- 1Department of Neurology, County Clinic Hospital, Braşov, Romania
- 2Faculty of Medicine, Transilvania University, Braşov, Romania
- 3Department Basic and Clinical Neuroscience, Parkinson Foundation Centre of Excellence, King's College London, Denmark Hill Campus, King's College Hospital, The Maurice Wohl Clinical Neuroscience Institute, London, United Kingdom
In Parkinson's disease (PD) patients, a wide range of ocular and visual disorders are present. Tear film instability, inflammation and dysfunction of the ocular surface, and the presence of symptoms of visual disturbance characterize dry eye, a multifactorial disease of the ocular surface. Based on a literature search, we discuss the frequency, pathogenesis, and influence on the quality of life of patients with dry eye in Parkinson's disease. Furthermore, we review the available means of diagnosis and management of dry eye. An improvement in awareness and recognition of dry eye is needed to provide suitable, personalized therapeutic options for PD patients, aiming to improve their quality of life, independence, and safety.
Introduction
Parkinson's disease (PD) is a neurodegenerative disorder presenting a wide range of non-motor symptoms including visual disturbances, with important implications for the quality of life of these patients. Visual disturbances in PD range from peripheral to central and include dry eye, diplopia, decreased blink rates, blepharitis, blepharospasm, visual hallucinations, retinal abnormalities, and convergence insufficiency (1). These disturbances lead to the appearance of ocular symptoms such as eye tearing, blurred vision, difficulty with reading, doubling of images, presence of passage hallucinations, impaired contrast sensitivity, and color vision and are frequently interconnected. For example, dopamine depletion and alpha-synuclein aggregation in the cell layers of the intra-retinal region have been shown to lead to a dysfunction in visual processing with impairment in color discrimination, contrast sensitivity, visual acuity, object, and motion perception (2, 3); double vision has been associated with the presence of visual hallucinations and convergence insufficiency (4). Dry eye disease was defined by the 2007 International Dry Eye Workshop (5) as “a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability with potential damage to the ocular surface that is accompanied by increased osmolarity of the tear film and inflammation of the ocular surface”. Symptoms of dry eyes include excess tearing, stinging or burning eyes, foreign body sensation, scratchiness, photophobia, and redness of the eye (6).
Dry eye disease has a prevalence of as high as 70% in PD patients (1, 7). Thus, patients with PD should be considered to be at increased risk of developing dry eyes. The aim of this study was to review the pathogenesis, clinical evaluation, impact on quality of life (QoL), and management of dry eye in Parkinson's disease.
Pathogenesis of dry eye in Parkinson's disease
Dysfunction of the tear-secreting glands and/or disorders of the ocular surface led to dry eye (8). The precorneal tear film is a hydrated gel, with its composition including water, electrolytes, mucins, soluble antimicrobial proteins (lactoferrin and lysosome), immunoglobulins, and growth factors that help regulate cellular processes (9, 10). A superficial lipid layer formed by hydrophilic polar lipids such as phospholipids and ceramides is adjacent to the aqueous-mucin layer (11). The aqueous-mucin layer is anchored by chemical attractions to the superficial corneal epithelium (Figure 1) (12). Its role is to protect and support the ocular surface.
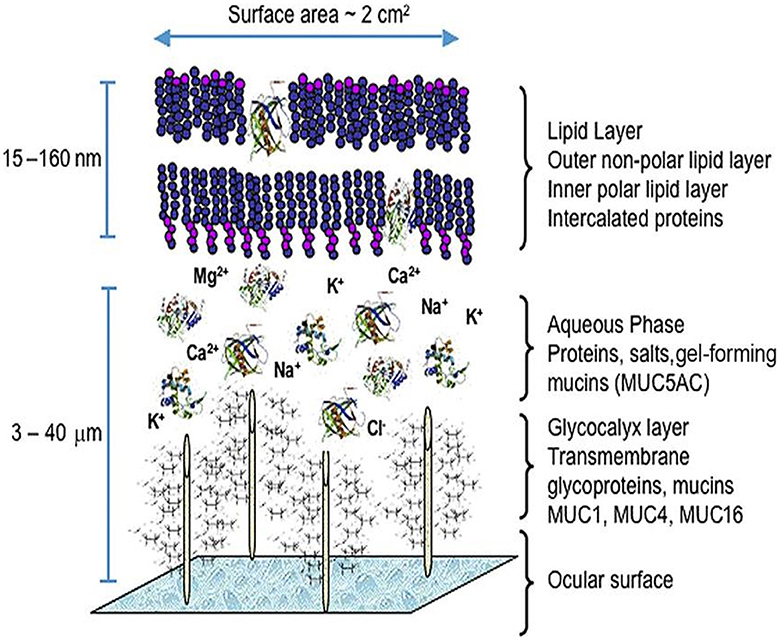
Figure 1. The precorneal tear film model. From Green-Church et al. (90), with permission.
The communication between the ocular surface (cornea and conjunctiva) and the tear-secreting glands (lacrimal glands and meibomian glands) occurs through a neural reflex arc: The sensory afferent information travels through the ophthalmic branch of the fifth cranial nerve (the trigeminal nerve) to the pons where the integration of signals with the input from cortical and other central nervous system centers is made. The efferent nerves are both parasympathetic (seventh cranial nerve—the facial nerve) and sympathetic (paraspinal sympathetic chain) fibers that travel to the lacrimal glands and are responsible for tear secretion (9). A decrease in aqueous tear secretion leads to an increase in tear film osmolarity and chronic inflammation that may severely affect the function and differentiation of the ocular surface epithelium (13).
