
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 08 November 2023
Sec. Sleep Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1264177
This article is part of the Research Topic The Complex Relationship Between Sleep, Rhythms, and Mood Disorders: Volume II View all 8 articles
 Ying Tao1,2†
Ying Tao1,2† Yi Qin1,2†
Yi Qin1,2† Sifan Chen1,2†
Sifan Chen1,2† Tian Xu1,2
Tian Xu1,2 Junhui Lin1,2
Junhui Lin1,2 Diansan Su1,2
Diansan Su1,2 Weifeng Yu1,2
Weifeng Yu1,2 Xuemei Chen1,2*
Xuemei Chen1,2*Background: Sleep is an important biological process and has been linked to many diseases; however, very little is known about which and how genes control and regulate sleep. Although technology has seen significant development, this issue has still not been adequately resolved. Therefore, we conducted a bibliometric analysis to assess the progress in research on sleep quality and associated genes over the past 2 decades. Through our statistical data and discussions, we aimed to provide researchers with better research directions and ideas, thus promoting the advancement of this field.
Methods: On December 29, 2022, we utilized bibliometric techniques, such as co-cited and cluster analysis and keyword co-occurrence, using tools such as CiteSpace, VOSviewer, and the Online Analysis Platform of Literature Metrology (http://bibliometric.com/), to conduct a thorough examination of the relevant publications extracted from the Web of Science Core Collection (WoSCC). Our analysis aimed to identify the emerging trends and hot spots in this field while also predicting their potential development in future.
Results: Cluster analysis of the co-cited literature revealed the most popular terms relating to sleep quality and associated genes in the manner of cluster labels; these included genome-wide association studies (GWAS), circadian rhythms, obstructive sleep apnea (OSA), DNA methylation, and depression. Keyword burst detection suggested that obstructive sleep apnea, circadian clock, circadian genes, and polygenic risk score were newly emergent research hot spots.
Conclusion: Based on this bibliometric analysis of the publications in the last 20 years, a comprehensive analysis of the literature clarified the contributions, changes in research hot spots, and evolution of research techniques regarding sleep quality and associated genes. This research can provide medical staff and researchers with revelations into future directions of the study on the pathological mechanisms of sleep-related diseases.
Sleep is a fundamental biological process that is conserved across a wide range of organisms from invertebrates to vertebrates, but the pathobiology and molecular mechanisms of sleep are still not fully understood. There was a strong interest in the genetics of sleep. In the 1930s, Geyer conducted a twin study in children and first showed that genetic factors were involved in the regulation of sleep. Since then, numerous high-quality studies on gene expression profiling indicate that there is a shared genetic basis for sleep across different species (1, 2). In other words, there is a belief that the mechanism responsible for sleep is evolutionarily conserved and that similar genetic pathways are involved in regulating sleep across different organisms. Nowadays, various new programs and techniques have been developed, leading to breakthroughs in the understanding of sleep function, circadian rhythms, and pathological mechanisms of sleep-related diseases (3–5).
However, the molecular and pathobiology mechanisms of sleep and sleep-related diseases are quite complex and least understood phenomena for a long time. Genetic analysis of sleep has only become a significant discipline in the past decade, with the focus expanding to drosophila, zebrafish, and worms. With the help of genetics, many sleep-related problems have been solved, for example, the identification of loci related to various sleep disorders such as obstructive sleep apnea (OSA), major depressive disorder (MDD), and insomnia disorder. As genetic technologies continue to develop, genes will be a powerful tool in solving these problems.
In this study, we utilized bibliometric citation analysis, which is widely employed to map and analyze the performance of specific fields, to gain insights into the temporal trends in the field of sleep quality and associated genes. It provides valuable predictions about the progress and development of specific fields based on factors such as publication volume and citation frequency (6, 7). The published studies have played a significant role in advancing specific research fields (8). To recognize the significance of the sleep and genetics topic and the effectiveness of bibliometric analysis, we conducted an analysis of publications related to this theme. Our objective was to assist researchers to identify the current trends and hot spots in this field, both in the past and future and to provide a comprehensive understanding of the evolution of this field.
The Web of Science core collection database was searched by retrieval form (TI = (gene OR genetic) OR KP = (gene OR genetic)) AND (TI = (sleep) OR KP=(sleep)) NOT (TI = (guideline OR recommendation OR consensus OR “case report” OR meta OR review)). The retrieval time was limited to January 01, 2003 to December 31, 2022. The inclusion criteria were studies related to the search, excluding reviews, letters, briefings, book reviews, etc., resulting in 3,996 articles. The data were used for visual analysis of authors, institutions, countries, journals, co-cited literature, and keywords. The detailed process of enrollment and screening is shown in Figure 1.
We used Web of Science (https://wcs.webofknowledge.com) to analyze search results and create a histogram that illustrates publication trends. We then converted the WoSCC data to the UTF-8 format and utilized the “total literature analysis” option to analyze publication trends across different countries and the “partnership analysis” option to analyze intercountry/regional relationships. Additionally, we utilized Web of Science to extract the histogram that displays the publication trend and analyze the research status, hot spots, and trend with the above software.
The publications' complete records and cited references were obtained from the WoSCC database and saved as a text file. We used VOSviewer1.6.18 (version 1.6.18, Leiden University, van Eck NJ and Waltman L), which can provide three kinds of visualization maps: network maps, overlay maps, and density maps, to analyze the information of authors, institutions, journals, countries, references, and keywords. We used CiteSpace 6.1.6 to calculate keywords burst and reference co-citation analysis. The graphs were created by the abovementioned software, and the layout was adjusted by Pajek and SCImago Graphica by using the above software to draw a visual map and analyze the research status, hot spots, and trend of sleep quality and associated genes.
Over the past 20 years, researchers have contributed 4,000 articles to the field of sleep, including editorial materials and reviews. The number of sleep-related publications has shown consistent growth, increasing from 73 in 2003 to 291 in 2022, while three minor dips in publication rates occurred from 2013 to 2014, from 2017 to 2018, and from 2020 to 2021. In 2022, the number of articles on sleep reached a peak at 291, with a significant increase of 37 publications from the previous year (Figure 2).
