
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 03 August 2023
Sec. Multiple Sclerosis and Neuroimmunology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1251667
This article is part of the Research Topic Aging in multiple sclerosis: from childhood to old age, in women and men View all 13 articles
 Lorena Lorefice1*
Lorena Lorefice1* Giuseppe Fenu2
Giuseppe Fenu2 Marzia Fronza1
Marzia Fronza1 Federica Murgia3
Federica Murgia3 Jessica Frau1
Jessica Frau1 Giancarlo Coghe1
Giancarlo Coghe1 Maria Antonietta Barracciu4
Maria Antonietta Barracciu4 Luigi Atzori3
Luigi Atzori3 Stefano Angioni5
Stefano Angioni5 Eleonora Cocco1
Eleonora Cocco1Background: Recent evidence has shown a significant association between menopause and multiple sclerosis (MS) progression. This study investigated the possible role of menopause in influencing MS from clinical and neuroradiological perspectives. Notably, the possible association between menopause and brain atrophy has been evaluated.
Materials and methods: This study included women with MS whose ages ranged from 45 to 55 years. Demographic and clinical characteristics were collected, and the reproductive phase was defined as non-menopausal or menopausal based on the final menstrual period. Thus, MS activity over the past year was reported as the annualised relapse rate (ARR), and MRI activity (defined as new T2 lesions and/or the presence of gadolinium-enhancing lesions at the last MRI assessment in comparison with the MRI performed within the previous 12 months) were compared between non-menopausal women (non-MW) and menopausal women (MW). Volume measurements of the whole brain (WB), white matter (WM), grey matter (GM), and cortical GM were estimated using the SIENAX software, and the possible relationship with menopausal status was assessed by regression analysis.
Results: The study included 147 women with MS. Eighty-four (57.1%) were MW, with a mean age of 48.5 ± 4.3 years at menopause onset and a mean duration of menopause of 4.1 ± 1.1 years. When compared for ARR, MW reported a lower rate than the non-MW (ARR of 0.29 ± 0.4 vs. 0.52 ± 0.5; p < 0.01). MRI activity was observed in 13.1% of MW and 20.6% of non-MW (p = 0.03). Lower cortical GM volumes (578.1 ± 40.4 mL in MW vs. 596.9 ± 35.8 mL in non-MW; p < 0.01) have also been reported. Finally, multivariate analysis showed a significant association of lower ARR (p = 0.001) and cortical GM volume (p = 0.002) with menopausal status after correction for chronological age and other variables.
Discussion: Menopause may be an adverse prognostic factor of MS. Our preliminary results suggest that menopause may facilitate cortical GM atrophy, probably due to a decline in the neuroprotective effects of estrogen, with negative effects on MS evolution.
One of the emerging topics in the field of gender medicine applied to multiple sclerosis (MS) is the issue of menopause (1), and its effects (often superimposed on those of aging) on various aspects of the disease (2). Menopause is a physiological event that marks the end of a woman’s reproductive competence (3). Characterised by irreversible interruption of menstruation, it occurs in the general population at an average age of approximately 50 years (4). Several immunologic changes have been described in postmenopausal women. These modifications, mainly driven by oestrogen deprivation, overlap with age-related changes, resulting in decreased CD4 T lymphocytes, B lymphocytes, natural killer (NK) cells cytotoxic activity, and increased proinflammatory responses, with effects on the risk of infection and autoimmunity (5). Notably, MS is characterised by great pathogenetic, clinical, and neuroradiological heterogeneity, with different disease outcomes in relation to the inflammatory and neurodegenerative mechanisms underlying the disease (6), window for therapeutic intervention (7), and type of therapeutic intervention (8). Numerous studies have shown a predominance of the disease in females of all ages, and recent studies have shown a shift in MS onset to older age, with a higher frequency of late-onset forms among women (9). For these forms, the possible effect of menopause on susceptibility to the disease should be considered, attributable to the postmenopausal proinflammatory state and deprivation of the neuroprotective effects of oestrogens and progestins (10, 11), which would act to reduce the resilience of the central nervous system (CNS) thereby facilitating the onset of MS in the presence of other predisposing factors (12). Recently, a large study has shown that women with MS have greater inflammatory activity in terms of relapse than men, up to