- 1Department of General Critical Care Medicine, Zhumadian Central Hospital, Zhumadian, China
- 2Department of Neurology, Zhumadian Central Hospital, Zhumadian, China
- 3Department of Scientific Research Management, Zhumadian Central Hospital, Zhumadian, China
- 4Department of Neurology, Jilin Province First Auto Work General Hospital, Jilin, China
Background: Stroke-associated pneumonia (SAP) is associated with a poor prognosis and a high mortality rate in stroke patients. However, the accuracy of early prediction of SAP is insufficient, and there is a lack of effective prognostic evaluation methods. Therefore, in this study, we investigated the predictive value of the Oxford Acute Severity of Illness Score (OASIS) in SAP to provide a potential reference index for the incidence and prognosis of SAP.
Methods: We recruited a total of 280 patients with acute ischemic stroke who had been diagnosed and treated in the Zhumadian Central Hospital between January 2021 and January 2023. These patients were divided into an SAP group (86 cases) and a non-SAP group (194 cases) according to SAP diagnostic criteria by expert consensus on the diagnosis and treatment of SAP. We collated general and clinical data from all patients, including the survival of SAP patients during the follow-up period. Multivariate logistic regression was used to analyze the risk factors for SAP. Kaplan–Meier and multivariate COX regression analyses were used to investigate the relationship between OASIS and the prognosis of SAP, and a receiver operating characteristic (ROC) curve was drawn to analyze the predictive value of OASIS for SAP.
Results: Our analyses identified body temperature, C-reactive protein, procalcitonin, OASIS, and a prolonged length of intensive care unit (ICU) stay as the main risk factors for SAP (all Ps < 0.05). Advanced age and an elevated OASIS were identified as the main risk factors for death in SAP patients (all Ps < 0.05). The risk of death in patients with OASIS of 31–42 points was significantly higher than that in patients with OASIS of 12–20 points (HR = 5.588, 95% CI = 1.531–20.401, P = 0.009). ROC curve analysis further showed that OASIS had a high predictive value for morbidity and the incidence of death in SAP patients.
Conclusion: OASIS can effectively predict the onset and death of SAP patients and provides a potential reference index for early diagnosis and the prediction of prognosis in patients with SAP. Our findings should be considered in clinical practice.
Introduction
Stroke is a common clinical disease. The results of a survey conducted in 31 provinces in China in 2020 showed the estimated prevalence (2.6%), incidence (505.2 per 100,000), and mortality (343.4 per 100,000) of stroke in people over 40 years of age in 2020, with ischemic stroke accounting for 86.8% of the total incidence (1). Stroke patients not only have to face high medical costs but also poor prognosis. According to statistics, the hospitalization cost of stroke in 2020 was as high as 58 billion yuan, of which patients paid ~19.8 billion yuan. The rate of death/discharge in hospital against medical advice was 9.2%, while ~12.5% (2.2 million people) of stroke survivors had stroke-related disability, and the disability rate at 3 and 12 months was 14.8 and 14.0%, respectively. The mortality rate of stroke at 3 and 12 months was 4.2 and 8.5%, respectively, and the recurrence rate of stroke at 3 and 12 months was 3.6 and 5.6%, respectively (2), indicating that it is urgent to improve stroke prevention and treatment strategies and strengthen assessment and early intervention in stroke prognosis in China, to improve stroke status. Stroke-associated pneumonia (SAP) is a common complication that often occurs within the 1st week after the onset of acute stroke. This condition predominantly refers to the symptoms of lung infection caused by nervous system damage and the reduced immune status that follows the onset of stroke. Previous epidemiological surveys have reported the incidence of SAP as 7–38% (3, 4). Furthermore, the risk of death within 30 days of stroke with SAP was 3-fold higher than in patients without SAP. This fact not only increases the difficulty of clinical treatment and prolongs the treatment period but also increases the incidence of severe disability (5). Over recent years, many researchers have designed SAP prediction models for different stroke patients using multivariate regression models, such as the acute ischemic stroke-associated pneumonia score (AIS-APS) and the intra cerebral hemorrhage-associated pneumonia score (ICH-APS) models, which are recommended by the Chinese Expert Consensus on the Diagnosis and Treatment of Stroke-related Pneumonia in 2019. However, the predictive accuracy of these models is not ideal, and there is a lack of predictive models to evaluate the prognosis of stroke when complicated by SAP (6, 7). Therefore, it is of great significance to continue developing early identification and accurate prognostic risk assessment methods for high-risk SAP patients to strengthen the individualized monitoring of stroke patients and implement targeted interventions to improve survival rates. Many severity scores have been used to evaluate the prognosis of critically ill patients. Of these, the acute physiology and chronic health evaluation II (APACHE II) model is the most common disease severity scoring system used in the ICU; this has had a significant effect on the prognostic evaluation of ICU patients (including those with acute stroke). However, the APACHE II system involves many parameters; most of these parameters involve laboratory tests. Furthermore, data collection can be laborious; this is not conducive to early and rapid diagnosis (8, 9). The Oxford Acute Severity of Illness Score (OASIS) is a scoring system that does not involve laboratory tests or imaging examinations. OASIS is widely used for the differential diagnosis of acute disease severity and has also been confirmed to have high identification and calibration efficiency for the prognosis of ICU patients. Consequently, OASIS can replace the more complex existing prediction systems (10, 11). However, few studies have investigated the predictive value of OASISs in the prognosis of patients with acute stroke. Moreover, nothing is known about the efficacy of OASISs when evaluating the incidence and prognosis of SAP. Therefore, in this study, we collected relevant data from patients with acute ischemic stroke and analyzed the effect of OASIS on the risk and prognosis of SAP. In addition, we investigated the predictive value of OASISs to provide reference guidelines for the prevention, treatment, and improved prognostic evaluation of patients with acute stroke complicated with SAP.
