
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 21 September 2023
Sec. Endovascular and Interventional Neurology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1226455
This article is part of the Research Topic Moyamoya disease – natural history and therapeutic challenges View all 9 articles
Objective: Regional cerebral oxygen saturation (rSO2) is linked with blood pressure. This study evaluated the influence of perioperative rSO2 monitoring on the prognosis of ischemic Moyamoya disease (MMD) patients undergoing anastomosis surgery.
Methods: In this prospective cohort, patients with unilateral ischemic MMD of Suzuki stage ≥3 were included. The decision of rSO2 was made by the clinician and the patient. The rSO2 group maintained intraoperative rSO2 levels through the modulation of blood pressure, inhaled oxygen concentration, carbon dioxide in arterial blood, and red blood cell transfusion. The non-rSO2 group used conventional anesthesia practices. Perioperative mean arterial pressure (MAP), rSO2 values, neurological complications, and postoperative results were assessed.
Results: A total of 75 eligible patients were categorized into a rSO2 monitoring group (n = 30) and a non-rSO2 monitoring group (n = 45). For the rSO2 group, the preoperative rSO2 was significantly lower on the affected side (P < 0.05). After anastomosis, this value notably increased (P = 0.01). A moderate relationship was observed between perioperative rSO2 and MAP before, during, and after surgery, with correlation coefficients (r) of 0.536, 0.502, and 0.592 (P < 0.05). Post-surgery MAP levels differed between the groups, with the rSO2 group showing decreased levels compared to pre-surgery and the non-rOS2 group displaying elevated levels. Notably, the rSO2 group reported shorter hospitalizations and decreased neurological complications. Patients with a hypertension history found postoperative MAP influencing hospital stay duration.
Conclusion: Perioperative rSO2 surveillance enhanced cerebral perfusion and minimized postoperative complications in ischemic MMD patients. Thus, rSO2 monitoring is advocated for MMD patients undergoing vascular anastomosis.
Moyamoya disease (MMD) is a chronic vascular brain disorder marked by progressive stenosis or occlusion of crucial cerebral arteries. Its clinical presentation is varied, including epilepsy, cognitive dysfunction, headache, and cerebral ischemia (1). The disease affects 1.14 out of 100,000 people in China (2), with women being twice as susceptible as men (3). It typically presents in children at ~5 years of age and adults in their 40s (4).
Surgery is the predominant treatment for MMD (5), with the superficial temporal anterior-middle cerebral arterial anastomosis being the standard approach for ischemic MMD (6). This surgery promotes blood supply from the unaffected parietal branch of the superficial temporal artery to the ischemic brain hemisphere (7), enhancing perfusion and setting up collateral circulation (8). However, perioperative complications such as cerebral hemorrhage and infarction remain a concern. It is essential to balance oxygen supply and demand in the brain's affected area during the perioperative period and swiftly address intraoperative cerebral hypoxia to prevent stroke (9). Traditionally, anesthesiologists have relied on experience for perioperative management, using preoperative mean arterial pressure (MAP), partial pressure of carbon dioxide in arterial blood (PaCO2), and other cerebral perfusion indicators. The perioperative MAP should be kept roughly 10% above the preoperative level to boost cerebral perfusion during vascular anastomosis (10). However, post-anastomosis, the affected brain region's blood flow can surge, risking vessel rupture and hemorrhage (11). Notably, if the empirical systolic blood pressure remains under 130 mmHg post-surgery and postoperative brain tissue perfusion is not improved (12), it fails to ameliorate the affected side's cerebral tissue perfusion. This can lead to complications such as hyperperfusion syndrome (CHS) (13), cerebral hemorrhage (14), and infarction (15). Thus, managing blood pressure is paramount during the perioperative phase (16).
Regional cerebral oxygen saturation (rSO2) offers non-invasive, real-time monitoring of local cerebral blood perfusion. It has been employed in various surgeries, including cardiac, orthopedic, and neurosurgery, displaying remarkable accuracy in gauging oxygen saturation and minimizing postoperative neurological complications (17). Prior research indicates that rSO2 reliably represents cerebral perfusion in MMD patients and can predict postoperative outcomes such as delirium, cerebrovascular reactive delirium (18), and CHS (19, 20). However, the utilization of rSO2 for perioperative blood pressure monitoring in MMD patients remains under-explored (1).
This study aimed to assess the impact of rSO2 monitoring in MMD patients undergoing unilateral superficial temporal artery to middle cerebral artery branch anastomosis.
This prospective cohort study included 110 patients with ischemic MMD who underwent direct anastomosis between the superficial temporal artery and the middle cerebral arterial branch for the first time between January 2019 and July 2021 in the Neurosurgery Department of Peking University International Hospital. The ethics committee of the university approved the study, and all patients provided signed informed consent for their participation.
