
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 12 September 2023
Sec. Headache and Neurogenic Pain
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1241676
This article is part of the Research Topic Headache and Neurogenic Pain – Case Report Collection 2022 View all 11 articles
Giant cell arteritis (GCA) is a kind of systemic vasculitis affecting individuals over 50 years old and is often the cause of new-onset headaches in older adults. Patients with GCA sometimes have rheumatic polymyalgia (PMR). The diagnosis of GCA generally depends on clinical manifestation, elevated erythrocyte sedimentation rate (ESR) or C-reactive protein, and positive imaging findings commonly obtained by ultrasound or temporal artery biopsy. In this study, we report a case of an 83-year-old woman with a new-onset headache and an elevated ESR. The result of the temporal artery ultrasound did not distinguish between vasculitis and atherosclerosis. The F18-fluorodeoxyglucose positron emission tomography and computed tomography (18F FDG PET-CT) were performed and suggested large vessel vasculitis with temporal artery involvement. In addition, polyarticular synovitis and bursitis were also revealed. Finally, the diagnosis of secondary headache attributed to CGA complicated with PMR was established. The patient experienced remission of symptoms after glucocorticoid therapy. PET can become a powerful tool for diagnosis and differential diagnosis when the ultrasound result is ambiguous and a biopsy is not obtained.
Giant cell arteritis (GCA) is a kind of systemic vasculitis involving the large and middle arteries, also known as temporal arteritis (1). The age of the population with GCA is generally over 50 years, and the annual incidence among individuals over 50 years old is ~10–20 per 100,000, which is highest in Nordic countries and rare in Asia; the average age at diagnosis is over 70 years (2). The prevalence rate of female patients is higher than that of male patients, and the ratio of male to female is 1:2.5–3 (3). GCA is closely related to rheumatic polymyalgia (PMR); 46–60% of GCA patients have comorbid PMR, and 16–21% of PMR patients have comorbid GCA (4). As the temporal artery and other cranial arteries are commonly involved, GCA is one of the causes of new-onset headache in middle-aged and elderly people (5). Although biopsy is still the golden standard of GCA, its low acceptance of invasive examination limits its uses. If the positive ultrasound result and other supportive evidence are absent, there would be a dilemma for differential diagnosis and optimal treatment. Here, we report a case of GCA complicated with PMR diagnosed by F18-fluorodeoxyglucose positron emission tomography and computed tomography (18F FDG PET-CT). Written informed consent for the personal medical information and images to be published was provided by a legally authorized representative.
The patient, an 83-year-old woman, went to the Neurologic Clinic, at Peking University People's Hospital on May 9, 2022 because of a paroxysmal headache for 10 days. The pain was located in the right temporal region and waxed and waned, sometimes accompanied by congestion and tears in the right eye. She had no fever, no dizziness, no nausea or vomiting, no blurred vision, no jaw and tongue claudication, and no general malaise. Blood pressure was 100/70 mmHg in the left upper limb but undetectable in the right upper limb by electronic sphygmomanometer. There was no obvious tenderness in the region of the right temporal artery. The routine blood examination showed moderate anemia (hemoglobin content of 85 g/L) and an erythrocyte sedimentation rate (ESR) of 101 mm/h. Considering the possibility of a secondary headache, she was admitted to the neurology ward on May 11, 2022. The patient had a history of neurological deafness, diabetes, and hypertension, but no family history of headache or cerebrovascular disease. Two months ago, an ultrasound examination showed that sclerotic plaque was formed at bilateral subclavian arteries with the intima thickened; the distal end of the right subclavian artery was severely stenosed (about 85%), and the distal part of the left subclavian artery was moderately stenosed (60–65%). A neurological physical examination suggested a positive right Babinski and Chaddock sign, no meningeal irritation sign, and other positive signs of the nervous system.