the age of 50 years. After that, the difference disappears, and the evolution of the disability worsens, becoming more similar in both the sexes (9, 13). Therefore, the possible effects of menopausal transition on the disease characteristics should be considered. Given the high number of women with MS among the aging population, it is crucial to understand the effects of menopause and its interaction with MS. Previously, in a longitudinal cohort of women with MS who were followed for approximately 10 years during the menopausal transition, Bove et al. showed that menopause represented an inflection point in their Expanded Disability Status Scale (EDSS) changes (14). In line with these findings, a multicentre study evaluating the effect of menopause on the clinical course of MS showed a significant decrease in annualised relapse rate (ARR) 2 years after menopause compared to the previous 2 years, while disability worsened (15). Conversely, Otero-Romero S et al. showed that menopause did not modify disability trajectories in a longitudinal cohort of women with MS who were followed from disease onset, after controlling for age and disease duration (16), thereby leaving controversial aspects to be investigated. Additionally, even less explored are the effects of menopause on neuroradiological activity and brain volume measurements in women with MS, which are significantly related to long-term disability (17). With regard to the latter point, a longitudinal study has recently shown that ovarian aging, as defined by anti-Müllerian hormone (AMH) levels, is associated with greater clinical disability and grey matter loss in women with MS (18) and is independent of the chronological age and disease duration, highlighting the crucial role of sex hormones in MS disease outcomes (19). In this framework, the present study aimed to evaluate, in a cohort of women with MS aged between 45 and 55 years, the possible impact of menopausal transition on clinical activity and MRI outcomes, and its effects on the whole brain (WB), white matter (WM), grey matter (GM), and cortical grey matter (cGM) volumes.
Women with relapsing–remitting MS (RRMS) (20) between the ages of 45 and 55 years were recruited from the Multiple Sclerosis Centre, Binaghi Hospital, University of Cagliari. Women were classified as as menopausal (MW) or non-menopausal (non-MW). Menopause onset was defined as the final menstrual period beyond which no menses occurred for 12 months (21) in association of neurovegetative menopausal symptoms (hot flushes). Women with surgical menopause were excluded, as were women exposed to oestrogen-progestin therapy (oral contraceptives) for up to 3 years before the final menstrual period or hormonal treatment during the menopausal transition. Demographic and clinical data [disease duration, disability level assessed by the EDSS (22), and disease-modifying therapy (DMT)], were recorded for each woman. MS clinical activity was defined as the presence of clinical relapse (new symptoms or the return of old symptoms for ≥24 h in the absence of an infection or fever). Thus, the annualized relapse rate (ARR), defined as the number of confirmed relapses in the last 12 months, was estimated after evaluation of medical records. MRI activity was defined as the presence of new or enlarged T2 lesions or gadolinium-enhancing T1 lesions at the last MRI assessment compared to the MRI performed within the previous 12 months (20). Quantitative MRI evaluations were performed for each patient, and brain volume measurements were estimated at the time of the last neurological assessment. Informed consent was obtained from all the participants after obtaining approval from the local ethics committee.
Brain volumes were measured using a 1.5 T scanner Siemens Magnetom Avanto (Siemens Medical Solutions, Erlangen, Germany). Three-dimensional magnetisation-prepared rapid gradient-echo (MPRAGE) was used to obtain 174 contiguous sagittal 3D-T1WI images with the following parameters: slice thickness = 1.3 mm, repetition time/echo time = 2400/3.6 ms, inversion time = 1,000 ms, flip angle = 8°, field of view = 24 cm, number of excitations = 1, and pixel matrix = 192 × 192. Brain volumes were measured for each participant on T1 W gradient echo images using SIENAX, a previously described cross-sectional version of the Structural Image Evaluation using Normalisation of Atrophy (SIENA) software to estimate the global brain volume normalised for head size, as well as the selective measurement of normalised WM, GM, and cortical GM volumes (23). All brain volume measurements were performed in a single session using the same MRI protocol.
