- 1Department of Neurology, Penang General Hospital, Penang, Malaysia
- 2Department of Multiple Sclerosis Therapeutics, Fukushima Medical University School of Medicine, Koriyama, Japan
- 3Multiple Sclerosis and Neuromyelitis Optica Center, Southern TOHOKU Research Institute for Neuroscience, Koriyama, Japan
Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD) is an inflammatory demyelinating disease of the central nervous system (CNS) with the presence of conformation-sensitive antibodies against MOG. The spectrum of MOGAD includes monophasic/relapsing optic neuritis, myelitis, neuromyelitis optica spectrum disorder (NMOSD) phenotype without aquaporin 4 (AQP4) antibodies, acute/multiphasic demyelinating encephalomyelitis (ADEM/MDEM)-like presentation, and brainstem and cerebral cortical encephalitis. There is no apparent female preponderance in MOGAD, and MOGAD can onset in all age groups (age at onset is approximately 30 years on average, and approximately 30% of cases are in the pediatric age group). While prevalence and incidence data have been available for AQP4+ NMOSD globally, such data are only beginning to accumulate for MOGAD. We reviewed the currently available data from population-based MOGAD studies conducted around the world: three studies in Europe, three in Asia, and one joint study in the Americas. The prevalence of MOGAD is approximately 1.3–2.5/100,000, and the annual incidence is approximately 3.4–4.8 per million. Among White people, the prevalence of MOGAD appears to be slightly higher than that of AQP4+ NMOSD. No obvious latitude gradient was observed in the Japanese nationwide survey. The data available so far showed no obvious racial preponderance or strong HLA associations in MOGAD. However, precedent infection was reported in approximately 20–40% of MOGAD cases, and this is worthy of further investigation. Co-existing autoimmune disorders are less common in MOGAD than in AQP4+ NMOSD, but NMDAR antibodies may occasionally be positive in patients with MOGAD. More population-based studies in different populations and regions are useful to further inform the epidemiology of this disease.
1. Introduction
Myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD) is an inflammatory demyelinating disease of the central nervous system (CNS), with the presence of conformation-sensitive antibodies against MOG detected by a cell-based assay (1). The MOG antibodies were initially detected primarily in a subgroup of patients with acute/multiphasic demyelinating encephalomyelitis (ADEM/MDEM) (2, 3). Subsequent studies showed that MOG antibodies were also present in patients with optic neuritis, myelitis, neuromyelitis optica spectrum disorder (NMOSD) phenotype without aquaporin 4 (AQP4) antibodies, and brainstem and cerebral cortical encephalitis (4–10). In addition to the unique clinical spectrum, MOGAD has some distinguishing immunopathological features, such as perivenous demyelinating lesions and the fusion pattern without astrocytic damage, MOG-dominant myelin loss in some lesions, and Th17-related cytokine upregulation (11). Therefore, MOGAD is now recognized as a clinical entity distinct from multiple sclerosis (MS) and AQP4 antibody-positive NMOSD (AQP4+ NMOSD).
Recently, the international diagnostic criteria for MOGAD were published (12), and the diagnosis of MOGAD is now made based on the presence of at least one of the core clinical demyelinating events (optic neuritis, myelitis, ADEM, cerebral monofocal or polyfocal deficits, brainstem or cerebellar deficits, and cerebral cortical encephalitis often with seizures), a positive MOG-IgG test, and the exclusion of alternative diagnoses including MS. When MOG antibody is low-positive in a patient with a core clinical demyelinating event, at least one supporting clinical or MRI feature should be met to make a diagnosis of MOGAD since alternative diagnoses including MS can be low-positive (if clear-positive, such a process is unnecessary). In some core clinical demyelinating events (e.g., cerebral monofocal or polyfocal deficits), the clinical and imaging findings are not necessarily strictly defined, and occasionally it may be difficult to diagnose MOGAD in such a case when the MOG antibody is low-positive.
Unlike AQP4+ NMOSD, which has a very high female-to-male ratio (up to 9:1), there is no apparent female preponderance in MOGAD (around 1.2:1). MOGAD can onset in all age groups, with a mean/median age at onset of approximately 28–30 years. Approximately 30% of MOGAD cases are in the pediatric age group, and MOGAD comprises approximately 35–40% of cases of acquired CNS demyelinating syndrome in the pediatric population (10, 13–16). Approximately 35–50% of MOGAD cases have a relapsing course, with the relapse risk being slightly higher (~60%) in the young adult-onset group (14–16). Optic neuritis is the most common onset phenotype (~40%). Age-related onset phenotype is a feature of MOGAD, where ADEM is more common in pediatric patients <10 years old, while myelitis and brainstem encephalitis are more common in adult patients (14–16).
Almost two decades since the discovery of AQP4 antibodies, there is now accumulated data on the prevalence and incidence of AQP4+ NMOSD globally, with over 30 population-based studies published or presented from all continents except Africa (17, 18). The data showed a racial preponderance where the prevalence is higher among East Asians (~5/100,000) and Black people (up to 10/100,000) when compared to White people (~1–1.5/100,000) and Austronesians (~1.5/100,000) (17–21). Despite being less well studied, there are now a number of population-based studies on the prevalence and incidence of MOGAD being reported, and it is time to review these data from around the world.