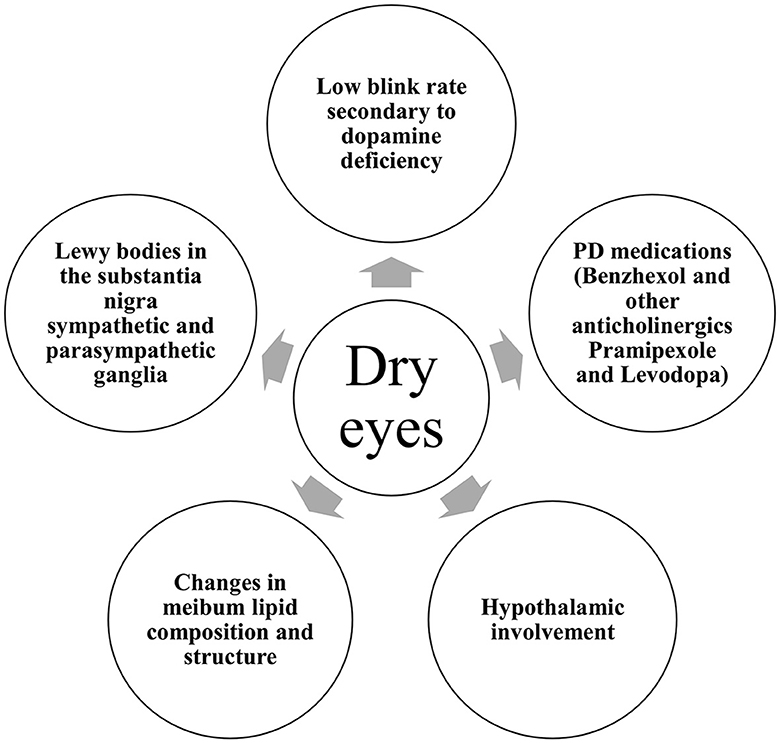
In Parkinson's disease, several mechanisms are incriminated (Figure 2). With disease progression, the accumulation of aggregated alpha-synuclein was hypothesized to spread from the nigrostriatal dopaminergic system to the hypothalamus and neocortex in a caudo-rostral pattern, leading to cell dysfunction, degeneration, and a subsequent decrease in striatal dopamine levels (14). The neurochemical control of blinking is exerted by the dopaminergic, GABAergic, and cholinergic systems of the brainstem. Thus, the decreased levels of dopamine in the central nervous system (CNS) of PD patients give rise to significantly decreased blink rates (15). Blinking is crucial for maintaining an adequate tear film on the surface of the eyes. Second, it is acknowledged that abnormalities in autonomic function are ubiquitous in PD (16). The superior salivatory nucleus and the lacrimal nucleus in the pons give rise to general visceral (parasympathetic and sensory) efferent fibers that are carried to the geniculate ganglion within the intermediate nerve. Preganglionic parasympathetic fibers exit the geniculate ganglion forming the greater superficial petrosal nerve that joins the deep petrosal nerve toward the pterygopalatine ganglion. From there, parasympathetic postganglionic fibers synapse with the lacrimal glands (17). From the hypothalamus, descending autonomic fibers regulated by the ventral striatum and limbic system travel to the superior salivatory nucleus. Thus, the dysfunction of the autonomic system caused by the presence of Lewy bodies in the substantia nigra as well as the sympathetic and parasympathetic ganglia might explain the lacrimation disturbances found in PD patients (18). Furthermore, changes in meibum lipid composition and structure could contribute to the increased susceptibility to dry eye in PD patients (19). Tear proteins involved in lipid metabolism, oxidative stress, and immune response were found to be altered in Parkinson's disease patients (20).
Finally, antiparkinsonian medications were associated with dry eye syndrome (21). Benzhexol, pramipexole, and levodopa are known to cause dryness in mucous membranes due to their anticholinergic effects. Other examples include orphenadrine, benztropine, bornaprine, procyclidine, benapryzine, and methixine (22).
Clinical evaluation of dry eye
Subjective assessment
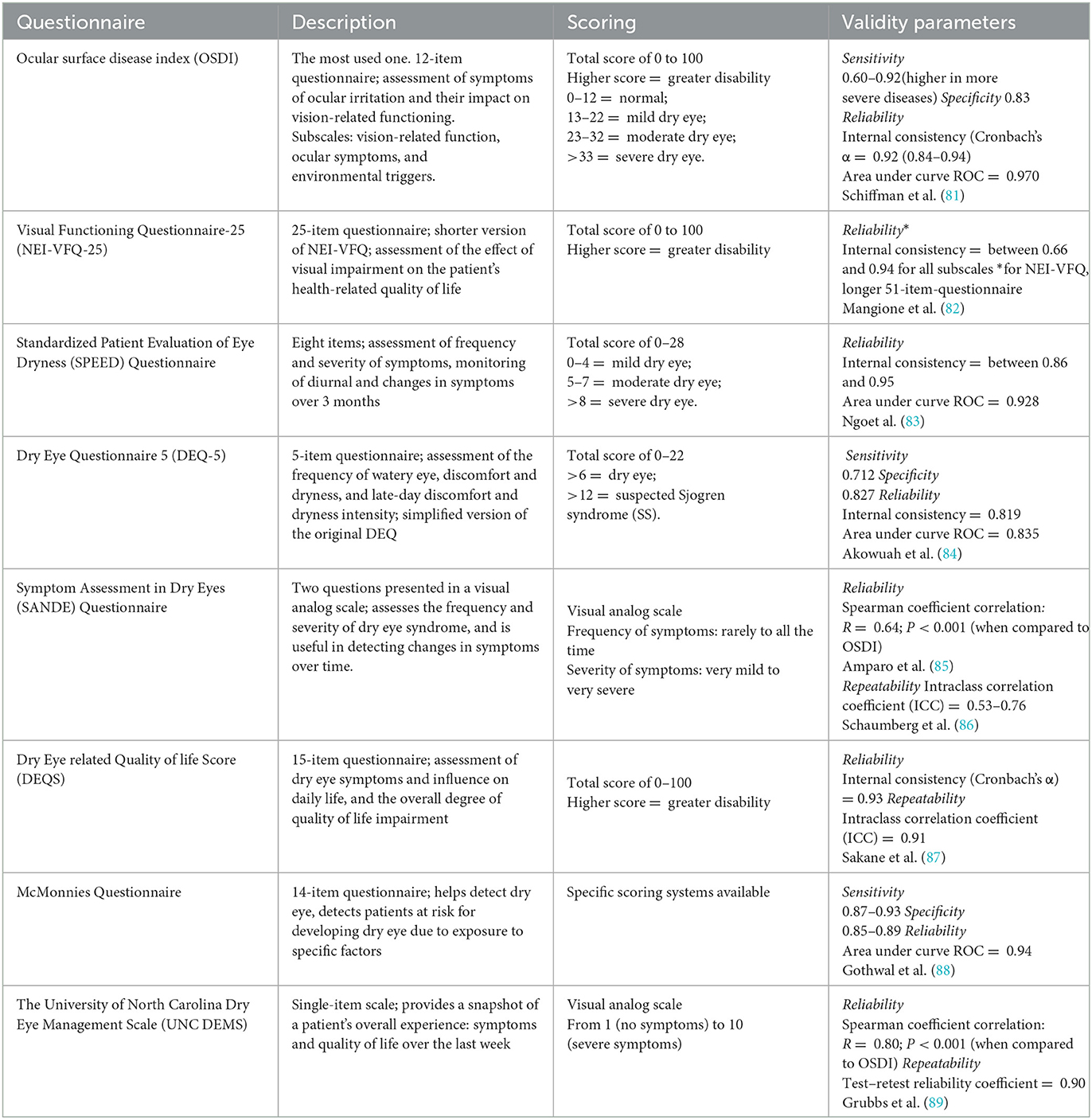
Dry eye can be assessed by using rating scales and questionnaires. The description, scoring system, and validity parameters of questionnaires can be found in Table 1. The Ocular Surface Disease Index (OSDI) is the most commonly used dry eye questionnaire. In PD patients, OSDI scores were significantly higher compared to healthy subjects (7).
Objective assessment
Tear function abnormalities in PD patients have been reported in the literature. Most authors reported more than one abnormal tear film test result, and, in some cases, the results were correlated with disease severity.
Slit lamp examination
A slit lamp examination can be used to diagnose moderate-to-severe dry eye by measuring the upper and lower tear menisci and by assessing the presence and grade of lid-parallel conjunctival folds (LIPCOFs), a sensitive predictor of dry eye. Based on the examination of conjunctival folds in the lower temporal quadrant, a grading system of three degrees has been proposed (23). The LIPCOF degree did not significantly differ between patients with Parkinson's disease and controls in a study by Nowacka et al. (7).
Therefore, more studies are needed in order to assess the usefulness of slit lamp examination for dry eye disease in PD.
Aqueous tear production (Schirmer test)
The insertion of a standardized filter paper strip into the lower conjunctival sac in order to measure the amount of wetting (millimeter units) after a period of 5 min is known as the Schirmer test. The screening threshold for dry eyes is 10 mm, with a value of 5 mm or less confirming the diagnosis (6).
