
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 11 October 2023
Sec. Stroke
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1249365
Background: Deep vein thrombosis (DVT) in lower extremities as a common complication of acute ischemic stroke (AIS) has long been studied. However, as the therapeutic options for AIS continue to advance, the pathogenic mechanisms behind DVT may change. Endovascular thrombectomy (EVT) has replaced intravenous thrombolysis and become the preferred treatment for AIS patients with large vessel occlusions. Therefore, it is important to update our understanding of DVT and its management. This study aimed to determine the prevalence and risk factors of DVT in AIS patients following EVT.
Methods: In this retrospective study, 245 AIS patients who had received EVT were recruited between January 2020 and December 2021. Within 10 days (median 4 days) of thrombectomy, DVT was diagnosed by ultrasonography. Demographic characteristics, clinical findings, and therapeutic procedures were compared between patients with and without DVT using univariate analysis. Cutoff points were defined for EVT time and plasma D-dimer concentration. Multivariable logistic regression was then used to determine the independent risk factors for DVT and evaluate their predictive power.
Results: The prevalence of DVT in AIS patients after EVT was 27.3%. Multifactorial logistic regression analysis showed that age (OR 1.036, 95% CI 1.001–1.073; P = 0.045), female sex (OR 3.015, 95% CI 1.446–6.289; P = 0.003), lower limb muscle strength less than grade three (OR 7.015, 95% CI 1.887–26.080; P = 0.004), longer EVT time (OR 1.012, 95% CI 1.004–1.020; P = 0.003), and higher D-dimer levels (OR 1.350, 95% CI 1.150–1.585; P < 0.001) were independently associated with higher DVT risk in AIS patients following EVT. The cutoff points for operative time of EVT and plasma D-dimer were 65.5 min and 1.62 mg/L, respectively, above which the risk for DVT was dramatically increased with OR > 4 in AIS patients.
Conclusion: AIS patients are at increased risk of developing DVT following EVT particularly if they have undergone prolonged thrombectomy procedures and exhibit high plasma levels of D-dimers. However, the results of our study need to be validated by a multicenter prospective study with a larger population of stroke patients.
Acute ischemic stroke (AIS) is one of the leading causes of disability and death both in China and worldwide, placing a heavy burden on both the healthcare system and families (1–3). One common complication in AIS patients is deep vein thrombosis (DVT), which is often diagnosed and monitored in the lower limbs. It was reported more than 40 years ago that up to 75% of stroke patients with hemiplegia without venous thromboembolism prophylaxis develop DVT in the lower limbs as evidenced by the uptake of 125I-labeled fibrinogen (4). Recently, duplex ultrasonography has become the standard method for diagnosing DVT, and the detected prevalence of DVT in low extremities ranged from 11.5 to 22.1% in stroke patients (5–10). DVT can lead to pulmonary embolism, which significantly damages the lungs and heart and is responsible for 13–25% of early deaths after stroke (11). DVT also leads to reduced mobility and prolonged hospitalization, which increases the risk of infection, pressure ulcers, and pneumonia. In addition, DVT can result in post-thrombotic syndrome, which causes chronic pain, swelling, and skin changes in the affected leg. This further impairs the patient's mobility and quality of life. Therefore, timely and effective diagnosis and treatment of DVT are necessary.
Occlusion of large proximal arteries supplying the brain leads to particularly poor functional outcomes in AIS patients (12). In 2015, several studies provided compelling evidence that endovascular thrombectomy (EVT) for stroke due to large vessel occlusion results in significantly better recanalization and clinical outcomes than intravenous thrombolysis alone (12). In 2019, the American Heart Association/American Stroke Association recommended EVT for AIS patients for up to 24 h after the onset of symptoms when imaging mismatch or mismatch between severity of clinical deficit and infarct volume is observed (13). EVT has now replaced intravenous thrombolysis and become a standard therapy for AIS patients with large vessel occlusions.
It should be noted that patients receiving EVT require general or local anesthesia during EVT and post-operative monitoring in the intensive care unit (ICU). The endovascular intervention may damage endothelial cells and induce thrombosis (14). The pathogenic mechanisms mediating DVT after EVT must be altered compared to those in previously studied stroke patients treated with and without intravenous thrombolysis. It is supposed that the prevalence of DVT in EVT-treated patients is higher. However, the exact prevalence/incidence and risk factors for DVT in EVT patients remain unclear. In this study, we aimed to answer this question by examining all stroke patients who received EVT in our hospital within 2 years.
Our project was a retrospective cohort study. The study protocol was approved by the Ethics Committee of Taizhou Hospital, Zhejiang Province, China (Registration number: K20181204). Between January 2020 and December 2021, all 310 AIS patients aged ≥18 years at our hospital received EVT according to the international and Chinese guidelines (13, 15).
