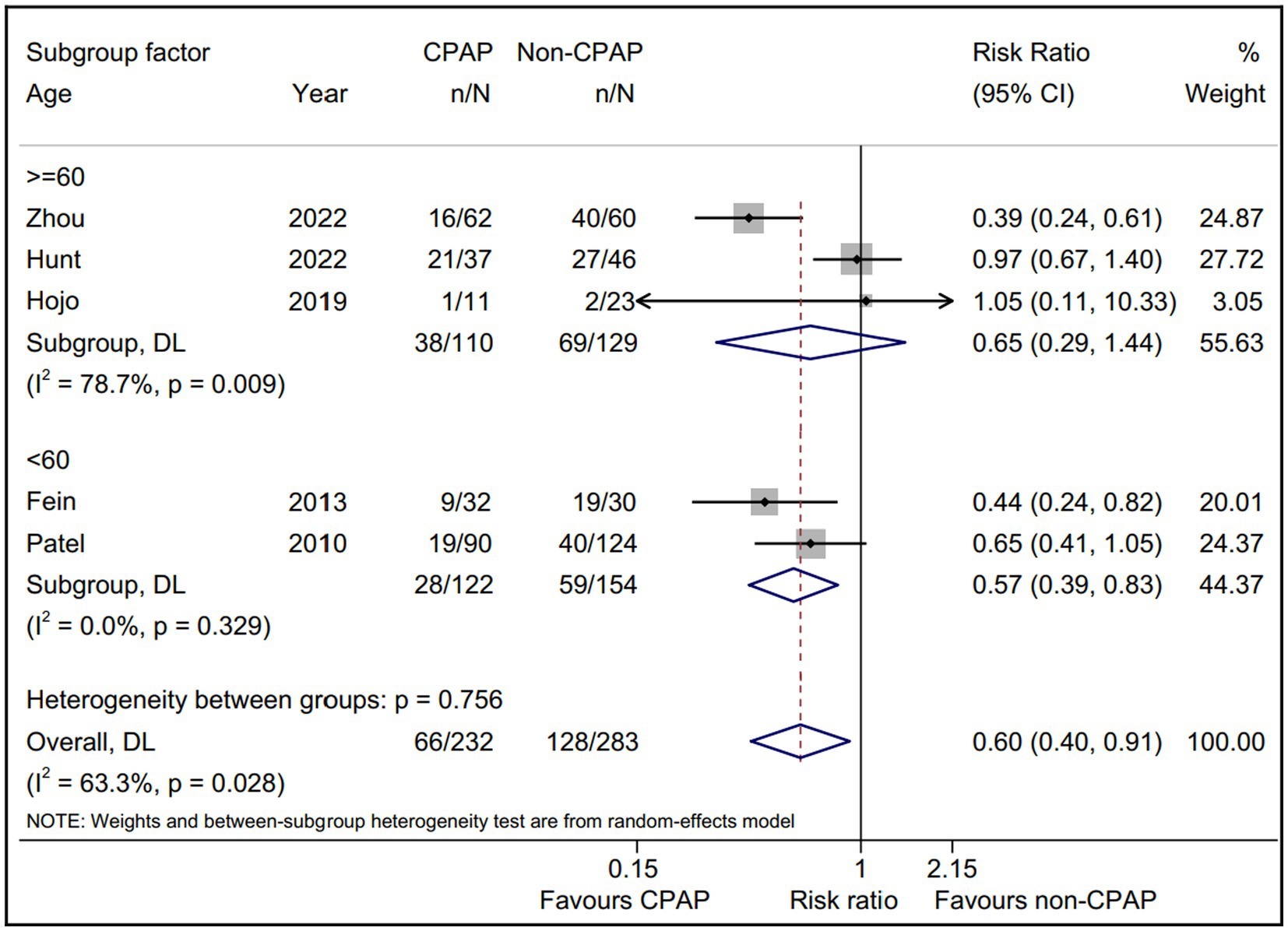
Figure 5. Forest plot of the age subgroup analysis for the AF recurrence between CPAP group and non-CPAP group. CPAP, continuous positive airway pressure; AF, atrial fibrillation.