Schirmer test scores were significantly lower in patients with Parkinson's disease than those in controls in a study by Demirci et al. (24) (6.52 ± 2.94 mm/5 min vs. 11.3 ± 6.16 mm/5 min). Similar results were obtained in three other studies (25–27) that followed the corneal parameters in PD patients compared to controls: 6.56 ± 4.75 mm/5 min vs. 12.81 ± 5.68 mm/5 min, 9.08 ± 4.46 mm/5 min vs. 17.16 ± 9.57 mm/5 min, and 4.3 ± 1.8 mm/5 min vs. 9.4 ± 3.0 mm/5 min, respectively. Schirmer's test scores were also found to be significantly affected in patients with PD compared to healthy subjects (13.20 ± 10.45 vs. 17.49 ± 11.16 mm) in a study by Nowacka et al. (7).
The Schirmer test, therefore, is a useful method for evaluating and diagnosing dry eye in Parkinson's disease patients.
Staining of the ocular surface
Vital staining of the ocular surface using different dyes, such as lissamine green, rose bengal, and fluorescein, has been widely used to assess the integrity of the conjunctival and corneal epithelial cells. The damaged epithelial cell stain was in a bright color (green for fluorescein and lissamine green and purple for rose bengal) after the instillation of a drop of dye solution under cobalt-blue-filtered light (28).
Reddy et al. (29) determined the degree of ocular surface staining with rose bengal, lissamine green, and fluorescein sodium in patients with PD and progressive supranuclear palsy (PSP) compared to healthy subjects. A high percentage of PD and PSP patients had abnormal staining compared to healthy controls (none). This is in concordance with a study by Demirci et al. (24) that found higher corneal fluorescein staining in PD patients than that in the control group.
Staining of the ocular surface may prove useful in diagnosing dry eye disease in PD, but more studies are needed.
Tear film stability (tear break-up time)
By applying a fluorescein strip to the lower conjunctival sac and examining it under cobalt-blue-filtered light, the tear break-up time or TBUT can be determined (30). TBUT represents the time measured between the last blink and the appearance of the first dark spot, and a value under 10 s is considered abnormal (31).
TBUT was significantly lower in patients with Parkinson's disease than in healthy subjects in various studies (18, 24, 25, 32). Biousse et al. (1) found that only TBUT was abnormal in PD patients compared to controls in terms of normal rose bengal staining and Schirmer test values. In another study, TBUT was not significantly different between the PD group and the control group, while Schirmer test results and meibomian gland function were significantly affected (7).
Studies are conflicting regarding the use of TBUT in properly diagnosing dry eye syndrome. More studies are needed in order to assess the usefulness of the test for Parkinson's disease patients.
Anterior segment optical coherence tomography
With high-resolution AS-OCT, cross-sectional images of the cornea can be obtained, allowing not only the examination of corneal layers (38) but also the measurement of corneal thickness and cross-sectional area, precise height, and volume of the tear meniscus (33).
Using AS-OCT, Ulusoy et al. (25) measured the thickness of each corneal sublayer in patients with Parkinson's disease in comparison to healthy individuals. They found that the thicknesses of the Bowman and stromal layers were significantly lower in PD patients. Furthermore, stromal thickness was negatively correlated with disease duration and severity and positively correlated with TBUT and Schirmer test scores. They concluded that reduced blinking rates and tear film dysfunction lead to corneal thinning in patients with PD.
Corneal thickness is an important indicator of corneal health. Central corneal thickness (CCT) was found to be significantly decreased in PD patients compared to healthy subjects in several studies (24).
Aksoy et al. (32) reported that the CCT, TBUT, and Schirmer test values decrease in correlation with disease severity (increasing Hoehn–Yahr scores). Demirci et al. (24) found corneal thickness to be significantly correlated with TBUT, blinking rates, and Schirmer test scores in PD patients.
Tamer et al. (18) measured the tear meniscus height in PD patients and controls and found abnormal tear meniscus height in 67.9% of the PD patients recruited in the study. Tear meniscus height was not linearly associated with the disease stage.
The use of optical coherence tomography to measure the thickness of corneal sublayers and tear meniscus proved to be a reliable method in evaluating the presence of dry eyes in Parkinson's disease patients.
Evaluation of blink rates
Blinking is necessary to maintain a healthy and regular tear film. Reduced blinking causes increased evaporation of aqueous components, resulting in subsequent contamination of the mucin layer and thinning of the tear film (34). Blinks are not only reduced but also are less effective with a decrease in amplitude and velocity in PD patients (35, 36). Due to impaired blinking, PD patients are at increased risk for dry eye. Inflammation, impairment of blinking, corneal sensitivity, and decreased tear secretion aggravate dry eye symptoms in PD (37).
PD patients were found to have significantly decreased blinking rates (BRs) compared to healthy controls (1, 18, 24, 27).
Tamer et al. (18) found blinking rates to be inversely correlated with total abnormal tear tests and with disease severity (H-Y scores). This is in concordance with other studies that also found a significant negative correlation between blink rates and H-Y scores in PD patients (24, 25, 32).
Fitzpatrick et al. (38) found significantly decreased blink rates in PD patients compared to healthy individuals during different everyday tasks such as reading a book or watching a video. They found no correlation between BR and disease severity, duration, or treatment.
Decreased blink rates, therefore, could be an important indicator that further tear tests are needed in order to properly diagnose dry eye syndrome in PD patients at risk.
Dry eye staging
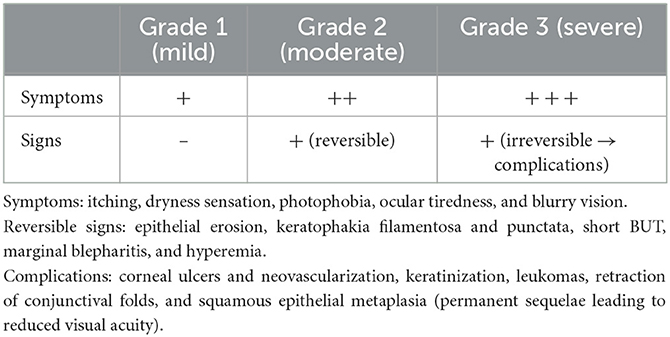
Based on the presence of symptoms and signs, dry eye can be classified into three grades of severity: grade 1 or mild, grade 2 or moderate, and grade 3 or severe (Table 2) (39).
Impact on the quality of life
In Parkinson's disease patients, both motor and non-motor symptoms (including ophthalmological problems) contribute significantly to a decreased quality of life (QoL). PD patients with dry eye experience several symptoms that further worsen QoL. These symptoms include dryness, itching, redness, ocular fatigue and pain, excessive tearing, and decreased visual acuity. The presence of ocular discomfort due to dry eye was associated with greater interference with activities of daily living and with higher scores on the OSDI (40).
In a study by Borm et al. (41), 53% of PD patients reported that the presence of ophthalmologic symptoms had a moderate-to-severe effect on their quality of life, compared with 16% of controls. The greatest interference was experienced while reading, driving a car, watching television, and working on a computer. In another study, Borm et al. (42) measured the impact on daily life using the VFQ-25 (Visual Functioning-25 questionnaire). In total, 44% of PD participants reported poor QoL due to the presence of relevant ophthalmological disturbances. The severity of visual disturbances is also correlated with an increased risk for falls as PD patients compensate for their motor and postural impairments with visual guidance (43, 44).