In this article, we review population-based studies of MOGAD to determine its prevalence and incidence in all regions of the world. We also review certain epidemiological aspects such as precedent infection/vaccination, autoimmune comorbidities, and seasonal variation, as well as the available data on HLA and other genetic associations in MOGAD.
2. Prevalence and incidence of MOGAD
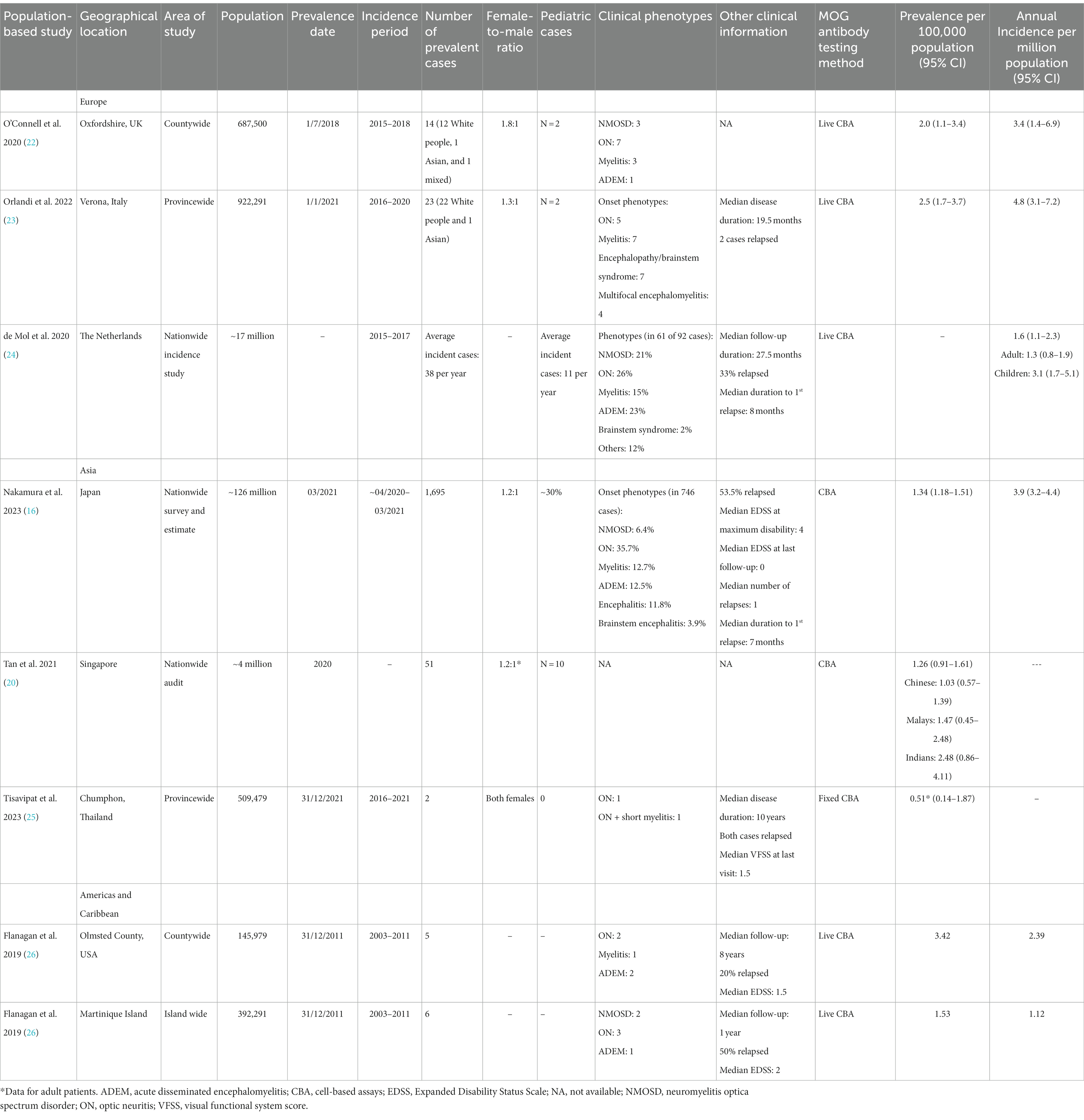
There have been a total of seven population-based studies that have provided data on the prevalence and incidence of MOGAD (as of June 2023). Three studies were conducted in Europe: Oxfordshire (UK) (22), Verona (Italy) (23), and the Dutch nationwide incidence study (24). The studies in Asia included a nationwide survey in Japan (16), a nationwide audit in Singapore (20), and a study in Chumphon (Thailand) (25). In the Americas and the Caribbean, there was a joint study in Olmsted County (Minnesota, USA) and Martinique Island, which was presented at a conference (ECTRIMS 2019) (26). The data from these studies are summarized and presented in Figure 1 and Table 1.