We comprehensively enrolled a total of 1,231 AF patients with OSA (628 in the CPAP therapy group and 603 in non-CPAP therapy group) from eight clinical studies. To the best of our knowledge, this study might be the first registered meta-analysis to compare the AF recurrence for AF patients with OSA underwent ablation between CPAP therapy group and non-CPAP therapy group. The main findings are as follows: (1) CPAP therapy may be an effective strategy on reduction of AF recurrence after ablation for AF patients with OSA; (2) The CPAP treatment strategy and the non-PAF proportion might be the potential determinants of outcome impact on AF recurrence for AF patients with OSA after ablation.
A significantly epidemiological association was presented between OSA and AF. The scientific statement from American Heart Association on the OSA and cardiovascular disease have highlighted that OSA is an predominantly independent and modifiable risk factor in the initiation and progression of AF (24). West et al. (25) even reported that the AF risk was expected to increase up to 3.31 among the individuals with severe OSA. At present, the latest ESC guideline for AF management suggested that radiofrequency ablation therapy has been considered as an effective strategy for rhythm control in symptomatic and drug-refractory AF patients (2). Whereas, challenges remain on the AF patients with multiple comorbidities, especially with the underrecognized and undertreated OSA. A meta-analysis with a total of 4,572 AF patients underwent catheter ablation conducted by Congrete et al. (14) reported that the AF recurrence rate in patients with OSA was as high as 1.70 in comparison with the patients without OSA, sugggesting that the OSA could significantly impair the effective of AF ablation.
Multiple abnormal pathophysiologies underlying the OSA were directly or indirectly responsible for the development and progression of AF, including inflammation, oxidative stress, vascular endothelia dysfunction, and sympathetic overactivity. OSA was characterized with nocturnal hypoxemia and hypercapnia due to the repeated upper airway collapse during sleep, which could markly promote the activation of infalmmation, oxidative stress, and vascular endothelia dysfunction, subsequently causing to the atrial electrical and structural remodeling, ultimately leading to the AF episode (26, 27). Moreover, acute apneic episodes in OSA could result in sympathetic overactivity, shortening atrial refractoriness, increasing triggered activity and promoting the AF initiation (28, 29).
CPAP has been reported to be an effective alternation to markly improve and correct the abnormal pathophysiologies underlying the OSA. Accumulated evidence indicated that the CPAP therapy could significantly reduce the AF episodes in OSA patients. A prospective cohort study conducted by Varga et al. (12) including ninety-three patients with OSA suggested that the prevalence of cardiac arrhythmias (such as AF) was significantly declined with three-month CPAP therapy (p = 0.03). A randomized controlled trial (NCT01165710) including 10,132 AF patients with two-year follow-up also indicated that the OSA patients underwent CPAP treatment were less likely to the progression to permanent AF compared with OSA patients without CPAP therapy (hazard ratio = 0.66; 95% CI, 0.46–0.94; p = 0.021) (13). However, the effective of the CPAP on AF patients with OSA after ablation is still controversial. An previous meta-analysis on the efficacy of AF patients with OSA underwent CPAP therapy after ablation highlighted that the patients with OSA not undergoing CPAP therapy had a 57% greater risk of AF recurrence compared with that undergoing CPAP therapy (30). Whereas, a recent randomized controlled trial showed the different outcomes. In paroxysmal AF patients with OSA, CPAP treatment failed to further reduce the risk of AF recurrence after ablation, and PVI could significantly reduced the AF burden in OSA patients without any statistical difference between CPAP therapy and non-CPAP therapy group (16). Therefore, we perfomed this meta-analysis to clarify the role of CPAP on the AF patients with OSA after ablation.
Our results suggested that compared with non-CPAP treatment group, CPAP treatment group was significantly associated with a lower AF recurrence rate, indicating that the CPAP could reduce the AF recurrence after ablation in AF patients with OSA. Sensitivity analysis and Egger’s test revealed that our results were relatively robust. Meanwhile, TSA result also indicated the firm evidence favoring CPAP group for the AF recurrence risk. This result further supported the pooled results.