All statistical analyses were performed using SPSS for Mac version 20.0 (SPSS Inc., Chicago, IL, United States). First, a descriptive analysis was performed, reporting demographic, clinical, and MRI data as means (quantitative variables) or percentages (qualitative variables). A t-test was used to compare demographics (age), clinical data (MS duration, EDSS score, and ARR), and MRI measurements of the WB, WM, GM, and cortical GM in non-MW and MW. Similarly, analysis of variance (ANOVA) was used to compare qualitative variables (presence of MRI activity in the last year and use of high-efficacy DMTs).
Therefore, regression analyses were performed to investigate the relationship of ARR and MRI activity (entered into the models as dependent variables) with menopausal status, while controlling for other demographic and clinical variables. Similarly, the relationship between MRI measurements of WB, WM, GM, and cortical GM volumes and menopausal status was explored using regression analyses. Statistical significance (p) was set at <0.05 for all assays.
The study included 147 relapsing remitting women with MS between the ages of 45 and 55, of whom 63 (42.9%) were non-MW and 84 (57.1%) were MW, with an average age of 48.5 ± 4.3 years at menopause onset. The mean age was 46.1 ± 3.1 years in non-MW and 52.6 ± 3.2 years in MW (p < 0.01), with disease duration of 15.5 ± 6.3 years and 18.2 ± 8 years, respectively (p < 0.05). Table 1 summarises the demographic and clinical characteristics of the patients included in this study, and also indicates the DMTs. In particular, high-efficacy DMTs were reported in 23.8% of