In patients suffering from dry eye, the prevalence of depression and anxiety is approximately three times higher than that in patients without dry eye disease (45). This is especially important because depression and anxiety are among the most frequently reported neuropsychiatric disturbances in PD with a prevalence of up to 90% (46). Thus, patients with dry eye and Parkinson's disease are at increased risk for depression and anxiety. Treatment of dry eye with over-the-counter lubricants of the ocular surface improved patient-reported satisfaction levels and QoL to as high as 75% for patients with mild symptoms and 65% for patients with severe symptoms (47). However, PD-related motor impairments might interfere with the self-administration of ocular products, with PD patients experiencing limited independence from being unable to handle eye drop instillation themselves, thus further decreasing their quality of life (48).
Management of dry eye in PD
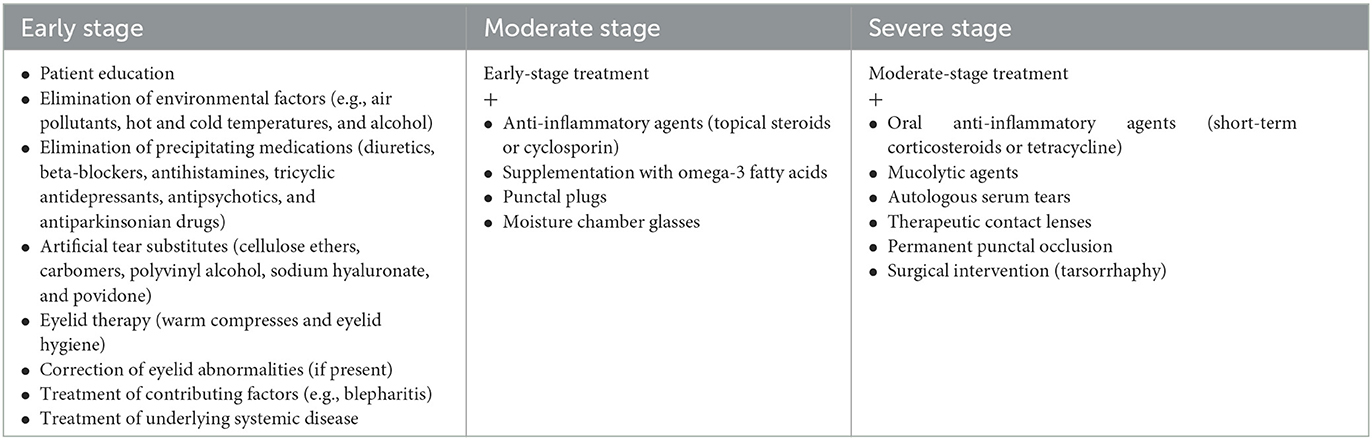
Dry eye management usually begins with conventional over-the-counter ocular surface lubricants in the early stages and can progress to advanced therapies in more severe cases. In some of the cases, new therapies may be added to previous ones to increase the efficacy of the treatment. Treatment options are summarized in Table 3 (6, 49). However, symptomatic treatment of dry eye has not yet been studied, specifically for Parkinson's disease patients.
Patient education
Patient education is an important step in the care management of people with chronic illnesses such as Parkinson's disease because it provides support and information to patients and caregivers while also improving self-care, treatment compliance, patient wellness, and physical function through exercise. Many patient education programs have been developed worldwide with various improvements in QoL in PD patients (50–54).
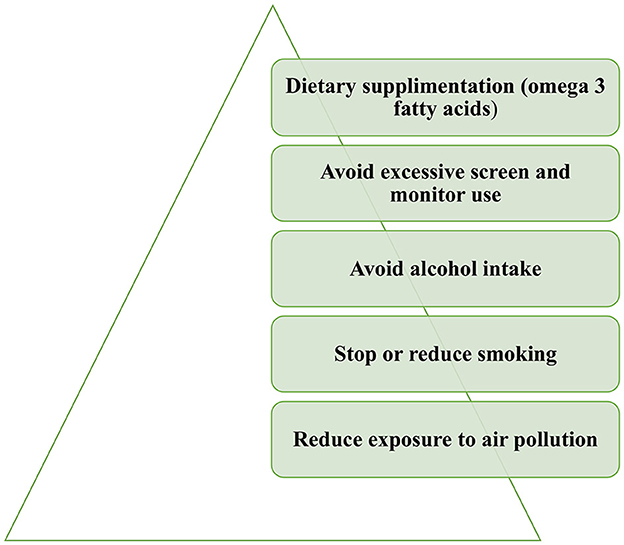
Proper patient education is also essential for dry eye. The implementation of certain lifestyle and behavioral changes could alleviate dry eye symptoms (Figure 3). Exposure to air pollution or other environmental irritants, including tobacco smoke, should be limited. Cigarette smoking was found to have adverse effects on tear protein and on the lipid layer of the tear film and is associated with dry eye (55, 56). Excessive monitor usage should also be avoided. Dietary changes should be implemented with the consumption of omega-3 fatty acids and the limitation of alcohol intake (57).
Elimination of precipitating medications
Antiparkinsonian medications such as levodopa, benzhexol, or pramipexole are known determinants of dry eye. Other implicated medications are also antipsychotics and antidepressants, as depression and psychosis are two major neuropsychiatric comorbidities in PD patients.
While the elimination of precipitating medications is recommended, that is not always the case for Parkinson's disease patients. Antiparkinsonian medication is crucial for the improvement of PD for both motor and non-motor symptoms. Clinicians should consider, if possible, switching between classes of antiparkinsonian medications if a certain treatment is considered to be the cause of dry eye symptoms. For example, amantadine was found to induce corneal endothelial toxicity in a dose-dependent manner (58), thus potentially being involved in treatment-induced dry eye.
On the other hand, levodopa replacement therapy has been shown to improve the blinking rates of PD patients (35). As discussed above, blinking is involved in the pathogenesis of dry eye, thus, an improvement in blinking might alleviate dry eye symptoms for these patients. The idea that patients should consciously increase their blink rates is difficult to achieve in the case of Parkinson's disease.
Artificial tear substitutes
Artificial tear substitutes are inorganic solutions containing electrolytes, surfactants, and viscosity agents that aim to lower the surface tension of the tear film, enhance tear volume by forming a hydrophilic layer on the ocular surface, and prevent bacterial growth, thus reducing the symptoms of dry eye (59). Cellulose ethers, carbomers, polyvinyl alcohol, sodium hyaluronate, or povidone are the main components of most artificial tear substitutes. Each tear substitute has its own properties; hence, treatment should be individualized according to each patient's deficit. In the general population, substitute treatment with added lubricants and osmoprotectants has been shown to increase patient satisfaction levels over a short period of time (60, 61). However, this may not be the case for Parkinson's disease patients. The application of artificial tear substitutes requires increased manual dexterity. As PD patients struggle with fine-motor dexterity tasks due to motor impairments, treatment adherence is expected to be low. It is well-known that treatment burden is a serious issue for both PD patients and their caregivers, causing poor adherence to treatment, poor quality of life, and poor health outcomes (62).