We analyzed the countries and institutions of the authors of these articles. Our findings revealed that 10 countries contributed more than 100 publications related to sleep (Table 1). The United States has the highest number of publications, with a staggering 1,804, which is far ahead compared to other countries. China ranks second with 549 publications. The United States has strong research cooperations with China, Japan, Germany, and the United Kingdom (Figure 3A). However, cooperation within Asia, Africa, and South America appears to be weak. The shaped world map (Figure 3B) shows the visual analysis of published regions by VOSviewer software. A total of 3,996 articles related to sleep quality and associated genes research have been published in 92 countries. The world map shows the cooperation relationship strength among countries, and some countries including China, Canada, Australia, Brazil, Japan, and South Korea have limited collaborations with other countries except the United States.
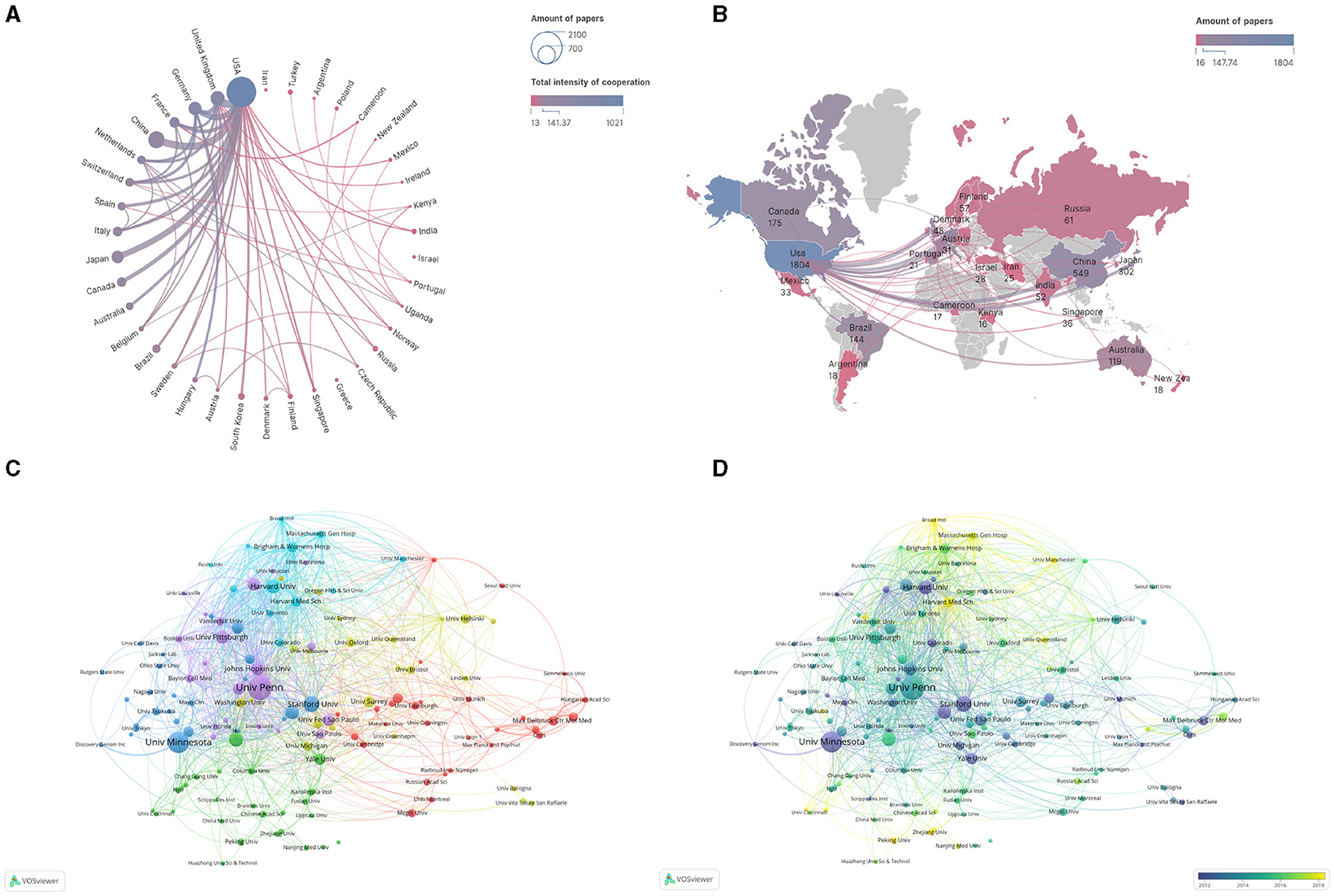
Figure 3. Cooperation maps among countries and institutions. (A) Cooperation relationships of countries. The size of points means the number of publications. The colors and the weight of the lines mean the intensity of cooperation. (B) World map of cooperation relationships among countries. The colors show the number of publications. (C) Cooperation relationships among institutions. Colors represent clusters automatically calculated by VOSviewer. (D) Time trend of cooperation among institutions. The colors correspond to the annual time periods of publications.
Regarding institutions, our analysis revealed a substantial amount of collaboration between institutions worldwide. Institutions contributing to sleep research are widely spread out and engage in a broad range of cooperations (Figure 3C). Brigham and Women's Hospital is the most willing to work with other institutions. Pennsylvania University, Harvard University, Stanford University, Yale University, and Minnesota University focus on sleep before 2012 with publications. Broad Institute, Harvard Medical School, Peking University, Zhejiang University, and Manchester University began to study sleep more recently (Figure 3D).
Fourteen institutions contributed more than fifty studies (Table 2). The University of Pennsylvania institution published the highest number of articles, 143, ranking first. This was followed by Minnesota University and Stanford University with 123 and 85 publications, respectively (Table 2).
Partnership among authors promotes global cooperation. A total of 20,615 authors contributed 3,996 articles. Tufik Sergio, Pack Allan I., Archer Simon N., Gozal David, and Cirelli Chiara have published at least 15 articles in this field (Table 3). They study on sleep earlier all before 2014. However, Tufik Sergio has a low ratio of total citations (TC) to publications, while Archer Simon N. and Cirelli Chiara have a high number of citation references with a ratio of more than 60 (Table 3). Saxena Richa, Keene Alex C., Li Yan, and Logan Ryan W. start to focus on sleep in recent years (Figure 4B).