The inclusion criteria were as follows: (1) patients with unilateral ischemic MMD seen on cerebral angiography; (2) patients without intracranial space-occupying lesions, cerebral hemorrhage, aneurysms, massive cerebral infarction, and other related diseases detected on CT imaging; (3) patients aged between 18 and 65 years and with any gender; (4) patients with ASA physical status classification scores of I and II; (5) patients with the Suzuki staging of ≥3 calculated on digital subtraction angiography (DSA) and the score of Mini-mental State Examination (MMSE) of >24 points; and (6) patients who furnished signed informed consent. The exclusion criteria were as follows: (1) patients who experienced severe immune system diseases or severe organ dysfunction, such as heart, liver, and kidney dysfunctions; (2) patients who underwent prior craniocerebral surgery; (3) patients with hemorrhagic MMD; (4) patients who had severe stenosis or occlusion of the subclavian artery and carotid artery; (5) patients with frontal skin infections in which the measuring of rSO2 could not have been done; (6) patients with bilateral ischemic MMD; and (7) changed surgical protocol or patients who withdrew voluntarily.
Seventy-five patients were included in the study analysis. The decision was made by the clinician and the participants whether monitoring of rSO2 is needed or not. The participants were divided into two groups according to whether rSO2 was monitored or not: the rSO2 monitoring group (the rSO2 group) and the non-rSO2 monitoring group (the non-rSO2 group).
Midazolam at a dosage of 0.02 mg/kg, propofol at a dosage of 1.5 mg/kg, sufentanil at a dosage of 0.4 μg/kg, and rocuronium at a dosage of 1 mg/kg were used for inducing anesthesia in both groups. When the bispectral index (BIS) reached 40–60 (the electrode was placed on the forehead contralateral side), endotracheal intubation and mechanical ventilation were connected. The end-tidal expiratory carbon dioxide pressure (PetCO2) was maintained between 35 and 45 mmHg by adjusting tidal volume and respiratory rate, and the inhaled oxygen concentration was adjusted to 60%. Anesthesia was maintained by intravenous infusion of propofol at the rate of 3–4 mg/(kg·h), remifentanil at the rate of 0.1–0.2 μg/(kg·min), continuous inhalation of sevoflurane at a percentage concentration of 0.8%−1.0%, and BIS was maintained between 40 and 60. During the operative procedure, rocuronium was given intravenously, and 10 μg sufentanil was given at the time of suturing the scalp. After completion of the scalp suture, all anesthetic drugs were withdrawn.
Fluid access was established, and the Allen test was negative on the right/left hand. Subsequently, the radial artery puncture catheterization was performed, and the MAP (intraoperative MAP), ECG, SPO2, body temperature, and BIS were determined. The rSO2 (Medtronic 5100C, America) was monitored in the rSO2 group. In the rSO2 group, rSO2 and MAP were monitored 1 day before surgery in the ward. The same trained anesthesiologist performed all rSO2 monitoring and postoperative follow-up. Method of rSO2 monitoring: alcohol was applied to the forehead for sterilization and dehydration. Then, patches for measuring rSO2 were placed on the forehead 1 cm away from the middle of the forehead and 1–2 cm above the eyebrow arch, and the rSO2 monitor was connected. The baseline rSO2 values were calibrated and monitored continuously for 3 h after stabilization of values for 5 min. MAP was measured from the right upper limb, with an interval of 5 min, and monitored for 3 h. The monitoring was performed on alternate days after entering the operating room. During surgery, blood pressure was adjusted in the rSO2 group with the observation of rSO2. The objective of monitoring rSO2 was to maintain no more than a 20% decrease in the baseline value or no more than a 55% decrease in the baseline absolute value during surgery.
When the rSO2 was lower than this level, the following treatment measures were adopted (21): (1) The connection and position of the rSO2 patch were checked; (2) The blood pressure was increased; (3) The concentration of inhaled O2 was raised; (4) If the above treatment measures did not improve the rSO2, blood gas was analyzed, and if anemia was present (<80 g/L), 2–4 U suspended red blood cells were transfused based on the oxygen saturation. The rSO2 was monitored for three consecutive days following the surgery. On D1, D2, and D3, all data were collected by the same anesthesiologist. In the non-rSO2 group, the anesthesiologist maintained the MAP before vascular anastomosis ~10% above the baseline value of the preoperative MAP. The intraoperative MAP was collected by the monitor. If the blood pressure decreased during the operation, ephedrine of 3–6 mg intravenously was administered to achieve the blood pressure rapidly, and also, noradrenaline was continuously administered to maintain the target blood pressure level. The endotracheal cannula was removed after the patient recovered from anesthesia, and the patient returned to the ward. For three consecutive days after surgery (D1, D2, and D3), patients in both groups were continuously monitored for 8 h (8 am to 4 pm) based on MAP or rSO2.