The inclusion criteria were as follows: age ≥ 18 years; AIS diagnosis confirmed by computed tomography (CT) or magnetic resonance imaging (MRI); and EVT performed within 24 h of symptom onset. The exclusion criteria were as follows: no ultrasound examination, hospitalization of <3 days, death during hospitalization, no recanalization after EVT, history of venous thromboembolism (VTE), and lower extremity agenesis.
DVT was diagnosed using a published protocol (5) by complete compression duplex ultrasonography of veins in both legs with a high-resolution 7.5 MHz linear-array transducer. The deep veins of the thigh, popliteal fossa, and calf were carefully examined at ~2 cm intervals in the transverse plane. On a more proximal plane, patients were examined in the supine position from the level of the inguinal ligament to the adductor canal. The popliteal vein was examined at its branch in the upper calf. The other calf veins were examined up to the level of the ankle. A diagnosis of deep vein thrombosis was made if duplex ultrasonography examination showed a loss of compressibility of the vein by ultrasound probe pressure, clot, or abnormal flow pattern (loss of phasic flow signal or loss of flow enhancement) with distal compression. The lack of visualization/flow measurements was considered insufficient for interpretation. Ultrasonography was performed in all AIS patients within 10 days of EVT or immediately if the patient complained of throbbing pain, swelling, or warmth in one leg. It should be noted that we included both proximal DVT and distal isolated DVT below the popliteal vein (e.g., posterior and anterior tibial veins, peroneal veins, and the muscular veins) in the study to accurately understand the effects of EVT on DVT in stroke patients.
During EVT, 307 and 1 patients underwent puncture at the right and left femoral artery in the groin, respectively. Two patients underwent puncture at the right flexor artery. The catheter was passed to the brain, and the clot was extracted. After EVT, the puncture site in the groin was compressed with a 1 kg sandbag for 6 h, and the punctured lower extremity was immobilized within 24 h of bed rest for 8 h. At 24 h after EVT or at any time within 24 h, neurological deficits progressed, and patients were reexamined with head CT and reassessed with the National Institute of Health Stroke Scale (NIHSS). When there were no hemorrhagic adverse events, regular treatment consisted of antiplatelet drugs that started 24 h after EVT. Three patients received anticoagulation with warfarin or low molecular heparin calcium starting 24 h after EVT because of an existing cardiac valve replacement. Other strategies of treatment included statins, management of blood glucose, blood pressure, or combinations of these treatments, following the Chinese guidelines for the early management of AIS patients (15).
As the bridge therapy with intravenous thrombolysis was considered to improve the recanalization of EVT (16), 68 of 310 AIS patients in our study received a standard intravenous thrombolytic therapy (0.9 mg/kg recombinant tissue-type plasminogen activator, alteplase, over 1 h with 10% of the first bolus) before EVT.
During hospitalization (≤14 days post-EVT), AIS patients were evaluated according to a Chinese guideline (17) and the international guideline (13) and carefully monitored for the risk of both DVT and bleeding. All patients were routinely examined for DVT in the lower extremities by duplex ultrasonography. Our standard protocol for post-thrombectomy management includes a single duplex scan for all stroke patients during their hospitalization. This scan is typically conducted within 2 weeks of EVT, with the timing determined by the individual patient's situation and the examination capacity of our ultrasound department. For example, the examination is scheduled after the patient has been transferred out of the ICU and extubated, taking place in either the stroke unit or the post-stroke unit (normal ward). The examination is only immediately performed if there is a suspicion of DVT. In this study, three patients underwent emergency ultrasound examination.
All the AIS patients who had received EVT had a high risk for both venous thrombosis/embolism and bleeding, so mechanical prophylaxis with intermittent pneumatic compression using the Flowtron® ACS900 Active Compression System (Arjo, Suzhou China) was performed after exclusion of contradictions according to the guidelines (13, 17). The patient wore compression garments (sleeves or boots) on the calves. The compression garment has multiple air chambers that inflate and deflate sequentially, creating an undulating motion that starts from the foot and moves upward, mimicking the natural muscle pump of the legs. Intermittent pneumatic compression (18 h/day) began after EVT, continued throughout the ICU stay, and proceeded to the normal ward if lower extremity muscle strength was less than grade 4 according to the Medical Research Council scale (grade 4: movement against gravity and moderate resistance through almost the entire range of motion) (18).
All clinical information was obtained from the digital history record system. We recorded age, sex, blood pressure, hypertension, diabetes, history of malignant tumors, bridging therapy (intravenous thrombolysis before EVT), EVT operation time (referred to puncture-to-recanalization time), pulmonary infection, respiratory failure, smoking, drinking, coronary heart disease, atrial fibrillation, central venous catheter, mechanical ventilation, time of tracheal intubation, duration of ICU stay, duration of hospital stay, anesthesia mode, endovascular thrombectomy site, lower extremity muscle strength less than grade 3 according to the Medical Research Council Scale (movement against gravity but not against resistance through almost the entire range of motion) (18), NIHSS and modified Rankin Scale (mRS) scores at admission and at discharge, serum creatinine (SCr), glucose, total cholesterol (TC), triglyceride (TG), blood urea nitrogen (BUN), low-density lipoprotein (LDL), high-density lipoprotein (HDL), fibrinogen, and D-dimer. All laboratory tests were conducted within 24 h of the EVT. In addition, we assessed the DVT type.