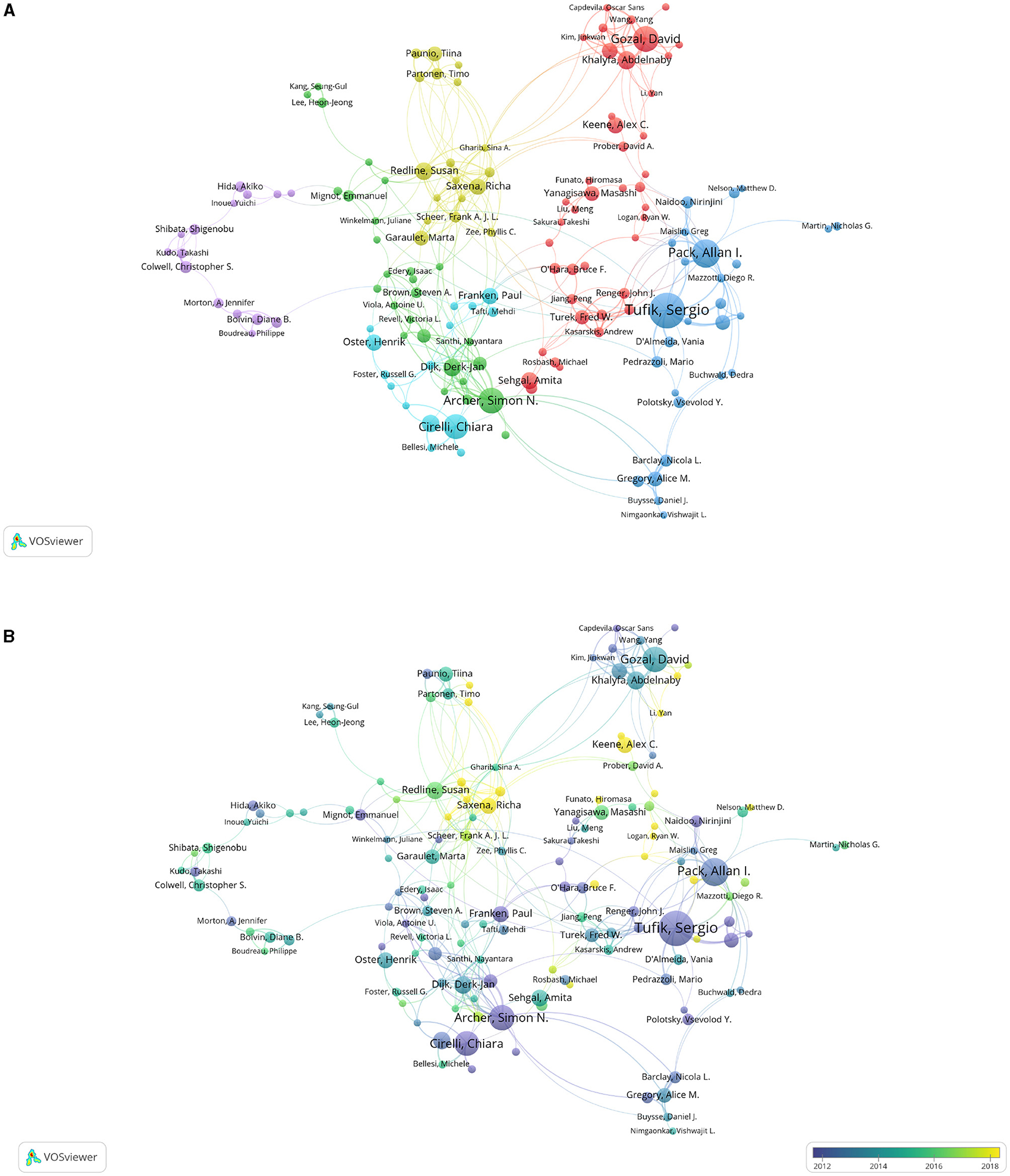
Figure 4. Cooperation maps among authors publishing articles on sleep. (A) Cooperation relationships among authors. The size of points means the number of publications. Colors represent clusters automatically calculated by VOSviewer. (B) Time trend of cooperation among authors. The colors correspond to the annual time periods of publications.
Most of the studies were conducted by more than one researcher. There seemed to be close cooperation among different authors worldwide. Gozal David and Khalyfa Abdelnaby have the strongest communication. There is no major cluster formed in the map, and the cooperations among authors are various (Figure 4A).
In total, 147 journals have published 2,497 articles on sleep. The journals with the top three publications are PLoS ONE (164), Sleep (159), and Scientific Reports (96) (Table 4). There are 32 articles related to sleep on Nature, Science, and Cell, but they have the highest ratio of total citations (Table 5). As shown in Figure 5A, the time trend map shows Molecular Therapy, Brain Research, and Neuroscience published articles on sleep earlier with more than 30 publications (Figure 5B). On the contrary, Circadian Clock in Brain Health and Disease, Frontiers in Genetics and iScience start to published research studies on sleep since 2021.