Advanced-stage therapies
In cases where artificial tear substitute treatment is insufficient, topical anti-inflammatory treatment may be efficient. Topical cyclosporine proved to have a high success rate for patients with mild-to-severe dry eye disease (63) and seemed to prevent the progression of dry eye symptoms over a period of 12 months (64). Topical corticosteroids have been reported to reduce corneal fluorescein staining and improve ocular irritation (65). Patients should be monitored, as prolonged treatment with corticosteroids may cause cataract formation and increased intraocular pressure.
In ocular surface diseases, obstruction of the lacrimal drainage to preserve the tears on the ocular surface can be achieved with punctal plugs, which are biocompatible silicone devices. The use of punctal plugs has been shown to decrease the use of tear substitutes and improve symptoms in dry eye patients (66). Complications of punctal plug use include partial migration or extrusion, which can cause local irritation or even canaliculitis and keratitis, loss, epiphora, punctal stenosis, and infectious complications (pyogenic granuloma) (67). Permanent punctal occlusion by laser or thermal cauterization can be beneficial in severe cases. An alternative for permanent punctal occlusion may be labial mucous membrane grafting, especially in patients with conjunctival cicatricial changes (68).
Moisture chamber glasses or spectacles (MCSs) are prosthetic devices that provide a comfortable and moister ocular environment by preventing the evaporation of tears and protecting the eyes from irritants such as wind, dust, or pollen. In a study by Shen et al. (69), significant improvements in ocular comfort and ocular parameters, tear meniscus height (TMH), non-invasive tear break-up time (NI-BUT), and tear film lipid layer thickness were found in the MCS group (dry eye subjects who wore MCSs for a period of 90 min) compared to the control group. MCSs are a feasible, non-invasive, alternative treatment for dry eye, especially for patients exposed to harsh environmental conditions.
The temporal or permanent closure of the eyelids (tarsorrhaphy) can be used in severe, refractory cases of dry eye. It allows a better distribution of the tear film on the surface of the eyes by decreasing the rate of evaporation of the tear film (70). It was proven to be very effective in the management of ocular surface problems, including dry eye, with a success rate as high as 90% and minor complications (71).
These treatments have not been specifically studied for Parkinson's disease patients.
Sjögren's syndrome and Parkinson's disease
Sjögren's syndrome (SS) is an autoimmune disorder characterized by the presence of dry mouth, dry eyes, and recurrent episodes of salivary gland enlargement due to keratoconjunctivitis sicca and focal lymphocytic sialadenitis (72). It has been associated with central nervous system abnormalities such as seizures, cognitive dysfunction, aseptic meningoencephalitis, focal cerebral deficits, multiple sclerosis-like symptoms, and movement disorders (73).
The risk of Parkinson's disease was found to be 1.37 times greater in patients with autoimmune rheumatic diseases than in controls in a nationwide population-based cohort study (74). Furthermore, the incidence of PD was higher in SS patients (2.5%; 215 out of 8,422 patients); thus, primary and secondary SS patients were considered to have a higher risk of developing Parkinson's disease (74). The pathogenesis of this phenomenon is unclear. It is thought to be due to an autoimmune process aimed against the basal ganglia that could involve anti-SSA and SSB antibodies or anti–beta2-glycoprotein IgG antibodies (75, 76).
Several cases of SS associated with Parkinsonism have been described in the literature (75, 77–79). In most cases, antiparkinsonian drugs did not improve the neurological signs and symptoms, while corticosteroid treatment variably improved the symptomatology in some cases.
A diagnosis of Sjögren's syndrome should be considered in patients with Parkinsonian features complaining of xerostomia and dry eye.
We recommend that dry eye assessment, a critical element of vision considered vital in Parkinson's disease, be added to the recently described dashboard system for the vitals of PD patients (80).
Conclusion
In Parkinson's disease patients' dry eye is a frequent complaint and has a negative impact on their health-related quality of life. Reduced blink rates due to dopamine depletion in the CNS, the presence of Lewy bodies in the substantia nigra, sympathetic and parasympathetic ganglia with subsequent autonomic system dysfunction, changes in meibum lipid composition and tear proteins, and changes in PD medications all play a role in the complex pathogenesis of this disorder. Various treatments for dry eye are available, but most of them, if not all, have not been specifically studied for Parkinson's disease patients. Instillation of artificial tear substitutes and removal of incriminated medications may not be feasible in PD patients. The care of these patients should always include an ophthalmologist as part of a multidisciplinary team. More studies are needed to explore this heterogenous syndrome in PD.
Author contributions
LU, SD, and CF-P worked on the conception and design of the article. LU carried out the search and drafted the article. KRC, CF-P, and SD revised the article for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by Grant Asociatia Pro Neurologia 1/2021.
Conflict of interest
CF-P received royalties from Elsevier and Springer Verlag, and honoraria from AbbVie and the International Parkinson's Disease and Movement Disorders Society, outside of the present study. KRC received grants (IIT) from Britannia Pharmaceuticals, AbbVie, UCB, GKC, EU Horizon 2020, Parkinson's UK, NIHR, Parkinson's Foundation, Wellcome Trust, Kirby Laing Foundation, MRC; royalties or licenses from Oxford (book), Cambridge publishers (book), MAPI institute (KPPS, PDSS 2); consulting fees, support for attending meetings or travel, and participated on data safety monitoring board or advisory board for AbbVie, UCB, GKC, Bial, Cynapsus, Lobsor, Stada, Zambon, Profile Pharma, Synovion, Roche, Theravance, Scion, Britannia, Acadia, 4D Pharma, and Medtronic, outside of the present study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Biousse V, Skibell BC, Watts RL, Loupe DN, Drews-Botsch C, Newman NJ, et al. Ophthalmologic features of Parkinson's disease. Neurology. (2004) 62:177–80. doi: 10.1212/01.WNL.0000103444.45882.D8
2. Weil RS, Schrag AE, Warren JD, Crutch SJ, Lees AJ, Morris HR, et al. Visual dysfunction in Parkinson's disease. Brain. (2016) 139:2827–43. doi: 10.1093/brain/aww175
3. Archibald NK, Clarke MP, Mosimann UP, Burn DJ. The retina in Parkinson's disease. Brain. (2009) 132:1128–45. doi: 10.1093/brain/awp068
4. Visser F, Vlaar AMM, Borm CDJM, Apostolov V, Lee YX, Notting IC, et al. Diplopia in Parkinson's disease: visual illusion or oculomotor impairment? J Neurol. (2019) 266:2457–64. doi: 10.1007/s00415-019-09430-w
5. Lemp MA, Baudouin C, Baum J, Dogru M, Foulks GN, Kinoshita S, et al. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international Dry Eye WorkShop. Ocular Surf. (2007) 5:75–92. doi: 10.1016/S1542-0124(12)70081-2
6. Módis L, Szalai E. Dry eye diagnosis and management. Exp Rev Ophthalmol. (2011) 6:67–79. doi: 10.1586/eop.10.89
7. Nowacka B, Lubiński W, Honczarenko K, Potemkowski A, Safranow K. Ophthalmological features of Parkinson disease. Med Sci Monitor. (2014) 20:2243–9. doi: 10.12659/MSM.890861
8. Bron AJ, Yokoi N, Gaffney E, Tiffany JM. Predicted phenotypes of dry eye: proposed consequences of its natural history. Ocular Surf. (2009) 7:78–92. doi: 10.1016/S1542-0124(12)70299-9
9. Pflugfelder SC, Solomon A, Stern ME, Palmer B. The diagnosis and management of dry eye: a twenty-five-year review. Cornea. (2000) 19:644–9. doi: 10.1097/00003226-200009000-00009
10. Van Setten G, Schultz G, Van Setten G, Van G, Schultz SG. Transforming growth factor-alpha is a constant component of human tear fluid. Arch Clin Exp Ophthalmol. (1994) 232:523–6. doi: 10.1007/BF00181994
11. Greiner JV, Glonek T, Korb DR, Booth R, Leahy CD. Phospholipids in meibomian gland secretion. Ophthalm Res. (1996) 28:44–9. doi: 10.1159/000267872
12. Pflugfelder SC, Liu Z, Monroy D, Li DQ, Carvajal ME, Price-Schiavi SA, et al. Detection of sialomucin complex (MUC4) in human ocular surface epithelium and tear fluid. Invest Ophthalmol Vis Sci. (2000) 41:1316–26.