The perioperative period (22) is referred to 7 days before surgery to 7 days after surgery. The outcome indicators were the postoperative cerebral hemorrhage, cerebral infarction, and incidence of CHS in the two groups.
The clinical manifestations of cerebral hemorrhage and CHS were similar, such as postoperative headache, seizure, and focal neurological deficit. (1) Diagnosis of cerebral hemorrhage: new intracranial blood was seen on a postoperative craniocerebral CT scan. (2) Diagnosis of CHS: (a) The diagnosis was done by observation of local neurological dysfunction and epilepsy. (b) CT/MRI perfusion imaging and SPECT scans showed that blood perfusion at the bypass site increased from the lowest value before surgery to the highest value after surgery and was locally clustered. (c) The diagnosis was performed by excluding local muscle swelling and compression (23). (3) Diagnosis of cerebral infarction: postoperative craniocerebral CT or MRI images showed new cerebral ischemic lesions. All patients received an MRI for outcome evaluation. The postoperative complications of all patients were analyzed by the same senior neurosurgeon and radiologist.
Data were obtained from the medical records, anesthesia information system, and rSO2 monitors of Peking University International Hospital. The general characteristics of 75 patients were collected, and operation time and MAP values were recorded for the preoperative period (3 h on the day before the surgery), intraoperative period (from the time when the skin was incised to the time of the end of suturing), postoperative period (from the end of the surgery to the time when leaving the surgery room), and postoperative three consecutive days (D1, D2, and D3). Hemoglobin content from the blood gas analysis before and after surgery was recorded for the two groups. The perioperative rSO2 mean values (D1, D2, and D3) were recorded in the rSO2 group. The data of cerebral infarction, CHS, cerebral hemorrhage, reoperation, and death were recorded from the postoperative period to the time of discharge.
SPSS version 20.0 statistical software was used to analyze the data. The Shapiro–Wilk test evaluated the normal distribution. The measurement data were expressed as mean ± standard deviation (mean ± SD). If they did not fit the normal distribution, the quartile method M (P25 and P75) was used. The intra-group comparison was performed by t-test and repeated measure analysis of variance between the two groups. For comparing the measurement data between groups, repeated measurement analysis of variance was used for complex conditions. The least significant difference (LSD) method was used for the comparison between the groups and the Kruskal–Wallis H method was used for testing if the application conditions were not met. Counting data were expressed as frequencies and percentages and compared using the chi-square tests. The Kolmogorov–Smirnov test was used to evaluate the data distribution. The Pearson method was used for correlation analysis. An alpha value of 0.05 and a P-value of <0.05 were considered to be statistically significant differences.
In this study, 110 patients were enrolled, and 15 patients were excluded due to severe immune system diseases or severe organ dysfunction (n = 2), craniocerebral surgery (n = 2), hemorrhagic MMD (n = 3), severe stenosis or occlusion of the subclavian artery and carotid artery (n = 3), frontal skin infections (n = 3), and bilateral ischemic Moyamoya disease. Finally, 95 patients were screened and divided into the rSO2 group (n = 38) and the non-rSO2 group (n = 57) according to whether the rSO2 was monitored or not. In the rSO2 group, the surgical protocol was changed in two patients, and six patients withdrew voluntarily from the study. In the non-rSO2 group, the surgical protocol was modified in seven patients, and five patients withdrew voluntarily from the study. Finally, 38 patients were included in the rSO2 group and 45 in the non-rSO2 group. The flow chart for the selection of patients is shown in Figure 1.
Seventy-five patients were included in the analysis, and all underwent direct anastomosis of the unilateral superficial temporal artery and middle cerebral arterial branch. The mean age of the patients in the rSO2 group was 47.33 ± 7.52 years, and the preoperative MAP was 97.28 ± 11.67 mmHg. The mean age of the patients in the non-rSO2 group was 46.30 ± 7.80 years, and the preoperative MAP was 98.57 ± 9.92 mmHg. A statistical difference in the general characteristics of patients was found between the two groups, except for gender (P > 0.05), as shown in Table 1.
The length of stay in the rSO2 group was 7.73 ± 2.23 days and in the non-rSO2 group was 9.04 ± 2.68 days with a statistically significant difference (P = 0.03). The length of stay in the two groups was correlated to rSO2 monitoring, history of hypertension, preoperative PaCO2, preoperative Hb, time of surgery, postoperative MAP D1, MAP D2, MAP D3, and preoperative PaO2. Utilizing the rSO2 monitored could have shortened the length of stay (P = 0.042). Patients with a history of hypertension, postoperative MAP D1, MAP D2, and MAP D3 affected the length of stay, and the rest were not significantly correlated, as shown in Table 2.
