- 1Centre for Molecular Neurosciences, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India
- 2Department of Physiology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India
- 3Department of Microbiology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India
- 4Department of Neurology, Kasturba Medical College, Manipal Academy of Higher Education, Manipal, Karnataka, India
Parkinson's disease (PD) presents with severe motor manifestations and a plethora of non-motor symptoms. Urinary dysfunctions are one of the most common non-motor symptoms of PD patients responsible for reduced quality of life. Urinary extracellular vesicles (EVs) are mostly considered to originate from the cells in the urogenital tract. In this study, we have performed urinary EV analysis in 29 PD cases with varied severity of urinary dysfunction and correlated it with the EV dynamics in 29 age-matched controls. In the studied cases, apart from urinary dysfunction, symptoms of depression, anxiety, cognitive dysfunction, sleep, and wakefulness were observed in >75% of the cases. No significant difference in urinary EV size, concentration and urinary EV protein concentration was observed between PD cases with urinary dysfunction and controls. However, a significant positive association was observed between urinary EV concentration and motor scores (p = 0.042), while no association was observed between urinary EV concentration and urinary dysfunction scores. Chronic stress induced by motor symptoms could be one of the reasons for excessive EV production in PD patients with urinary dysfunctions. Large-scale studies on the association of urinary EV dynamics with motor and non-motor symptoms may provide additional information on urinary dysfunction in PD.
Introduction
Parkinson's disease (PD) presents with basic motor manifestations of bradykinesia, unequal tremor, and rigidity. Apart from motor symptoms, a plethora of non-motor symptoms (NMS) is well-associated with PD. The NMS are frequent, may be observed far before the actual onset of motor symptoms, and may correlate to a variable extent with the severity of motor symptoms (1, 2). The NMS could be observed throughout the course of PD and is considered the most important determinant of quality of life for PD patients. They are also responsible for the increased cost of care and hospitalization in the late stages of PD (3, 4). Urinary dysfunctions are one of the most common non-motor symptoms of Parkinson's disease patients responsible for reduced quality of life (5, 6). A recent systematic review and meta-analysis revealed urinary dysfunction in >60% of PD cases (6).
The term “extracellular vesicles” (EVs) refers to particles that are naturally released from cells, are delimited by a lipid bilayer, and cannot replicate independently (7). EVs are known to be released from all cell types. EVs contain proteins, lipids, mRNAs, miRNAs, and several other biomolecules depending on the parental cell source (8, 9). Since urine is available in plenty non-invasively, it is an easy source for exploring disease associations. Urinary extracellular vesicles are mostly considered to originate from the cells in the urogenital tract. Thus, urinary EV dynamics may be altered with the pathology of kidneys, bladder, and male or female urogenital tracts (10, 11). As PD is well-associated with urinary dysfunction, identifying the urinary EV dynamics in PD cases with urinary dysfunction and comparing it with age-matched controls could provide new insights into the pathophysiology of this NMS. In this study, we have performed EV analysis in 29 PD cases with varied severity of urinary dysfunction and correlated it with the EV dynamics in 29 age-matched controls.
Materials and methods
Study subjects and their clinical diagnosis
This study was a prospective cross-sectional single-center study performed following approval from the Kasturba Medical College Institutional Ethics Committee (# IEC 43/2021). Parkinson's-plus syndromes like multiple system atrophy, progressive supranuclear palsy, corticobasal degeneration and secondary Parkinsonism due to multiple causes were excluded. Following informed consent, 29 patients were recruited for PD cases and the demographic data were collected. Of the 29 cases, 26 cases examined were Hoehn and Yahr stage 2 with tremor rigidity affecting bilaterally, and walking/gait difficulties but without a history of falls. Only three cases were stage 1 with unilateral motor involvement. All the PD cases except two naive cases were on levodopa and carbidopa therapy thrice daily. All PD patients were assessed only in the “on” period for logistic reasons. On and off state refers to the motor fluctuation with the gradual decrease in the levodopa effect. On period refers to when motor symptoms are well-controlled. The off period refers to when the motor symptoms return.
Identifying motor symptoms
Along with bradykinesia, the presence of rigidity, 4–6 Hz resting tremor, or postural instability along with three supportive features were considered for PD diagnosis. The Movement Disorders Society—Unified Parkinson's Disease Rating Scale (UPDRS) characterizes the extent and burden of the disease and defines the disease course. Part 3 of the UPDRS is for motor examination and the specific disability is marked on a 0–4 scale which requires about 10 min for completion.