The statistical analysis of the gathered data was conducted using SPSS software for Windows (Version 26.0, IBM, Armonk, USA). The data for continuous variables were described as mean ± SD, and categorical variables were presented as frequencies. Continuous variables were compared between independent groups by the T-test or Mann–Whitney U-test depending on whether the continuous variables were normally distributed. Categorical variables were compared by the Pearson χ2 test. The independent variables with a P-value of < 0.05 in the univariate analysis were included in the multivariable analysis. A multivariable logistic regression study was used to determine the risk factors of DVT and relevant odds ratios (ORs) with 95% confidence intervals. To further investigate the possible influence of female sex on the association between risk factors and the development of DVT, the interaction between operation time, D-dimer, age or lower extremity muscle strength, and female sex was assessed in the logistic regression. To identify the optimal cutoff points for D-dimer plasma level and EVT operation time in predicting DVT, the receiver operator characteristic curve (ROC curve) and the Youden index (J) method were used. The optimal cutoff point was defined with the maximal Youden index (19). A P-value of < 0.05 was considered to be statistically significant.
From January 2020 to December 2021, 310 AIS patients in our hospital received EVT according to the international and Chinese guidelines (13, 15). Sixty-five patients were excluded due to incomplete information, lack of recanalization at EVT, and other exclusion criteria (Figure 1). Ultimately, 245 patients were enrolled in this study. All patients were administered mechanical prophylaxis using intermittent pneumatic compression immediately after EVT but did not take anticoagulant medications, except that three patients received warfarin or low molecular heparin calcium after EVT because of pre-existing cardiac valve replacement. All patients underwent ultrasonography within 1 to 10 days post-EVT, with a median of 4 days. On the basis of ultrasound findings, DVT was detected in 67 of 245 cases (27.3%), all of whom had undergone right femoral artery puncture during EVT. The prevalence was higher than the overall prevalence previously observed in patients with acute stroke (including ischemic and hemorrhagic stroke) (5–10). Most patients were asymptomatic, and only three of them developed swelling of the lower extremities. No patient developed pulmonary embolism. The most commonly affected blood vessel was the muscular calf vein, which was affected in 57 of the 67 cases (85.07%). This observation was consistent with a published study of asymptomatic DVT in stroke patients (10). The other veins that showed DVT were the posterior tibial vein (5/67), fibular vein (2/67), popliteal vein (1/67), and femoral vein (1/67). In one case (1/67), DVT occurred in multiple vessels.
Of these 67 patients, 29 patients (43.28%) had DVT in the right lower extremity, which was more than 14 patients (20.90%) in the left lower extremity and 24 patients (35.82%) in both lower extremities (Table 1). Of note, 30 (44.77%) patients had lower limb muscle strength less than grade 3 on the left side, 21 (31.34%) on the right side, and 7 (10.45%) on both sides. Nine patients had no lower limb weakness (Table 1). We wondered whether immobilization of the right lower limb during and after surgery contributed to the formation of DVT. We analyzed the correlation between the localization of DVT and the location of lower limb muscle strength less than grade 3. We found that the location of DVT was significantly related to the side of muscle weakness [χ2 (6) = 14.783, P = 0.022] (Table 1). Thus, the location of the puncture site in the right groin did not seem to be the main influencing factor for DVT.
To determine the predictors of DVT after EVT, we compared the demographic and baseline characteristics, clinical findings, and therapeutic procedures of patients with and without DVT. Using univariate variable analysis, we found that female, older age, lower extremity muscle weakness <grade 3 (the Medical Research Council scale), respiratory failure, pulmonary infection, central venous catheter, mechanical ventilation, longer duration of EVT surgery, longer duration of ICU monitoring, longer duration of tracheal intubation, and higher plasma D-dimer level positively correlated with an increased risk of developing DVT in AIS patients after EVT (P-values for all parameters analyzed <0.05; Table 2). Not surprisingly, the severity of neurological deficits (as shown by NIHSS) was higher in AIS patients who developed DVT on admission than in control patients without DVT, and DVT also negatively affected recovery, as shown by NIHSS and mRS of AIS patients at discharge and 90 days after onset of stroke (P-values for all parameters analyzed <0.05; Table 2).