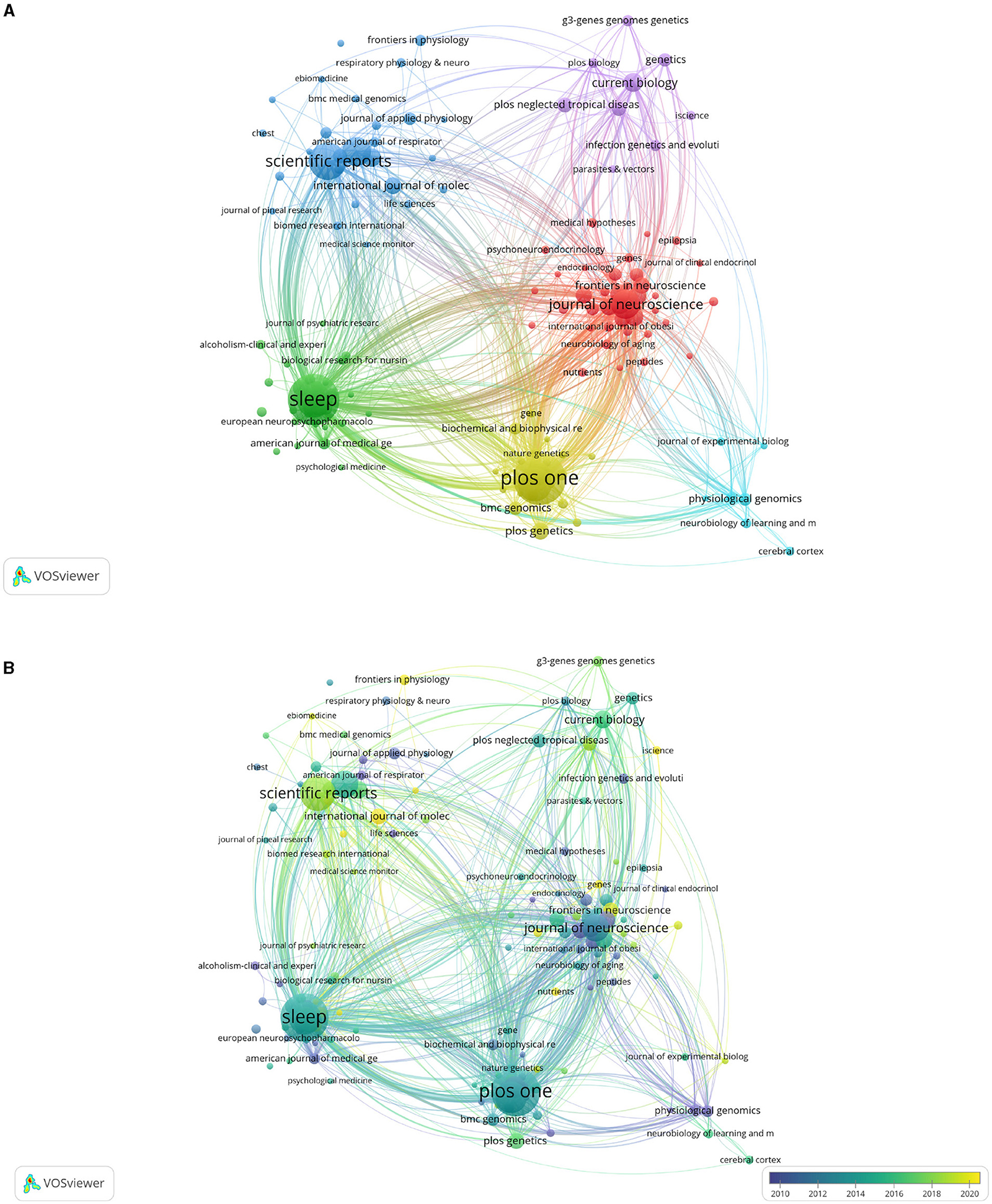
Figure 5. Maps of journals that published articles on sleep. (A) Cluster map of journals. The size of points means the number of publications on sleep. Colors represent clusters automatically calculated by VOSviewer. (B) Time trend of journals publishing articles on sleep. The colors correspond to the annual time periods of publications.
Subsequently, we utilized clustered network analysis to delve deeper into the co-citations and conduct a thorough investigation of their interconnections. As shown in Figure 6A, the co-cited references were clustered into 12 major cluster labels: shift work, transposon, sleep duration, microarray, GWA, lethargus, per3, F-box protein, miRNA, non-viral vector, insomnia, and major depressive disorder. A timeline view of the distinct co-citations is shown in Figure 6B to present all the cited literature more clearly. Each ball represents a cited article. The size of the ball is proportional to the number of citations. The connected ball indicates that these articles are cited together. With purple representing relatively old citations and yellow representing more recent citations, we found that in sleep quality and associated genes research, mental illness has been a hot topic since 2005. The study of insomnia and sleep duration is an emerging area of research after 2015 and has received increasing attention in recent years. What is interesting to us is that GWAS has been the focus of recent research, and we look forward to finding more interesting genes related to sleep through this method.
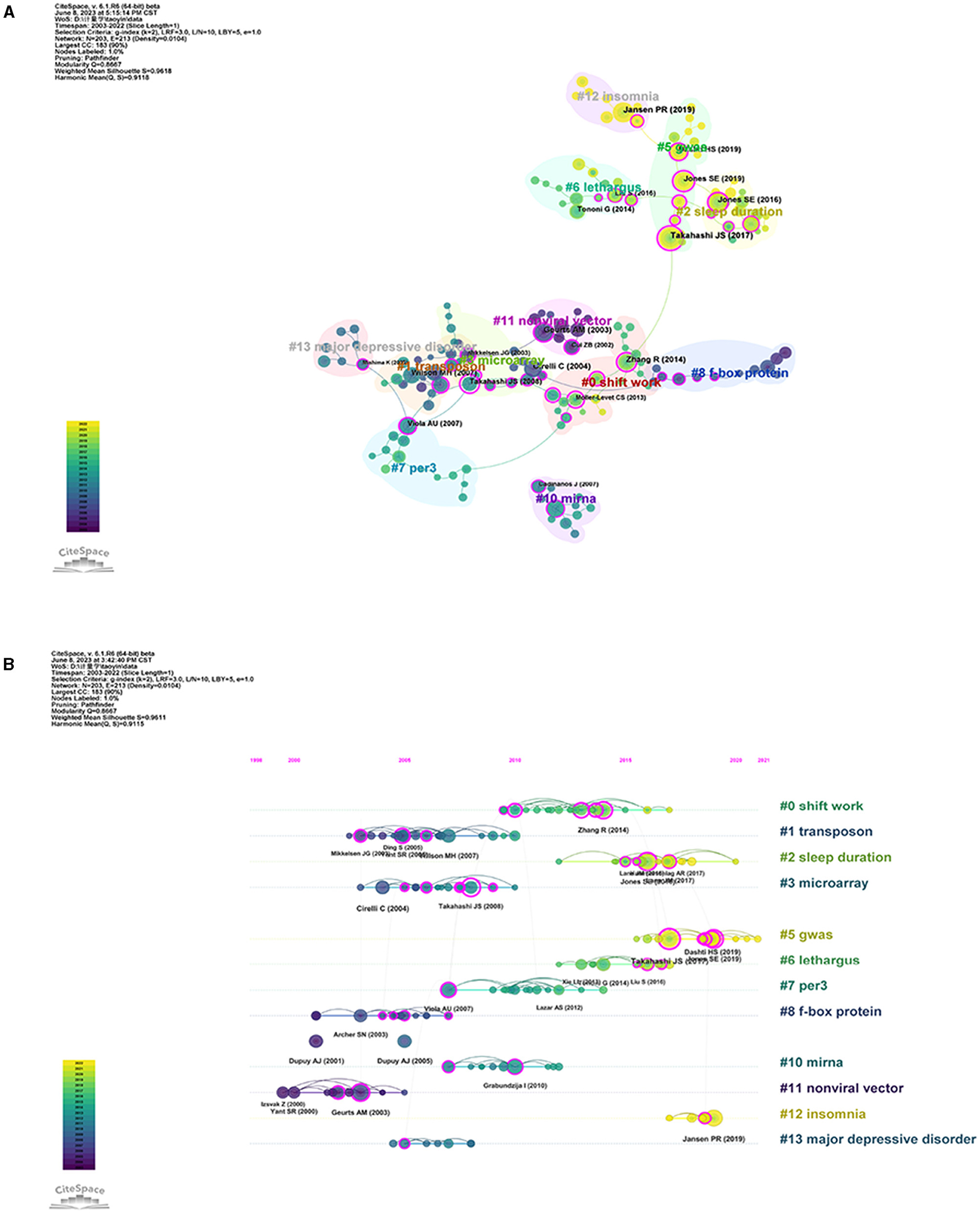
Figure 6. Co-cited references in sleep publications. (A) Visualization network of co-cited references regarding sleep articles. The size of points means the number of being cited. The colors padded correspond to the annual time periods of publications. (B) Timelines of keywords in co-cited references.