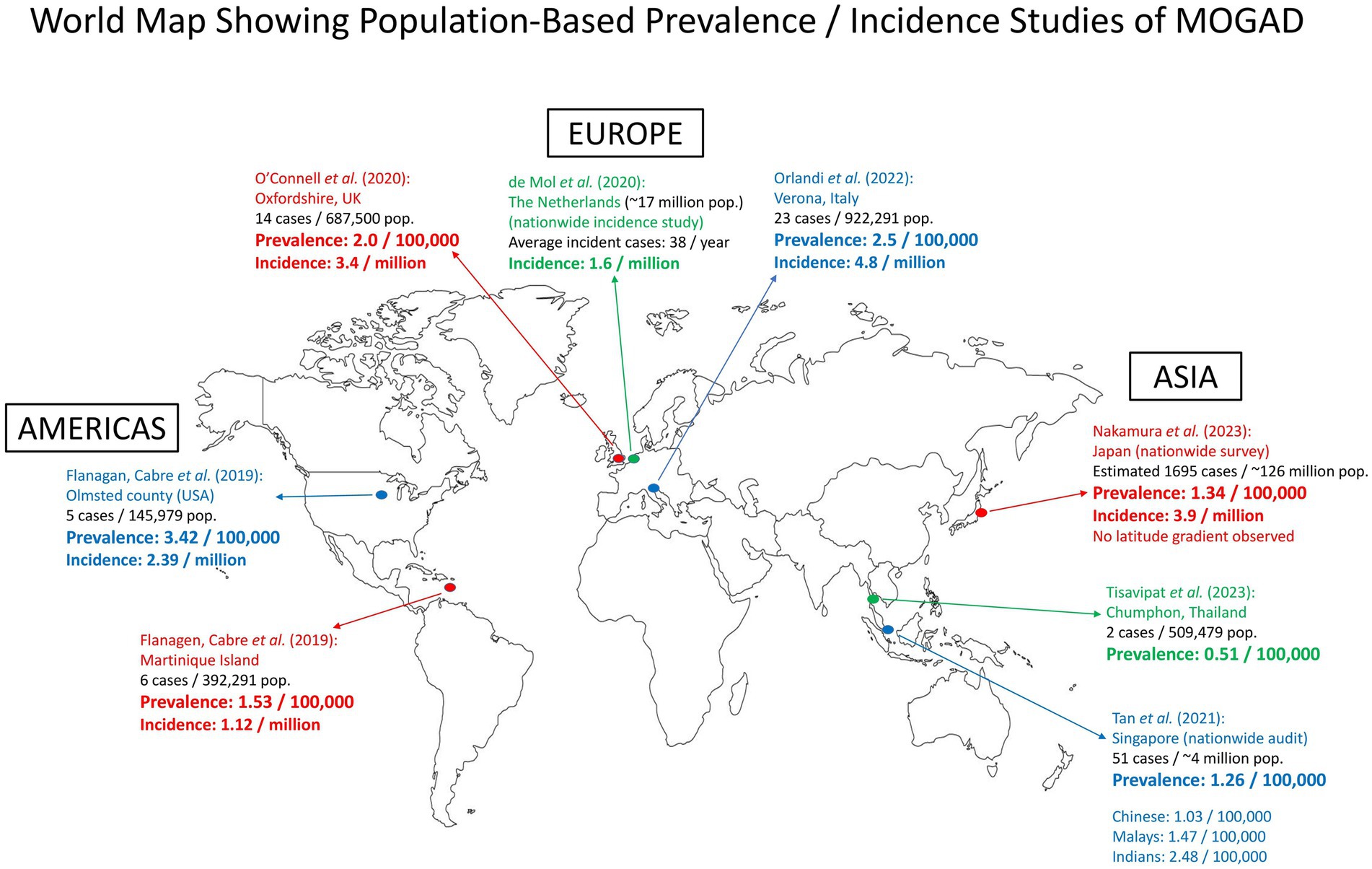
Figure 1. Map showing population-based prevalence/incidence studies of MOGAD around the world. There were three studies in Europe, three in Asia, and one joint study in the Americas and the Caribbean. pop., population.
It is of note that all these studies were conducted before the international diagnostic criteria for MOGAD were proposed (12). With the new diagnostic criteria, future epidemiological data may be more standardized and accurate. It is also important to note that the majority of the studies were based on the results of MOG antibodies detected in sera. However, some patients with MOGAD are known to be MOG antibody-positive only in the cerebrospinal fluid (CSF) (27, 28), and they were not included in the prevalence and incidence calculations.
2.1. Europe
The first population-based prevalence study of MOGAD in Europe was reported from Oxfordshire (UK), with a prevalence of 2.0/100,000 (total of 14 patients, with 12 White people) and an annual incidence of 3.4 per million (22). In that study, among White people, the prevalence of MOGAD was 1.9/100,000 (12 cases), while the prevalence of AQP4-IgG+ NMOSD was 1.0/100,000 (6 cases). This study shows that in White people, MOGAD appears to be two times more common than AQP4+ NMOSD.
The MOGAD study from Verona (Italy) reported a prevalence of 2.5/100,000 (in 23 patients; 22 White people and 1 Asian) and an annual incidence of 4.4 per million (23). This prevalence in Verona is quite consistent with that reported from Oxfordshire.