Because different predictors of DVT in stroke patients identified in the univariate analysis might influence each other, we performed a logistic regression analysis to limit the confounding effects of various variables. The potential predictors, sex, age, lower extremity muscle weakness, respiratory failure, pulmonary infection, central venous catheter, duration of EVT surgery, and plasma level of D-dimer, listed in Table 2, entered the regression analysis. We observed the following variables that independently predicted DVT in AIS patients after EVT: age (OR 1.036, P = 0.045), female (OR 3.015, P = 0.003), lower limb muscle strength less than grade 3 (OR 7.015, P = 0.004), longer EVT time (OR 1.012, P = 0.003), and higher D-dimer levels (OR 1.350, P < 0.001) (Table 3).
We wondered whether age, lower limb muscle strength, EVT time, and D-dimer levels differently predicted the development of DVT in women and men. Therefore, we repeated the logistic analysis with these predictors alone, and their combinations with sex (age*sex, lower limb muscle strength*sex, EVT time*sex, and D-dimer level*sex) as independent variables. We observed that the interactions between these tested risk factors and sex were not statistically significant (see Supplementary Table 1; P > 0.05), which suggested that sex does not alter the associations between age, lower limb muscle strength, EVT time and D-dimer levels, and the development of DVT.
As described above, our study revealed that aging, female sex, lower limb paralysis, and higher plasma D-dimer levels were associated with the development of DVT, which is consistent with previous research findings (5–8). In the specific cohort of stroke patients with occlusion in a large cerebral artery and receiving thrombectomy, we identified the operative time of EVT as another independent risk factor for DVT. As EVT potentially alters the coagulation and thrombolytic activity in stroke patients (14, 20, 21), the threshold of D-dimer level in predicting/diagnosing DVT should be updated. In the following study, we used the ROC curve and defined the cutoff point for the operative time of EVT as 65.5 min with a sensitivity and specificity of 70.1 and 62.9%, respectively (Table 4; Figure 2A). Similarly, the cutoff point for D-dimer concentration was 1.62 mg/L with a sensitivity of 71.6% and specificity of 70.2% (Table 4; Figure 2B), which is consistent with published results with the optimal cutoff points at 1.52, 1.59, and 1.66 mg/L for predicting DVT in acute stroke patients (9, 22, 23). We also defined the cutoff point for age as 60.5 years with a sensitivity and specificity of 89.6 and 34.3%, respectively (Table 4; Figure 2C). Due to the low specificity, we calculated age as a continuous variable in the subsequent statistical analysis.
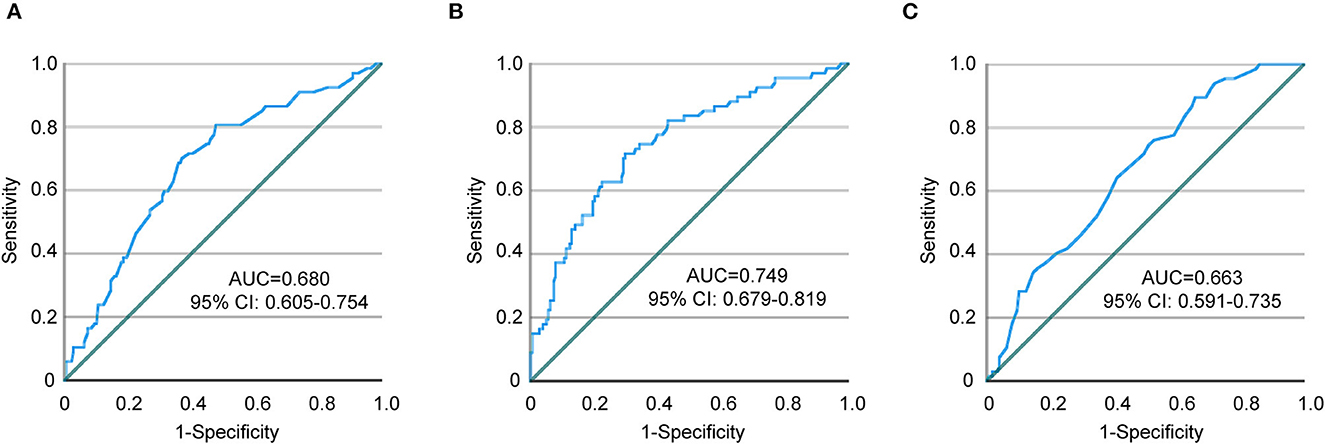
Figure 2. ROC curves showing a relationship between endovascular thrombectomy time, D-dimer, age, and DVT. (A) Endovascular thrombectomy time, (B) D-dimer, and (C) Age. ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval.
We then repeated the logistical regression to assess the predicting power of prolonged operative time during EVT (≥65.5 min) and higher plasma D-dimer level (≥1.62 mg/L) for the development of DVT. Interestingly, prolonged operative time and higher plasma D-dimer levels were consistently the independent risk factors for the development of DVT in stroke patients after thrombectomy (Table 5). The odd ratios (>4) were even larger than those calculated from the continuous variables EVT time and D-dimers (see Table 3). Age was excluded from the list of risk factors in this analysis (P > 0.05). Respiratory failure, pulmonary infection, and central venous catheter were not independent predictors for the development of DVT (P > 0.05).