Materials and methods
Data sources and study population
We recruited patients with acute ischemic stroke who had been hospitalized in the Department of Critical Care Medicine of Zhumadian Central Hospital between January 2021 and January 2023. Patients who met the following conditions were enrolled: (1) met the diagnostic criteria for acute ischemic stroke in the 2018 Chinese Guidelines for the Diagnosis and Treatment of Acute Ischemic Stroke (12) and had been diagnosed by head computed tomography (CT) or magnetic resonance imaging (MRI); (2) admission to ICU within 24 h of onset; (3) first stroke or previous stroke without obvious sequelae; (4) aged > 18 years; and (5) provided informed consent for this study. Patients were excluded if they had been discharged within 24 h after onset or had died, had pulmonary infection or other pulmonary diseases before admission, had recent surgical history or trauma, had other diseases affecting neurological function or other serious systemic diseases, had an incomplete clinical dataset, or had been discharged abnormally. A total of 280 patients were finally included as research objects; of these, 86 SAP patients who met the SAP diagnostic criteria by the expert consensus on the diagnosis and treatment of stroke-associated pneumonia (13) were included in the SAP group; the remaining 194 patients were included in the non-SAP group (Figure 1).
We collated a range of relevant data from each patient, including demographics (age, sex, BMI, and history of past illness), vital signs on admission (heart rate, respiratory rate, mean arterial pressure, and body temperature), Glasgow coma scale (GCS) and National Institutes of Health Stroke Scale (NIHSS) scores, length of stay before ICU admission, emergency surgery before admission to the ICU, mechanical ventilation and daily urine volume on the 1st day of ICU, the length of ICU stay, complications (SAP, encephaledema, and epilepsy) and the 28-day survival of SAP patients. Laboratory tests included white blood cell count (WBC), C-reactive protein (CRP), and procalcitonin (PCT).
All patients signed informed consent forms, and this study was approved by the Medical Ethics Committee of Zhumadian Center Hospital (Approval number: 2021 - 05 - KY 001).
Evaluations and calculations
The GCS was used to evaluate the level of consciousness of patients on the 1st day of ICU admission. The lower the score, the more serious the disturbance of consciousness. A score of 15 points indicated conscious consciousness, 13–14 points indicated a mild disturbance of consciousness, 9–12 points indicated a moderate disturbance of consciousness, 3–8 points indicated coma, and < 3 points indicated deep coma or brain death (14). The NIHSS score was used to evaluate the degree of neurological deficit in stroke patients; the higher the score, the more serious the neurological deficit; 0–1 was normal or nearly normal, 1–4 referred to mild stroke/minor stroke, 5–15 referred to moderate stroke, and 15–20 referred to moderate or severe stroke. A score of 21–42 was defined as a severe stroke (15).
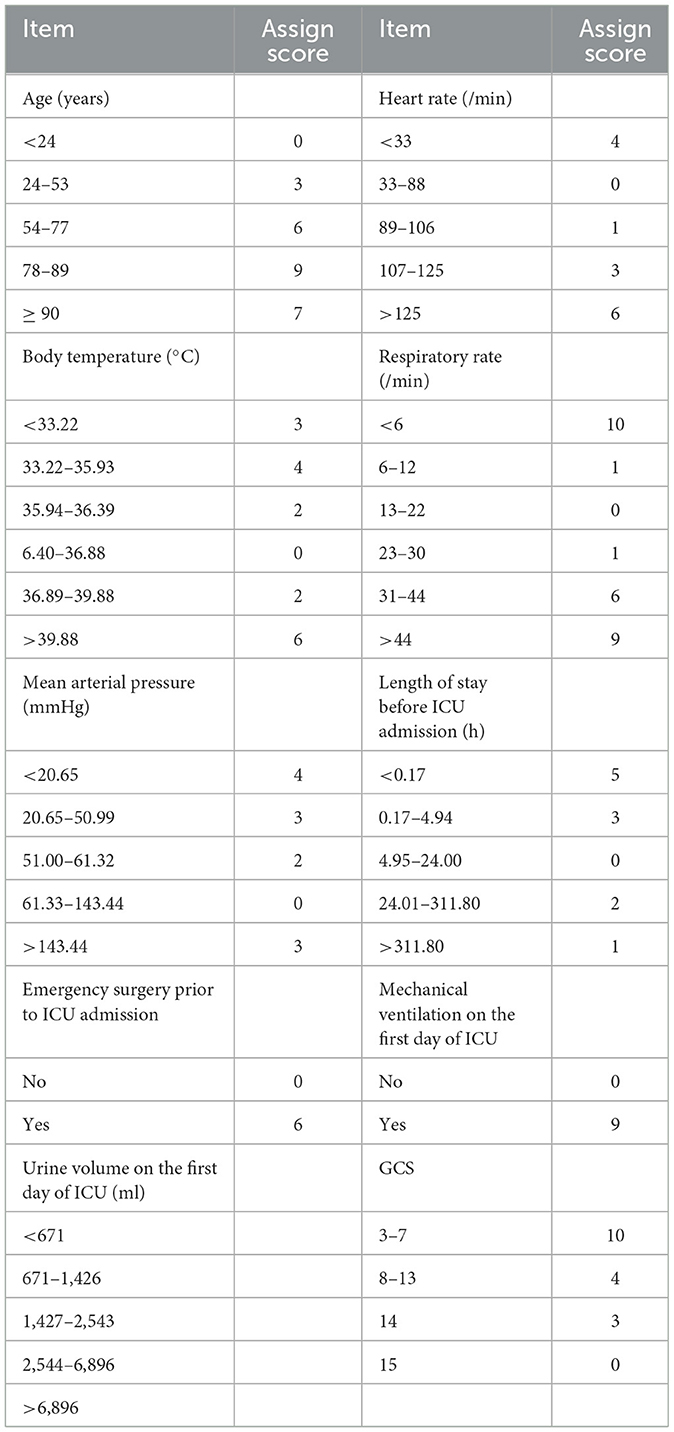
The OASIS scoring system was first developed by Johnson et al. in 2013 to assess the severity of acute illness in patients without the need for laboratory indicators. Over recent years, the OASIS scoring system has been applied to the diagnosis and prognosis evaluation of acute diseases. The OASIS scoring system features 10 parameters: age, heart rate, respiratory rate, mean arterial pressure (MAP), body temperature, GCS, length of stay before ICU admission, emergency surgery prior to ICU admission, urine volume, and mechanical ventilation on the 1st day of ICU, and its scoring criteria are shown in Table 1. The total score represents the summation of each score; the higher the total score, the more severe the disease. If there were multiple records of the same item on the 1st day of ICU evaluation, the most serious record prevailed (11).