In the Dutch nationwide MOGAD incidence study, the data from a single centralized laboratory that performed MOG antibody tests were analyzed. From 2015 to 2017, the average annual incidence was 1.6 per million (1.3 per million in adults, and a higher incidence of 3.1 per million in children) (24). The study investigators noted that these incidence estimates were minimum figures, and increased awareness of the antibody tests among treating physicians would likely lead to a higher incidence. For instance, in 2017, the annual incidence was 2.4 per million (children: 4.7 per million).
There were also two population-based studies conducted in Catalonia and Portugal on the prevalence and incidence of NMOSD that included AQP4 antibody-positive cases and MOG antibody-positive cases (29, 30). However, these cases were the ones that strictly fulfilled the 2015 criteria of the International Panel on NMO Diagnosis, and therefore, for MOG antibody-positive cases, only those with an NMO phenotype were included. In Catalonia, 12% of the NMOSD cases were MOG antibody-positive, giving a prevalence of 0.11/100,000 (29). In the Portuguese nationwide study, 67/180 (37%) of NMOSD cases were MOG antibody-positive, giving a prevalence of 0.65/100,000 (30). Suffice it to say, if all phenotypes of MOGAD (e.g., those with monophasic or relapsing optic neuritis or the ones with brain syndromes) were included, the prevalence would be higher.
2.2. Asia
The Japanese nationwide MOGAD survey (Japan’s population in 2020 was 126 million) published recently identified 877 MOGAD cases, including 258 new cases, and estimated there were 1,695 MOGAD cases nationwide. This gave an estimated prevalence of 1.34/100,000 and an annual incidence of 3.9 per million (16). The survey also showed that there was no obvious latitude gradient observed between the northern and southern parts of Japan for MOGAD prevalence. This study was based on data gleaned from questionnaires sent to neurology, pediatric neurology, and neuro-ophthalmology departments (N = 3,790) across the country, which is different from a conventional population-based prevalence and incidence study. In addition, the response rate in the primary survey was relatively low (36.4%). These may be the limitations of the survey.
In the multi-racial island nation of Singapore at the equator, a nationwide audit estimated a prevalence of 1.26/100,000 (20). When breaking down according to the racial groups, the prevalence was 1.03/100,000 among Chinese, 1.47/100,000 among Malays, and 2.48/100,000 among Indians, suggesting a potential influence of race on the prevalence. On the other hand, multi-center studies from Australia and the UK largely did not show significant racial preponderance (15, 31), though, in the UK study, there was a slightly increased proportion of South Asian patients in the young pediatric group (<12 years), while in the Australian cohort, there was a slightly increased proportion of South Asian patients in the adult group. Longitudinal studies and data from other regions will be useful to clarify this.
A recent study from the Chumphon province in Thailand reported two cases of MOGAD in adult females, giving a prevalence of 0.51/100,000 among the adult population (25). In the same study, the prevalence of MS was 0.77/100,000, while the prevalence of AQP4+ NMOSD was 3.08/100,000. More population-based studies of MOGAD from the diverse regions of Asia are awaited.
2.3. The Americas and the Caribbean
There was a joint study from Olmsted County (USA) and Martinique Island that was presented at the ECTRIMS 2019 congress but utilized earlier data (prevalence date: 2011) (26). In Olmsted County, the prevalence was 3.42/100,000 and the annual incidence was 2.39 per million. On Martinique Island in the Caribbean, the prevalence was 1.53/100,000 and the annual incidence was 1.12 per million. A follow-up study from these two areas will help provide updated data on MOGAD prevalence and incidence in the Americas and the Caribbean.
In São Paulo (Brazil), a study estimated the prevalence of MOGAD by first determining the ratio of MS to MOGAD in a university referral center and then extrapolating it by using the known MS prevalence (in 1997) in that region. With this method, the prevalence of MOGAD was estimated to be 0.4/100,000 (32). Nevertheless, there could be an underestimation as the center only treats adult patients but not pediatric patients. In a multi-center study in the province of Quebec (Canada) that involved seven major adult and pediatric academic centers, a total of 45 MOGAD cases were identified, giving a minimum prevalence of 0.52/100,000 (33). This was also likely an underestimation because some cases might not have been tested for MOG antibodies or referred to those specialized centers.
3. Precedent infection and vaccination
An important clinical aspect of MOGAD is that approximately 20–40% of the cases had infectious prodromes or precedent infections. Those precedent infections included the common cold, pharyngolaryngitis, bronchitis, pneumonia, acute gastroenteritis, and infections related to influenza, mycoplasma, streptococcus, and chlamydia (16, 31, 34–36). The Japanese study showed that this precedent infection was more frequent in the pediatric-onset group (39% for those <10 years) than in the adult-onset group (13.5%) (16). It is interesting to see how infectious diseases are involved in the pathogenesis of MOGAD (immune activation, molecular mimicry, etc.). Additionally, there were also a small number of MOGAD cases that onset after vaccination. The vaccines reported included those for influenza, Japanese encephalitis, measles/rubella, diphtheria/tetanus/pertussis, and COVID-19 (16, 34, 36–38).