Identifying non-motor symptoms
Non-motor rating scale was used to assess the extent of various motor and NMSs symptoms. The NMS section comprises 52 items gathered into 13 distinct domains: five items in depression, six in cognition, three in apathy, four in psychosis, four items in anxiety, four in impulse control and related disorders, three in urinary, two in orthostatic hypotension, two in sexual, six in sleep and wakefulness, four in gastrointestinal, four in pain and five in others. Scoring was done for frequency (0–4, 0 being never to 4 being most of the time) and severity (0 to 4, zero being not present to 4 being severe). These two values were multiplied to get the total score. The maximum possible score is 832 points.
Urine collection
From each PD patient, a urine sample was collected in a 20 ml sterile container labeled with the sample number. For comparing the dynamics of extracellular vesicles, urine was collected from 29 age-matched control, healthy cases.
Isolation of urinary extracellular vesicles
Twenty ml of a urine sample from the sterile container was transferred to a 50 ml Falcon tube. The first centrifugation was done at 2,500 rcf for 30 min at 4°C. Pellet was discarded and to the supernatant, an equal quantity of 12% 2x polyethylene glycol was added and incubated at 4°C for 1 h. After 1 h, centrifugation was carried out at 3,300 rcf for 1 h at 4°C. The pellet was resuspended in sterile 1 ml PBS and an equal quantity of 5% 2x polyethylene glycol was added and incubated at 4°C for 1 h. After 1 h, centrifugation was carried out at 3,300 rcf for 1 h at 4°C. The supernatant was discarded and the pellet was collected.
Evaluation of size distribution and concentration of EVs
Purified EVs were characterized according to Minimum Information for the Study of Extracellular Vesicles (MISEV) 2018 guidelines (7). The size distribution and the concentration of urinary EVs were determined by NanoSight LM10 (Malvern Instruments, UK). Detailed procedures for sample analysis were provided in our earlier report (12).
Morphology of EVs
The morphology of the urinary EVs was evaluated using transmission electron microscopy by applying a few drops of isolated EVs to 300 mesh carbon-coated grids at room temperature. These grids were stained with 0.5% uranyl acetate, and air dried for 10 min at room temperature. The images were collected using JEM 2100, a multipurpose 120 KV analytical transmission microscope.
Evaluation of molecular marker expression in the urinary EVs
The molecular indicators for EVs, such as positive markers TSG 101 and CD 63 proteins, and negative marker GRP 94, were evaluated through western blotting. As a positive control for TSG101, CD63, and GRP94, human embryonic kidney (HEK293) cells were used. Briefly, following the lysis of EV and cell samples in RIPA buffer, total proteins in the isolated EVs were estimated using the BCA method. Proteins were separated through SDS-PAGE and transferred to the PVDF membrane. The presence of TSG101, CD63, and GRP94 was detected using rabbit polyclonal antibodies PA 531260, SAB 4301607, and SAB 2101094 (all from Invitrogen), respectively with appropriate secondary antibodies. The initial protein loading amount was confirmed with monoclonal β-actin (MA 1140, Invitrogen).
Statistical analysis
Statistical analysis was performed using GraphPad Prism. Results were expressed as either Mean ± SEM or percentage. The student unpaired t-test was done to compare results between the two groups. Linear regression analysis was performed to find the association between different parameters.
Results
The study population included 29 idiopathic Parkinson's disease cases and 29 age-matched controls. Individual patient characteristics are provided in Table 1. There was a total of 12 female PD patients. The mean age of 29 controls was 61.2 ± 1.98 years while the mean age of 29 PD cases was 60.2 ± 1.7 years with no significant difference between the two groups. PD cases were confirmed based on the motor examination as per UPDRS part 3.
Non-motor symptoms of Parkinson's disease patients
The NMS was evaluated for all 29 PD cases. Symptoms such as depression, anxiety, cognitive dysfunction, urinary dysfunction, sleep, and wakefulness were observed in >75% of the cases while apathy, gastrointestinal issues, and pain were demonstrated in >50% of the cases. Symptoms of psychosis, impulse control-related disorders, and orthostatic hypotension were observed in <50% of the cases.