The C-reactive protein (CRP) was elevated (39.1 mg/L). Cytokeratin 19 fragment was slightly elevated (3.32 ng/ml, reference < 3.30 ng/ml) among the tumor marker measurements. The urine and stool routine, blood biochemical analysis, antinuclear antibody, anti-ENA antibody, ANCA, Coomb's test, rheumatoid factor, immunoglobulin, galactomannan test (1,3)-β-D-glucan and galactomannan test, T-cell spot test for tuberculosis infection, PPD test, anti-double-stranded DNA antibody, serum and urine immunofixation electrophoresis, IL-2/4/6/10, IFN-γ, TNF-α, cytomegalovirus, EB virus, and adenovirus nucleic acid detection showed no markedly abnormal findings.
The brain MR (Figures 1A–F) showed several lacunar foci in bilateral basal ganglia and thalamus (especially on the left), leukoencephalopathy, and atrophy. An MRI angiogram (Figure 1G) suggested intracranial arteriosclerosis and mild to moderate stenosis of the M1 segment of the left middle cerebral artery. Temporal artery ultrasound (TAU) showed hypoechogenic non-smooth thickening of the bilateral superficial temporal artery wall with the presence of a few punctate strong echo plaques (Figure 2), which could not tell vasculitis from atherosclerosis clearly. After a consultation with a rheumatologist, the patient underwent an 18F FDG PET-CT examination. The results showed that FDG uptake increased unevenly in the temporal arteries, ascending aorta, aortic arch, brachiocephalic trunk, descending aorta, abdominal aorta, bilateral subclavian arteries, axillary arteries, carotid arteries, femoral arteries, popliteal arteries, and tibial arteries (SUVmax: 1.4–5.3). Vessel walls were slightly thickened, and strip calcification was seen in part of them (Figure 3). In addition, increased FDG uptake (SUVmax: 4.2) could be seen in atlantoaxial, shoulder, wrist, hip, knee, sternoclavicular joints, and lumbar spinous processes, with synovium thickened or synovial capsule enlarged (Figure 4). In the meantime, the patient presented another clinical clue that she had bilateral shoulder joint, knee joint, hip, and waist pain in the past 2 years. Finally, the diagnosis of GCA complicated with PMR was established.
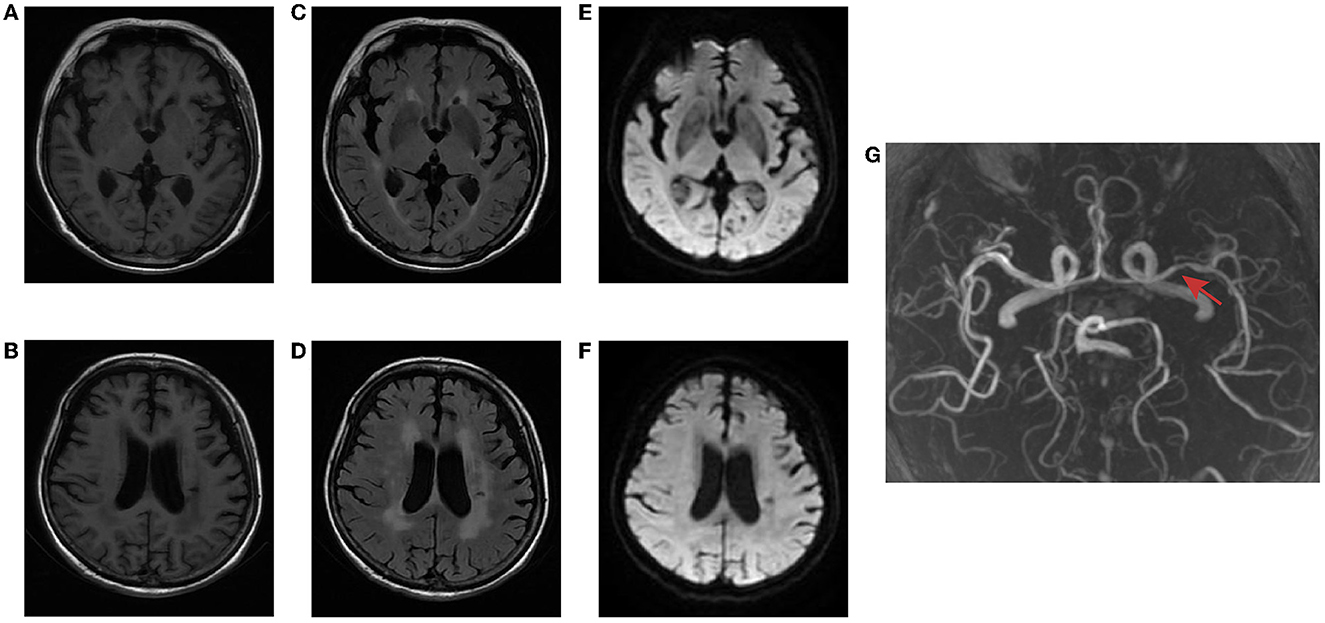
Figure 1. Brain MR of the patient. T1-weighted imaging (A, B) and FLAIR (C, D) showed several lacunar foci in bilateral basal ganglia and thalamus, leukoencephalopathy, and atrophy. No hyperintense on DWI (E, F). MRI angiogram suggested stenosis of the left middle cerebral artery (red arrow) (G).