Keyword analysis plays a crucial role in identifying research hot spots and trends, offering valuable insights into future directions. The VOSviewer software was used to perform co-occurrence cluster analysis on the keywords of the article. The minimum occurrence times of each keyword were set to 10 and 164 keywords were screened from the 6,973 keywords to form a visual map (Figure 7A). There were six clusters summarized (Figure 7B), including sleep, circadian rhythm, obstructive sleep apnea, genetics, orexin, and transposon.
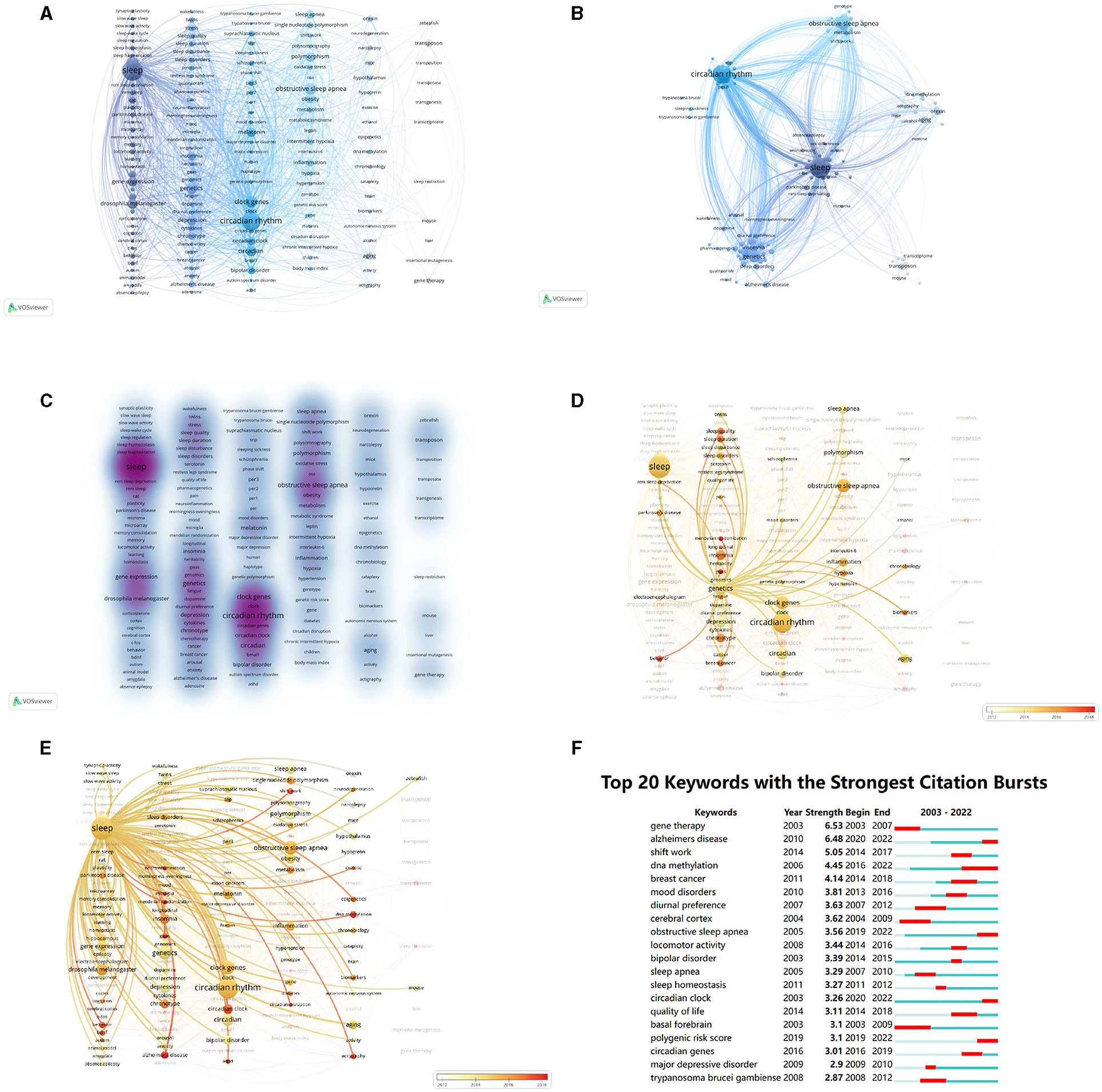
Figure 7. Keywords in sleep publications. (A) Visualization network of keywords with more than 10 occurrences. (B) Density map of keywords with more than 10 occurrences. (C) Cluster map of keywords. The size of points means the occurrences of keywords. The lines mean the relationships between two keywords. Colors represent clusters automatically calculated by VOSviewer. (D) Time trend of keywords related to “genetics”. The colors correspond to the annual time periods of publications. (E) Time trend of keywords related to “sleep”. The colors correspond to the annual time periods of publications. (F) Top 20 keywords with the strongest citation bursts analyzed by CiteSpace. The red areas mean the burst periods of keywords.
In the keyword clustering heat map, the color depth is proportional to the number of studies, and the darker the color, the higher the popularity of the keyword, the more studies. “Sleep” and “circadian rhythm” received the most studies (Figure 7C).
By examining the bursts of keywords over time, valuable insights into the research trajectory and emerging areas of focus within the field of study can be gained. Among the identified keywords, “basal forebrain” and “cerebral cortex”, associated with brain regions, emerged as the earliest bursting keywords. Subsequently, keywords such as “diurnal preference,” “shift work,” “mood disorder,” “bipolar disorder,” and “sleep apnea” gained prominence. More recent bursts of interest were observed in keywords such as “Alzheimer's disease,” “DNA methylation,” “circadian clock,” “circadian gene,” “obstructive sleep apnea,” and “polygenic risk score” (Figures 7D, E).