The severity of motor and non-motor symptoms
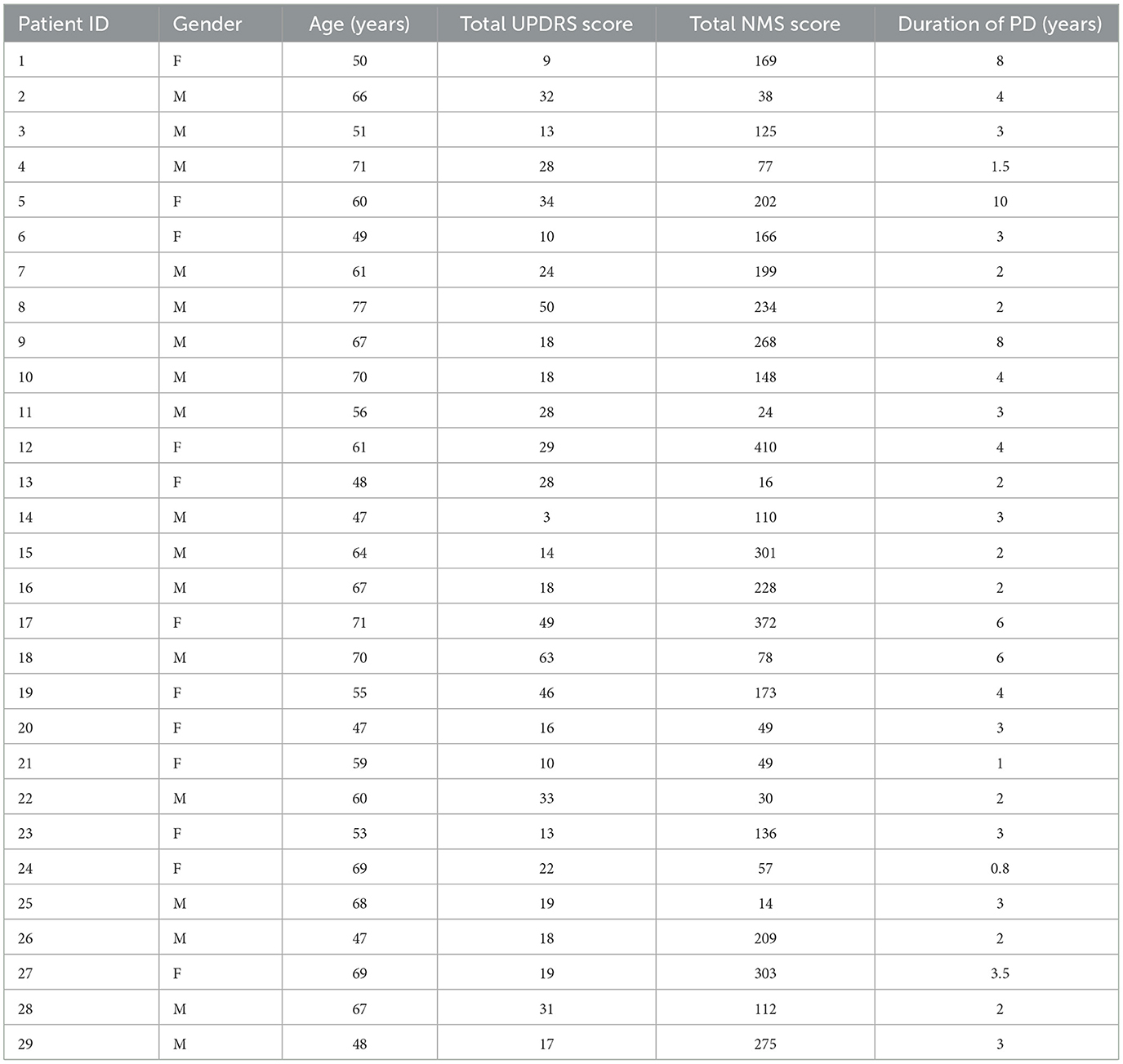
The severity of various motor symptoms is demonstrated in Figure 1A. The mean UPDRS part 3 score for 29 PD cases was 24.6 ± 2.6. The severity of muscle rigidity, finger tapping, hand movements, pronation supination, toe-tapping, and rest tremor amplitudes was highly demonstrated compared to other symptoms. Speech disturbance and mask facies were present in several cases but in lower severity. Gait and posture disturbance was found among a lesser number of cases.