Subgroup analysis was conducted to identify the potential determinants for the AF recurrence rate between the CPAP therapy group and non-CPAP therapy group for the AF patients with OSA. A statistically significant intervention-covariate interaction was identified in study design subgroup, suggesting that the previous observational studies showed a benefit outcome in CPAP group and RCT did not. Whereas, only one RCT was eligible in our meta-analysis, which could lead to potential bias due to limitated sample size (16). Noteworthily, another statistically significant interaction was identified in non-PAF proportion subgroup, revealing that the benefit for CPAP could be achieved in >50% subgroup (RR = 0.49; p = 0.000) while it could not in ≤50% subgroup (RR = 0.97; p = 0.866). The results suggested that the higher proportion of non-PAF was associated with the more benefits for CPAP on the AF patients with OSA after ablation, which could partly account for the negative result of the only RCT without non-PAF patients (16). Importantly, this subgroup result provided a basis for a RCT to further evaluate the role of AF type on the effect of CPAP in AF patients with OSA after ablation.
In addition, a borderline statistically significance (p = 0.049 for interaction) was presented between first ablation then CPAP subgroup (RR = 0.41) and first CPAP then ablation subgroup (RR = 0.67), which indicated that first ablation then CPAP strategy might be superior to first CPAP then ablation strategy for the AF patients with OSA after ablation. The potential explanations were as follows. CPAP was reported to markedly improve oxidative stress, inflammation and sympathetic overactivity in OSA, which means that it could suppress the trigger activities from atrium to a certain extent (26). Therefore, we made a reasonable speculation that the potential focal activities might be masked with CPAP treatment prior to ablation, leading to a incomplete elimiatation of the ectopic focus within the atrium during ablation, ultimately causing that first CPAP then ablation strategy showed a inferior results in comparison with first ablation then CPAP strategy. Similarly, this subgroup result might be expected to be a preliminary basis to clarify the role of CPAP treatment strategy via a prospective RCT with large cohorts.
Increasing studies have demonstrated that a higher volume for intervention group was significantly associated with a better outcome, which indicated the “practice makes perfect” principle (31, 32). Similarly, a potentially significant trend (p = 0.062) was displayed in sample size subgroup with, suggesting that the larger sample size in CPAP treatment group tended to be associated with the lower AF recurrence. Moreover, the remaining three subgroups, including follow-up time, ablation strategy, and ablation energy, showed the consistent trend to the pooled results without significantly statistical interaction, respectively. These subgroup results suggested that the benefits of CPAP on AF patients with OSA after ablation were not affected by the three subgroup items.
Age is an independent risk factor for the AF progress and prognosis, and aging is reported to be associated with a high AF recurrence after ablation therapy (33, 34). Whereas, no data to date revealed the effect of age on the AF recurrence with/without CPAP therapy for OSA patients after ablation treatment. In this study, we found that the young AF patients with OSA (<60 years) could acquire benefits from CPAP therapy, and the elderly ones (≥60 years) did not, further suggesting that aging might play a detrimental role on the CPAP therapy for AF patients with OSA after ablation. This subgroup results also indicated that the young AF patients with OSA underwent ablation might be a more suitable subjects with CPAP therapy, which was expected to provide a theoretical basis for personalized treatment AF combined OSA.
There are several limitations in our study. First, in the most of our eligible studies, the diagnosis of AF recurrence was based on the periodic and occasional ECG or 24 h Holter recording, as well as the AF symptoms, whereas only one study used the continuous monitoring of AF burden with a loop recorder for the diagnosis of AF recurrence. Therefore, the asymptomatic AF recurrence might been easily ignored, leading to potentially underestimate the AF recurrence and fail to accurately evaluate the effective of CPAP on the AF patients with OSA after ablation. More studies with a loop recorder should be performed to further assess the role of CPAP. Second, the AF type was also an key factor determined the ablation prognosis. Whereas, in this study, we found that the higher proportion of non-PAF and the more benefits with CPAP on the AF patients with OSA after ablation. Due to limitation of eligible studies and available data, the result should be interpreted with caution until confirmed in larger studies. Third, our study belongs to the meta-analysis, and some potential biases might affect our results. Whereas, sensitivity analysis and publication bias test (such as Egger’s test) both suggested our robust results. Meanwhile, TSA result also provide the firm evidence to support the pooled results. Finally, only one RCT with limitated sample size was eligible in this study, which may lead to the possible bias and restrict us to draw a substantial conclusion. Therefore, more RCTs with large sample size are needed for further demonstrating these results.