In this study, a comprehensive analysis was conducted on 312 keywords that occurred more than 10 times, yielding noteworthy results. The top five frequently occurring keywords were identified as follows: “circadian rhythm” (185 occurrences), “sleep deprivation” (162 occurrences), “circadian rhythms” (148 occurrences), “obstructive sleep apnea” (106 occurrences), and “clock genes” (89 occurrences). The keywords formed five distinct clusters representing key areas of research interest, including sleep, obstructive sleep apnea, circadian rhythm, genetics, and epilepsy (Figure 7F).
In our study, bibliometric mapping was used to visualize the development of sleep quality and associated genes research from 2002 to 2022. Our findings revealed a continuous increase in scientific output in the field of genetics and sleep over the past two decades. The ranking of publication numbers of countries, universities, authors, and journals helped researchers to search for cooperations, to seek positions, or to publish their works conveniently. Analyzing the collaborative relationships among different countries/regions and institutions can also reflect the academic exchange in this field. Recent advancements in sleep research studies have been facilitated by experimental techniques such as genome-wide association studies (GWAS) analysis. Additionally, newly emergent research hot spots, including obstructive sleep apnea, circadian clock, and circadian genes, were indicated by keyword burst detection. However, despite the substantial amount of research in this area, there was a lack of studies focusing on predicting and clarifying current research hot topics and frontiers. To address this gap, we cataloged the attributes of existing studies and analyzed the results of cluster labeling and burst detection techniques to develop a nuanced understanding of the current state of research and identify promising avenues for future exploration in this field.
GWAS, which is the study of related single nucleotide polymorphisms (SNPs) for a given phenotype with large human populations, have been applied in different sleep subtypes since 2015, especially in sleep duration research studies. SNPs near PAX8 gene, responsible for regulating thyroid-specific genes, were identified in multiple GWAS studies on self-reported and objective sleep duration (9–12). Mutations in this gene were found to potentially increase sleep duration by 2–3 min (13, 14). Vaccinia-related kinase 2 (VRK2), associated with schizophrenia (15), was another gene that had been robustly replicated in the previous GWAS (10). GWAS helped scientists identify lots of new intriguing loci related to sleep such as DRD2, SLC6A3, or GABRR1 in the neuron system (11), but more efforts are needed as few novel loci from GWAS are strongly replicated (14).
Apart from SNPs, epigenetic processes including DNA methylation (DNAm), which means the methyl modification of cytosine by DNA methyltransferase, have been implicated in influencing sleep (16). The loss of DNAm of differentially methylated positions showed an association with insufficient sleep in a cross-sectional genome-wide analysis (17). Circadian clock genes were reported to be regulated by DNAm. For example, the expression level of Per1 was increased during perinatal development by demethylating within the SCN (18), while Cry2 showed significantly increased methylation in older mice (19). Moreover, researchers have reported that the methylation level of some genes may correlate with sleep disorders. Forkhead Box P3 (FOXP3) and endothelial nitric oxide synthase (eNOS) gene DNAm levels have been suggested to play a crucial role in pediatric OSA (20). Moreover, DNAm modules in MAPT, which is a key regulator of Tau proteins in the brain, were implicated in sleep duration in children (21). However, there is still an ongoing debate in this area. A meta-analysis in 2022 showed DNA methylation, at birth or in childhood, was not associated with sleep (22). Consequently, there is an urgent need for epigenome-wide association studies to validate the reliability of specific epigenetic alterations in relation to sleep.
Circadian rhythms, which serve as an endogenous time clock, are present in a wide range of living organisms (23). The neuronal network and molecular mechanisms of circadian rhythms were well studied. The core regulatory loop of circadian rhythms was controlled by the transcription factors brain and muscle ARNT-like protein 1 (BMAL1) and circadian locomotor output cycles kaput (CLOCK), which promoted the expression of period and cryptochrome genes (Per1, Per2, Cry1, and Cry2). Consequently, the accumulation of heterodimer PER/CRY inhibited their own transcription, forming a self-regulatory feedback loop. This network regulated the expression of countless other genes and was vital for cell physiology and metabolism (24, 25). Recently, some research studies showed these circadian clock genes had multiple functions outside the suprachiasmatic nucleus (SCN), a major core brain region that controls timekeeping, to influence sleep (26). For example, sleep deprivation for 6 h increased the expression of per1 in forebrain lysates (26) and the expression of per2 in the whole brain, liver, and kidney but not in SCN (27). Moreover, the expression of Bmal1 in the skeletal muscle was essential and capable of effectively regulating the total amount of sleep (28). As for Cry1 and Cry2, double knockout mice showed increased non-rapid eye movement (non-REM) sleep (29). The molecular mechanism of circadian genes in regulating sleep is still under investigation.
Obstructive sleep apnea (OSA), which is caused by recurrent episodes of upper airway collapse and obstruction during sleep, is a prevalent sleep-disordered breathing (30). It often manifests through symptoms such as loud snoring, nocturnal awakenings, and excessive daytime sleepiness. The prevalence of moderate OSA in the general population ranged from 9 to 38%, with a higher occurrence among men (30). There are many risk factors that affect the OSA, including unmodifiable risk factors like sex, age, and race, and modifiable risk factors such as obesity, endocrine disorders, or obstruction (31). The genetic etiology of OSA was little known. Previous studies using different methods, such as genetic expression analysis and Mendelian randomization analysis, found some genes may be associated with OSA such as PCNA, PSMC6 (32), LEPR, MMP-9, and GABBR1 (33). Recently, one GWAS study of OSA using FinnGen found high genetic correlations between OSA and obesity (34). Another twin study showed OSA also had a common genetic background with hypertriglyceridemia (35). Moreover, genetic analyses also showed patients with OSA have higher C-reactive protein and tumor necrosis factor-alpha levels (36) and were susceptible to diseases such as atrial fibrillation (37), but the mechanism of these associations was not clear.
Sleep disorders were associated with a higher risk of developing depressed and suicidal thoughts in those with major depressive disorder (MDD). MDD could be influenced by genetic factors, with a heritability of 40–50% based on twin studies. New research studies indicate that depression and sleep disorders share pathogenesis. According to a study conducted by Ma et al., circadian rhythm disturbances might contribute to depression (38). Park et al. utilized a non-parametric model-free0000000000 multivariate dimensionality reduction (MDR) approach and found that circadian genes, TIMELESS rs4630333 and CSNK1E rs135745, were significantly associated with MDD and bipolar disorder (39). Moreover, genetic variations in adenosine metabolism were associated with depression, sleep, and attention disturbances. MyD88, essential for microglial activation, affected microglial homeostasis functions and lowered the serotonergic neuronal output, leading to insomnia and depressive behavior.