Follow-up and endpoints
Patients with SAP were followed-up for 28 days (the 1st day of follow-up was the day of SAP diagnosis daily). The endpoints for SAP patients included progression-free survival (PFS) and overall survival (OS). PFS was defined as the time from the beginning of SAP intervention to the observation of disease progression or death from any cause. OS was defined as the time from SAP diagnosis to the occurrence of death from any cause.
Statistical analysis
All data were analyzed using SPSS (version 26.0) statistical software. The Shapiro–Wilk test was used to evaluate the normality of measurement data; if the data conformed to the normal distribution, then the mean ± standard deviation (SD) are given, and the independent sample t-test was used to compare the two groups. Measurement data that were not normally distributed are expressed as medians and inter-quartile ranges (IQR), and the Mann–Whitney test was used to compare the two groups. Count data are expressed as a frequency and percentage, and the chi-square (χ2) test was used to compare the two groups. Multiple logistic regression was used to analyze the influencing factors of patients with acute stroke complicated with SAP. A Kaplan–Meier survival curve was used to analyze the correlation between OASISs, PFS, and OS in SAP patients, and multiple COX regression was used to analyze the factors that influence prognosis and survival in SAP patients. Receiver operating characteristic (ROC) curve analysis was used to analyze the value of OASISs to predict the incidence and prognosis of SAP. A P-value of < 0.05 was considered to indicate statistical significance.
Results
Comparison of general clinical data between the non-SAP and SAP groups
Table 2 depicts the analysis of general and clinical data from patients in the SAP and non-SAP groups. There were no significant differences between the two groups with regard to gender, age, BMI, past medical history, heart rate, respiratory rate, mean arterial pressure, GCS score, NIHSS score, emergency operation before ICU admission, urine volume on the 1st day of ICU admission, length of stay before ICU admission, proportion of encephaledema and epilepsy, and WBC upon admission (all P > 0.05). However, age, body temperature, the levels of CRP and PCT, and OASIS at admission were significantly higher in the SAP group when compared to the non-SAP group (Z = 2.158, P = 0.031; Z = 4.410; Z = 4.410, P = 0.000; t = 3.350, P = 0.001; t = 2.973, P = 0.003; t = 7.017, P = 0.000). Furthermore, the length of ICU stay was significantly longer in the SAP group (Z = 2.338, P = 0.000).
Analysis of risk factors for acute stroke patients complicated by SAP
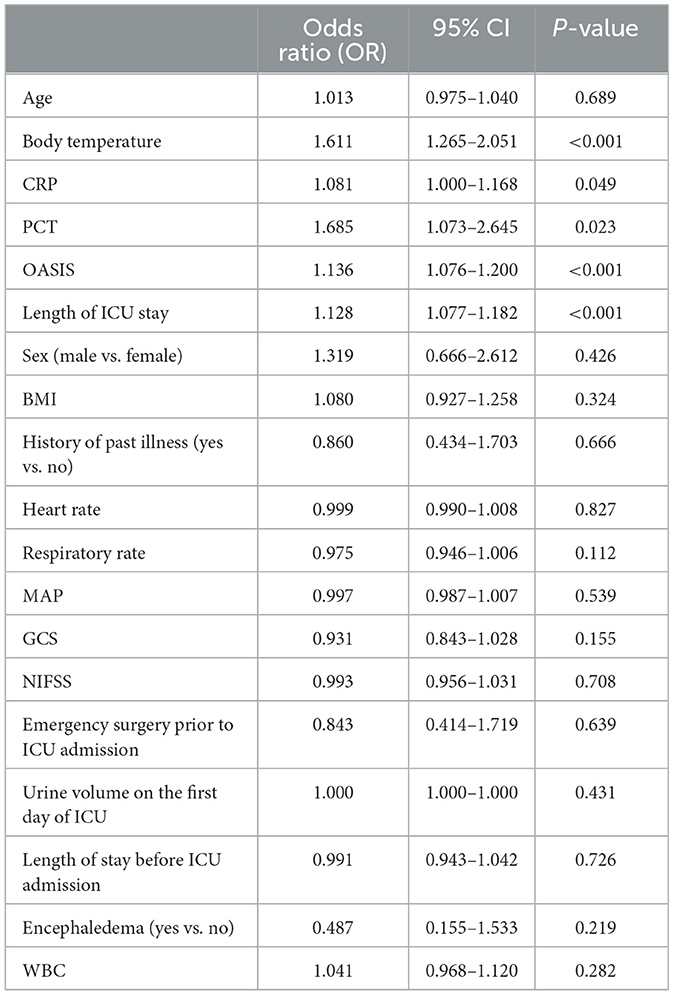
SAP occurred was defined as the dependent variable (yes = 1, no = 0) and multiple logistic regression analysis was conducted using age, body temperature, CRP and PCT levels, OASIS, and length of ICU stay as independent variables. Sex, BMI, history of past illness, heart rate, respiratory rate, MAP, GCS, NIHSS score, emergency surgery before ICU admission, urine volume on the 1st day of ICU admission, length of stay before ICU admission, proportion of encephaledema and epilepsy, and WBC on admission were utilized as covariates. Analysis showed that the risk factors for acute stroke patients with SAP in acute stroke patients were body temperature, CRP and PCT levels, OASIS, and length of ICU stay at admission. The higher the levels of CRP and PCT, the higher the risk of SAP [odds ratio (OR) = 1.611, P < 0.001; OR = 1.081, P = 0.049; OR = 1.685, P = 0.023]; furthermore, a higher OASIS indicated a higher risk of SAP (OR = 1.136, P < 0.001) (Table 3).
Predictive diagnosis of acute stroke patients with SAP
Multiple logistic regression analysis found that body temperature, CRP and PCT levels, and OASIS at admission were all risk factors for SAP in acute stroke patients. Next, we considered whether these factors could hold predictive value for SAP. ROC curve analysis showed that body temperature, and CRP and PCT levels had an average predictive value for SAP in acute stroke patients (AUCs of 0.665, 0.623, and 0.585, respectively; Figure 2A), while OASIS had a high predictive value (AUC: 0.717, Figure 2B). To improve the predictive value of OASIS, we next analyzed the combined predictive value of OASIS + CRP + PCT and found that the predictive value of this combination of parameters (AUC: 0.742, Figure 2C) was higher than that for OASIS; however, this was not significant (Z = 1.558, P = 0.119). The 95% confidence interval range of the AUCs for each variable is shown in Figure 2D.