No significant difference in preoperative MAP was found between the two groups perioperatively (P = 0.62). Within the rSO2 group, the intraoperative MAP and postoperative MAP decreased significantly compared to the preoperative MAP (P < 0.01). Within the non-rSO2 group, the intraoperative and postoperative MAPs differed significantly from the preoperative MAP (P = 0.00), as shown in Table 3.
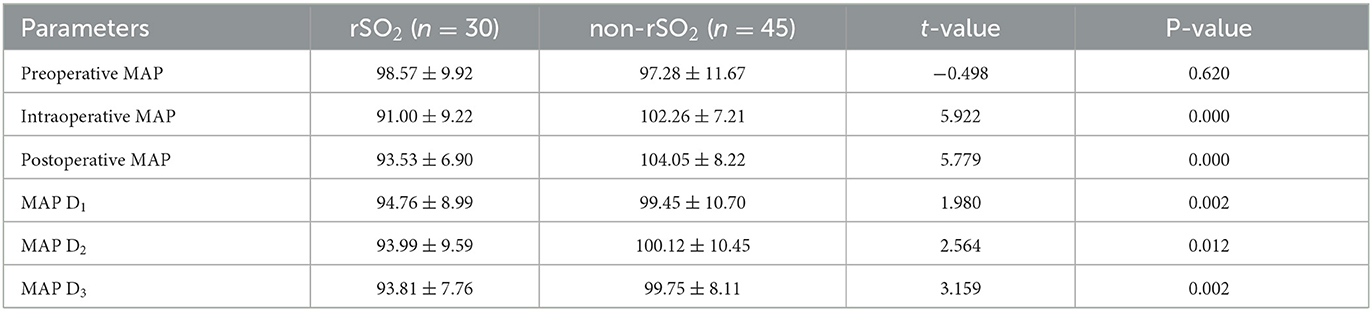
Table 3. Comparison of MAP of perioperative periods between the rSO2 group and the non-rSO2 group (mean ± SD).
The preoperative rSO2 in the rSO2 group was significantly lower on the affected side than on the healthy side (P = 0.00). The rSO2 of the affected side was higher than that of the healthy side after vascular anastomosis, and the difference was statistically significant (P = 0.01). The rSO2 on the affected side was higher than that on the same side before surgery (P < 0.05).
The rSO2 of D1, D2, and D3 on the affected side were significantly higher than those on the healthy side for 3 days after surgery (P < 0.01; Table 4).
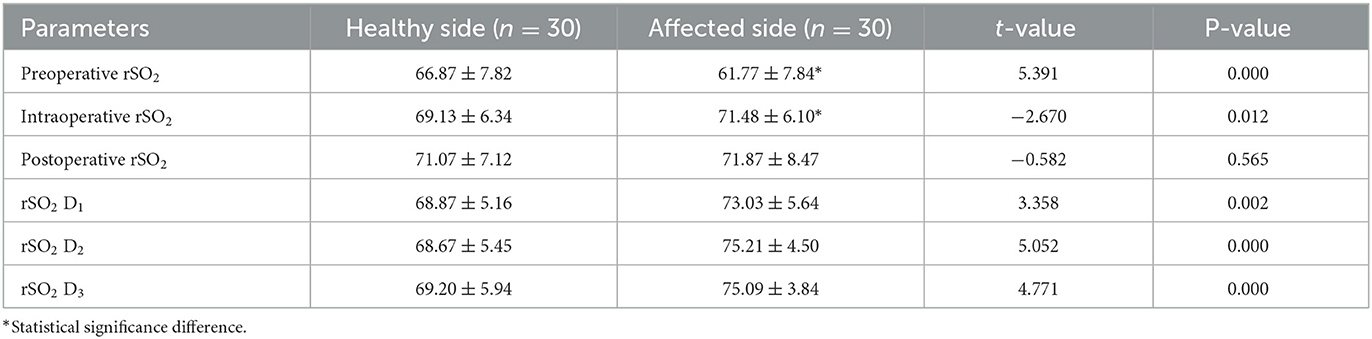
Table 4. Comparison of the rSO2 between the affected side and the healthy side in the rSO2 group at each time of the perioperative period (mean ± SD).
The mean value of rSO2 showed a moderately positive correlation to the corresponding MAP before, during, and after surgery, and the correlation coefficients (r) were 0.536, 0.502, and 0.592, respectively (P < 0.05), as shown in Figures 2A–C.