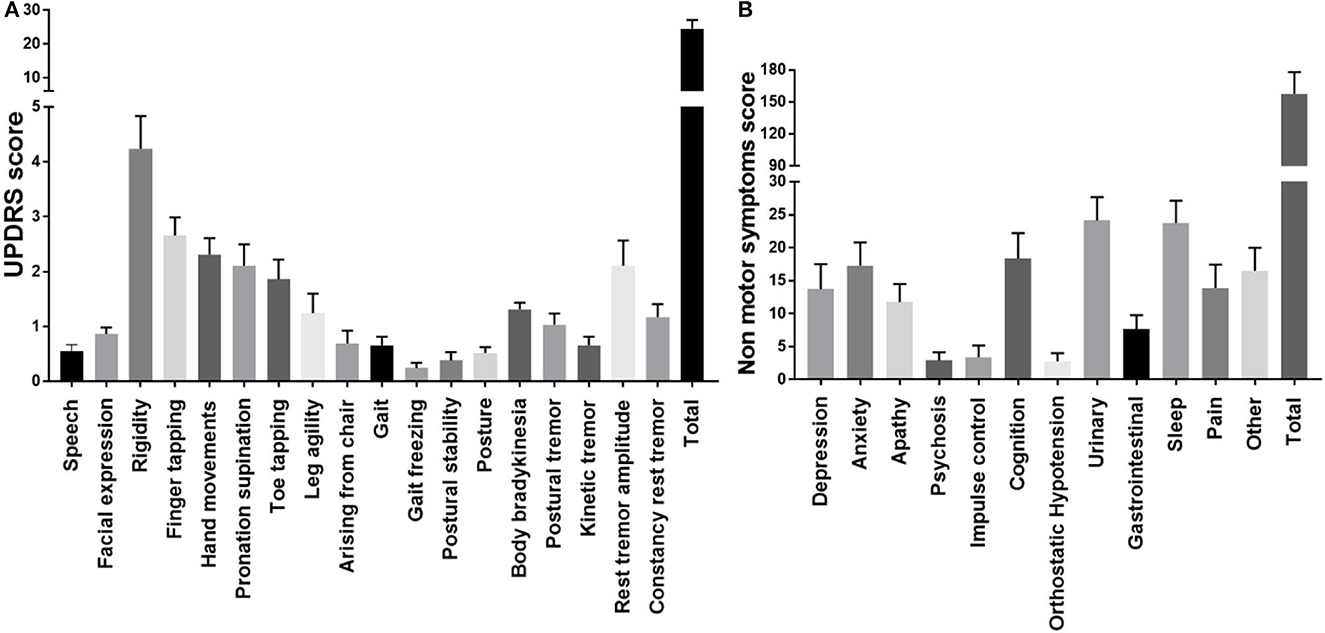
Figure 1. The severity of motor and non-motor symptoms in Parkinson's disease patients. (A) Severity of motor symptoms in PD cases. (B) Severity of non-motor symptoms in PD cases.
The severity of NMS is demonstrated in Figure 1B. The mean NMS score was 161.66. The most severe NMS observed was urinary symptoms (24.14 ± 3.542) which include urinary urgency (urgent need to empty bladder); urinary frequency (had to empty the bladder more than every 2 h) and nocturia (had to empty the bladder more than twice overnight). The next most severe NMS was observed in the sleep domain (23.72 ± 3.392). These include insomnia sleep behavior, sleeping during waking hours, restlessness, periodic limb movements, waking due to snoring, and gasping. As seen in the graph below severity of anxiety, cognitive difficulties, and others (physical fatigue, mental fatigue, unintentional weight loss, impaired olfaction, and excessive sweating) were also high.
Evaluation of urinary extracellular vesicles
As mentioned in the methodology, urine samples were collected from all 29 PD cases and 29 age-matched controls. Isolated urinary extracellular vesicles (uEVs) were analyzed for the concentration and size distribution of EVs (Figure 2A), the ultrastructure of EVs (Figure 2B) and the molecular markers of the EVs (Figure 2C). As per the guidelines of MISEV 2018, evaluation of particle number and size (through nanoparticle tracking analysis, etc.), the ultrastructure of the isolated particle (using transmission electron microscopy, etc.) and three molecular markers (one cytosolic EV marker such as TSG101, one transmembrane EV marker such as CD63 and a negative EV marker such as GRP 94) is essential to confirm the identity of the isolated particles as EVs (7).