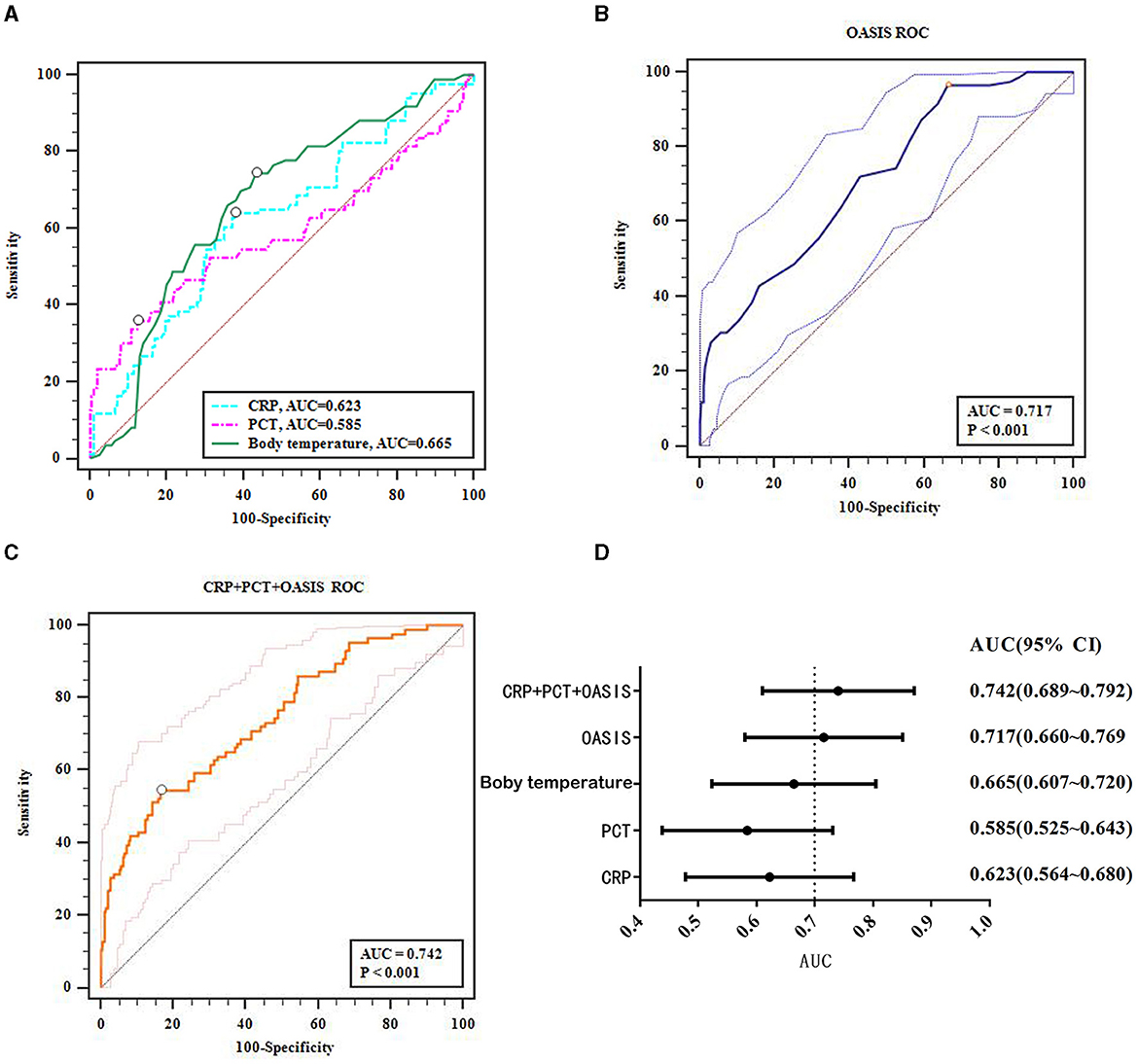
Figure 2. ROC curves analyze the predictive value of the major factors that affect the occurrence of SAP. (A) ROC results for CRP, PCT, temperature; (B) OASIS ROC; (C) CRP + PCT + OASIS ROC; (D) AUC of the major factors that affect the occurrence of SAP.
Relationship between OASIS and the prognosis of patients with acute stroke complicated with SAP
The highest and lowest OASISs for the 86 SAP patients were 42 points and 12 points, respectively; these scores were divided into three groups according to the score range: a high-OASIS group (31–42 points), a mid-OASIS group (21–30 points), and a low-OASIS group (12–20 points) (16). Kaplan–Meier analysis showed that the OS of the high-OASIS group was significantly shorter than that of the mid-OASIS group and the low-OASIS group (P = 0.012; P = 0.003) and that there was no significant difference between the mid-OASIS and low-OASIS groups (P = 0.155) (Figure 3A), thus indicating that OASIS was related to OS. However, there was no significant difference in PFS among the three groups (P = 0.503) (Figure 3B); thus, OASIS was not correlated with PFS.