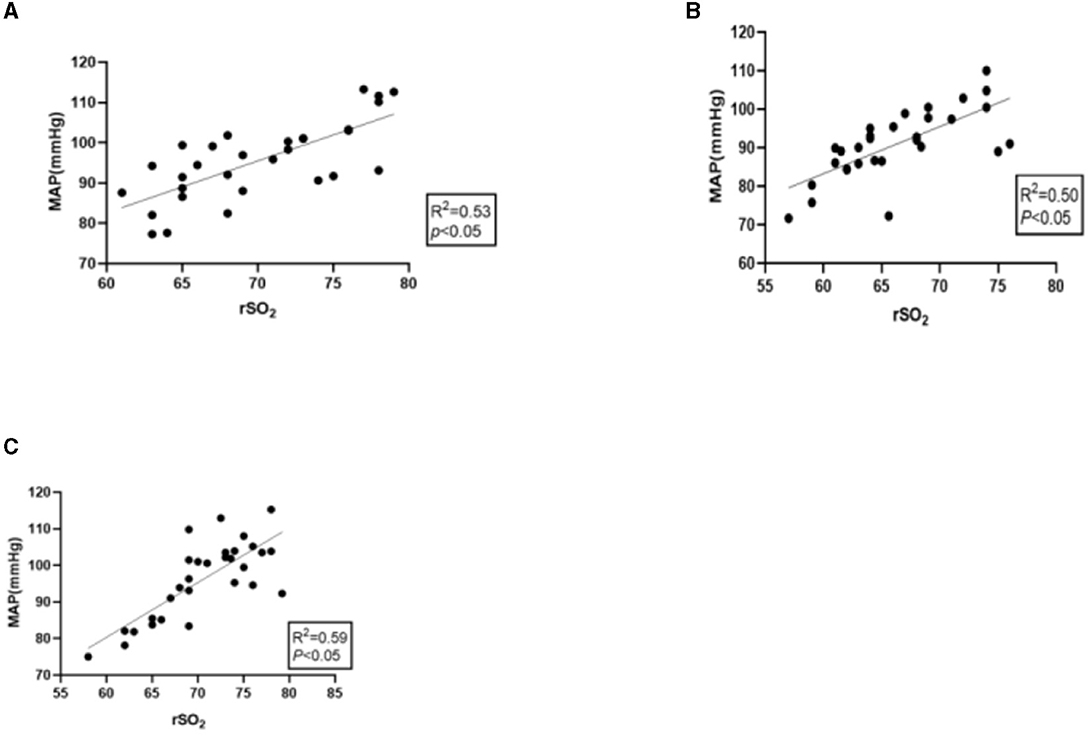
Figure 2. (A) Correlation between preoperative MAP and mean rSO2. (B) Correlation between intraoperative MAP and mean rSO2. (C) Correlation between postoperative MAP and mean rSO2.
One case had CHS (3.3%), and one case experienced cerebral hemorrhage (3.3%) in the rSO2 group between the time after surgery and the time of discharge. Two cases had cerebral hemorrhage (4.4%), and 10 cases had CHS (33.3%) in the non-rSO2 group. No cerebral infarction, reoperation, or death occurred in the two groups. Fisher's test showed that neurological complication in the rSO2 group were significantly less than those in the non-rSO2 group (P = 0.036).
Postoperative complications such as CHS and cerebral hemorrhage in patients with ischemic MMD were correlated with rSO2 and the management of MAP during and for three consecutive days after surgery (D1, D2, and D3; P < 0.05), as shown in Table 5.
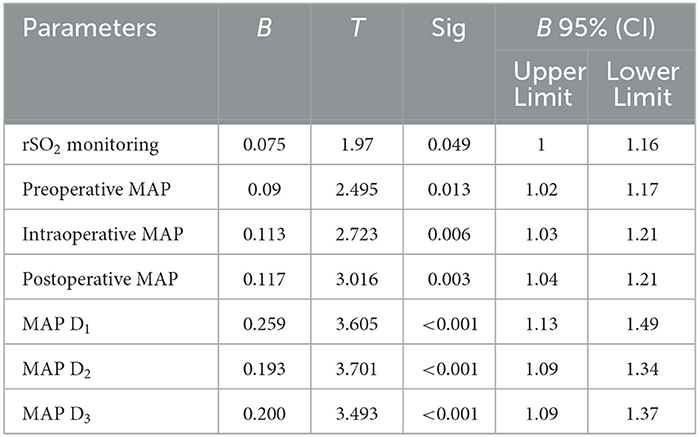
Table 5. Multivariate logistic regression analysis of MMD postoperative complications with rSO2 and MAP at different time periods.