Figure 2. Characterization of isolated urinary EVs. (A) Nanoparticle tracking analysis of urinary EVs. (B) Ultrastructural evaluation of EVs using transmission electron microscopy. (C) Molecular markers of EVs TSG101, CD 63, and GRP 94 in isolated urinary EVs.
Detailed analysis of urinary EVs demonstrated a mean size of 211.4 ± 6.1 in controls and 202.5 ± 4.6 for PD cases without any significant difference between the groups. Ultrastructural evaluation of the EVs using transmission electron microscopy identified abundant ~200 nm-sized round or oval-shaped particles (Figure 2B). Evaluating molecular markers through western blotting of the isolated particles demonstrated the positive staining for TSG 101 and CD63, while negative for GRP 94 (Figure 2C). The positive control used confirmed the functionality of the GRP 94 antibody while β-actin immunostaining confirmed comparable loading of proteins for all the markers. These details confirmed that isolated particles from urine were indeed extracellular vesicles.
Urinary EV size, concentration, and protein concentration in controls and PD cases with urinary dysfunction
Detailed analysis of EV size demonstrated a mean size of 211.4 ± 6.1 in controls and 202.5 ± 4.6 for PD cases without any significant difference between the groups (Figure 3A). Analysis of urinary EV concentration demonstrated 83.74 ± 18.14 billion in controls and 78.39 ± 22.71 billion in PD patients demonstrating no significant difference between the groups (Figure 3B). Evaluation of urinary EV protein concentration demonstrated 92.56 ± 10.34 μg/ml for controls and 89.78 ± 8.95 μg/ml in PD patients demonstrating no significant difference between the groups (Figure 3C).
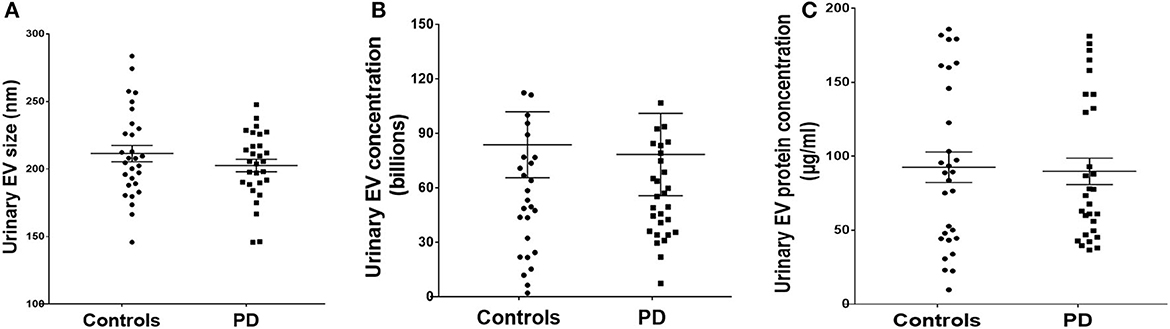
Figure 3. Urinary EV dynamics of PD and controls. (A) Demonstrates EV size, (B) demonstrates EV concentration, and (C) demonstrates EV protein concentration in control and PD cases with urinary dysfunction.
Relationships of urinary EV size, concentration, and EV protein concentration with the severity of motor and non-motor symptoms of PD patients
Linear regression analysis of urinary EV concentration separately with motor and NMS scores demonstrated a positive correlation (with motor symptoms r2 = 0.144, p = 0.042, Figure 4A) while no correlations with NMS (r2 = 0.03, p = 0.4, Figure 4B). The association of the severity of motor symptoms with urinary EV concentration is a very surprising finding and requires large-scale studies for the essential conclusion. However, urinary EV size and EV protein concentrations demonstrated no significant association with motor and NMS (data not shown).