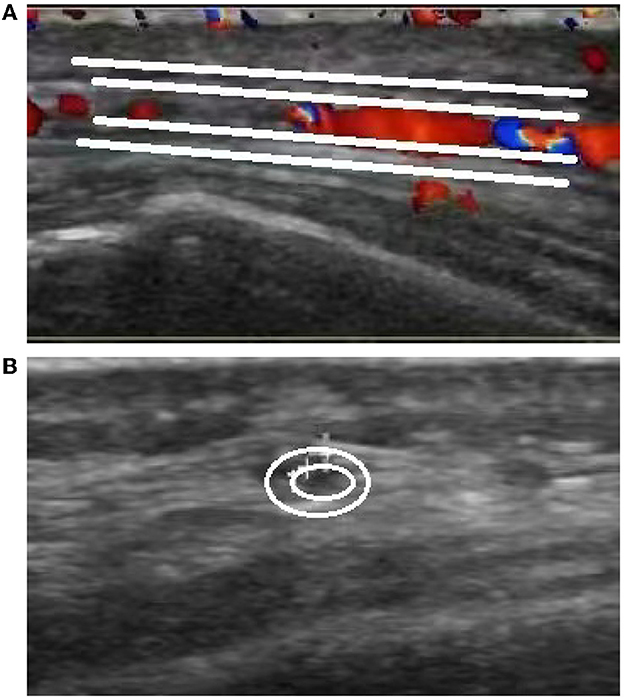
Figure 2. Ultrasound images of the temporal artery. Long-axis section of the temporal artery (A) exhibits irregular wall morphology characterized by thickening and the presence of a few punctate strong echo plaques. Short-axis section of the temporal artery (B) demonstrates non-uniform thickening with a non-smooth appearance.
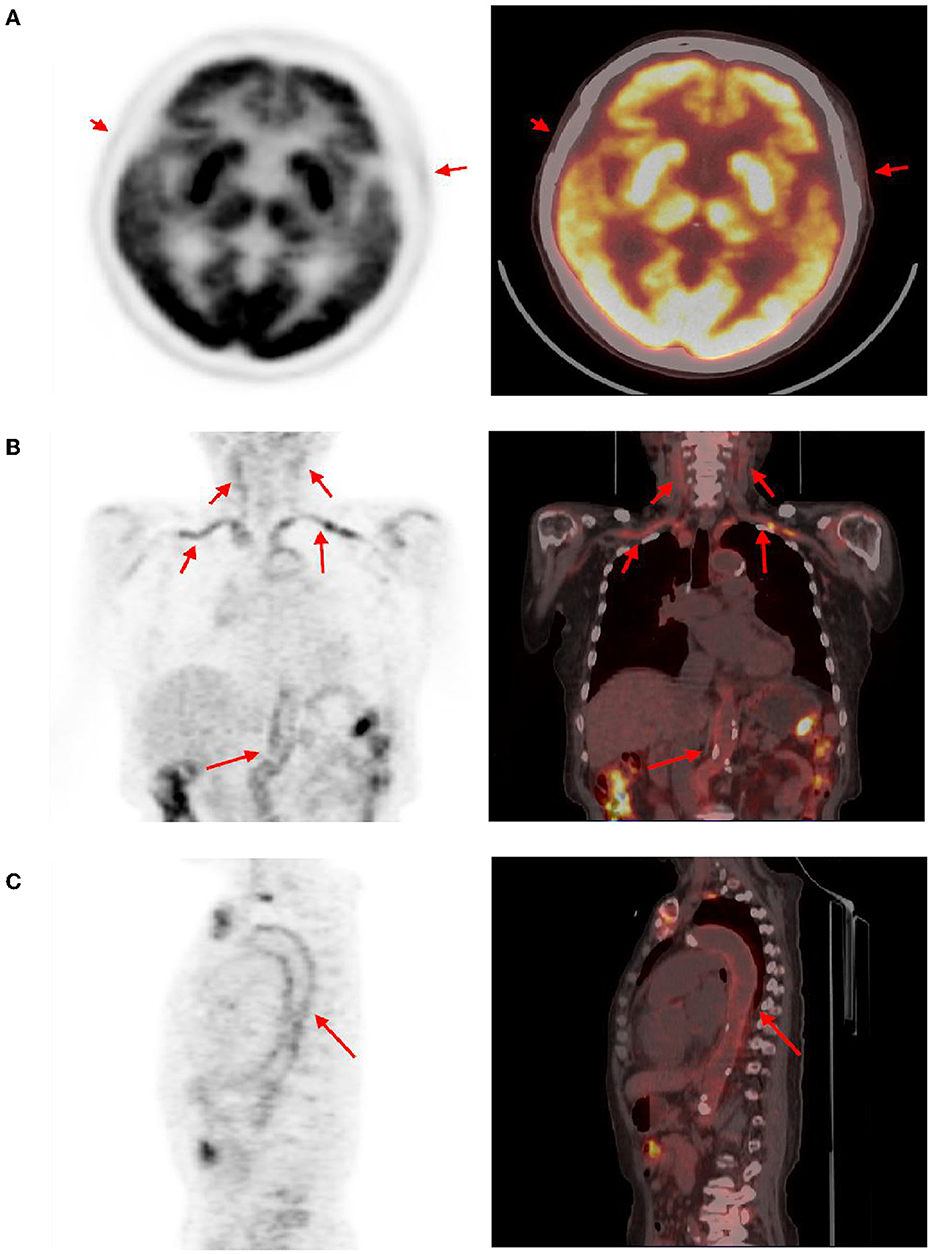
Figure 3. Abnormal findings of arteries in PET-CT. The temporal arteries (A), subclavicular arteries, brachial trunk, axillary arteries, carotid arteries (B), aortic arch, descending aorta, abdominal aorta (C), and several other arteries showed an uneven increase in FDG uptake (red arrow, SUVmax distribution in 1.4–5.3), and corresponding CT showed slightly thicken vascular wall. Calcification can be seen in some artery walls.