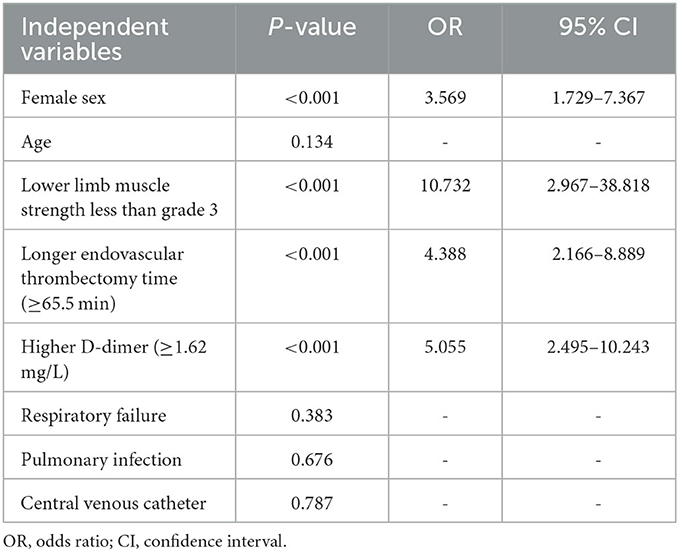
Table 5. Multivariable logistic regression analysis to determine the predictive power of long EVT time and high D-dimer level for DVT.
DVT has been studied as a common complication in stroke patients over the past 50 years (24). In the era of thrombectomy, we are now addressing this old but new question. AIS patients after endovascular thrombectomy were at potentially high risk for DVT. Prolonged surgical time for thrombectomy, along with other known risk factors, such as age, female sex, lower extremity muscle weakness, and higher plasma D-dimer levels, was strongly associated with the development of DVT.
Our study showed that the prevalence of DVT in ischemic stroke patients within 10 days of thrombectomy was 27.3% (67/245), which is higher than the overall prevalence of 11.5 to 22.1% in stroke patients (including ischemic and hemorrhagic stroke, with and without intravenous thrombolysis) within 14 days of disease onset (5–10). Our prevalence included both proximal and distal DVT, and 85% of patients had DVT in the muscular calf veins. Compared with proximal DVT, distal DVT is less likely to cause severe clinical sequelae, i.e., pulmonary embolism (25). However, it does not mean that distal DVT is clinically insignificant. It is still possible for thrombosis in the muscular calf veins to progress further into the deep venous trunk and lead to more severe DVT (26, 27).
Disease severity may be a reason for the higher prevalence of DVT in our study. Our patients had higher NIHSS scores (with a median of 14) compared with other populations studied (5–10). It is widely recognized that paralysis in stroke patients promotes the development of DVT (24). We also observed that lower extremity muscle weakness was the strongest independent predictor of DVT (see Tables 3, 5). The loss of venous pump function caused by muscle contusion may contribute to DVT formation. In clinical practice, we encourage patients to actively move both their paralyzed and non-paralyzed extremities and to get out of bed whenever possible. Additionally, we advise caregivers to passively move the patient's paralyzed extremities.
Given the potential cause–effect relationship between paralysis and DVT, we were curious about whether the immobilization of lower limbs during and after EVT surgery could lead to the development of DVT. Surprisingly, a transfemoral puncture in the right groin did not alter the correlation between the localization of DVT and the side of muscle weakness. This suggests that immobilization of the right lower extremity during and after EVT surgery does not contribute significantly to the formation of DVT. Nevertheless, it is reasonable to choose an optimal vascular access for EVT. For endovascular interventions in patients with acute coronary syndromes or ischemic stroke, the transradial approach has been shown to be a safe alternative to the transfemoral approach (28–30).
As far as we know, we are the first to report that the time from puncture to reperfusion in the EVT procedure is an independent risk factor for the development of DVT in stroke patients, with a longer duration associated with a higher risk. A longer time from puncture to reperfusion in stroke patients has been already associated with poor recovery within 3 months and an increase in malignant cerebral edema and symptomatic intracranial hemorrhage within 24 h after successful EVT (31–33). Our study indicated that prolonged performance of EVT not only affects the brain locally but also systemically affects the coagulation system. The facilitated development of DVT may subsequently hinder the rehabilitation of paralyzed legs and lead to poor recovery of stroke patients described above (31). However, it is unclear whether and how EVT itself promotes thrombosis in stroke patients. Experiments in artificial blood vessels and animal models showed that EVT has the potential to damage endothelial cells and vessel walls, which is exacerbated by repeated stentriever passages (14, 20). The damaged endothelial cells and blood walls release tissue factors and subsequently activate the coagulation cascade (34). Indeed, a small study showed that the procoagulant activity of tissue factor and plasminogen activator inhibitor-1 increased after EVT in patients with ischemic stroke (21).