Blood pressure is regulated to ensure good cerebral perfusion when the brain region is affected. However, blood pressure cannot directly reflect the blood perfusion in encephalopathy, which may be an indirect indicator (24). The rSO2 is the measure of blood oxygen saturation in local tissues, including the brain tissue, and is monitored non-invasively.
Many previous studies (4, 25–27) found that frequent intraoperative monitoring of rSO2 had a certain predictive value of intraoperative cerebral ischemia and postoperative neurological complications. Samra et al. (28) found that when rSO2 was reduced by <20% of the baseline value, the occurrence of postoperative neurological complications was significantly reduced, and the rSO2 level had high sensitivity (80%) and specificity (82.2%) in predicting neurological complications.
The patients in the rSO2 group were monitored for rSO2 on the day before surgery, and the rSO2 value on the affected side was lower than that on the healthy side, suggesting that vascular lesions could have led to abnormal cerebral perfusion. The sensitivity of rSO2 level in monitoring cerebral perfusion had another viewpoint. The results of the present study showed a moderate positive correlation between perioperative rSO2 and MAP, which suggests that empirical blood pressure management is necessary. In a normal population with conditions of reasonable blood volume, no serious cardiac dysfunction, and no serious anemia, the improvement of cerebral perfusion can be achieved by adjusting the patient's blood pressure using only vasoactive drugs. However, in patients with ischemic MMD, it might not be beneficial to simply elevate blood pressure due to the fragile vascular mass. At this point, an individualized blood pressure regulation program may be more appropriate for such patients when cerebral perfusion decreases and rSO2 decreases. In addition to increasing blood pressure, cerebral perfusion in the affected area of the brain can also be improved through comprehensive treatments, such as increasing inhaled O2 concentration, adjusting PaCO2, and transfusing red blood cells (29) to reduce the incidence of CHS and cerebral hemorrhage caused by the elevated blood pressure.
After vascular anastomoses, rSO2 on the affected side increased even more than that on the healthy side, demonstrating that blood from the superficial temporal artery on the affected side entered the cerebral ischemic area and improved the cerebral tissue blood perfusion. The rSO2 was monitored continuously for 3 days after surgery in the rSO2 group, and the value of rSO2 on the diseased side was similar to the value at the time of vascular opening after vascular anastomosis, which may have been due to the poor compensatory capacity of cerebral vessels in the affected area, the increased cerebral blood flow shunt, and the ischemic area reaching a new equilibrium point after a certain period. The rSO2 value could reflect the cerebral perfusion on the affected side in real-time and let the anesthesiologist adjust accordingly, which could reduce the occurrence of postoperative neurological complications, ensuring surgical benefit, and improving the postoperative outcome of patients (all patients in the rSO2 group received an intraoperative reduction of BP due to the rSO2 measurement). The results of He et al. (29) show that the length of hospital stay in the non-rSO2 group was longer than that in the rSO2 group, which was consistent with our result of multivariate analysis. We also found that the length of stay of MMD patients was related to hypertension, so controlling the patients' blood pressure might have a positive effect on reducing the length of stay.
Complications such as CHS syndrome and cerebral hemorrhage were low in this study, which is a decrease compared to previous studies (30, 31). The empirical method of maintaining blood pressure ~10% above the preoperative MAP before vascular anastomosis could not better provide cerebral perfusion on the affected side, even with the occurrence of cerebral perfusion syndrome and cerebral hemorrhage after the opening of the artery clamp, which was similar to the outcome of some study (11, 32). Blood pressure management should be individualized to reduce the occurrence of complications (32). Through logistic regression analysis, we found that rSO2 and perioperative BP control were directly correlated with postoperative neurological complications, and the BP in the rSO2 group was significantly lower than that in the non-rSO2 group, and the complications were also fewer as well. This finding suggested that intervention using rSO2 monitoring could assist to detect and adjust in cerebral perfusion and oxygen supply in real-time, leading to an improved prognosis for patients, as well as a shorter hospital stay, which was consistent with the results of Li et al. (33).
This study had a small sample size, and short-term follow-up and the postoperative BP management and treatment of patients in the rSO2 group were adjusted by the surgeon according to the value of rSO2. Unfortunately, we did not collect the specific management methods.
Moreover, since no monitoring of rSO2 was performed in the non-rSO2 group, no comparison of the brain oxygenation itself can be made thus putting in question whether the reduced number of complications and shorter length of stay were due to better oxygenation or purely due to the lower BP, requiring a more rigorous study design analyzing a larger sample of patients and longer follow-up to confirm or negate the value of rSO2 monitoring.
The current study outcome found that the intraoperative use of rSO2 monitoring was associated with a positive impact on the quality of recovery of patients after vascular reconstruction for ischemic MMD.