4. Autoimmune comorbidities
Co-existing autoimmune diseases are observed in up to 7–10% of MOGAD patients, with Sjogren syndrome, rheumatoid arthritis, ulcerative colitis, thyroid disorder, psoriasis, and NMDAR encephalitis being commonly reported (16, 31, 34, 35, 39), but the frequency appears to be lower than that in AQP4+ NMOSD (40). In a laboratory study, the serum and/or CSF of 376 patients positive for MOG antibodies were tested for co-existent neuronal surface antibodies. A total of 14 (3.7%) patients were dual positive for MOG and NMDAR antibodies, making NMDAR antibodies the most frequent co-existent neuronal surface antibodies in MOGAD (41). In the Japanese nationwide survey, 15 (31%) of 48 selected MOGAD patients tested were positive for NMDAR antibodies (16). A systematic review showed that there have been more than 200 cases reported in the literature of either MOGAD co-existing with NMDAR encephalitis or dual positivity of MOG and NMDAR antibodies in encephalitis or MOGAD patients (42).
5. HLA and other genetic associations
For AQP4+ NMOSD, multiple studies have shown different HLA associations (both risk and protective HLA alleles) in different racial groups (Asians, White people, and Latin Americans) (19, 43–46). For MOGAD, two studies (the Netherlands and the UK) did not find any strong HLA associations (43, 47). However, a study in Guangzhou (China) found that pediatric-onset MOGAD was associated with the DQB1*05:02-DRB1*16:02 haplotype, though no HLA association was found for adult-onset MOGAD (48). Another study in Guangzhou also revealed three non-HLA susceptibility loci (BANK1, RNASET2, and TNIP1) for MOGAD (49).
6. Seasonal variation
Three studies [Tohoku region (Japan), Verona (Italy), and Quebec (Canada)] reported an autumn-winter predominance for the onset of MOGAD (23, 33, 50). However, a joint study from Germany and the Kanto region in Japan did not find such a trend (51). That study reported the lowest incidence of MOGAD onset during autumn in both the German and Kanto cohorts. A UK study did not observe seasonal variation in MOGAD onset either (52). More studies are needed to further clarify this aspect.
7. Conclusion
We reviewed the currently available data from population-based MOGAD studies conducted around the world. The prevalence of MOGAD is approximately 1.3–2.5/100,000, and the annual incidence is approximately 3.4–4.8 per million. As disease awareness increases, and with the ease of availability of MOG antibody assays, the prevalence is expected to rise in the future. Moreover, through the application of the international diagnostic criteria of MOGAD (12), the epidemiological data are expected to be more accurate.
Among White people, the prevalence of MOGAD appears to be slightly higher than that of AQP4+ NMOSD. Conversely, in populations or regions where the prevalence of AQP4+ NMOSD is higher (such as in Japan), MOGAD appears comparatively less common. So far, there has been no obvious racial preponderance observed in MOGAD, though the slight increase in prevalence among South Asians requires further investigation. The role of precedent infection observed in a proportion of MOGAD cases is also worthy of further research. More population-based studies in different populations and regions of the world will be very useful to further inform the epidemiology of this unique inflammatory demyelinating disease of the CNS.
Author contributions
JH and KF conceived and designed the study, collected and analyzed the data, drafted the manuscript, critically revised the manuscript for intellectual content, and approved the final manuscript.
Funding
The article processing fee is funded by The Sumaira Foundation.
Conflict of interest
KF serves on scientific advisory boards or as a consultant for Biogen, Mitsubishi-Tanabe, Novartis, Chugai, Roche, Alexion, VielaBio/Horizon Therapeutics, UCB, Merck Biopharma, Japan Tobacco, Argenx, and Abbvie; has received funding for travel or speaker honoraria from Chugai, Roche, Biogen, Novartis, Alexion, Teijin, Mitsubishi-Tanabe, AsahiKasei, Eisai, Takeda, and Bayer; serves on editorial boards of Clinical and Experimental Neuroimmunology, Frontiers in Neurology, Neurology: Neuroimmunology and Neuroinflammation, Multiple Sclerosis Journal, Multiple Sclerosis and Related Disorders, Neuroimmunology Reports and European Journal of Neurology, and advisory board of Sri Lanka Journal of Neurology; and has been funded by the Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Technology of Japan and by the Grants-in-Aid for Scientific Research from the Ministry of Health, Welfare and Labor of Japan.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reindl, M, and Waters, P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat Rev Neurol. (2019) 15:89–102. doi: 10.1038/s41582-018-0112-x
2. O’Connor, KC, McLaughlin, KA, De Jager, PL, Chitnis, T, Bettelli, E, Xu, C, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med. (2007) 13:211–7. doi: 10.1038/nm1488
3. Brilot, F, Dale, RC, Selter, RC, Grummel, V, Kalluri, SR, Aslam, M, et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann Neurol. (2009) 66:833–42. doi: 10.1002/ana.21916
4. Kitley, J, Woodhall, M, Waters, P, Leite, MI, Devenney, E, Craig, J, et al. Myelin-oligodendrocyte glycoprotein antibodies in adults with a neuromyelitis optica phenotype. Neurology. (2012) 79:1273–7. doi: 10.1212/WNL.0b013e31826aac4e