non-MW compared to 17.8% of MW (p < 0.05). Table 2 shows the characteristics of non-MW and MW with clinical and neuroradiological activity in the last year of the disease and presents the comparison data of the brain MRI measurements obtained by an independent t-test. In particular, in the last year, an ARR of 0.52 ± 0.5 in non-MW vs. 0.29 ± 0.4 in MW (p < 0.01) was reported, with MRI activity observed in 20.6% of non-MW vs. 13.1% of MW (p < 0.05). Regression analysis was performed to evaluate the factors that influenced clinical activity, as indicated by the ARR: an inverse relationship was observed between chronological age (p = 0.028) and menopausal status (p = 0.001). Analogously, an inverse relationship that tends towards significance was observed between MRI activity and chronological age (p = 0.064), while no relationship was reported with menopausal status (Table 3). Multivariate analysis was then performed considering WB, WM, GM, and cortical GM as dependent variables while controlling for age, disease duration, EDSS score, and menopausal status, included in the model as independent variables. A significant association between lower cortical GM volume and menopausal status (p = 0.002) was reported, independent of other demographic (age) and clinical variables (MS duration and EDSS) (Table 4).
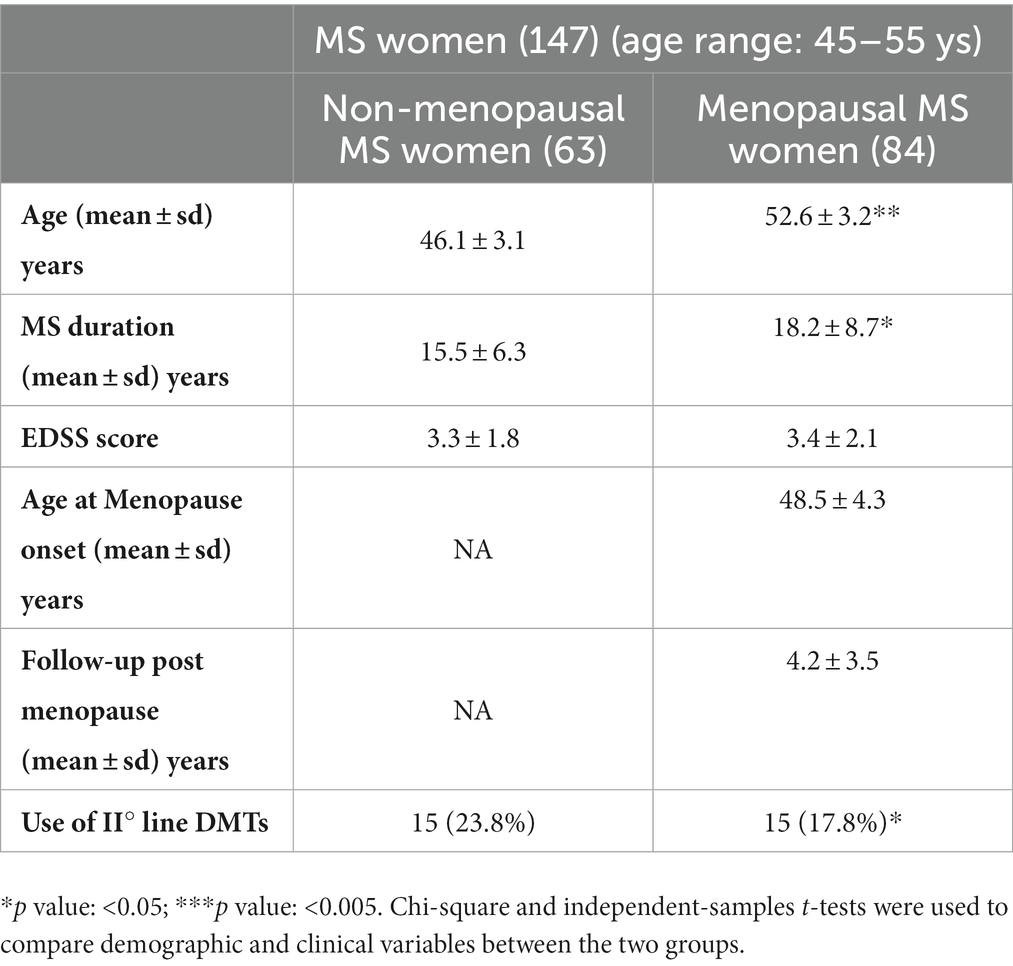
Table 1. Demographic and clinical features of MS women categorized in relation to menopausal status.
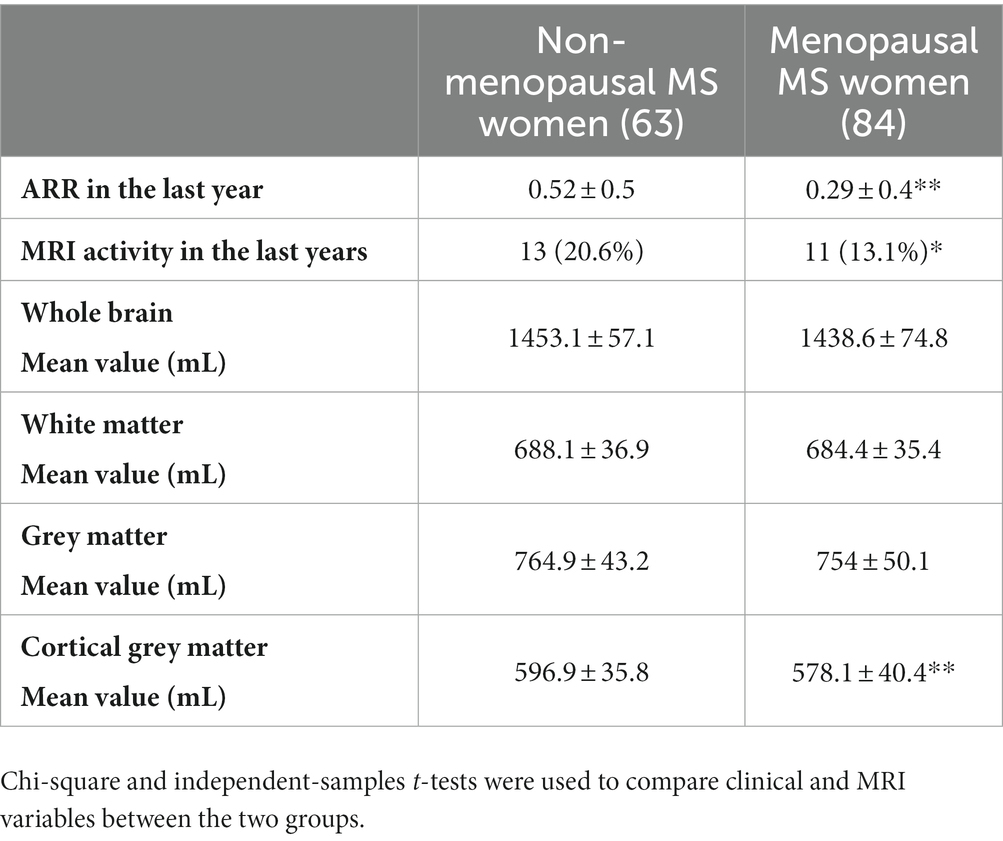
Table 2. Annualized Relapse Rate, MRI activity and brain volume measurements in menopausal and non-menopausal MS women.