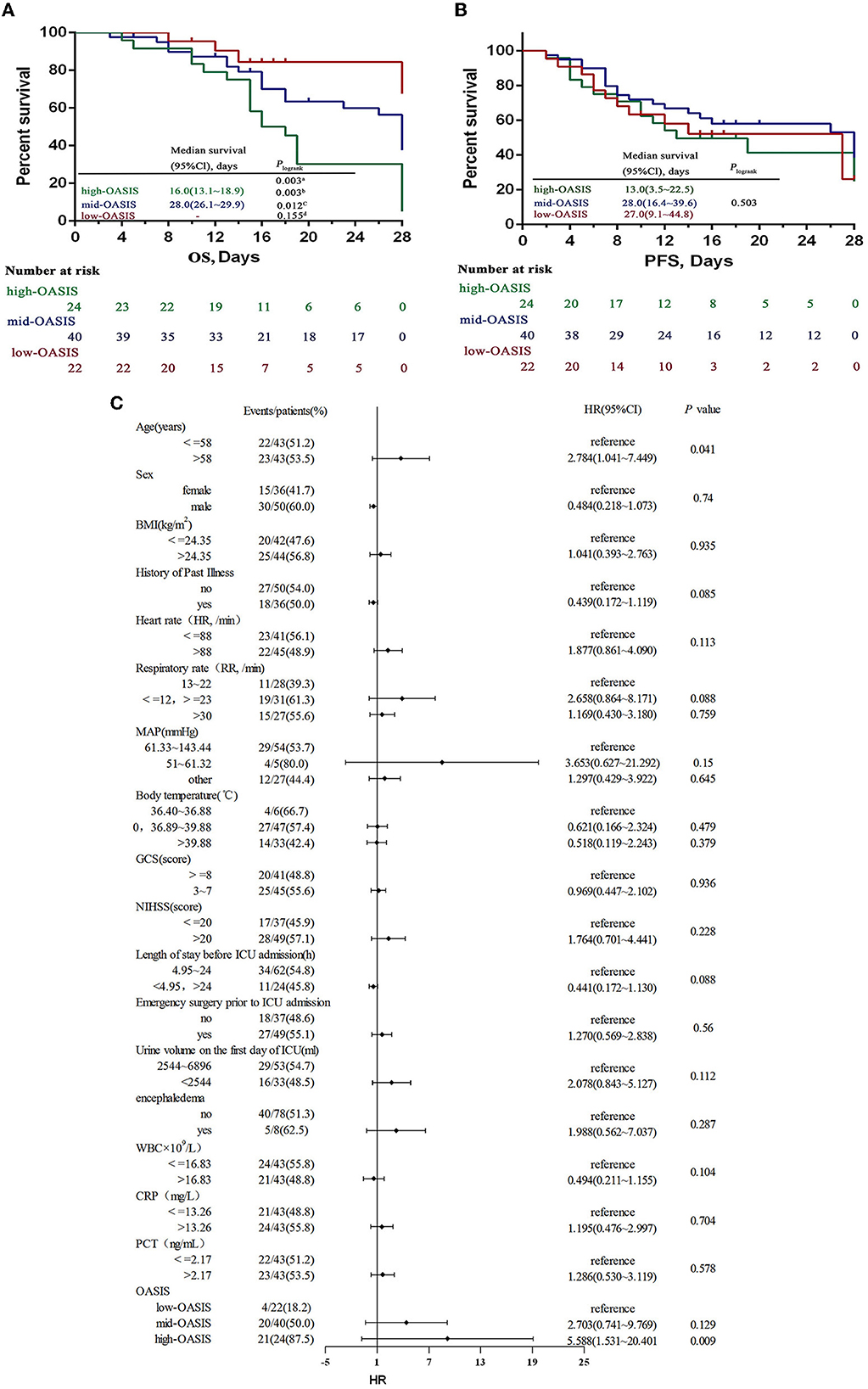
Figure 3. OS (A) and PFS (B) by different OASIS groups and ROC curves (C) for OASIS. OS overall survival, PFS progression-free survival. aThree-group comparison: P < 0.05, bComparison between high-OASIS and low-OASIS: P < 0.05, cComparison between high-OASIS and med-OASIS: P < 0.05, dComparison between med-OASIS and low-OASIS: P < 0.05. -The analyses failed because of <50% died in the low-oasis group.
OASIS has an independent effect on OS in SAP patients
A COX regression model was established to analyze the risk factors for prognosis and survival in patients with acute stroke complicated by SAP. Death was used as the state variable and OS was used as the time variable; various other factors were used as covariates: age, sex, BMI, history of past illness, heart rate, respiratory rate, MAP, body temperature, GCS, NIHSS, length of stay before ICU admission, emergency surgery prior to ICU admission, urine volume on the 1st day of ICU, encephaledema, WBC, CRP and PCT levels, and OASIS. Prior to COX regression analysis, the continuity covariates were transformed into categorical variables; age, BMI, WBC, and CRP and PCT levels were demarcated by the median value. Furthermore, heart rate, respiratory rate, MAP, body temperature, GCS, length of stay before ICU admission, and urine volume on the 1st day of ICU were classified by referring to the scoring standards of various parameters in the OASIS model; NIHSS classification refers to the NIHSS grading standards. Analysis showed that age and OASIS were independent risk factors that affect the prognostic OS in SAP patients (P = 0.041; P < 0.001); the risk of death was 2.784-fold higher in patients over 58 years of age than in patients under 58 years of age (95% CI: 1.041–7.449), 5.588-fold higher in patients in the high-OASIS group than those in the low-OASIS group (95% CI: 1.531–20.401), and 2.703-fold higher in patients in the mid-OASIS group than in the low-OASIS group (95% CI: 0.741–9.769) (Figure 3C). These data proved that OASIS was associated with SAP prognosis and that an elevated OASIS was a major independent risk factor for death in patients with SAP.
Prognostic diagnosis of OASIS in SAP patients
ROC curve analysis was used to explore the diagnostic value of OASIS in predicting the death of SAP patients. Analysis showed that OASIS had a high predictive diagnostic value (AUC = 0.769, P < 0.001) with a sensitivity of 77.88% and a specificity of 68.29% (Figure 4).
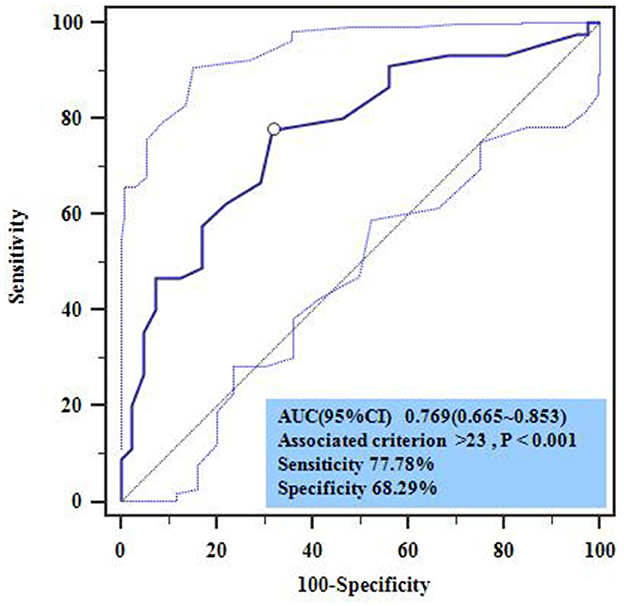
Figure 4. Multivariate cox regression analysis of prognostic death in SAP patients. HR, hazard ratio; high-OASIS 31–42 scores; med-OASIS 21–30 scores; low-OASIS 12–20 scores.