13. Lemp MA, Bron AJ, Baudouin C, Bentez Del Castillo JM, Geffen D, Tauber J, et al. Tear osmolarity in the diagnosis and management of dry eye disease. Am J Ophthalmol. (2011) 151:792–8. doi: 10.1016/j.ajo.2010.10.032
14. Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. (2004) 318:121–34. doi: 10.1007/s00441-004-0956-9
16. van Deursen DN, van den Heuvel OA, Booij J, Berendse HW, Vriend C. Autonomic failure in Parkinson's disease is associated with striatal dopamine deficiencies. J Neurol. (2020) 267:1922–30. doi: 10.1007/s00415-020-09785-5
17. Brackmann DE, Fetterman BL. Cranial nerve VII: facial nerve. In:G. G. Christopher, , editor. Textbook of Clinical Neurology. 3rd ed. W.B. Saunders (Philadelphia, PA), (2007). p. 185–198.
18. Tamer C, Melek IM, Duman T, Öksüz H. Tear film tests in Parkinson's disease patients. Ophthalmology. (2005) 112:1795. doi: 10.1016/j.ophtha.2005.04.025
19. Blinchevsky S, Ramasubramanian A, Borchman D, Sayied S, Venkatasubramanian K. Meibum lipid composition and conformation in parkinsonism. EC Ophthalmol. (2021) 12:20–9.
20. Boerger M, Funke S, Leha A, Roser AE, Wuestemann AK, Maass F, et al. Proteomic analysis of tear fluid reveals disease-specific patterns in patients with Parkinson's disease – A pilot study. Parkinson Relat Disord. (2019) 63:3–9. doi: 10.1016/j.parkreldis.2019.03.001
21. Ekker MS, Janssen S, Seppi K, Poewe W, de Vries N, Theelen MT, et al. Ocular and visual disorders in Parkinson's disease: common but frequently overlooked. Parkinson Relat Disord. (2017) 40:1–10. doi: 10.1016/j.parkreldis.2017.02.014
22. Wong J, Lan W, Ong LM, Tong L. Non-hormonal systemic medications and dry eye. Ocular Surf. (2011) 9:212–26. doi: 10.1016/S1542-0124(11)70034-9
23. Höh H, Schirra F, Kienecker C, Ruprecht KW. Lid-parallel conjunctival folds are a sure diagnostic sign of dry eye. Ophthalmologe. (1995) 92:802–8.
24. Demirci S, Gunes A, Koyuncuoglu HR, Tok L, Tok O. Evaluation of corneal parameters in patients with Parkinson's disease. Neurol Sci. (2016) 37:1247–52. doi: 10.1007/s10072-016-2574-1
25. Ulusoy EK, Ulusoy DM. Evaluation of corneal sublayers thickness and corneal parameters in patients with Parkinson's disease. Int J Neurosci. (2021) 131:939–45. doi: 10.1080/00207454.2020.1761353
26. Sari ES, Koç R, Yazici A, Sahin G, Çakmak H, Kocatürk T, et al. Tear osmolarity, break-up time and schirmer's scores in Parkinson's disease. Turk Oftalmol Dergisi. (2015) 45:142–5. doi: 10.4274/tjo.46547
27. Çomoglu SS, Güven H, Acar M, Öztürk G, Koçer B. Tear levels of tumor necrosis factor-alpha in patients with Parkinson's disease. Neurosci Lett. (2013) 553:63–7. doi: 10.1016/j.neulet.2013.08.019
28. Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. (1995) 21:221–32.
29. Reddy VC, Patel SV, Hodge DO, Leavitt JA. Corneal sensitivity, blink rate, and corneal nerve density in progressive supranuclear palsy and Parkinson disease. Cornea. (2013) 32:631–5. doi: 10.1097/ICO.0b013e3182574ade
30. Gulati A, Dana R. Keratoconjunctivitis sicca: clinical aspects. In:Foster CS, Azar DT, Dohlman CS, , editors. The Cornea (Scientific Foundations and Clinical Practice). Philadelphia, PA: Lippincott Williams & Wilkins (2005). p. 603–27.
31. Lemp MA, Hamill JR. Factors affecting tear film breakup in normal eyes. Arch Ophthalmol. (1973) 89:103–5. doi: 10.1001/archopht.1973.01000040105007
32. Aksoy D, Ortak H, Kurt S, Cevik E, Cevik B. Central corneal thickness and its relationship to Parkinson's disease severity. Can J Ophthalmol. (2014) 49:152–6. doi: 10.1016/j.jcjo.2013.12.010
33. Milner MS, Beckman KA, Luchs JI, Allen QB, Awdeh RM, Berdahl J, et al. Dysfunctional tear syndrome: dry eye disease and associated tear film disorders - new strategies for diagnosis and treatment. Curr Opin Ophthalmol. (2017) 27:3–47. doi: 10.1097/01.icu.0000512373.81749.b7
34. Holly FJ. Physical chemistry of the normal and disordered tear film. Transact Ophthalmol Soc United Kingdom (1962). (1985) 104 (Pt 4):374–80.