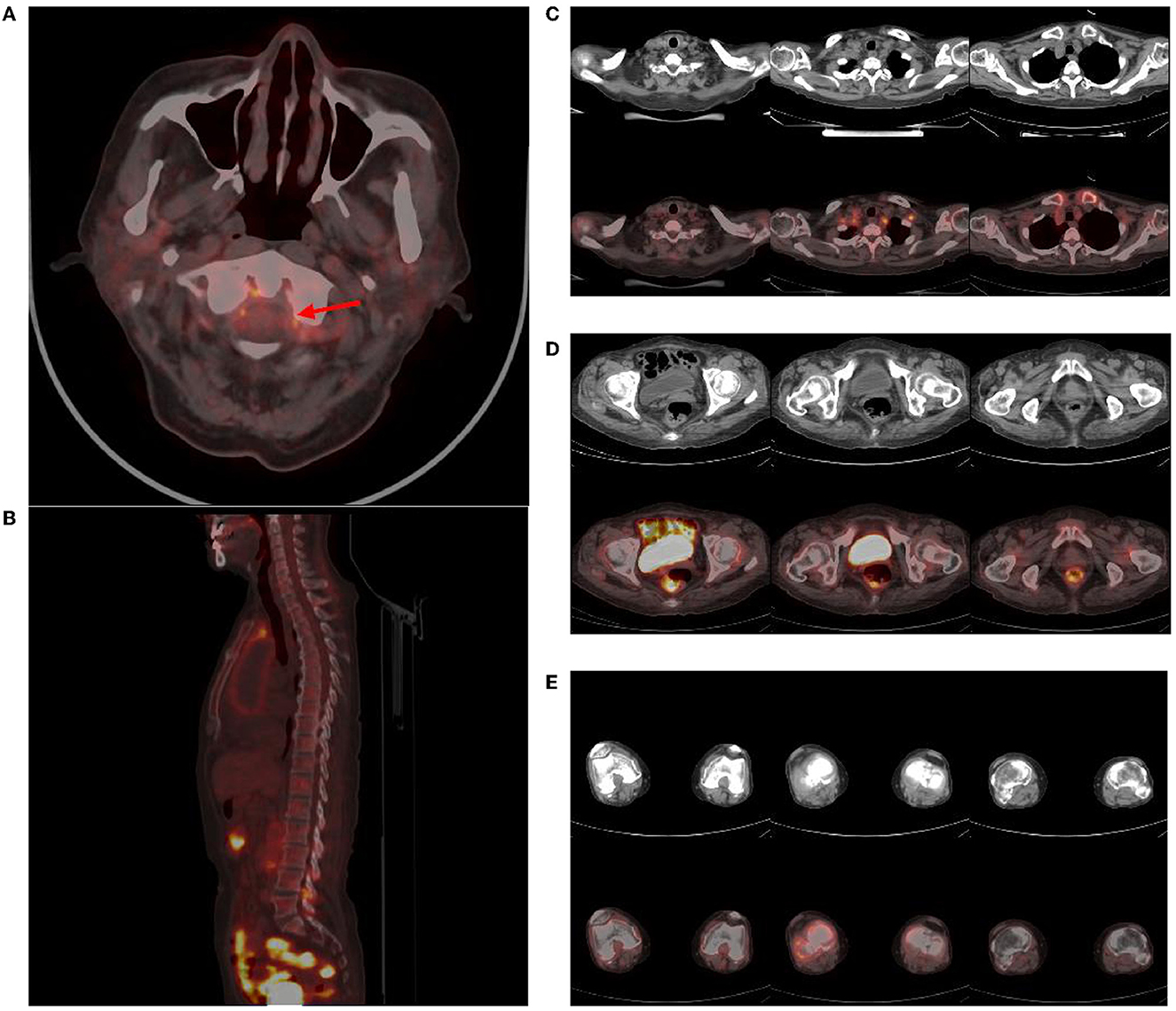
Figure 4. Abnormal findings of joints in PET-CT. Atlantoaxial joint (red arrow) (A), lumbar spinous processes (B), sternoclavicular and shoulder joints (C), hip joints (D), knee joints (E), and several other regions showed an increase in FDG uptake (SUVmax: 4.2), and corresponding CT showed synovial thickening or bursae enlargement.
She received 40 mg of intravenous methylprednisolone daily for 7 days and then 50 mg of oral prednisone daily. The headache and limb pain were significantly relieved. ESR decreased from 101 to 25 mm/h, and CRP decreased from 39.1 to 2.7 mg/L. The disease was kept under good control at follow-up.
This case involved an elderly woman with new-onset headache, accompanied by an elevation of ESR and CRP. Except for the positive right Babinski and Chaddock sign, which could be explained by old lesions of the brain revealed by the MRI, no other positive sign was found. The secondary headache attributed to GCA was suspected. On the basis of the GCA diagnostic criteria put forward by the American College of Rheumatology (ACR) in 1990 (6), ACR and the European League Against Rheumatism (EULAR) introduced the “halo sign” of TAU and high FDG uptake of large arteries in FDG-PET into the diagnostic criteria in 2022. After the introduction of TAU and PET, the new criteria have a sensitivity of 87% and a specificity of 95% (7). A halo sign is defined as a hypo- or iso-echogenic dark aspect of the vessel walls (8). The TAU in this case showed hypoechogenic uneven thickening of the artery wall with punctate plaques, which did not fit the typical halo sign and failed to distinguish between vasculitis and atherosclerosis (9, 10). According to EULAR's recommendations on the application of imaging in the clinical practice of large vessel vasculitis, a temporal artery biopsy (TAB) or further imaging examination was needed to confirm the diagnosis (11).