Sleep-related disorders are complex phenotypes influenced by both genetic and environmental factors, leading to significant individual variations (40). In contrast, common genetic-related diseases such as albinism and hemophilia are less affected by environmental factors and are easier to study. Genes involved in the regulation of sleep-related disorders are complex, and genetic research in this area is still in its early stages. Many questions remain unanswered. Many other diseases are often associated with sleep-related disorders. Studying the genes related to sleep-related disorders greatly aids in understanding the mechanisms of other diseases, such as mental illnesses and binge eating disorder (BED) (41), which are closely linked to sleep disorders. Unlike other diseases, sleep-related disorders do not affect specific organs, making them more challenging to study (3).
Although many genes related to sleep disorders have been identified through existing technological means, it is difficult to replicate the genes obtained in different laboratories, and many genes cannot be validated. Furthermore, because sleep-related disorders also exhibit environmental susceptibility, it is important to take into account regional and ethnic differences when conducting genetic association analyses (40).
In conclusion, bibliometric analysis offered an objective and quantitative method for assessing publication performance between countries, researchers, and research institutions. Our results showed considerable interest in the field of sleep quality and associated genes research in recent years, particularly the study of GWAS, circadian rhythms, OSA, and depression. The relationship between genes and sleep disorders has gained increasing attention. Recent literature has gradually revealed the specific mechanisms involved, providing potential avenues for prevention, treatment, and intervention of sleep disorders.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
YT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing—original draft. YQ: Data curation, Software, Writing—original draft. SC: Conceptualization, Software, Validation, Writing—original draft. TX: Data curation, Writing—review and editing. JL: Formal analysis, Writing—review and editing. DS: Supervision, Writing—review and editing. WY: Supervision, Writing—review and editing. XC: Funding acquisition, Writing—review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (Nos. 81874371 and 82201369), Shanghai Engineering Research Center of Perioperative Organ Support and Function Preservation (20DZ2254200), and Shanghai Municipal Key Clinical Specialty (shslczdzk03601 to WY).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cirelli C, Gutierrez CM, Tononi G. Extensive and divergent effects of sleep and wakefulness on brain gene expression. Neuron. (2004) 41:35–43. doi: 10.1016/S0896-6273(03)00814-6
2. Mackiewicz M, Shockley KR, Romer MA, Galante RJ, Zimmerman JE, Naidoo N, et al. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. (2007) 31:441–57. doi: 10.1152/physiolgenomics.00275.2006
3. Schulz H. The history of sleep research and sleep medicine in Europe. J Sleep Res. (2022) 31:e13602. doi: 10.1111/jsr.13602
4. Allada R, Siegel JM. Unearthing the phylogenetic roots of sleep. Curr Biol. (2008) 18:R670–R9. doi: 10.1016/j.cub.2008.06.033
5. Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. (1983) 6:179–85. doi: 10.1093/sleep/6.3.179
6. Fei X, Wang S, Li J, Zeng Q, Gao Y, Hu Y. Bibliometric analysis of research on Alzheimer's disease and non-coding RNAs: opportunities and challenges. Front Aging Neurosci. (2022) 14:1037068. doi: 10.3389/fnagi.2022.1037068
7. Kokol P, Blazun Vosner H, Zavrsnik J. Application of bibliometrics in medicine: a historical bibliometrics analysis. Health Info Libr J. (2021) 38:125–38. doi: 10.1111/hir.12295
8. Nie Y, Wen L, Song J, Wang N, Huang L, Gao L, et al. Emerging trends in epigenetic and childhood trauma: bibliometrics and visual analysis. Front Psychiatry. (2022) 13:925273. doi: 10.3389/fpsyt.2022.925273
9. Gottlieb DJ, Hek K, Chen TH, Watson NF, Eiriksdottir G, Byrne EM, et al. Novel loci associated with usual sleep duration: the CHARGE Consortium Genome-Wide Association Study. Mol Psychiatry. (2015) 20:1232–9. doi: 10.1038/mp.2014.133
10. Jones SE, Tyrrell J, Wood AR, Beaumont RN, Ruth KS, Tuke MA, et al. Genome-wide association analyses in 128,266 individuals identifies new morningness and sleep duration loci. PLoS Genet. (2016) 12:e1006125. doi: 10.1371/journal.pgen.1006125
11. Dashti HS, Jones SE, Wood AR, Lane JM, van Hees VT, Wang H, et al. Genome-wide association study identifies genetic loci for self-reported habitual sleep duration supported by accelerometer-derived estimates. Nat Commun. (2019) 10:1100. doi: 10.1038/s41467-019-08917-4
12. Doherty A, Smith-Byrne K, Ferreira T, Holmes MV, Holmes C, Pulit SL, et al. GWAS identifies 14 loci for device-measured physical activity and sleep duration. Nat Commun. (2018) 9:5257. doi: 10.1038/s41467-018-07743-4
13. Webb JM, Fu YH. Recent advances in sleep genetics. Curr Opin Neurobiol. (2021) 69:19–24. doi: 10.1016/j.conb.2020.11.012
14. Garfield V. Sleep duration: a review of genome-wide association studies (GWAS) in adults from 2007 to 2020. Sleep Med Rev. (2021) 56:101413. doi: 10.1016/j.smrv.2020.101413
15. Steinberg S, de Jong S, Irish Schizophrenia Genomics C, Andreassen OA, Werge T, Borglum AD, et al. Common variants at VRK2 and TCF4 conferring risk of schizophrenia. Hum Mol Genet. (2011) 20:4076–81. doi: 10.1093/hmg/ddr325
16. Qureshi IA, Mehler MF. Epigenetics of sleep and chronobiology. Curr Neurol Neurosci Rep. (2014) 14:432. doi: 10.1007/s11910-013-0432-6
17. Lahtinen A, Puttonen S, Vanttola P, Viitasalo K, Sulkava S, Pervjakova N, et al. A distinctive DNA methylation pattern in insufficient sleep. Sci Rep. (2019) 9:1193. doi: 10.1038/s41598-018-38009-0
18. Ji Y, Qin Y, Shu H, Li X. Methylation analyses on promoters of mPer1, mPer2, and mCry1 during perinatal development. Biochem Biophys Res Commun. (2010) 391:1742–7. doi: 10.1016/j.bbrc.2009.12.146
19. Zhang L, Lin QL, Lu L, Yang CC, Li YL, Sun FL, et al. Tissue-specific modification of clock methylation in aging mice. Eur Rev Med Pharmacol Sci. (2013) 17:1874–80.