5. Sato, DK, Callegaro, D, Lana-Peixoto, MA, Waters, PJ, de Haidar Jorge, FM, Takahashi, T, et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology. (2014) 82:474–81. doi: 10.1212/WNL.0000000000000101
6. Fujimori, J, Takai, Y, Nakashima, I, Sato, DK, Takahashi, T, Kaneko, K, et al. Bilateral frontal cortex encephalitis and paraparesis in a patient with anti-MOG antibodies. J Neurol Neurosurg Psychiatry. (2017) 88:534–6. doi: 10.1136/jnnp-2016-315094
7. Ogawa, R, Nakashima, I, Takahashi, T, Kaneko, K, Akaishi, T, Takai, Y, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm. (2017) 4:e322. doi: 10.1212/NXI.0000000000000322
8. Banks, SA, Morris, PP, Chen, JJ, Pittock, SJ, Sechi, E, Kunchok, A, et al. Brainstem and cerebellar involvement in MOG-IgG-associated disorder versus aquaporin-4-IgG and MS. J Neurol Neurosurg Psychiatry. (2021) 92:384–90. doi: 10.1136/jnnp-2020-325121
9. Matsumoto, Y, Misu, T, Mugikura, S, Takai, Y, Nishiyama, S, Kuroda, H, et al. Distinctive lesions of brain MRI between MOG-antibody-associated and AQP4-antibody-associated diseases. J Neurol Neurosurg Psychiatry. (2021) 92:682–4. doi: 10.1136/jnnp-2020-324818
10. Marignier, R, Hacohen, Y, Cobo-Calvo, A, Pröbstel, AK, Aktas, O, Alexopoulos, H, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. (2021) 20:762–72. doi: 10.1016/S1474-4422(21)00218-0
11. Takai, Y, Misu, T, Fujihara, K, and Aoki, M. Pathology of myelin oligodendrocyte glycoprotein antibody-associated disease: a comparison with multiple sclerosis and aquaporin 4 antibody-positive neuromyelitis optica spectrum disorders. Front Neurol. (2023) 14:1209749. doi: 10.3389/fneur.2023.1209749
12. Banwell, B, Bennett, JL, Marignier, R, Kim, HJ, Brilot, F, Flanagan, EP, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: international MOGAD panel proposed criteria. Lancet Neurol. (2023) 22:268–82. doi: 10.1016/S1474-4422(22)00431-8
13. Hacohen, Y, Absoud, M, Deiva, K, Hemingway, C, Nytrova, P, Woodhall, M, et al. Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurol Neuroimmunol Neuroinflamm. (2015) 2:e81. doi: 10.1212/NXI.0000000000000081
14. Cobo-Calvo, A, Ruiz, A, Rollot, F, Arrambide, G, Deschamps, R, Maillart, E, et al. Clinical features and risk of relapse in children and adults with myelin oligodendrocyte glycoprotein antibody-associated disease. Ann Neurol. (2021) 89:30–41. doi: 10.1002/ana.25909
15. Satukijchai, C, Mariano, R, Messina, S, Sa, M, Woodhall, MR, Robertson, NP, et al. Factors associated with relapse and treatment of myelin oligodendrocyte glycoprotein antibody-associated disease in the United Kingdom. JAMA Netw Open. (2022) 5:e2142780. doi: 10.1001/jamanetworkopen.2021.42780
16. Nakamura, M, Ogawa, R, Fujimori, J, Uzawa, A, Sato, Y, Nagashima, K, et al. Epidemiological and clinical characteristics of myelin oligodendrocyte glycoprotein antibody-associated disease in a nationwide survey. Mult Scler. (2023) 29:530–9. doi: 10.1177/13524585231156736
17. Hor, JY, Asgari, N, Nakashima, I, Broadley, SA, Leite, MI, Kissani, N, et al. Epidemiology of neuromyelitis optica spectrum disorder and its prevalence and incidence worldwide. Front Neurol. (2020) 11:501. doi: 10.3389/fneur.2020.00501
18. Papp, V, Magyari, M, Aktas, O, Berger, T, Broadley, SA, Cabre, P, et al. Worldwide incidence and prevalence of neuromyelitis optica: a systematic review. Neurology. (2021) 96:59–77. doi: 10.1212/WNL.0000000000011153
19. Hor, JY, Wong, CK, Ew, JV, Idris, S, Tan, HJ, and Wong, DWJ. Neuromyelitis optica spectrum disorder in Asia: epidemiology and risk factors. Neurol Clin Neurosci. (2021) 9:274–81. doi: 10.1111/ncn3.12478
20. Tan, K, Yeo, T, Yong, KP, Thomas, T, Wang, F, Tye, J, et al. Central nervous system inflammatory demyelinating diseases and neuroimmunology in Singapore – epidemiology and evolution of an emerging subspecialty. Neurol Clin Neurosci. (2021) 9:259–65. doi: 10.1111/ncn3.12479
21. Houzen, H, Kano, T, Kondo, K, Takahashi, T, and Niino, M. Prevalence, incidence and clinical features of neuromyelitis optica spectrum disorders in northern Japan. J Neurol Neurosurg Psychiatry. (2023) 94:494–5. doi: 10.1136/jnnp-2022-330274
22. O’Connell, K, Hamilton-Shield, A, Woodhall, M, Messina, S, Mariano, R, Waters, P, et al. Prevalence and incidence of neuromyelitis optica spectrum disorder, aquaporin-4 antibody-positive NMOSD and MOG antibody-positive disease in Oxfordshire, UK. J Neurol Neurosurg Psychiatry. (2020) 91:1126–8. doi: 10.1136/jnnp-2020-323158
23. Orlandi, R, Mariotto, S, and Gajofatto, A. Prevalence, incidence, and season distribution of MOG antibody-associated disease in the province of Verona, Italy. Mult Scler Relat Disord. (2022) 63:103884. doi: 10.1016/j.msard.2022.103884
24. de Mol, CL, Wong, YYM, van Pelt, ED, Wokke, BHA, Siepman, TAM, Neuteboom, RF, et al. The clinical spectrum and incidence of anti-MOG-associated acquired demyelinating syndromes in children and adults. Mult Scler. (2020) 26:806–14. doi: 10.1177/1352458519845112
25. Tisavipat, N, Jitpratoom, P, Siritho, S, Prayoonwiwat, N, Apiwattanakul, M, Boonyasiri, A, et al. The epidemiology and burden of neuromyelitis optica spectrum disorder, multiple sclerosis, and MOG antibody-associated disease in a province in Thailand: a population-based study. Mult Scler Relat Disord. (2023) 70:104511. doi: 10.1016/j.msard.2023.104511
26. Flanagan, E, Cabre, P, St Sauver, J, Chen, J, Fryer, J, Smith, C, et al. The epidemiology of myelin oligodendrocyte glycoprotein antibody associated disorders. Mult Scler. (2019) 25:160.