D-dimer is a small protein fragment present in the blood after a blood clot has been degraded by fibrinolysis. D-dimer is an important diagnostic marker for venous thromboembolism (35). We observed that plasma D-dimer levels increased within 24 h after EVT and independently predicted DVT. The plasma level of D-dimer has been extensively investigated as a predictor for DVT in stroke patients. Interestingly, the cutoff point for D-dimer in predicting DVT in our study (1.62 mg/L) is very similar to the cutoff points (1.52, 1.59, and 1.66 mg/L) found in three other studies that measured D-dimer levels in acute stroke patients upon admission (9, 22, 23). This suggests that a high level of D-dimer in plasma may be a reliable biological marker for diagnosing DVT. It is worth noting that the subjects in these three studies did not undergo EVT, indicating that thrombectomy may not significantly affect the plasma level of D-dimer.
The prevention of venous thromboembolism is challenging. To date, there is no standard clinical practice guideline. The clinical trial of peri-EVT treatment with aspirin and/or unfractionated heparin was discontinued because these measures significantly increased the risk of intracranial hemorrhage (36). We have used intermittent pneumatic compression to prevent DVT in most stroke patients after EVT because this method has been shown to reduce the risk of DVT and potentially improve survival in a large number of patients who are immobile after stroke (37). Indeed, the prevalence of proximal DVT in the stroke patients we studied was very low [of 245 stroke patients after EVT, only one patient developed DVT in the popliteal vein (1/245; 0.41%) and one in the femoral vein (1/245; 0.41%)], compared with published prevalence, e.g., 8.30% (6) and 2.43% (10), which could significantly reduce the life-threatening risk of DVT. However, another study including stroke patients with and without intermittent pneumatic compression is needed to evaluate the therapeutic efficacy of mechanical prophylaxis in preventing DVT after EVT.
Our study has several limitations. First, because of emergent mechanical thrombectomy, ultrasound was not performed prior to EVT to determine whether the patient had already developed DVT at the time of admission. Nevertheless, the observation that the prevalence of DVT was related to the time of thrombectomy is plausible because the prolonged time of thrombectomy was unlikely due to the preexistence of DVT. Our finding is scientifically important because it demonstrated that thrombectomy may induce a thrombotic event although the causal relationship and its clinical significance require further investigation. Second, the study population was chosen from a single institution with a limited number and diversity of patients. Third, it was a retrospective study, and some data may not have been collected. In addition, we did not perform further validation to determine whether people with all these risk factors are more likely to develop DVT.
Our study shows that ischemic stroke patients who receive endovascular mechanical thrombectomy may be at increased risk of developing deep vein thrombosis, especially if they are old (≥60.5 years), female, have weak lower extremity muscles (<grade 3 in the Medical Research Council scale), have had the thrombectomy for a long time (≥65.5 min), and have a high plasma level of D-dimer (≥1.62 mg/L). Prophylaxis for deep vein thrombosis should be given as soon as possible after thrombectomy. However, the results of our study need to be validated by a multicenter prospective study with a larger population of stroke patients.
The original contributions presented in the study are included in the article and Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by Ethics Committee of Taizhou Hospital, Zhejiang Province, China. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin because the project was a retrospective study, collecting data from a digital history recording system.
LH, FW, and YL conceived and designed the study. W-YQ and YL designed the statistical analysis. LH, T-HT, and J-MY collected and analyzed the data and prepared the figure and tables. LH, FW, YL, J-MY, W-YQ, and XP-X wrote and revised the manuscript. All authors approved the final version of the manuscript.
The study was supported by grants from the Zhejiang Provincial Basic and Public Welfare Research Program (Grant Number: LGF21H020005) and the Zhejiang Provincial Medicine and Health Research Foundation (Grant Number: 2021RC141).