35. Agostino R, Bologna M, Dinapoli L, Gregori B, Fabbrini G, Accornero N, et al. Voluntary, spontaneous, and reflex blinking in Parkinson's disease. Mov Disord. (2008) 23:669–75. doi: 10.1002/mds.21887
36. Korošec M, Zidar I, Reits D, Evinger C, VanderWerf F. Eyelid movement during blinking in patients with Parkinson's disease. Mov Disord. (2006) 21:1248–51. doi: 10.1002/mds.20930
37. Örnek N, Da? E, Örnek K. Corneal sensitivity and tear function in neurodegenerative diseases. Curr Eye Res. (2015) 40:423–8. doi: 10.3109/02713683.2014.930154
38. Fitzpatrick E, Hohl N, Silburn P, O'Gorman C, Broadley SA. Case-control study of blink rate in Parkinson's disease under different conditions. J Neurol. (2012) 259:739–44. doi: 10.1007/s00415-011-6261-0
39. Murube J, Németh J, Höh H, Kaynak-Hekimhan P, Horwath-Winter J, Agarwal A, et al. The triple classification of dry eye for practical clinical use. Eur J Ophthalmol. (2005) 15:660–7. doi: 10.1177/112067210501500602
40. Sayegh RR, Yu Y, Farrar JT, Kuklinski EJ, Shtein RM, Asbell PA, et al. Ocular discomfort and quality of life among patients in the dry eye assessment and management study. Cornea. (2021) 40:869–76. doi: 10.1097/ICO.0000000000002580
41. Borm CDJM, Visser F, Werkmann M, De Graaf D, Putz D, Seppi K, et al. Seeing ophthalmologic problems in Parkinson disease: results of a visual impairment questionnaire. Neurology. (2020) 94:e1539–47. doi: 10.1212/WNL.0000000000009214
42. Borm CDJM, Werkmann M, De Graaf D, Visser F, Hofer A, Peball M, et al. Undetected ophthalmological disorders in Parkinson's disease. J Neurol. (2022) 269:3821–32. doi: 10.1007/s00415-022-11014-0
43. Caudron S, Guerraz M, Eusebio A, Gros JP, Azulay JP, Vaugoyeau M, et al. Evaluation of a visual biofeedback on the postural control in Parkinson's disease. Neurophysiol Clin. (2014) 44:77–86. doi: 10.1016/j.neucli.2013.10.134
44. Ivers RQ, Cumming RG, Mitchell P, Attebo K. Visual impairment and falls in older adults: the blue mountains eye study. J Am Geriatr Soc. (1998) 46:58–64. doi: 10.1111/j.1532-5415.1998.tb01014.x
45. Wan KH, Chen LJ, Young AL. Depression and anxiety in dry eye disease: a systematic review and meta-analysis. Eye. (2016) 30:1558–67. doi: 10.1038/eye.2016.186
46. Timmer MHM, van Beek MHCT, Bloem BR, Esselink RAJ. What a neurologist should know about depression in Parkinson's disease. Pract Neurol. (2017) 17:359–68. doi: 10.1136/practneurol-2017-001650
47. Nichols KK, Bacharach J, Holland E, Kislan T, Shettle L, Lunacsek O, et al. Impact of dry eye disease on work productivity, and patients' satisfaction with over-the-counter dry eye treatments. Investig Ophthalmol Vis Sci. (2016) 57:2975–82. doi: 10.1167/iovs.16-19419
48. Guo LW, Akpek EK. The negative effects of dry eye disease on quality of life and visual function. Turk J Med Sci. (2020) 50:1611–5. doi: 10.3906/sag-2002-143
49. American Academy of Ophthalmology Cornea and External Disease Panel. Preferred Practice Pattern. Guidelines for Dry Eye Syndrome. San Francisco, CA: American Academy of Ophthalmology (2013).
50. Montgomery EB, Lieberman A, Singh G, Fries JF, Keller W. Patient education and health promotion can be in Parkinson's disease: a randomized controlled trial. Am J Med. (1994) 97:429–35. doi: 10.1016/0002-9343(94)90322-0
51. Simons G, Thompson SBN, Smith Pasqualini MC. An innovative education programme for people with Parkinson's disease and their carers. Parkinson Relat Disord. (2006) 12:478–85. doi: 10.1016/j.parkreldis.2006.05.003
52. A'Campo LEI, Wekking EM, Spliethoff-Kamminga NGA, le Cessie S, Roos RAC. The benefits of a standardized patient education program for patients with Parkinson's disease and their caregivers. Parkinson Relat Disord. (2010) 16:89–95. doi: 10.1016/j.parkreldis.2009.07.009
53. Macht M, Gerlich C, Ellgring H, Schradi M, Rusiñol ÀB, Crespo M, et al. Patient education in Parkinson's disease: formative evaluation of a standardized programme in seven European countries. Patient Educ Couns. (2007) 65:245–52. doi: 10.1016/j.pec.2006.08.005
54. Lindskov S, Westergren A, Hagell PA. controlled trial of an educational programme for people with Parkinson's disease. J Clin Nurs. (2007) 16:368–76. doi: 10.1111/j.1365-2702.2007.02076.x
55. Altinors DD, Akça S, Akova YA, Bilezikçi B, Goto E, Dogru M, et al. Smoking associated with damage to the lipid layer of the ocular surface. Am J Ophthalmol. (2006) 141:1016–21. doi: 10.1016/j.ajo.2005.12.047
56. Grus FH, Sabuncuo P, Augustin A, Pfeiffer N. Effect of smoking on tear proteins. Graefe's Arch Clin Exp Ophthalmol. (2002) 240:889–92. doi: 10.1007/s00417-002-0539-y
57. Lee BS, Kabat AG, Bacharach J, Karpecki P, Luchs J. Managing dry eye disease and facilitating realistic patient expectations: a review and appraisal of current therapies. Clin Ophthalmol. (2020) 14:119–26. doi: 10.2147/OPTH.S228838
58. Chang KC, Jeong JH, Kim MK, Wee WR, Lee JH, Jeon BS, et al. The effect of amantadine on corneal endothelium in subjects with Parkinson's disease. Ophthalmology. (2010) 117:1214–9. doi: 10.1016/j.ophtha.2009.10.039
59. Prashanth CN, Nagaraju R, Sujatha R. Artificial tear substitutes: which one & when? J. Evol Med Dental Sci. (2013) 2:4332–7. doi: 10.14260/jemds/843
60. Davitt WF, Bloomenstein M, Christensen M, Martin AE. Efficacy in patients with dry eye after treatment with a new lubricant eye drop formulation. J Ocular Pharmacol Ther. (2010) 26:347–53. doi: 10.1089/jop.2010.0025
61. Baudouin C, Cochener B, Pisella PJ, Girard B, Pouliquen P, Cooper H, et al. Randomized, phase III study comparing osmoprotective carboxymethylcellulose with sodium hyaluronate in dry eye disease. Eur J Ophthalmol. (2012) 22:751–61. doi: 10.5301/ejo.5000117
62. Tan QY, Cox NJ, Lim SER, Coutts L, Fraser SDS, Roberts HC, et al. The experiences of treatment burden in people with Parkinson's disease and their caregivers: a systematic review of qualitative studies. J Parkinsons Dis. (2021) 11:1597–617. doi: 10.3233/JPD-212612
63. Perry HD, Solomon R, Donnenfeld ED, Perry AR, Wittpenn JR, Greenman HE, et al. Evaluation of topical cyclosporine for the treatment of dry eye disease. Arch Ophthalmol. (2008) 126:1046–50. doi: 10.1001/archopht.126.8.1046
64. Rao SN. Topical cyclosporine 0.05% for the prevention of dry eye disease progression. J Ocular Pharmacol Ther. (2010) 26:157–63. doi: 10.1089/jop.2009.0091
65. Pflugfelder SC, Maskin SL, Anderson B, Chodosh J, Holland EJ, de Paiva CS, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. (2004) 138:444–57. doi: 10.1016/j.ajo.2004.04.052
66. Marcet MM, Shtein RM, Bradley EA, Deng SX, Meyer DR, Bilyk JR, et al. Safety and efficacy of lacrimal drainage system plugs for dry eye syndrome a report by the american academy of ophthalmology. Ophthalmology. (2015) 122:1681–7. doi: 10.1016/j.ophtha.2015.04.034
67. Best AL Labetoulle M and Legrand M M'garrech M Barreau E Rousseau A. Punctal and canalicular plugs: indications, efficacy and safety. J Francais d'Ophtalmol. (2019) 42:e95–104. doi: 10.1016/j.jfo.2018.12.003
68. Osaki TH, Sant'Anna AE, Osaki MH. An alternative for permanent punctal occlusion: labial mucous membrane graft in the management of severe dry eye. Ophthal Plast Reconstr Surg. (2017) 33:395–96. doi: 10.1097/IOP.0000000000000950
69. Shen G, Qi Q, Ma X. Effect of moisture chamber spectacles on tear functions in dry eye disease. Optomet Vis Sci. (2016) 93:158–64. doi: 10.1097/OPX.0000000000000778
70. Valim V, Trevisani VFM, Sousa de Vilela JM, Belfort VS. Current approach to dry eye disease. Clin Rev Aller Immunol. (2015) 49:288–97. doi: 10.1007/s12016-014-8438-7
71. Cosar CB, Cohen EJ, Rapuano CJ, Maus M, Penne RP, Flanagan JC, et al. Tarsorrhaphy clinical experience from a cornea practice. Cornea. (2001) 20:787–91. doi: 10.1097/00003226-200111000-00002
72. Alexander E, Provost TT. Sjögren's syndrome. Association of cutaneous vasculitis with central nervous system disease. Arch Dermatol. (1987) 123:801–10. doi: 10.1001/archderm.1987.01660300123025
73. Alexander E. Central nervous system (CNS) manifestations of primary Sjögren's syndrome: an overview. Scand J Rheumatol. (1986) 61(Suppl):161–5.