27. Carta, S, Cobo Calvo, Á, Armangué, T, Saiz, A, Lechner, C, Rostásy, K, et al. Significance of myelin oligodendrocyte glycoprotein antibodies in CSF: a retrospective multicenter study. Neurology. (2023) 100:e1095–108. doi: 10.1212/WNL.0000000000201662
28. Matsumoto, Y, Kaneko, K, Takahashi, T, Takai, Y, Namatame, C, Kuroda, H, et al. Diagnostic implications of MOG-IgG detection in sera and cerebrospinal fluids. Brain. (2023). doi: 10.1093/brain/awad122
29. Sepúlveda, M, Aldea, M, Escudero, D, Llufriu, S, Arrambide, G, Otero-Romero, S, et al. Epidemiology of NMOSD in Catalonia: influence of the new 2015 criteria in incidence and prevalence estimates. Mult Scler. (2018) 24:1843–51. doi: 10.1177/1352458517735191
30. Santos, E, Rocha, AL, Oliveira, V, Ferro, D, Samões, R, Sousa, AP, et al. Neuromyelitis optica spectrum disorders: a nationwide Portuguese clinical epidemiological study. Mult Scler Relat Disord. (2021) 56:103258. doi: 10.1016/j.msard.2021.103258
31. Ramanathan, S, Mohammad, S, Tantsis, E, Nguyen, TK, Merheb, V, Fung, VSC, et al. Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry. (2018) 89:127–37. doi: 10.1136/jnnp-2017-316880
32. Silva, GD, Apóstolos-Pereira, SL, and Callegaro, D. Estimated prevalence of AQP4 positive neuromyelitis optica spectrum disorder and MOG antibody associated disease in São Paulo, Brazil. Mult Scler Relat Disord. (2023) 70:104488. doi: 10.1016/j.msard.2022.104488
33. Boudjani, H, Fadda, G, Dufort, G, Antel, J, Giacomini, P, Levesque-Roy, M, et al. Clinical course, imaging, and pathological features of 45 adult and pediatric cases of myelin oligodendrocyte glycoprotein antibody-associated disease. Mult Scler Relat Disord. (2023) 76:104787. doi: 10.1016/j.msard.2023.104787
34. Jarius, S, Ruprecht, K, Kleiter, I, Borisow, N, Asgari, N, Pitarokoili, K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. (2016) 13:280. doi: 10.1186/s12974-016-0718-0
35. Cobo-Calvo, A, Ruiz, A, Maillart, E, Audoin, B, Zephir, H, Bourre, B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults. Neurology. (2018) 90:e1858–69. doi: 10.1212/WNL.0000000000005560
36. Azumagawa, K, Nakashima, I, Kaneko, K, Torisu, H, Sakai, Y, Kira, R, et al. A nation-wide survey of Japanese pediatric MOG antibody-associated diseases. Brain and Dev. (2021) 43:705–13. doi: 10.1016/j.braindev.2021.01.008
37. Kim, KH, Kim, SH, Park, NY, Hyun, JW, and Kim, HJ. Onset of various CNS inflammatory demyelinating diseases following COVID-19 vaccinations. Mult Scler Relat Disord. (2022) 68:104141. doi: 10.1016/j.msard.2022.104141
38. Francis, AG, Elhadd, K, Camera, V, Ferreira dos Santos, M, Rocchi, C, Adib-Samii, P, et al. Acute inflammatory disease of the central nervous system after SARS-CoV-2 vaccination. Neurol Neuroimmunol Neuroinflamm. (2023) 10:e200063. doi: 10.1212/NXI.0000000000200063
39. Cross, H, Sabiq, F, Ackermans, N, Mattar, A, Au, S, Woodhall, M, et al. Myelin oligodendrocyte glycoprotein (MOG) antibody positive patients in a multi-ethnic Canadian cohort. Front Neurol. (2021) 11:525933. doi: 10.3389/fneur.2020.525933
40. Kunchok, A, Flanagan, EP, Snyder, M, Saadeh, R, Chen, JJ, Weinshenker, BG, et al. Coexisting systemic and organ-specific autoimmunity in MOG-IgG1-associated disorders versus AQP4-IgG+ NMOSD. Mult Scler. (2021) 27:630–5. doi: 10.1177/1352458520933884
41. Kunchok, A, Flanagan, EP, Krecke, KN, Chen, JJ, Caceres, JA, Dominick, J, et al. MOG-IgG1 and co-existence of neuronal autoantibodies. Mult Scler. (2021) 27:1175–86. doi: 10.1177/1352458520951046