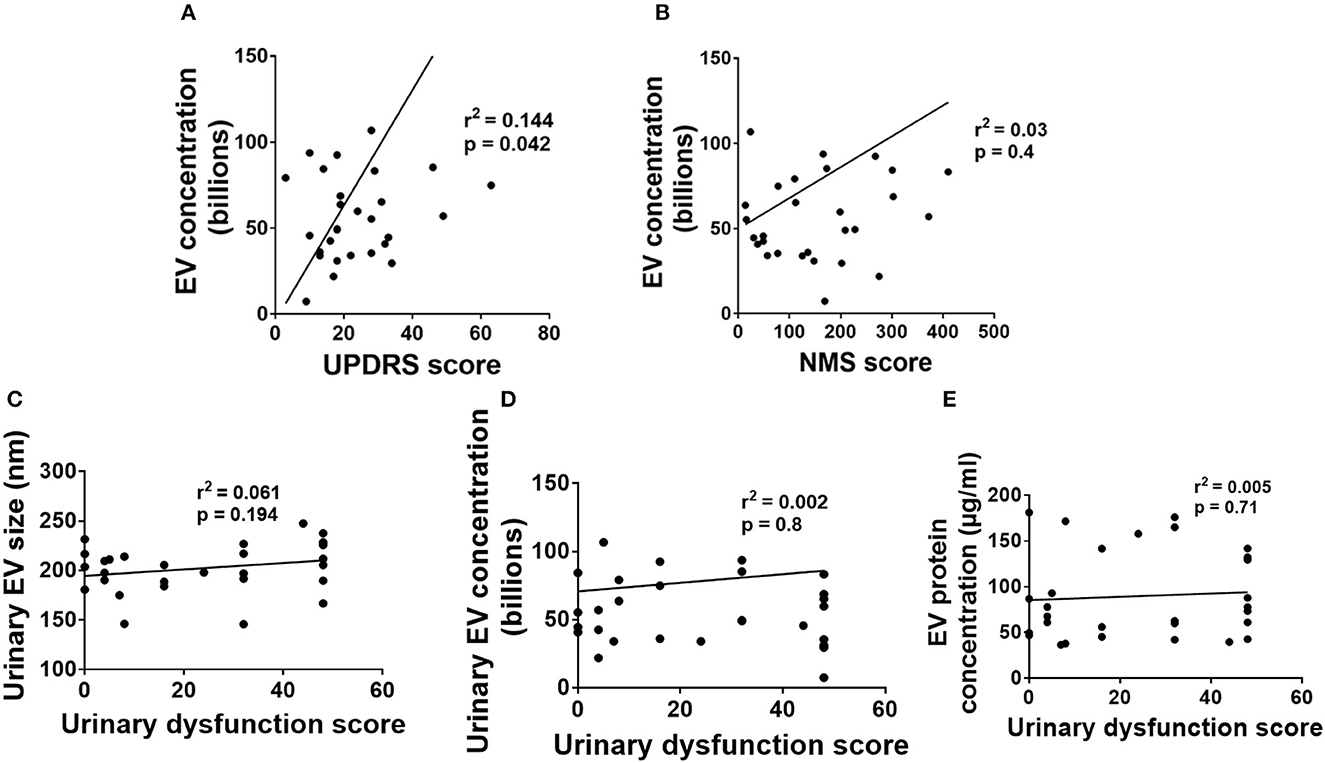
Figure 4. Association of urinary EV dynamics with PD symptoms. (A) Urinary EV concentration vs. UPDRS score. (B) Urinary EV concentration vs. nonmotor symptom score. (C) Urinary EV size with urinary dysfunction scores. (D) Urinary EV concentration with urinary dysfunction scores. (E) Urinary EV protein concentration with urinary dysfunction scores.
Relationships of urinary EV size, concentration, and EV protein concentration with urinary dysfunctions in PD patients
We have performed linear regression analysis for urinary EV data with urinary dysfunction scores. Linear regression analysis of urinary EV size with urinary dysfunction scores demonstrated no correlations between them (p > 0.05, Figure 4C). Also, linear regression analysis of urinary EV concentration with urinary dysfunction scores demonstrated no correlations between them (p > 0.05, Figure 4D). Further, linear regression analysis of urinary EV protein concentration with urinary dysfunction scores also demonstrated no correlations between them (p > 0.05, Figure 4E).
Discussion
The high severity of motor symptoms is a well-established reality in PD. As per UPDRS part 3, the clinical criteria for diagnosis of PD requires the presence of bradykinesia and 1 of the following features: rigidity, 4–6 Hz resting tremor, or postural instability, in addition, to three supportive features. Varied extents of NMS in PD were demonstrated in different studies (13–16). Our results suggest that all the PD patients demonstrated different NMS with varying severity although urinary symptoms as the most severe NMS followed by sleep disturbance.