Our study suggests that CPAP therapy might be an effective strategy on reduction of AF recurrence after ablation for AF patients with OSA. The CPAP treatment strategy and the non-PAF proportion might be the possible determinants on AF recurrence for AF patients with OSA after ablation. More RCTs with large sample size should be designed and performed to further demonstrate our results.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
FL: Data curation, Methodology, Writing – original draft. C-JH: Data curation, Methodology, Writing – review & editing. C-HD: Writing – review & editing. R-XW: Validation, Writing – review & editing. HL: Conceptualization, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like show sincere appreciation to the reviewers for critical comments on this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1269945/full#supplementary-material
1. Chugh, SS, Havmoeller, R, Narayanan, K, Singh, D, Rienstra, M, Benjamin, EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. (2014) 129:837–47. doi: 10.1161/CIRCULATIONAHA.113.005119
2. Hindricks, G, Potpara, T, Dagres, N, Arbelo, E, Bax, JJ, Blomström-Lundqvist, C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
3. Dai, L, Wang, X, and Xiao, Y. Role of chemosensitivity: possible pathophysiological mediator of obstructive sleep apnea and type 2 diabetes. Sleep Med. (2023) 101:490–6. doi: 10.1016/j.sleep.2022.12.004
4. Salman, LA, Shulman, R, and Cohen, JB. Obstructive sleep apnea, hypertension, and cardiovascular risk: epidemiology, pathophysiology, and management. Curr Cardiol Rep. (2020) 22:6. doi: 10.1007/s11886-020-1257-y
5. Mochol, J, Gawrys, J, Gajecki, D, Szahidewicz-Krupska, E, Martynowicz, H, and Doroszko, A. Cardiovascular disorders triggered by obstructive sleep apnea-a focus on endothelium and blood components. Int J Mol Sci. (2021) 22:5139. doi: 10.3390/ijms22105139
6. Moula, AI, Parrini, I, Tetta, C, Lucà, F, Parise, G, Rao, CM, et al. Obstructive sleep apnea and atrial fibrillation. J Clin Med. (2022) 11:1242. doi: 10.3390/jcm11051242
7. He, H, Lachlan, T, Chandan, N, Lim, VG, Kimani, P, Ng, GA, et al. Obstructive sleep apnoea and cardiac arrhythmias (OSCA) trial: a nested cohort study using injectable loop recorders and Holter monitoring in patients with obstructive sleep apnoea. BMJ Open. (2023) 13:e070884. doi: 10.1136/bmjopen-2022-070884
8. Matiello, M, Nadal, M, Tamborero, D, Berruezo, A, Montserrat, J, Embid, C, et al. Low efficacy of atrial fibrillation ablation in severe obstructive sleep apnea patients. Europace. (2010) 12:1084–9. doi: 10.1093/europace/euq128
9. Brundel, B, Ai, X, Hills, MT, Kuipers, MF, Lip, GYH, and de Groot, NMS. Atrial fibrillation. Nat Rev Dis Primers. (2022) 8:21. doi: 10.1038/s41572-022-00347-9
10. Martynowicz, H, Wieczorek, T, Macek, P, Wojakowska, A, Poręba, R, Gać, P, et al. The effect of continuous positive airway pressure and mandibular advancement device on sleep bruxism intensity in obstructive sleep apnea patients. Chron Respir Dis. (2022) 19:147997312110523. doi: 10.1177/14799731211052301
11. Bock, JM, Vungarala, S, Karim, S, and Somers, VK. Obstructive sleep apnea as a cardiovascular risk factor-beyond CPAP. Can J Cardiol. (2021) 37:756–65. doi: 10.1016/j.cjca.2021.01.027