Several studies have shown that natural menopause may contribute to a more rapid decline in women with MS, resulting in a turning point in the worsening of MS (14–16). These studies evaluated the impact of menopausal transition on clinical activity and EDSS changes; however, its effects on MRI activity and neurodegenerative aspects remain poorly explored. In this context, our study aimed to evaluate the impact of menopause on the course of MS, and explore the effects on MRI inflammatory activity, which is defined as an increase in lesion burden and presence of gadolinium-enhancing lesions, and the impact on brain atrophy, a principal surrogate indicator of neurodegeneration and predictor of long-term MS outcomes. In line with the results of previous studies, a relationship between lower ARR with chronological age and menopausal status was observed. At the same time, lower MRI activity appears to be associated with increasing age but is independent of menopause. It is now known that aging affects many aspects of MS (2). On the one hand, the peripheral immune response decreases, resulting in immunosenescence and making inactive plaques predominant; on the other hand, inflammation becomes compartmentalised and thus more challenging to detect, while neurodegenerative processes become more evident (24). Therefore, it is difficult to distinguish the effects exclusively linked to aging from those of menopause, which have similar effects on many aspects of immunity, brain damage, and disease evolution. Previously, Graves et al. reported that ovarian aging, as indicated by lower AMH levels, was associated with both clinical and radiographic metrics of MS severity, as shown by the relationship with lower grey matter volume after adjustments for chronological age and disease duration (18). Similarly, our study revealed an association between lower cortical grey volume and menopausal status, independent of chronological age and duration of MS, suggesting an increased level of neurodegenerative pathological processes after this reproductive biological transition. It is known that oestrogen levels decrease with menopause (3), and in line with this decrease, their neuroprotective effects decline (25). As shown in experimental autoimmune encephalomyelitis (EAE) models, oestrogen preserves synaptic transmission and has a role in sparing neurons and synapses in the brain and myelin and axons in the spinal cord (26, 27). Oestrogen exerts neuroprotective effects through various mechanisms. First, oestrogen has a suppressive effect on neuroinflammation and strongly inhibits microglial activation. In addition, a direct neuroprotective effect on the mitochondria, with increased aerobic glycolysis, respiratory efficiency, ATP generation, Ca2+ load tolerance, and antioxidant effects, has been reported (28, 29). EAE studies with various oestrogen treatments have led to clinical disease defence, as well as protection from CNS inflammation, axonal loss, demyelination, and promotion of remyelination processes (30). Thus, the reduction in the anti-inflammatory role of oestrogen after menopause could cause inflammatory damage to axons and myelin, contributing to brain damage and the accumulation of disability. Moreover, oestrogen depletion associated with menopausal transition facilitates the propensity for cardiovascular disease (4) thereby increasing the risk of aging-related comorbidities, and the impact of these comorbidities on brain damage should be considered (31). Beyond this, the effects of menopausal transition on frailty, conceived as a marker of the depletion of the organism’s homeostatic reserves (32), and on brain resilience (33) to various types of brain chronic damages (MS related or not) remain unexplored.