Urinary extracellular vehicles provide a great opportunity to non-invasively understand certain cellular pathologies associated with various disorders. Since Parkinson's disease is associated with severe motor and non-motor symptoms including urinary tract dysfunction, we used this opportunity to understand the urinary EV dynamics in PD. It is assumed that urinary dysfunction in PD is caused by neurogenic dysfunction and not by urogenital dysfunction. Normal micturition requires active functioning of the bladder and urethral sphincters, which are controlled by the central nervous system through coordinated network activity of the sympathetic and parasympathetic system with the somatic nervous system to maintain urinary continence (17). Neurodegeneration in the autonomic nervous system and higher micturition centers could be responsible for neurogenic lower urinary tract dysfunction in PD. Since urinary extracellular vesicles are mostly considered to originate from the cells in the urogenital tract, it is reasonable that no significant difference in urinary EV size, concentration, and urinary EV protein concentration was observed between PD cases with urinary dysfunction and controls.
Also, urinary dysfunction in PD is well-associated with the degeneration of nigrostriatal dopaminergic neurons in early and untreated patients (18). Exploring basic features such as size, number, and total protein content of urinary EVs could be useful for identifying unestablished links between motor and non-motor symptoms with urinary dysfunctions. While some specific molecular biomarker studies have been performed earlier in urinary EVs from PD cases (19), the available literature does not demonstrate the implications of motor and non-motor symptoms of PD and its medications such as levodopa and carbidopa on EV secretions in urinary dysfunctions. Although the sample size is small, our study observed a significant positive correlation between motor symptoms and urinary EV concentration while no definitive association was observed with non-motor symptoms, including urinary dysfunction. We believe stress induced by motor symptoms could be a possible reason for excessive EV production in PD patients as stress is an inducer of EVs (20).
Limitations of the study include a relatively small sample size and patients were assessed only in the “on” period for logistic reasons. Future studies on a large population of PD cases with urinary dysfunction could provide a definitive link between motor symptoms and urinary EV concentration. Also, implications of the duration of PD and severity of motor and non-motor symptoms on the urinary EV concentration could be extensively evaluated. Also, future studies on the molecular composition of urinary EVs may help to delineate causal factors for urinary dysfunction such as intrinsic lower urinary tract factors vs. PD-dependent pathology.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Kasturba Medical College Institutional Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SR: Conceptualization, Formal analysis, Writing—original draft, Writing—review & editing. NK: Writing—review & editing, Methodology. AA: Writing—review & editing, Methodology. DP: Conceptualization, Methodology, Writing—review & editing, DJ: Conceptualization, Methodology, Writing—review & editing, DU: Conceptualization, Funding, Methodology, Formal analysis, Writing—original draft, Writing—review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. S. N. Nandi foundation trust, sanctioned to DU.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stern MB, Lang A, Poewe W. Toward a redefinition of Parkinson's disease. Mov Disord. (2012) 27:54–60. doi: 10.1002/mds.24051
2. Coelho M, Ferreira JJ. Late-stage Parkinson disease. Nat Rev Neurol. (2012) 8:435–42. doi: 10.1038/nrneurol.2012.126
3. Heimrich KG, Schönenberg A, Santos-García D, Mir P, Coppadis Study Group and Prell. The impact of nonmotor symptoms on health-related quality of life in Parkinson's disease: a network analysis approach. J Clin Med. (2023) 12:2573. doi: 10.3390/jcm12072573