TAU is convenient and easy to perform, and it is the preferred imaging method when GCA is clinically suspected (11). According to the literature, the halo sign of TAU has a sensitivity of 77% and a specificity of 96% for the diagnosis of GCA (12). TAB is still the gold standard for diagnosing GCA (13), but biopsy is invasive, and its acceptance by patients is low. Pooled data showed that the sensitivity of TAB was similar to TAU findings, but the positive rate of biopsy showed a decreasing trend year by year (14). The patient refused an invasive examination. In recent years, studies have found that FDG-PET had high sensitivity and specificity for the diagnosis of GCA, which were 80–90% and 73–98%, respectively (15). In this case, there was no typical halo sign, which made the involvement of the temporal artery doubtful. In pathological process, functional changes often precede structural changes, and FDG-PET theoretically has a higher sensitivity to early vascular inflammation with only functional involvement. In addition, although ESR was elevated in this elderly woman, other rheumatic and immune-related indicators and pathogenic agent tests were negative. Therefore, the possibility of tumors could not be ruled out, and PET is more helpful for the differential diagnosis. Therefore, PET-CT was finally chosen.
The results of PET-CT showed that the aorta, its main branches, and even the distal arteries showed high uptake of linear FDG. Combined with other clinical features, according to the classification criteria of ACR/EULAR in 2022, the diagnosis of GCA was established (7). Meanwhile, PET-CT revealed no signs of a tumor. Although the result of TAU was ambiguous, abnormal FDG uptake of the temporal artery in PET-CT confirmed that PET could detect vessel wall inflammation before significant changes in anatomical morphology. Therefore, PET can also show the extent of disease involvement earlier and more systematically. In addition, PET can also quantitatively display the inflammation level of the blood vessel wall, and different stages of the disease can be displayed as standard uptake values (SUV) of different degrees on PET, so as to better reflect the activity of the disease (16).
Notably, polyarticular synovitis and bursitis were also detected, suggesting PMR, which was overlooked in the initial clinical inquiry related to headache. PMR lacks specific diagnostic methods, and its diagnosis depends on clinical criteria and exclusion diagnoses. PET can not only detect the presence of GCA but also reveal the abnormal FDG uptake characteristic of PMR (17). The study by Yamashita et al. suggested that when PET showed that two or more parts of the ischial tubercle, greater trochanter, and lumbar spinous process were involved, the sensitivity and specificity of diagnosing PMR were 85.7 and 88.2%, respectively. The sensitivity and specificity of the lumbar spinous process alone can also reach 78.6 and 82.4%, respectively. Other parts, such as sternoclavicular joints, shoulder joints, elbow joints, wrist joints, hip joints, knee joints, and others, also have abnormal manifestations but lack specificity (18). Other studies have also summarized the PET imaging characteristics of PMR (19, 20). Compared to ultrasound, PET imaging results are more specific (21). In this case, several regions including the spinous process of the lumbar spine were involved. Based on the patient's clinical manifestations and hematological indicators, the diagnosis of GCA complicated with PMR was established (22). The patient's response to treatment also supports the diagnosis.
The diagnosis and the treatment process, in this case, were not perfect. It can be seen from the figure that the subject's brain tissue showed high uptake of 18F-FDG, while the walls of the cerebral arteries were too thin to be detectable against the background. The patient's MRA showed stenosis of the left middle cerebral artery, and there were many old lesions in the left cerebral hemisphere. Therefore, it was not clear whether the intracranial artery was involved. Kinoshita et al. reported a case of cerebral infarction caused by GCA with middle cerebral artery involvement. After 3 months of prednisolone therapy, the patient's middle cerebral artery stenosis improved (23). Perhaps follow-up observation or other imaging examinations, such as a high-resolution MRI angiogram, can make up for the defect of PET in the evaluation of intracranial blood vessels.