The present study has several limitations. First, the effects of menopausal transition on MS evolution were evaluated by comparing groups of MW and non-MW in the same age range, but not longitudinally in the same cohort. Second, most women with MS were treated with DMTs, which may have improved the course of the disease, making it more difficult to detect the effects of the menopausal transition. However, we chose not to exclude treated patients to avoid selection bias (such as the inclusion of only benign or stable MS). Furthermore, MRI data were not available for healthy controls to determine whether the association between menopause and lower GM volume was specific to women with MS. Similarly, we did not collect data of male MS patients and controls, which would have helped distinguish the effects of menopause on clinical and MRI measurements from those of aging and andropause. Furthermore, normative values for brain volumes have never been established, but only specific cut-off values capable of distinguishing between ‘physiological’ and ‘pathological’ brain volume loss in MS patients assessed longitudinally (34). However, these values are not applicable to our study since we did not longitudinally evaluate the brain volumes.
Finally, it should be emphasised that the menopausal transition process is gradual and begins even before the final menstrual period; in addition, the duration of menopause was different in the group of WM examined, while hormonal changes, which can affect the disease’s immunity, inflammation, and neurodegenerative aspects, were not evaluated in this study (5).
Menopause may represent an adverse prognostic factor for MS evolution, inducing a worsening of disability and neurodegenerative aspects of MS. Our preliminary results suggest that menopause could facilitate cortical GM atrophy, probably due to a decline in the neuroprotective effects of oestrogen. In this context, further studies are needed to evaluate the impact of menopause on disease evolution. In particular, it is crucial to define studies that consider homogeneous groups of MS women, also exposed to the same type of DMT, and studies with a longitudinal design, including healthy women in the same biological phase, to define better how menopause interacts with MS and to discriminate the clinical, neuroradiological, and immunological effects induced by aging and aging-related comorbidities (35). Additionally, the effects of sex on immunosenescence and brain resilience should be further investigated with a view to facilitate an approach increasingly focused on gender medicine.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Multiple Sclerosis Centre, University of Cagliari. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LL conceptualized the study and wrote, reviewed, and edited the manuscript. GF, MF, JF, GC, FM, and MB were responsible for resources and data curation. LA, SA, and EC supervised the study. All authors contributed to the article and approved the submitted version.
LL, GF, JF, GC, and EC received honoraria for consultancy or speaking from Biogen, Novartis, Sanofi, Genzyme, Serono and Teva and Almirall.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lorefice, L, D’Alterio, MN, Firinu, D, Fenu, G, and Cocco, E. Impact of menopause in patients with multiple sclerosis: current perspectives. IJWH. (2023) 15:103–9. doi: 10.2147/IJWH.S334719
2. Graves, JS, Krysko, KM, Hua, LH, Absinta, M, Franklin, RJM, and Segal, BM. Ageing and multiple sclerosis. Lancet Neurol. (2023) 22:66–77. doi: 10.1016/S1474-4422(22)00184-3
3. Hall, JE. Endocrinology of the menopause. Endocrinol Metab Clin N Am. (2015) 44:485–96. doi: 10.1016/j.ecl.2015.05.010