12. Varga, PC, Rosianu, HS, Vesa, ŞC, Hancu, BGD, Beyer, R, and Pop, CM. The impact of continuous positive airway pressure on cardiac arrhythmias in patients with sleep apnea. J Res Med Sci. (2020) 25:42. doi: 10.4103/jrms.JRMS_677_18
13. Holmqvist, F, Guan, N, Zhu, Z, Kowey, PR, Allen, LA, Fonarow, GC, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-results from the outcomes registry for better informed treatment of atrial fibrillation (ORBIT-AF). Am Heart J. (2015) 169:647–654.e2. doi: 10.1016/j.ahj.2014.12.024
14. Congrete, S, Bintvihok, M, Thongprayoon, C, Bathini, T, Boonpheng, B, Sharma, K, et al. Effect of obstructive sleep apnea and its treatment of atrial fibrillation recurrence after radiofrequency catheter ablation: a meta-analysis. J Evid Based Med. (2018) 11:145–51. doi: 10.1111/jebm.12313
15. Zhou, Y, Yan, M, Yuan, J, Wang, Y, and Qiao, S. Continuous positive airway pressure treatment decreases the risk of atrial fibrillation recurrence in patients with obstructive sleep apnea after radiofrequency ablation. Int Heart J. (2022) 63:716–21. doi: 10.1536/ihj.22-129
16. Hunt, TE, Traaen, GM, Aakerøy, L, Bendz, C, Øverland, B, Akre, H, et al. Effect of continuous positive airway pressure therapy on recurrence of atrial fibrillation after pulmonary vein isolation in patients with obstructive sleep apnea: a randomized controlled trial. Heart Rhythm. (2022) 19:1433–41. doi: 10.1016/j.hrthm.2022.06.016
17. Brok, J, Thorlund, K, Gluud, C, and Wetterslev, J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. (2008) 61:763–9. doi: 10.1016/j.jclinepi.2007.10.007
18. Hojo, R, Fukamizu, S, Miyazawa, S, Kawamura, I, Sakurada, H, and Hiraoka, M. The relationship between obstructive sleep apnea and recurrence of atrial fibrillation after pulmonary vein isolation using a contact force-sensing catheter. J Interv Card Electrophysiol. (2019) 54:209–15. doi: 10.1007/s10840-018-0489-x
19. Fein, AS, Shvilkin, A, Shah, D, Haffajee, CI, das, S, Kumar, K, et al. Treatment of obstructive sleep apnea reduces the risk of atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol. (2013) 62:300–5. doi: 10.1016/j.jacc.2013.03.052
20. Naruse, Y, Tada, H, Satoh, M, Yanagihara, M, Tsuneoka, H, Hirata, Y, et al. Concomitant obstructive sleep apnea increases the recurrence of atrial fibrillation following radiofrequency catheter ablation of atrial fibrillation: clinical impact of continuous positive airway pressure therapy. Heart Rhythm. (2013) 10:331–7. doi: 10.1016/j.hrthm.2012.11.015
21. Neilan, TG, Farhad, H, Dodson, JA, Shah, RV, Abbasi, SA, Bakker, JP, et al. Effect of sleep apnea and continuous positive airway pressure on cardiac structure and recurrence of atrial fibrillation. J Am Heart Assoc. (2013) 2:e000421. doi: 10.1161/JAHA.113.000421
22. Patel, D, Mohanty, P, di Biase, L, Shaheen, M, Lewis, WR, Quan, K, et al. Safety and efficacy of pulmonary vein antral isolation in patients with obstructive sleep apnea: the impact of continuous positive airway pressure. Circ Arrhythm Electrophysiol. (2010) 3:445–51. doi: 10.1161/CIRCEP.109.858381
23. Jongnarangsin, K, Chugh, A, Good, E, Mukerji, S, Dey, S, Crawford, T, et al. Body mass index, obstructive sleep apnea, and outcomes of catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. (2008) 19:668–72. doi: 10.1111/j.1540-8167.2008.01118.x