42. Molazadeh, N, Bose, G, Lotan, I, and Levy, M. Autoimmune diseases and cancers overlapping with myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD): a systematic review. Mult Scler J Exp Transl Clin. (2022) 8:20552173221128170. doi: 10.1177/20552173221128170
43. Bruijstens, AL, Wong, YYM, van Pelt, DE, van der Linden, PJE, Haasnoot, GW, Hintzen, RQ, et al. HLA association in MOG-IgG- and AQP4-IgG-related disorders of the CNS in the Dutch population. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e702. doi: 10.1212/NXI.0000000000000702
44. Matsushita, T, Masaki, K, Isobe, N, Sato, S, Yamamoto, K, Nakamura, Y, et al. Genetic factors for susceptibility to and manifestations of neuromyelitis optica. Ann Clin Transl Neurol. (2020) 7:2082–93. doi: 10.1002/acn3.51147
45. Romero-Hidalgo, S, Flores-Rivera, J, Rivas-Alonso, V, Barquera, R, Villarreal-Molina, MT, Antuna-Puente, B, et al. Native American ancestry significantly contributes to neuromyelitis optica susceptibility in the admixed Mexican population. Sci Rep. (2020) 10:13706. doi: 10.1038/s41598-020-69224-3
46. Alvarenga, MP, do Carmo, LF, Vasconcelos, CCF, Alvarenga, MP, Alvarenga-Filho, H, de Melo Bento, CA, et al. Neuromyelitis optica is an HLA associated disease different from multiple sclerosis: a systematic review with meta-analysis. Sci Rep. (2021) 11:152. doi: 10.1038/s41598-020-80535-3
47. Grant-Peters, M, Dos Passos, GR, Yeung, HY, Jacob, A, Huda, S, Leite, MI, et al. No strong HLA association with MOG antibody disease in the UK population. Ann Clin Transl Neurol. (2021) 8:1502–7. doi: 10.1002/acn3.51378
48. Sun, X, Qiu, W, Wang, J, Wang, S, Wang, Y, Zhong, X, et al. Myelin oligodendrocyte glycoprotein-associated disorders are associated with HLA subtypes in a Chinese paediatric-onset cohort. J Neurol Neurosurg Psychiatry. (2020) 91:733–9. doi: 10.1136/jnnp-2019-322115
49. Shu, Y, Ma, X, Chen, C, Wang, Y, Sun, X, Zhang, L, et al. Myelin oligodendrocyte glycoprotein-associated disease is associated with BANK1, RNASET2 and TNIP1 polymorphisms. J Neuroimmunol. (2022) 372:577937. doi: 10.1016/j.jneuroim.2022.577937
50. Akaishi, T, Fujimori, J, Takahashi, T, Misu, T, Takai, Y, Nishiyama, S, et al. Seasonal variation of onset in patients with anti-aquaporin-4 antibodies and anti-myelin oligodendrocyte glycoprotein antibody. J Neuroimmunol. (2020) 349:577431. doi: 10.1016/j.jneuroim.2020.577431
51. Liu, J, Mori, M, Zimmermann, H, Brandt, A, Havla, J, Tanaka, S, et al. Anti-MOG antibody-associated disorders: differences in clinical profiles and prognosis in Japan and Germany. J Neurol Neurosurg Psychiatry. (2021) 92:377–83. doi: 10.1136/jnnp-2020-324422
Keywords: myelin oligodendrocyte glycoprotein, MOG antibody-associated disease, neuromyelitis optica spectrum disorder, population study, prevalence, incidence, epidemiology
Citation: Hor JY and Fujihara K (2023) Epidemiology of myelin oligodendrocyte glycoprotein antibody-associated disease: a review of prevalence and incidence worldwide. Front. Neurol. 14:1260358. doi: 10.3389/fneur.2023.1260358
Edited by:
Sasitorn Siritho, Bumrungrad International Hospital, ThailandReviewed by:
Sara Mariotto, University of Verona, ItalyPatrick Joseph Waters, University of Oxford, United Kingdom
Nanthaya Tisavipat, Mayo Clinic, United States
Copyright © 2023 Hor and Fujihara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jyh Yung Hor, aG9yanlAeWFob28uY29t; Kazuo Fujihara, ZnVqaWthenVAbWVkLnRvaG9rdS5hYy5qcA==
 Jyh Yung Hor
Jyh Yung Hor Kazuo Fujihara
Kazuo Fujihara