Discussion
SAP is a serious complication in stroke patients, which can affect the prognosis of patients and increase the risk of disability and death, thus generating a heavy burden for both family and society. Although previous researchers have investigated the early diagnosis of SAP, there is no consensus of opinion. Furthermore, these existing prediction methods differ widely, thus reducing the accuracy of early SAP prediction and the lack of prevention or timely anti-infection treatment, thus increasing the risk of adverse events. Therefore, actively investigating early diagnosis and prognostic risk assessment methods for SAP is a key strategy to reduce the incidence of adverse prognostic events in stroke.
In the present study, we analyzed general and clinical data from patients in the SAP group and the non-SAP group. Analysis showed that age, body temperature, CRP and PCT levels, and OASISs of patients in the SAP group were significantly higher than those in the non-SAP group; furthermore, the length of ICU stay was longer in the SAP group. CRP and PCT levels, and OASIS were identified as the main risk factors for patients with ischemic stroke complicated by SAP, thus indicating that the onset of SAP is significantly affected by the patient's age, body temperature at admission, CRP and PCT levels, and OASIS. As age increases, organ function and immunity decline, thus making the body vulnerable to invasion by exogenous substances; this could increase the risk of pulmonary infection (17). However, stroke is often accompanied by an inflammatory response that could lead to an imbalance in the system that regulates body temperature; this may increase body temperature, reduce immunity, and increase the risk of pathogenic infection and invasion of the lungs, thus aggravating the inflammatory response and immune response disorders. Collectively, these events may lead to a further increase in body temperature and the levels of CRP and PCT, thus increasing the risk of pneumonia (18). OASIS is a comprehensive evaluation index based on 10 parameters, including age, body temperature, and urine volume on the 1st day of ICU. The higher the OASIS, the more severe the disease. Stroke patients with more severe diseases have relatively poor body and immune function, thus increasing the risk of pneumonia. In a previous study, Szylińska et al. showed that advanced age, elevated CRP level, and prolonged hospital stay were all associated with the onset of SAP and represented independent risk factors for SAP (19). In another study, Kuo et al. showed that age, body temperature, pulse rate, and NIHSS score on admission were the main predictors of post-stroke pneumonia (20). Other studies have also pointed out that the older the patient, the higher the incidence of SAP; preoperative neutrophil count, lymphocyte count, and the levels of PCT and CRP were all identified as risk factors for SAP, thus indicating that PCT and CRP can be used as early predictive indicators of SAP (21, 22), thus supporting our current findings. However, OASIS has rarely been included in the multivariate regression analysis of SAP in previous studies. This may be because OASIS is widely recommended to evaluate acute conditions, but less attention has been paid to its application for acute complications. However, several parameters in the OASIS model, including age, body temperature, and heart rate, have been confirmed by most researchers to be closely related to the incidence of SAP, thus indicating that OASISs may affect the incidence of SAP. Our current findings confirmed that OASISs are closely related to the risk of SAP. It is worth noting that the effect of age on the incidence of SAP in our logistic regression analysis was not obvious; it is possible that this was related to our small sample size or interactions between OASIS and age.
Based on the risk factors of SAP, many researchers have attempted to establish SAP risk models using a range of risk factors, including the A2D2 score scale, the preventive antibacterial therapy in acute ischemic stroke score scale, and the AIS-APS scale (5). However, these scoring systems have limitations that need to be considered. For example, these systems can only be applied in a limited number of scenarios. Furthermore, some scoring systems involve a number of laboratory parameters, thus creating limitations for early rapid prediction. The OASIS model is widely used in the ICU because it is applicable to all types of acute diseases and does not involve laboratory indicators; however, its diagnostic efficiency can vary. A previous study reported that the OASIS model exhibited a good discriminative value to differentiate qSOFA (quick sequential organ failure assessment)-negative patients with sepsis from those without sepsis (AUC: 0.753; 95% CI: 0.741–0.765) (23). Another study reported the general diagnostic value of the OASIS model for sepsis after cardiac surgery (AUC: 0.650; 95% CI: 0.638–0.661) (24). In this study, ROC curve analysis was used to analyze the predictive value of body temperature, PCT and CRP levels, and OASIS for SAP; analysis revealed a general predictive value for body temperature, and PCT and CRP levels (AUCs: 0.665 0.585, 0.623; 95% CIs: 0.607–0.720, 0.525–0.643, 0.564–0.680); OASIS had a higher value for predicting SAP (AUC: 0.717; 95% CI: 0.660–0.769). In addition, we also analyzed the combined predictive value of OASIS + CRP + PCT and found that the predictive value of OASIS + CRP + PCT (AUC: 0.742; 95% CI: 0.889–0.792) was higher than that of OASIS alone although this was not significant (Z = 1.558, P = 0.119). Considering the clinical practice, we believe that although the combination of OASIS + CRP + PCT improves the diagnostic value partly, it takes a long time and is not conducive to rapid diagnosis because it involves laboratory indicators and needs to collect CRP and PCT of patients. Therefore, OASIS can predict the risk of SAP and represents an objective and simple model that does not require biochemical parameters and is worthy of clinical promotion.