In conclusion, during the surgical procedure in patients undergoing superficial temporal anterior-middle cerebral arterial branch anastomosis for ischemic MMD, the application of MAP and rSO2 monitoring reduced the MAP of patients during the middle and early postoperative period and was associated with the occurrence of postoperative neurological complications compared with the patients who had only MAP detection. It is recommended to monitor rSO2 as a routine procedure for patients with ischemic MMD undergoing bypass surgery, which would benefit the patients.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the institutional review Committee of Peking University International Hospital approved the study (KYSQ2019-058-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
XC and XQ collected the clinical data and drafted the manuscript. JW analyzed and interpreted the data. RW and LY critically revised the important knowledge content. XG and JW analyzed the data statistically. All authors read and approved the manuscript.
This study was supported by the National Natural Science Foundation of China (NSFC # 82171887).
The authors thank RW and JW for their support in data collection during the second multiple-valve operation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhang X, Xiao W, Zhang Q, Xia D, Gao P, Su J, et al. Progression in Moyamoya disease: clinical features, neuroimaging evaluation, and treatment. Curr Neuropharmacol. (2022) 20:292–308. doi: 10.2174/1570159X19666210716114016
2. Zhang D, Huang L, Huang Z, Zhou Q, Yang X, Gu H, et al. Epidemiology of Moyamoya disease in China: a nationwide hospital-based study. Lancet Reg Health West Pac. (2022) 11:100331. doi: 10.1016/j.lanwpc.2021.100331
3. Shang S, Zhou D, Ya J, Li S, Yang Q, Ding Y, et al. Progress in Moyamoya disease. Neurosurg Rev. (2020) 43:371–82. doi: 10.1007/s10143-018-0994-5
4. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. (2009) 360:1226–37. doi: 10.1056/NEJMra0804622
5. Smith ER, Scott RM. Surgical management of moyamoya syndrome. Skull Base. (2005) 15:15–26. doi: 10.1055/s-2005-868160
6. Ando T, Shimada Y, Fujiwara S, Yoshida K, Kobayashi M, Kubo Y, et al. Revascularisation surgery improves cognition in adult patients with moyamoya disease. J Neurol Neurosurg Psychiatry. (2020) 91:332–4. doi: 10.1136/jnnp-2019-321069
7. Smith ER, Scott RM. Progression of disease in unilateral moya-moya syndrom. Progression of disease in unilateral moyamoya syndrome. Neurosurg Focus. (2008) 24:E17. doi: 10.3171/FOC/2008/24/2/E17
8. Mayeku J, Lopez-Gonzalez MA. Current surgical options for Moyamoya disease. Cureus. (2020) 12:e11332. doi: 10.7759/cureus.11332
9. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. (1969) 20:288–99. doi: 10.1001/archneur.1969.00480090076012
10. Parray T, Martin TW, Siddiqui S. Moyamoya disease: a review of the disease and anesthetic management. J Neurosurg Anesthesiol. (2011) 23:100–9. doi: 10.1097/ANA.0b013e3181f84fac
11. Li Sh, Yu Rg, He F, Qiu PL, Fiu W. Influencing factors of regional cerebral oxygen saturation during cardiopulmonary bypass. Shanghai Med J. (2016) 39:202–7.
12. Thudium M, Ellerkmann RK, Heinze I, Hilbert T. Relative cerebral hyperperfusion during cardiopulmonary bypass is associated with risk for postoperative delirium: a cross-sectional cohort study. BMC Anesthesiol. (2019) 19:35. doi: 10.1186/s12871-019-0705-y
13. Yu J, Zhang J, Li J, Zhang J, Chen J. Cerebral hyperperfusion syndrome after revascularization surgery in patients with moyamoya disease: systematic review and meta-analysis. World Neurosurg. (2020) 135:357–66. doi: 10.1016/j.wneu.2019.11.065
14. Tokairin K, Kazumata K, Uchino H, Ito M, Ono K, Tatezawa R, et al. Postoperative intracerebral hemorrhage after combined revascularization surgery in Moyamoya disease: profiles and clinical associations. World Neurosurg. (2018) 120:e593–600. doi: 10.1016/j.wneu.2018.08.132
15. Cho H, Jo KI, Yu J, Yeon JY, Hong SC, Kim JS. Low flow velocity in the middle cerebral artery predicting infarction after bypass surgery in adult Moyamoya disease. J Neurosurg. (2017) 126:1573–7. doi: 10.3171/2016.3.JNS152256