24. Yeghiazarians, Y, Jneid, H, Tietjens, JR, Redline, S, Brown, DL, el-Sherif, N, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. (2021) 144:e56–67. doi: 10.1161/CIR.0000000000000988
25. West, SD, and Thompson, A. Continuous positive airway pressure as potential treatment of atrial fibrillation. BMJ. (2022) 376:o349. doi: 10.1136/bmj.o349
26. Orrù, G, Storari, M, Scano, A, Piras, V, Taibi, R, and Viscuso, D. Obstructive sleep apnea, oxidative stress, inflammation and endothelial dysfunction-an overview of predictive laboratory biomarkers. Eur Rev Med Pharmacol Sci. (2020) 24:6939–48. doi: 10.26355/eurrev_202006_21685
27. May, AM, Van Wagoner, DR, and Mehra, R. OSA and cardiac arrhythmogenesis: mechanistic insights. Chest. (2017) 151:225–41. doi: 10.1016/j.chest.2016.09.014
28. Huang, B, Liu, H, Scherlag, BJ, Sun, L, Xing, S, Xu, J, et al. Atrial fibrillation in obstructive sleep apnea: neural mechanisms and emerging therapies. Trends Cardiovasc Med. (2021) 31:127–32. doi: 10.1016/j.tcm.2020.01.006
29. Yang, X, Zhang, L, Liu, H, Shao, Y, and Zhang, S. Cardiac sympathetic denervation suppresses atrial fibrillation and blood pressure in a chronic intermittent hypoxia rat model of obstructive sleep apnea. J Am Heart Assoc. (2019) 8:e010254. doi: 10.1161/JAHA.118.010254
30. Li, L, Wang, ZW, Li, J, Ge, X, Guo, LZ, Wang, Y, et al. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnea with and without continuous positive airway pressure treatment: a meta-analysis of observational studies. Europace. (2014) 16:1309–14. doi: 10.1093/europace/euu066
31. Lador, A, Peterson, LE, Swarup, V, Schurmann, PA, Makkar, A, Doshi, RN, et al. Determinants of outcome impact of vein of Marshall ethanol infusion when added to catheter ablation of persistent atrial fibrillation: a secondary analysis of the VENUS randomized clinical trial. Heart Rhythm. (2021) 18:1045–54. doi: 10.1016/j.hrthm.2021.01.005
32. Li, F, Sun, JY, Wu, LD, Zhang, L, Qu, Q, Wang, C, et al. The long-term outcomes of ablation with vein of Marshall ethanol infusion vs. ablation alone in patients with atrial fibrillation: a meta-analysis. Front Cardiovasc Med. (2022) 9:871654. doi: 10.3389/fcvm.2022.871654
33. Bahnson, TD, Giczewska, A, Mark, DB, Russo, AM, Monahan, KH, al-Khalidi, HR, et al. Association between age and outcomes of catheter ablation versus medical therapy for atrial fibrillation: results from the CABANA trial. Circulation. (2022) 145:796–804. doi: 10.1161/CIRCULATIONAHA.121.055297
Keywords: continuous positive airway pressure, obstructive sleep apnea, atrial fibrillation, ablation, recurrence
Citation: Li F, He C-J, Ding C-H, Wang R-X and Li H (2023) Continuous positive airway pressure therapy might be an effective strategy on reduction of atrial fibrillation recurrence after ablation in patients with obstructive sleep apnea: insights from the pooled studies. Front. Neurol. 14:1269945. doi: 10.3389/fneur.2023.1269945
Received: 26 August 2023; Accepted: 26 October 2023;
Published: 09 November 2023.
Edited by:
Helena Martynowicz, Wroclaw Medical University, PolandReviewed by:
Wiktor Kuliczkowski, Wroclaw Medical University, PolandCopyright © 2023 Li, He, Ding, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Li, OTkxMTI2M0AxNjMuY29t; Ru-Xing Wang, cnV4aW5nd0BhbGl5dW4uY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.