The authors thank all patients for contributing to this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1249365/full#supplementary-material
1. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Tu WJ, Zhao Z, Yin P, Cao L, Zeng J, Chen H, et al. Estimated burden of stroke in China in 2020. JAMA Netw Open. (2023) 6:e231455. doi: 10.1001/jamanetworkopen.2023.1455
3. Tu WJ, Wang LD, Yan F, Peng B, Hua Y, Liu M, et al. China stroke surveillance report 2021. Mil Med Res. (2023) 10:33. doi: 10.1186/s40779-023-00463-x
4. McCarthy ST, Turner JJ, Robertson D, Hawkey CJ, Macey DJ. Low-dose heparin as a prophylaxis against deep-vein thrombosis after acute stroke. Lancet. (1977) 2:800–1. doi: 10.1016/S0140-6736(77)90728-0
5. Liu LP, Zheng HG, Wang DZ, Wang YL, Hussain M, Sun HX, et al. Risk assessment of deep-vein thrombosis after acute stroke: a prospective study using clinical factors. CNS Neurosci Ther. (2014) 20:403–10. doi: 10.1111/cns.12227
6. Ha SH, Kim YJ, Heo SH, Chang DI, Kim BJ. Prediction of deep vein thrombosis by ultrasonography and D-dimer in Asian patients with ischemic stroke. BMC Neurol. (2020) 20:257. doi: 10.1186/s12883-020-01842-w
7. Li SY, Feng L, Xiao MJ, Chen SY, He JC, Wang Z. Derivation and validation of a clinical prediction scale for isolated distal deep venous thrombosis in patients after acute ischemic stroke. J Stroke Cerebrovasc Dis. (2017) 26:2087–92. doi: 10.1016/j.jstrokecerebrovasdis.2017.04.027
8. Li F, Wei C, Huo S, Liu X, Du J. Predictors of deep-vein thrombosis for acute stroke at admission to a rehabilitation unit: a retrospective study. Front Neurol. (2023) 14:1137485. doi: 10.3389/fneur.2023.1137485
9. Mori T, Yoshioka K, Tanno Y. Frequency of deep vein thrombosis at admission for acute stroke and associated factors: a cross-sectional study. Thromb J. (2021) 19:62. doi: 10.1186/s12959-021-00315-5
10. Wang Y, Shi Y, Dong Y, Dong Q, Ye T, Fang K. Clinical risk factors of asymptomatic deep venous thrombosis in patients with acute stroke. Clin Appl Thromb Hemost. (2019) 25:1076029619868534. doi: 10.1177/1076029619868534
11. Kelly J, Rudd A, Lewis R, Hunt BJ. Venous thromboembolism after acute stroke. Stroke. (2001) 32:262–7. doi: 10.1161/01.STR.32.1.262
12. Maingard J, Foo M, Chandra RV, Leslie-Mazwi TM. Endovascular treatment of acute ischemic stroke. Curr Treat Options Cardiovasc Med. (2019) 21:89. doi: 10.1007/s11936-019-0781-9
13. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the Early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
14. Teng D, Pannell JS, Rennert RC, Li J, Li Y-S, Wong VW, et al. Endothelial trauma from mechanical thrombectomy in acute stroke: in vitro live-cell platform with animal validation. Stroke. (2015) 46:1099–106. doi: 10.1161/STROKEAHA.114.007494
15. Liu L, Chen W, Zhou H, Duan W, Li S, Huo X, et al. Chinese stroke association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol. (2020) 5:159–76. doi: 10.1136/svn-2020-000378
16. Podlasek A, Dhillon PS, Butt W, Grunwald IQ, England TJ. To bridge or not to bridge: summary of the new evidence in endovascular stroke treatment. Stroke Vasc Neurol. (2022) 7:179–81. doi: 10.1136/svn-2021-001465
17. 中国健康促进基金会血栓与血管专项基金专家委员会[Expert Committee on Special Fund for Thrombosis and Vascular disease of China Health Promotion Foundation]. 医院内静脉血栓栓塞症防治与管理建议[Recommendations for the prevention and management of venous thromboembolism in hospitals]. Natl Med J China. (2018) 98:1383–8. doi: 10.3760/cma.j.issn.0376-2491.2018.18.003
18. Compston A. Aids to the investigation of peripheral nerve injuries. Medical research council: nerve injuries research committee. His majesty's stationery office: 1942; pp. 48 (iii) and 74 figures and 7 diagrams; with aids to the examination of the peripheral nervous system. By Michael O'Brien for the guarantors of brain. Saunders elsevier: 2010; pp. [8] 64 and 94 figures. Brain. (2010) 133:2838–44. doi: 10.1093/brain/awq270
19. Fluss R, Faraggi D, Reiser B. Estimation of the youden index and its associated cutoff point. Biom J. (2005) 47:458–72. doi: 10.1002/bimj.200410135
20. Hernandez D, Cuevas JL, Gramegna LL, Requena M, Pinana C, de Dios M, et al. Increased number of passes and double stent retriever technique induces cumulative injury on arterial wall after mechanical thrombectomy in a Swine model. Transl Stroke Res. (2022) 14:425–33. doi: 10.1007/s12975-022-01044-1
21. Welch JC, Erkmen K, Gentile N. Changes in procoagulant blood biomarkers after mechanical thrombectomy. J Stroke Cerebrovasc Dis. (2021) 30:105772. doi: 10.1016/j.jstrokecerebrovasdis.2021.105772