4. Duncan GW, Khoo TK, Yarnall AJ, O'Brien JT, Coleman SY, Brooks DJ, et al. Health-related quality of life in early Parkinson's disease: the impact of nonmotor symptoms. Mov Disord. (2014) 29:195–202. doi: 10.1002/mds.25664
5. Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson's disease and are we missing them? Mov Disord. (2010) 25:2493–500. doi: 10.1002/mds.23394
6. Li FF, Cui YS, Yan R, Cao SS, Feng T. Prevalence of lower urinary tract symptoms, urinary incontinence and retention in Parkinson's disease: a systematic review and meta-analysis. Front Aging Neurosci. (2022) 14:977572. doi: 10.3389/fnagi.2022.977572
7. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. (2018) 7:1535750. doi: 10.1080/20013078.2018.1535750
8. Upadhya D, Shetty AK. Extracellular vesicles as therapeutics for brain injury and disease. Curr Pharm Des. (2019) 25:3500–5. doi: 10.2174/1381612825666191014164950
9. Upadhya D, Shetty AK. Promise of extracellular vesicles for diagnosis and treatment of epilepsy. Epilepsy Behav. (2021) 121(Pt B):106499. doi: 10.1016/j.yebeh.2019.106499
10. Karpman D, Ståhl AL, Arvidsson I. Extracellular vesicles in renal disease. Nat Rev Nephrol. (2017) 13:545–62. doi: 10.1038/nrneph.2017.98
11. Merchant ML, Rood IM, Deegens JKJ, Klein JB. Isolation and characterization of urinary extracellular vesicles: implications for biomarker discovery. Nat Rev Nephrol. (2017) 13:731–49. doi: 10.1038/nrneph.2017.148
12. Reddy SK, Ballal AR, Shailaja S, Seetharam RN, Raghu CH, Sankhe R, et al. Small extracellular vesicle-loaded bevacizumab reduces the frequency of intravitreal injection required for diabetic retinopathy. Theranostics. (2023) 13:2241–55. doi: 10.7150/thno.78426
13. Karri M, Ramasamy B, Kalidoss R. Prevalence of non-motor symptoms in Parkinson's disease and its impact on quality of life in tertiary care center in India. Ann Indian Acad Neurol. (2020) 23:270–4. doi: 10.4103/aian.AIAN_10_19
14. Hughes KC, Gao X, Baker JM, Stephen C, Kim IY, Valeri L, et al. Non-motor features of Parkinson's disease in a nested case-control study of US men. J Neurol Neurosurg Psychiatry. (2018) 89:1288–95. doi: 10.1136/jnnp-2018-318275
15. Ravan A, Ahmad FM, Chabria S, Gadhari M, Sankhla CS. Non-motor symptoms in an Indian cohort of Parkinson's disease patients and correlation of progression of non-motor symptoms with motor worsening. Neurol India. (2015) 63:166–174 doi: 10.4103/0028-3886.156276
16. Boeve BF, Molano JR, Ferman TJ, Lin S-C, Bieniek K, Tippmann-Peikert M, et al. Validation of the Mayo Sleep Questionnaire to screen for REM sleep behavior disorder in a community-based sample. J Clin Sleep Med. (2013) 9:475–80. doi: 10.5664/jcsm.2670
17. Yoshimura N, Chancellor MB. Neurophysiology of lower urinary tract function and dysfunction. Rev Urol. (2003) 5:S3–10.
18. Wang J, Cao R, Huang T, Liu C, Fan Y. Urinary dysfunction is associated with nigrostriatal dopaminergic degeneration in early and untreated patients with Parkinson's disease. Parkinsons Dis. (2020) 2020:4981647. doi: 10.1155/2020/4981647
19. Fraser KB, Rawlins AB, Clark RG, Alcalay RN, Standaert DG, Liu N. Parkinson's Disease Biomarker Program Consortium; West AB. Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson's disease. Mov Disord. (2016) 31:1543–50. doi: 10.1002/mds.26686
Keywords: extracellular vesicles, Parkinson's disease, motor symptoms, urinary dysfunctions, neurodegenerative disease
Citation: Roy S, Kashyap NN, Anchan AS, Punja D, Jasti DB and Upadhya D (2023) Urinary extracellular vesicle dynamics in Parkinson's disease patients with urinary dysfunction. Front. Neurol. 14:1250832. doi: 10.3389/fneur.2023.1250832
Received: 13 July 2023; Accepted: 30 October 2023;
Published: 17 November 2023.
Edited by:
Huifang Shang, Sichuan University, ChinaReviewed by:
Gabriele Zanirati, Pontifical Catholic University of Rio Grande do Sul (PUCRS), BrazilIdrish Ali, Monash University, Australia
Copyright © 2023 Roy, Kashyap, Anchan, Punja, Jasti and Upadhya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dinesh Upadhya, ZGluZXNoLnVwYWRoeWFAbWFuaXBhbC5lZHU=
 Santanu Roy
Santanu Roy Namita N. Kashyap
Namita N. Kashyap Abigail Sheldon Anchan
Abigail Sheldon Anchan Dhiren Punja
Dhiren Punja Dushyanth Babu Jasti4
Dushyanth Babu Jasti4 Dinesh Upadhya
Dinesh Upadhya