74. Chang CC, Lin TM, Chang YS, Chen WS, Sheu JJ, Chen YH, et al. Autoimmune rheumatic diseases and the risk of Parkinson disease: a nationwide population-based cohort study in Taiwan. Ann Med. (2018) 50:83–90. doi: 10.1080/07853890.2017.1412088
75. Walker RH, Spiera H, Brin MF, Warren Olanow C. Parkinsonism associated with Sjögren's Syndrome: three cases and a review of the literature. Mov Disord. (1999) 14:262–8. doi: 10.1002/1531-8257(199903)14:2<262::AID-MDS1011>3.0.CO;2-6
76. Hassin-Baer S, Levy Y, Langevitz P, Nakar S, Ehrenfeld M. Anti-β2-glycoprotein I in Sjogren's syndrome is associated with parkinsonism. Clin Rheumatol. (2007) 26:743–7. doi: 10.1007/s10067-006-0398-8
77. Visser LH, Koudstaal PJ, Van De Merweb JP. Hemiparkinsonism in a patient with primary Sjögren's syndrome. A case report and a review of the literature. Clin Neurol Neurosurg. (1993) 95:141–5. doi: 10.1016/0303-8467(93)90009-6
78. Nishimura H, Tachibana H, Makiura N, Okuda B, Sugita M. Corticosteroid-responsive parkinsonism associated with primary Sjögren's syndrome. Clin Neurol Neurosurg. (1994) 96:327–31. doi: 10.1016/0303-8467(94)90124-4
79. Créange A, Brugieres P, Voisine MC, Degos JD. Primary Sjögren's syndrome presenting as progressive parkinsonism. Mov Disord. (1997) 12:121–3. doi: 10.1002/mds.870120124
80. Chaudhuri KR, Titova N, Qamar MA, Mură?an I, Falup-Pecurariu C. The dashboard vitals of Parkinson's: not to be missed yet an unmet need. J Pers Med. (2022) 12:1994. doi: 10.3390/jpm12121994
81. Schiffm an RM, Christianson MD, Gordon J, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. (2000) 118:615–21. doi: 10.1001/archopht.118.5.615
82. Mangione CM, Lee PP, Pitts J, Gutierrez P, Berry S, Hays RD, et al. Psychometric properties of the National Eye Institute Visual Function Questionnaire (NEI-VFQ). Arch Ophthalmol. (1998) 116:1496–504. doi: 10.1001/archopht.116.11.1496
83. Ngo W, Situ P, Keir N, Korb D, Blackie C, Simpson T, et al. Psychometric properties and validation of the standard patient evaluation of eye dryness questionnaire. Cornea. (2013) 32:1204–10. doi: 10.1097/ICO.0b013e318294b0c0
84. Akowuah PK, Adjei-Anang J, Nkansah EK, Fummey J, Osei-Poku K, Boadi P, et al. Comparison of the performance of the dry eye questionnaire (DEQ-5) to the ocular surface disease index in a non-clinical population. Contact Lens Anterior Eye. (2022) 45:101441. doi: 10.1016/j.clae.2021.101441
85. Amparo F, Schaumberg DA, Dana R. Comparison of two questionnaires for dry eye symptom assessment: the ocular surface disease index and the symptom assessment in dry eye. Ophthalmology. (2015) 122:1498–503. doi: 10.1016/j.ophtha.2015.02.037
86. Schaumberg DA, Gulati A, Mathers WD, Clinch T, Lemp MA, Nelson JD, et al. Development and validation of a short global dry eye symptom index. Ocular Surf. (2007) 5:50–7. doi: 10.1016/S1542-0124(12)70053-8
87. Sakane Y, Yamaguchi M, Yokoi N, Uchino M, Dogru M, Oishi T, et al. Development and validation of the dry eye-related quality-of-life score questionnaire. JAMA Ophthalmol. (2013) 131:1331–8. doi: 10.1001/jamaophthalmol.2013.4503
88. Gothwal VK, Pesudovs K, Wright TA, McMonnies CW. McMonnies questionnaire: enhancing screening for dry eye syndromes with rasch analysis. Investig Ophthalmol Vis Sci. (2010) 51:1401–7. doi: 10.1167/iovs.09-4180
89. Grubbs J, Huynh K, Tolleson-Rinehart S, Weaver MA, Williamson J, Lefebvre C, et al. Instrument development of the UNC dry eye management scale. Cornea. (2014) 33:1186–92. doi: 10.1097/ICO.0000000000000243
90. Green-Church KB, Butovich I, Willcox M, Borchman D, Paulsen F, Barabino S, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid protein interactions in health and disease. Invest Ophthalmol Vis Sci. (2011) 52:1979–93. doi: 10.1167/iovs.10-6997d
Keywords: dry eye, Parkinson's disease, keratoconjunctivitis sicca, visual disturbances, diagnosis, management
Citation: Ungureanu L, Chaudhuri KR, Diaconu S and Falup-Pecurariu C (2023) Dry eye in Parkinson's disease: a narrative review. Front. Neurol. 14:1236366. doi: 10.3389/fneur.2023.1236366
Received: 07 June 2023; Accepted: 14 July 2023;
Published: 01 August 2023.
Edited by:
Pedro J. Garcia-Ruiz, University Hospital Fundación Jiménez Díaz, SpainReviewed by:
Takayasu Mishima, Fukuoka University, JapanPravin Khemani, Swedish Medical Center, United States
Copyright © 2023 Ungureanu, Chaudhuri, Diaconu and Falup-Pecurariu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Larisa Ungureanu, dW5ndXJlYW51ZWxlbmFsYXJpc2FAZ21haWwuY29t
 Larisa Ungureanu
Larisa Ungureanu K. Ray Chaudhuri
K. Ray Chaudhuri Stefania Diaconu
Stefania Diaconu Cristian Falup-Pecurariu
Cristian Falup-Pecurariu