4. Santoro, N. The menopausal transition. Am J Med. (2005) 118:8–13. doi: 10.1016/j.amjmed.2005.09.008
5. Giefing-Kröll, C, Berger, P, Lepperdinger, G, and Grubeck-Loebenstein, B. How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell. (2015) 14:309–21. doi: 10.1111/acel.12326
6. Filippi, M, Bar-Or, A, Piehl, F, Preziosa, P, Solari, A, Vukusic, S, et al. Multiple sclerosis. Nat Rev Dis Primers. (2018) 4:43. doi: 10.1038/s41572-018-0041-4
7. Cocco, E, Sardu, C, Spinicci, G, Musu, L, Massa, R, Frau, J, et al. Influence of treatments in multiple sclerosis disability: a cohort study. Mult Scler. (2015) 21:433–41. doi: 10.1177/1352458514546788
8. Iaffaldano, P, Lucisano, G, Caputo, F, et al. Long-term disability trajectories in relapsing multiple sclerosis patients treated with early intensive or escalation treatment strategies. Ther Adv Neurol Disord. (2021) 14:175628642110195. doi: 10.1177/17562864211019574
9. Koch-Henriksen, N, Thygesen, LC, Stenager, E, Laursen, B, and Magyari, M. Incidence of MS has increased markedly over six decades in Denmark particularly with late onset and in women. Neurology. (2018) 90:e1954–63. doi: 10.1212/WNL.0000000000005612
10. Bove, RM, Healy, B, Augustine, A, Musallam, A, Gholipour, T, and Chitnis, T. Effect of gender on late-onset multiple sclerosis. Mult Scler. (2012) 18:1472–9. doi: 10.1177/1352458512438236
11. Pluchino, N, Cubeddu, A, Giannini, A, Merlini, S, Cela, V, Angioni, S, et al. Progestogens and brain: an update. Maturitas. (2009) 62:349–55. doi: 10.1016/j.maturitas.2008.11.023
12. Olsson, T, Barcellos, LF, and Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat Rev Neurol. (2017) 13:25–36. doi: 10.1038/nrneurol.2016.187
13. Magyari, M, and Koch-Henriksen, N. Quantitative effect of sex on disease activity and disability accumulation in multiple sclerosis. J Neurol Neurosurg Psychiatry. (2022) 93:716–22. doi: 10.1136/jnnp-2022-328994
14. Bove, R, Healy, BC, Musallam, A, Glanz, BI, de Jager, PL, and Chitnis, T. Exploration of changes in disability after menopause in a longitudinal multiple sclerosis cohort. Mult Scler. (2016) 22:935–43. doi: 10.1177/1352458515606211
15. Baroncini, D, Annovazzi, PO, de Rossi, N, Mallucci, G, Torri Clerici, V, Tonietti, S, et al. Impact of natural menopause on multiple sclerosis: a multicentre study. J Neurol Neurosurg Psychiatry. (2019) 90:1201–6. doi: 10.1136/jnnp-2019-320587
16. Otero-Romero, S, Midaglia, L, Carbonell-Mirabent, P, Zuluaga, M, Galán, I, Río, J, et al. Menopause does not modify disability trajectories in a longitudinal cohort of women with clinically isolated syndrome and multiple sclerosis followed from disease onset. Eur J Neurol. (2022) 29:1075–81. doi: 10.1111/ene.14782
17. Barnett, M, Bergsland, N, Weinstock-Guttman, B, Butzkueven, H, Kalincik, T, Desmond, P, et al. Brain atrophy and lesion burden are associated with disability progression in a multiple sclerosis real-world dataset using only T2-FLAIR: the NeuroSTREAM MSBase study. Neuroimage Clin. (2021) 32:102802. doi: 10.1016/j.nicl.2021.102802
18. Graves, JS, Henry, RG, Cree, BAC, Lambert-Messerlian, G, Greenblatt, RM, Waubant, E, et al. Ovarian aging is associated with gray matter volume and disability in women with MS. Neurology. (2018) 90:e254–60. doi: 10.1212/WNL.0000000000004843
19. Murgia, F, Giagnoni, F, Lorefice, L, Caria, P, Dettori, T, D’Alterio, MN, et al. Sex hormones as key modulators of the immune response in multiple sclerosis: a review. Biomedicine. (2022) 10:3107. doi: 10.3390/biomedicines10123107
20. Lublin, FD, Reingold, SC, Cohen, JA, Cutter, GR, Sorensen, PS, Thompson, AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. (2014) 83:278–86. doi: 10.1212/WNL.0000000000000560