22. Harvey RL, Roth EJ, Yarnold PR, Durham JR, Green D. Deep vein thrombosis in stroke. the use of plasma D-dimer level as a screening test in the rehabilitation setting. Stroke. (1996) 27:1516–20. doi: 10.1161/01.STR.27.9.1516
23. Balogun IO, Roberts LN, Patel R, Pathansali R, Kalra L, Arya R. Clinical and laboratory predictors of deep vein thrombosis after acute stroke. Thromb Res. (2016) 142:33–9. doi: 10.1016/j.thromres.2016.04.002
24. Warlow C, Ogston D, Douglas AS. Venous thrombosis following strokes. Lancet. (1972) 1:1305–6. doi: 10.1016/S0140-6736(72)91034-3
25. Stein PD, Matta F, Musani MH, Diaczok B. Silent pulmonary embolism in patients with deep venous thrombosis: a systematic review. Am J Med. (2010) 123:426–31. doi: 10.1016/j.amjmed.2009.09.037
26. Zhang P, Bian Y, Xu F, Lian L, Zhu S, Tang Z, et al. The incidence and characteristics of venous thromboembolism in neurocritical care patients: a prospective observational study. Clin Appl Thromb Hemost. (2020) 26:1076029620907954. doi: 10.1177/1076029620907954
27. Henry JC, Satiani B. Calf muscle venous thrombosis: a review of the clinical implications and therapy. Vasc Endovascular Surg. (2014) 48:396–401. doi: 10.1177/1538574414541704
28. Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. (2011) 377:1409–20. doi: 10.1016/S0140-6736(11)60404-2
29. Shaban S, Rastogi A, Phuyal S, Huasen B, Haridas A, Zelenak K, et al. The association of transradial access and transfemoral access with procedural outcomes in acute ischemic stroke patients receiving endovascular thrombectomy: a meta-analysis. Clin Neurol Neurosurg. (2022) 215:107209. doi: 10.1016/j.clineuro.2022.107209
30. Marlowe FJA, Powell E. Evaluating the safety and efficacy of transradial approach for thrombectomy in posterior circulation stroke. a systematic literature review and meta-analysis. Interv Neuroradiol. (2022). doi: 10.1177/15910199221107259
31. Ma G, Yu Z, Jia B, Xian Y, Ren Z, Mo D, et al. Time to endovascular reperfusion and outcome in acute ischemic stroke: a nationwide prospective registry in China. Clin Neuroradiol. (2022) 32:997–1009. doi: 10.1007/s00062-022-01178-7
32. Pu M, Chen J, Chen Z, Li Z, Li Z, Tang Y, et al. Predictors and outcome of malignant cerebral edema after successful reperfusion in anterior circulation stroke. J Stroke Cerebrovasc Dis. (2023) 32:107139. doi: 10.1016/j.jstrokecerebrovasdis.2023.107139
33. Li Y, van Landeghem N, Demircioglu A, Kohrmann M, Dammann P, Oppong MD, et al. Predictors of symptomatic intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke patients with anterior large vessel occlusion-procedure time and reperfusion quality determine. J Clin Med. (2022) 11:7433. doi: 10.3390/jcm11247433
34. Smith SA, Travers RJ, Morrissey JH. How it all starts: initiation of the clotting cascade. Crit Rev Biochem Mol Biol. (2015) 50:326–36. doi: 10.3109/10409238.2015.1050550
35. Kuwashiro T, Toyoda K, Oyama N, Kawase K, Okazaki S, Nagano K, et al. High plasma D-dimer is a marker of deep vein thrombosis in acute stroke. J Stroke Cerebrovasc Dis. (2012) 21:205–9. doi: 10.1016/j.jstrokecerebrovasdis.2010.06.009
36. van der Steen W, van de Graaf RA, Chalos V, Lingsma HF, van Doormaal PJ, Coutinho JM, et al. Safety and efficacy of aspirin, unfractionated heparin, both, or neither during endovascular stroke treatment (MR CLEAN-MED): an open-label, multicentre, randomised controlled trial. Lancet. (2022) 399:1059–69. doi: 10.1016/S0140-6736(22)00014-9
37. Collaboration CT, Dennis M, Sandercock P, Reid J, Graham C, Forbes J, et al. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. (2013) 382:516–24. doi: 10.1016/S0140-6736(13)61050-8
Keywords: acute ischemic stroke, endovascular thrombectomy, deep vein thrombosis, risk factors, D-dimer (DD)
Citation: Han L, Yang J-M, Qian W-Y, Xu X-P, Tung T-H, Liu Y and Wang F (2023) Risk factors for lower extremity deep vein thrombosis in acute stroke patients following endovascular thrombectomy: a retrospective cohort study. Front. Neurol. 14:1249365. doi: 10.3389/fneur.2023.1249365
Received: 28 June 2023; Accepted: 19 September 2023;
Published: 11 October 2023.
Edited by:
Wen-Jun Tu, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Xiuli Yan, First Affiliated Hospital of Jilin University, ChinaCopyright © 2023 Han, Yang, Qian, Xu, Tung, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Wang, d2FuZ2ZlbmdAZW56ZW1lZC5jb20=; Yang Liu, YS5saXVAbXgudW5pLXNhYXJsYW5kLmRl
†These authors have contributed equally to this work and share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.