Other studies have shown that SAP patients often have a worse prognosis, such as prolonged hospital stay and significantly increased hospital mortality, thus exerting a significant impact on medical resources and the economy. At present, the early prediction of SAP prognosis is of great significance for formulating early intervention measures, improving prognosis, and reducing mortality (25, 26). However, the current research on the prognostic value of stroke complicated with SAP was limited to laboratory indicators (27), which brought a lot of work to the assessment and difficult to make an early assessment quickly and accurately, which may delay the intervention. Therefore, a concise, rapid, and accurate stroke prognosis assessment method is of great significance for reducing the adverse prognosis of stroke patients. As an important reference index for the differential diagnosis of acute diseases, the OASIS model not only has a high value for the differential diagnosis of patient conditions but also has a certain reference value for predicting the prognosis of acute diseases. In a previous study, Chen et al. showed that OASIS was significantly correlated with the hospital mortality of sepsis patients (including patients with stroke-related sepsis) (OR: 1.07; 95% CI: 1.06–1.08) and had a certain ability to predict death (AUC: 0.652; 95% CI: 0.636–0.668) (28). Zhu et al. showed that OASIS had a high predictive value for 28-day mortality in patients with sepsis (AUC: 0.753; 95% CI: 0.742–0.764) (29), and it was also associated with death in machine-ventilated stroke patients (P < 0.001) (30). Huang et al. found that OASIS was correlated with the prognosis of ICU patients with respiratory failure (including patients with stroke-related respiratory failure) (OR: 1.06; 95% CI: 1.05–1.08) and also had some predictive value for prognostic mortality (AUC: 0.664; 95% CI: 0.644–0.685) (31). However, the current studies for OASIS in stroke prognosis lack pertinency, and the predictive value for stroke with SAP prognosis has not been reported previously. In the present study, survival analysis was used to analyze the relationship between OASISs and the survival rates of SAP patients; analysis showed that OASIS was not related to PFS but was related to OS. Patients with an OASIS of 31–42 had a significantly shorter OS than those with a score of 21–30 and 12–20 (P < 0.05). To further verify the relationship between OASIS and OS, multiple COX regression analysis was performed by adding confounding factors, such as age and sex; results showed that age and OASIS were independent risk factors for the OS of SAP patients. The mortality risk for SAP patients older than 58 years was significantly higher than that for patients younger than 58 years (OR: 2.784; 95% CI: 1.041–7.449). The mortality risk of SAP patients with an OASIS of 31–42 was 5.588-fold higher than those with scores of 12–20 scores (95% CI: 1.531–20.401). Patients with scores of 21–30 had a 2.703-fold higher risk than those with scores of 12–20 (95% CI: 0.741–9.769). These findings suggested that OASIS is closely related to death in SAP patients and that increasing age elevates the risk of death in SAP patients. Because the OASIS system includes age parameters, this study only considered the predictive diagnostic value of OASIS in terms of the death of SAP patients. ROC curve analysis showed that OASIS had a high predictive value (AUC: 0.769; 95% CI: 0.665–0.853) with a sensitivity of 77.88% and a specificity of 68.29%; the best cutoff value of OASIS for predicting death was 23 points. Thus, we have confirmed that OASIS can predict the prognosis death of SAP patients efficiently and also suggests that clinical attention should be given to SAP patients aged >58 years; in such patients, early intervention measures should be actively taken to reduce the incidence of a poor prognosis.
However, our study has some limitations that need to be considered. First, the subjects in this study had ischemic stroke and had been treated in the General Intensive Care Department of Zhumadian Central Hospital; thus, our data are not fully representative of the general population. Second, the cases in this study were all patients with cerebral ischemic stroke patients; thus, we only considered a specific condition with limited applicability. Third, the sample size was insufficient, with only 86 SAP patients and 24 high-OASIS patients; this may be the reason for the high mortality rate of SAP. These deficiencies need to be addressed in future research.
Conclusion
The analysis demonstrated that OASIS can effectively predict the onset and death of patients with SAP and provide a potential reference index for early diagnostic and prognostic predictions for patients with SAP that can readily be translated to the clinic. Our findings have great significance in the prevention of SAP in patients with ischemic stroke, thus increasing survival rates and improving prognosis.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Zhumadian Central Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XW contributed to the conception or design of the work, acquisition, analysis, and interpretation of data for the work. HS guided article design, drafting, the work, and revised it critically for important intellectual content. JX and YL contributed to the case collection and experiments operation. YS and YY contributed to the manuscript final revision and supervision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tu WJ, Zhao Z, Yin P, Cao L, Zeng J, Chen H, et al. Estimated burden of stroke in China in 2020. JAMA Netw Open. (2023) 6:e231455. doi: 10.1001/jamanetworkopen.2023.1455
2. Tu WJ Wang LD Special Special Writing Group of China Stroke Surveillance Report. China stroke surveillance report 2021. Milit Med Res. (2023) 10:33. doi: 10.1186/s40779-023-00463-x
3. Teh WH, Smith CJ, Barlas RS, Wood AD, Bettencourt-Silva JH, Clark AB, et al. Impact of stroke-associated pneumonia on mortality, length of hospitalization, and functional outcome. Acta Neurol Scand. (2018) 138:293–300. doi: 10.1111/ane.12956
4. Yu YJ, Weng WC, Su FC, Peng TI, Chien YY, Wu CL, et al. Association between pneumonia in acute stroke stage and 3-year mortality in patients with acute first-ever ischemic stroke. J Clin Neurosci. (2016) 33:124–8. doi: 10.1016/j.jocn.2016.02.039
5. Fang F, Shu Y, Xiao Z. Clinical research progress in stroke-associated pneumonia. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2019) 31:1429–34. doi: 10.3760/cma.j.issn.2095-4352.2019.11.023