21. Harlow, SD, Gass, M, Hall, JE, Lobo, R, Maki, P, Rebar, RW, et al. Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. (2012) 97:1159–68. doi: 10.1210/jc.2011-3362
22. Kurtzke, JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. (1983) 33:1444–52. doi: 10.1212/WNL.33.11.1444
23. Smith, SM, Zhang, Y, Jenkinson, M, Chen, J, Matthews, PM, Federico, A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. NeuroImage. (2002) 17:479–89. doi: 10.1006/nimg.2002.1040
24. Giovannoni, G, Popescu, V, Wuerfel, J, Hellwig, K, Iacobaeus, E, Jensen, MB, et al. Smouldering multiple sclerosis: the ‘real MS’. Ther Adv Neurol Disord. (2022) 15:17562864211066751. doi: 10.1177/17562864211066751
25. Bove, R, and Gilmore, W. Hormones and MS: risk factors, biomarkers, and therapeutic targets. Mult Scler. (2018) 24:17–21. doi: 10.1177/1352458517737396
26. Benedek, G, Zhang, J, Bodhankar, S, Nguyen, H, Kent, G, Jordan, K, et al. Estrogen induces multiple regulatory B cell subtypes and promotes M2 microglia and neuroprotection during experimental autoimmune encephalomyelitis. J Neuroimmunol. (2016) 293:45–53. doi: 10.1016/j.jneuroim.2016.02.009
27. Spence, RD, and Voskuhl, RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol. (2012) 33:105–15. doi: 10.1016/j.yfrne.2011.12.001
28. Brinton, RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. (2008) 31:529–37. doi: 10.1016/j.tins.2008.07.003
29. Vegeto, E, Benedusi, V, and Maggi, A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol. (2008) 29:507–19. doi: 10.1016/j.yfrne.2008.04.001
30. Tiwari-Woodruff, S, and Voskuhl, RR. Neuroprotective and anti-inflammatory effects of estrogen receptor ligand treatment in mice. J Neurol Sci. (2009) 286:81–5. doi: 10.1016/j.jns.2009.04.023
31. Lorefice, L, Frau, J, Coghe, G, Pitzalis, R, Gessa, I, Contu, F, et al. Assessing the burden of vascular risk factors on brain atrophy in multiple sclerosis: a case- control MRI study. Mult Scler Relat Disord. (2019) 27:74–8. doi: 10.1016/j.msard.2018.10.011
32. Belvisi, D, Canevelli, M, Baione, V, Buscarinu, MC, Pellicciari, G, Fantozzi, R, et al. Operationalization of a frailty index in patients with multiple sclerosis: a cross-sectional investigation. Mult Scler. (2021) 27:1939–47. doi: 10.1177/1352458520987541
33. Stampanoni Bassi, M, Iezzi, E, Pavone, L, Mandolesi, G, Musella, A, Gentile, A, et al. Modeling resilience to damage in multiple sclerosis: plasticity meets connectivity. Int J Mol Sci. (2019) 21:143. doi: 10.3390/ijms21010143
34. de Stefano, N, Stromillo, ML, Giorgio, A, Bartolozzi, ML, Battaglini, M, Baldini, M, et al. Establishing pathological cut-offs of brain atrophy rates in multiple sclerosis. J Neurol Neurosurg Psychiatry. (2015) 87:jnnp-2014-309903–9. doi: 10.1136/jnnp-2014-309903
Keywords: aging, brain atrophy, menopause, multiple sclerosis, neurodegeneration, oestrogen deprivation
Citation: Lorefice L, Fenu G, Fronza M, Murgia F, Frau J, Coghe G, Barracciu MA, Atzori L, Angioni S and Cocco E (2023) Menopausal transition in multiple sclerosis: relationship with disease activity and brain volume measurements. Front. Neurol. 14:1251667. doi: 10.3389/fneur.2023.1251667
Received: 02 July 2023; Accepted: 24 July 2023;
Published: 03 August 2023.
Edited by:
Alessandra Lugaresi, University of Bologna, ItalyReviewed by:
Eva Havrdova, Charles University, CzechiaCopyright © 2023 Lorefice, Fenu, Fronza, Murgia, Frau, Coghe, Barracciu, Atzori, Angioni and Cocco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lorena Lorefice, bG9yZW5hLmxvcmVmaWNlQGhvdG1haWwuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.