PET has certain advantages in assisting the diagnosis of GCA and PMR, such as higher sensitivity and specificity, quantitative evaluation of the inflammation level, and evaluation of the disease extent involved. Of course, factors such as high costs and radiation exposure also limit its promotion. Although it cannot be used as a routine inspection, PET can become a powerful tool for diagnosis when the TAU result is negative and the TAB is not agreed upon.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Ethics Committee of Peking University People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
HJ, ZL, and HG contributed to the conception and design of the study. DW wrote the initial manuscript. XZ, LP, and MC contributed to the clinical analysis. LY contributed to the imaging analysis. HJ contributed to the manuscript's revision. All authors read and approved the submitted version.
This study was supported by Liu Zunjing Talent Introduction Research Initiation Fund under Award Number 2022-T-02.
Special thanks are given to Dr. Limin Ren and Dr. Wenxin Cai, rheumatologists at Peking University People's Hospital, who suggested the patient undergo PET-CT and helped us to diagnose giant cell arteritis complicated with rheumatic polymyalgia after the completion of the PET-CT.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. (2003) 349:160–9. doi: 10.1056/NEJMra022694
2. Sharma A, Mohammad AJ, Turesson C. Incidence and prevalence of giant cell arteritis and polymyalgia rheumatica: a systematic literature review. Semin Arthritis Rheum. (2020) 50:1040–8. doi: 10.1016/j.semarthrit.2020.07.005
3. Lacy A, Nelson R, Koyfman A, Long B. High risk and low prevalence diseases: giant cell arteritis. Am J Emerg Med. (2022) 58:135–40. doi: 10.1016/j.ajem.2022.05.042
4. Kermani TA, Warrington KJ. Polymyalgia rheumatica. Lancet. (2013) 381:63–72. doi: 10.1016/S0140-6736(12)60680-1
5. Ward TN, Levin M. Headache in giant cell arteritis and other arteritides. Neurol Sci. (2005) 26(Suppl. 2):s134–7. doi: 10.1007/s10072-005-0426-5
6. Hunder GG, Bloch DA, Michel BA, Stevens MB, Arend WP, Calabrese LH, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. (1990) 33:1122–8. doi: 10.1002/art.1780330810
7. Ponte C, Grayson PC, Robson JC, Suppiah R, Gribbons KB, Judge A, et al. 2022 American College of Rheumatology/EULAR classification criteria for giant cell arteritis. Ann Rheum Dis. (2022) 81:1647–53. doi: 10.1136/ard-2022-223480
8. Noumegni SR, Hoffmann C, Cornec D, Gestin S, Bressollette L, Jousse-Joulin S. Temporal artery ultrasound to diagnose giant cell arteritis: a practical guide. Ultrasound Med Biol. (2021) 47:201–13. doi: 10.1016/j.ultrasmedbio.2020.10.004
9. Greigert H, Ramon A, Tarris G, Martin L, Bonnotte B, Samson M. Temporal artery vascular diseases. J Clin Med. (2022) 11:275. doi: 10.3390/jcm11010275
10. Fernandez-Fernandez E, Monjo-Henry I, Bonilla G, Plasencia C, Miranda-Carus ME, Balsa A, et al. False positives in the ultrasound diagnosis of giant cell arteritis: some diseases can also show the halo sign. Rheumatology. (2020) 59:2443–7. doi: 10.1093/rheumatology/kez641
11. Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. (2018) 77:636–43. doi: 10.1136/annrheumdis-2017-212649
12. Duftner C, Dejaco C, Sepriano A, Falzon L, Schmidt WA, Ramiro S. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: a systematic literature review and meta-analysis informing the EULAR recommendations. RMD Open. (2018) 4:e000612. doi: 10.1136/rmdopen-2017-000612