6. Zhang R, Ji R, Pan Y, Jiang Y, Liu G, Wang Y, et al. External validation of the prestroke independence, sex, age, national institutes of health stroke scale score for predicting pneumonia after stroke using data from the China National Stroke Registry. J Stroke Cerebrovasc Dis. (2017) 26:938–43. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.043
7. Zapata-Arriaza E, Moniche F, Blanca PG, Bustamante A, Escudero-Martínez I, Uclés O, et al. External validation of the ISAN, A2DS2, and AIS-APS scores for predicting stroke-associated pneumonia. J Stroke Cerebrovasc Dis. (2018) 27:673–6. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.059
8. Cheng Y, Chen Q, Wu S, Zhu R, Wu C. Impact and predictive value of time-dependent acute physiology and chronic health evaluation II score on death risk in patients with severe stroke: data analysis based on MIMIC-III. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2021) 33:1237–42. doi: 10.3760/cma.j.cn121430-20210409-00533
9. Deniz M, Ozgun P, Ozdemir E. Relationships between RDW, NLR, CAR, and APACHE II scores in the context of predicting the prognosis and mortality in ICU patients. Eur Rev Med Pharmacol Sci. (2022) 26:4258–67. doi: 10.26355/eurrev_202206_29063
10. Qingui C, Wanmei H, Haichong Z, Lishan Z, Mian Z. Comparison of predicitive value of SAPS II and OASIS scoring systems in ICU patients' short-term outcomes. Chin J Crit Care Intens Care Med. (2018) 4:159–63. doi: 10.3877/cma.j.issn.2096-1537.2018.02.011
11. Johnson AE, Kramer AA, Clifford GD. A new severity of illness scale using a subset of acute physiology and chronic health evaluation data elements shows comparable predictive accuracy. Crit Care Med. (2013) 41:1711–8. doi: 10.1097/CCM.0b013e31828a24fe
12. Neurology C S O, Society C S. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin J Neurol. (2018) 51:666–82. doi: 10.3760/cma.j.issn.1006-7876.2018.09.004
13. Smith CJ, Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in Stroke Consensus Group. Stroke. (2015) 46:2335–40. doi: 10.1161/STROKEAHA.115.009617
14. Mehta R, Chinthapalli K. Glasgow coma scale explained. BMJ. (2019) 365:l1296. doi: 10.1136/bmj.l1296
15. Cheng Z, Geng X, Rajah GB, Gao J, Ma L, Li F, et al. NIHSS consciousness score combined with ASPECTS is a favorable predictor of functional outcome post endovascular recanalization in stroke patients. Aging Dis. (2021) 12:415–24. doi: 10.14336/AD.2020.0709
16. Sideris M, McCaughey T, Hanrahan JG, Arroyo-Manzano D, Zamora J, Jha S, et al. Risk of obstetric anal sphincter injuries (OASIS) and anal incontinence: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. (2020) 252:303–12. doi: 10.1016/j.ejogrb.2020.06.048
17. You Q, Bai D, Wu C, Wang H, Chen X, Gao J, et al. Risk factors for pulmonary infection in elderly patients with acute stroke: a meta-analysis. Heliyon. (2022) 8:e11664. doi: 10.1016/j.heliyon.2022.e11664
18. Xie J, Zhang XH, Zhu WY. Values for serum procalcitonin, C-reactive protein, and soluble triggering receptor expressed on myeloid cells-1 in predicting survival of patients with early-onset stroke-associated pneumonia. Genet Mol Res. (2015) 14:4716–23. doi: 10.4238/2015.May.11.4
19. Szylińska A, Bott-Olejnik M, Wańkowicz P, Karoń D, Rotter I, Kotfis K, et al. Novel index in the prediction of pneumonia following acute ischemic stroke. Int J Environ Res Public Health. (2022) 19:15306. doi: 10.3390/ijerph192215306
20. Kuo YW, Huang YC, Lee M, Lee TH, Lee JD. Risk stratification model for post-stroke pneumonia in patients with acute ischemic stroke. Eur J Cardiovasc Nurs. (2020) 19:513–20. doi: 10.1177/1474515119889770
21. Shi G, Li M, Zhou R, Wang X, Xu W, Yang F, et al. Procalcitonin related to stroke-associated pneumonia and clinical outcomes of acute ischemic stroke after IV rt-PA treatment. Cell Mol Neurobiol. (2022) 42:1419–27. doi: 10.1007/s10571-020-01031-w
22. Al-Khaled M. The multifactorial etiology of stroke-associated pneumonia. J Neurol Sci. (2019) 400:30–1. doi: 10.1016/j.jns.2019.02.042
23. Chen QG, Xie RJ, Chen YZ, Zeng M. Clinical value of Oxford acute severity of illness score in identifying quick sequential organ failure assessment-negative patients with sepsis. Zhonghua Jie He He Hu Xi Za Zhi. (2018) 41:701–8. doi: 10.3760/cma.j.issn.1001-0939.2018.09.010
24. Ruihuan S, Xu W, Zhongyuan L. The value of various severity assessment scoring systems in sepsis diagnosis and long-term prognosis prediction after cardiac surgery. Chin J Cardiothor Surg. (2022) 29:219–27. doi: 10.7507/1007-4848.202007060
25. Hannawi Y, Hannawi B, Rao CP, Suarez JI, Bershad EM. Stroke-associated pneumonia: major advances and obstacles. Cerebrovasc Dis. (2013) 35:430–43. doi: 10.1159/000350199
26. Chen Y, Yang H, Wei H, Chen Y, Lan M. Stroke-associated pneumonia: a bibliometric analysis of worldwide trends from 2003 to 2020. Medicine. (2021) 100:e27321. doi: 10.1097/MD.0000000000027321
27. Adiguzel A, Arsava EM, Topcuoglu MA. Temporal course of peripheral inflammation markers and indexes following acute ischemic stroke: prediction of mortality, functional outcome, and stroke-associated pneumonia. Neurol Res. (2022) 44:224–31. doi: 10.1080/01616412.2021.1975222
28. Chen Q, Zhang L, Ge S, He W, Zeng M. Prognosis predictive value of the Oxford Acute Severity of Illness Score for sepsis: a retrospective cohort study. PeerJ. (2019) 7:e7083. doi: 10.7717/peerj.7083
29. Zhu Y, Zhang R, Ye X, Liu H, Wei J. SAPS III is superior to SOFA for predicting 28-day mortality in sepsis patients based on Sepsis 30 criteria. Int J Infect Dis. (2022) 114:135–41. doi: 10.1016/j.ijid.2021.11.015
30. Zhu Y, Zhang J, Wang G, Yao R, Ren C, Chen GQ, et al. Machine learning prediction models for mechanically ventilated patients: analyses of the MIMIC-III database. Front Med. (2021) 8:662340. doi: 10.3389/fmed.2021.662340
Keywords: stroke-associated pneumonia, Oxford Acute Severity of Illness Score, stroke, diagnosis, prognosis
Citation: Wang X, Xia J, Shan Y, Yang Y, Li Y and Sun H (2023) Predictive value of the Oxford Acute Severity of Illness Score in acute stroke patients with stroke-associated pneumonia. Front. Neurol. 14:1251944. doi: 10.3389/fneur.2023.1251944
Received: 03 July 2023; Accepted: 14 August 2023;
Published: 04 September 2023.
Edited by:
Wen-Jun Tu, Peking Union Medical College, ChinaReviewed by:
Wenjia Chai, Capital Medical University, ChinaEiichiro Nagata, Tokai University Isehara Hospital, Japan
Qi Liu, Yunnan University, China
Copyright © 2023 Wang, Xia, Shan, Yang, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Sun, aGFpeGl3dUAxMjYuY29t
 Ximei Wang1
Ximei Wang1 Haiyan Sun
Haiyan Sun