20. Perikleous E, Steiropoulos P, Tzouvelekis A, Nena E, Koffa M, Paraskakis E, et al. Methylation in pediatric obstructive sleep apnea: an overview of preliminary findings. Front Pediatr. (2018) 6:154. doi: 10.3389/fped.2018.00154
21. Koopman-Verhoeff ME, Mulder RH, Saletin JM, Reiss I, van der Horst GTJ, Felix JF, et al. Genome-wide DNA methylation patterns associated with sleep and mental health in children: a population-based study. J Child Psychol Psychiatry. (2020) 61:1061–9. doi: 10.1111/jcpp.13252
22. Sammallahti S, Koopman-Verhoeff ME, Binter AC, Mulder RH, Cabre-Riera A, Kvist T, et al. Longitudinal associations of DNA methylation and sleep in children: a meta-analysis. Clin Epigenetics. (2022) 14:83. doi: 10.1186/s13148-022-01298-4
23. Young MW, Kay SA. Time zones: a comparative genetics of circadian clocks. Nat Rev Genet. (2001) 2:702–15. doi: 10.1038/35088576
24. Neves AR, Albuquerque T, Quintela T, Costa D. Circadian rhythm and disease: relationship, new insights, and future perspectives. J Cell Physiol. (2022) 237:3239–56. doi: 10.1002/jcp.30815
25. Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. (2011) 121:2133–41. doi: 10.1172/JCI46043
26. Bolsius YG, Zurbriggen MD, Kim JK, Kas MJ, Meerlo P, Aton SJ, et al. The role of clock genes in sleep, stress and memory. Biochem Pharmacol. (2021) 191:114493. doi: 10.1016/j.bcp.2021.114493
27. Curie T, Maret S, Emmenegger Y, Franken P. In vivo imaging of the central and peripheral effects of sleep deprivation and suprachiasmatic nuclei lesion on PERIOD-2 protein in mice. Sleep. (2015) 38:1381–94. doi: 10.5665/sleep.4974
28. Ehlen JC, Brager AJ, Baggs J, Pinckney L, Gray CL, DeBruyne JP, et al. Bmal1 function in skeletal muscle regulates sleep. Elife. (2017) 6:26557. doi: 10.7554/eLife.26557
29. Wisor JP, O'Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, et al. A role for cryptochromes in sleep regulation. BMC Neurosci. (2002) 3:20. doi: 10.1186/1471-2202-3-20
30. Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. (2017) 34:70–81. doi: 10.1016/j.smrv.2016.07.002
31. Rundo JV. Obstructive sleep apnea basics. Cleve Clin J Med. (2019) 86(Suppl. 1):2–9. doi: 10.3949/ccjm.86.s1.02
32. Song CM, Lee CH, Rhee CS, Min YG, Kim JW. Analysis of genetic expression in the soft palate of patients with obstructive sleep apnea. Acta Otolaryngol. (2012) 132(Suppl. 1):S63–8. doi: 10.3109/00016489.2012.660729
33. Veatch OJ, Bauer CR, Keenan BT, Josyula NS, Mazzotti DR, Bagai K, et al. Characterization of genetic and phenotypic heterogeneity of obstructive sleep apnea using electronic health records. BMC Med Genomics. (2020) 13:105. doi: 10.1186/s12920-020-00755-4
34. Strausz S, Ruotsalainen S, Ollila HM, Karjalainen J, Kiiskinen T, Reeve M, et al. Genetic analysis of obstructive sleep apnoea discovers a strong association with cardiometabolic health. Eur Respir J. (2021) 57:2003091. doi: 10.1183/13993003.03091-2020
35. Meszaros M, Tarnoki AD, Tarnoki DL, Kovacs DT, Forgo B, Lee J, et al. Obstructive sleep apnea and hypertriglyceridaemia share common genetic background: results of a twin study. J Sleep Res. (2020) 29:e12979. doi: 10.1111/jsr.12979
36. Yi M, Zhao W, Tan Y, Fei Q, Liu K, Chen Z, et al. The causal relationships between obstructive sleep apnea and elevated CRP and TNF-alpha protein levels. Ann Med. (2022) 54:1578–89. doi: 10.1080/07853890.2022.2081873
37. Chen W, Cai X, Yan H, Pan Y. Causal effect of obstructive sleep apnea on atrial fibrillation: a mendelian randomization study. J Am Heart Assoc. (2021) 10:e022560. doi: 10.1161/JAHA.121.022560
38. Ma W, Song J, Wang H, Shi F, Zhou N, Jiang J, et al. Chronic paradoxical sleep deprivation-induced depression-like behavior, energy metabolism and microbial changes in rats. Life Sci. (2019) 225:88–97. doi: 10.1016/j.lfs.2019.04.006
39. Park M, Kim SA, Yee J, Shin J, Lee KY, Joo EJ. Significant role of gene-gene interactions of clock genes in mood disorder. J Affect Disord. (2019) 257:510–7. doi: 10.1016/j.jad.2019.06.056
40. Billings ME, Hale L, Johnson DA. Physical and social environment relationship with sleep health and disorders. Chest. (2020) 157:1304–12. doi: 10.1016/j.chest.2019.12.002
Keywords: sleep, gene, GWAS, CiteSpace, VOSviewer, bibliometric analysis, co-citation analysis
Citation: Tao Y, Qin Y, Chen S, Xu T, Lin J, Su D, Yu W and Chen X (2023) Emerging trends and hot spots of sleep and genetic research: a bibliometric analysis of publications from 2002 to 2022 in the field. Front. Neurol. 14:1264177. doi: 10.3389/fneur.2023.1264177
Received: 20 July 2023; Accepted: 04 October 2023;
Published: 08 November 2023.
Edited by:
Agata Gabryelska, Medical University of Lodz, PolandReviewed by:
A. N. M. Mamun-Or-Rashid, Doshisha University, JapanCopyright © 2023 Tao, Qin, Chen, Xu, Lin, Su, Yu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuemei Chen, ZWRlbHdlaXNzX250QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.