13. Gribbons KB, Ponte C, Craven A, Robson JC, Suppiah R, Luqmani R, et al. Diagnostic assessment strategies and disease subsets in giant cell arteritis: data from an international observational cohort. Arthritis Rheumatol. (2020) 72:667–76. doi: 10.1002/art.41165
14. Rubenstein E, Maldini C, Gonzalez-Chiappe S, Chevret S, Mahr A. Sensitivity of temporal artery biopsy in the diagnosis of giant cell arteritis: a systematic literature review and meta-analysis. Rheumatology. (2020) 59:1011–20. doi: 10.1093/rheumatology/kez385
15. Slart R, Writing Group, Reviewer Group, Members of EANM Cardiovascular, Members of EANM Infection & Inflammation, Members of Committees, SNMMI Cardiovascular, et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. (2018) 45:1250–69. doi: 10.1007/s00259-018-3973-8
16. Tezuka D, Haraguchi G, Ishihara T, Ohigashi H, Inagaki H, Suzuki J, et al. Role of FDG PET-CT in Takayasu arteritis: sensitive detection of recurrences. JACC Cardiovasc Imaging. (2012) 5:422–9. doi: 10.1016/j.jcmg.2012.01.013
17. Kubota K, Yamashita H, Mimori A. Clinical value of FDG-PET/CT for the evaluation of rheumatic diseases: rheumatoid arthritis, polymyalgia rheumatica, and relapsing polychondritis. Semin Nucl Med. (2017) 47:408–24. doi: 10.1053/j.semnuclmed.2017.02.005
18. Yamashita H, Kubota K, Takahashi Y, Minaminoto R, Morooka M, Ito K, et al. Whole-body fluorodeoxyglucose positron emission tomography/computed tomography in patients with active polymyalgia rheumatica: evidence for distinctive bursitis and large-vessel vasculitis. Mod Rheumatol. (2012) 22:705–11. doi: 10.3109/s10165-011-0581-x
19. Wakura D, Kotani T, Takeuchi T, Komori T, Yoshida S, Makino S, et al. Differentiation between polymyalgia rheumatica (PMR) and elderly-onset rheumatoid arthritis using 18F-fluorodeoxyglucose positron emission tomography/computed tomography: is enthesitis a new pathological lesion in PMR? PLoS ONE. (2016) 11:e0158509. doi: 10.1371/journal.pone.0158509
20. Takahashi H, Yamashita H, Kubota K, Miyata Y, Okasaki M, Morooka M, et al. Differences in fluorodeoxyglucose positron emission tomography/computed tomography findings between elderly onset rheumatoid arthritis and polymyalgia rheumatica. Mod Rheumatol. (2015) 25:546–51. doi: 10.3109/14397595.2014.978936
21. Caporali R, Montecucco C, Epis O, Bobbio-Pallavicini F, Maio T, Cimmino MA. Presenting features of polymyalgia rheumatica (PMR) and rheumatoid arthritis with PMR-like onset: a prospective study. Ann Rheum Dis. (2001) 60:1021–4. doi: 10.1136/ard.60.11.1021
22. Dasgupta B, Cimmino MA, Kremers HM, Schmidt WA, Schirmer M, Salvarani C, et al. 2012 Provisional classification criteria for polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative. Arthritis Rheum. (2012) 64:943–54. doi: 10.1016/j.ymed.2012.09.009
Keywords: headache, giant cell arteritis, rheumatic polymyalgia, PET, case report
Citation: Wang D, Liu Z, Guo H, Yang L, Zhang X, Peng L, Cheng M and Jiang H (2023) Headache attributed to giant cell arteritis complicated with rheumatic polymyalgia diagnosed with F18-fluorodeoxyglucose positron emission tomography and computed tomography: a case report. Front. Neurol. 14:1241676. doi: 10.3389/fneur.2023.1241676
Received: 17 June 2023; Accepted: 14 August 2023;
Published: 12 September 2023.
Edited by:
Simona Sacco, University of L'Aquila, ItalyReviewed by:
Ozlem Coskun, Gazi University, TürkiyeCopyright © 2023 Wang, Liu, Guo, Yang, Zhang, Peng, Cheng and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Jiang, amgxOTkxQHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.