16. Funaki T, Takahashi JC, Houkin K, Kuroda S, Takeuchi S, Fujimura M, et al. Angiographic features of hemorrhagic Moyamoya disease with high recurrence risk: a supplementary analysis of the Japan Adult Moyamoya Trial. J Neurosurg. (2018) 128:777–84. doi: 10.3171/2016.11.JNS161650
17. Yu Y, Zhang K, Zhang L, Zong H, Meng L, Han R. Cerebral near-infrared spectroscopy (NIRS) for perioperative monitoring of brain oxygenation in children and adults. Cochrane Database Syst Rev. (2018) 1:CD010947. doi: 10.1002/14651858.CD010947.pub2
18. Han C, Gao TX, Zhang HD, Ma W, Li Y, Li B, et al. Wavelet analysis of cerebral oxygenation signal measured by near-infrared spectroscopy in Moyamoya disease. World Neurosurg. (2023) 172:e12–8. doi: 10.1016/j.wneu.2022.10.074
19. Wang X, Feng K, Liu H, Liu Y, Ye M, Zhao G, et al. Regional cerebral oxygen saturation and postoperative delirium in endovascular surgery: a prospective cohort study. Trials. (2019) 20:504. doi: 10.1186/s13063-019-3586-y
20. Iwaki K, Takagishi S, Arimura K, Murata M, Chiba T, Nishimura A, et al. A novel hyperspectral imaging system for intraoperative prediction of cerebral hyperperfusion syndrome after superficial temporal artery-middle cerebral artery anastomosis in patients with Moyamoya disease. Cerebrovasc Dis. (2021) 50:208–15. doi: 10.1159/000513289
21. Ito K, Ookawara S, Ueda Y, Miyazawa H, Uchida T, Kofuji M, et al. Cerebral oxygenation improvement is associated with hemoglobin increase after hemodialysis initiation. Int J Artif Organs. (2020) 43:695–700. doi: 10.1177/0391398820910751
22. Pinto BB, Chew M, Buse GL, Walder B. The concept of peri-operative medicine to prevent major adverse events and improve outcome in surgical patients: a narrative review. Eur J Anaesthesiol. (2019) 36:889–903. doi: 10.1097/EJA.0000000000001067
23. Wang S, Han J, Cheng L, Li N. Risk factors and preventive measures of cerebral hyperperfusion syndrome after carotid artery interventional therapy. Exp Ther Med. (2017) 14:2517–20. doi: 10.3892/etm.2017.4796
24. Zhang Y, Bao X-Y, Duan L, Yang W-Z, Li D-S, Zhang Z-S, et al. Encephaloduroarteriosynangiosis for pediatric Moyamoya disease: long-term follow-up of 100 cases at a single center. J Neurosurg Pediatr. (2018) 22:173–80. doi: 10.3171/2018.2.PEDS17591
25. Cura Z, Oc B, Arun O, Oc M, Duman I, Duman A. Effects of sevoflurane and propofol anesthesia on cerebral oxygenation in patients undergoing carotid endarterectomy. Turk Neurosurg. (2022) 32:76–82. doi: 10.5137/1019-5149.JTN.33776-21.2
26. Juliana N, Abu Yazit NA, Kadiman S, Hafidz KM, Azmani S, Teng NIMF, et al. Intraoperative cerebral oximetry in open heart surgeries reduced postoperative complications: a retrospective study. PLoS ONE. (2021) 16:e0251157. doi: 10.1371/journal.pone.0251157
27. Zugni N, Guadrini L, Rasulo F. Noninvasive neuromonitoring in the operating room and its role in the prevention of delirium. Best Pract Res Clin Anaesthesiol. (2021) 35:191–206. doi: 10.1016/j.bpa.2020.09.006
28. Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. (2000) 93:964–770. doi: 10.1097/00000542-200010000-00015
29. He S, Duan R, Liu Z, Ye X, Yuan L, Li T, et al. Characteristics of cognitive impairment in adult asymptomatic moyamoya disease. BMC Neurol. (2020) 20:322. doi: 10.1186/s12883-020-01898-8
30. Zhang Y, Tan J, Li P, Zhang X, Yang Y, Liu Y, et al. The perioperative application of continuous cerebral autoregulation monitoring for cerebral protection in elderly patients. Ann Palliat Med. (2021) 10:4582–892. doi: 10.21037/apm-21-707
31. Ding L, Chen DX Li Q. Effects of electroencephalography and regional cerebral oxygen saturation monitoring on perioperative neurocognitive disorders: a systematic review and meta-analysis. BMC Anesthesiol. (2020) 20:254. doi: 10.1186/s12871-020-01163-y
32. Shi Z, Wu L, Wang Y, Zhang H, Yang Y, Hang C. Risk factors of postoperative cerebral hyperperfusion syndrome and its relationship with clinical prognosis in adult patients with moyamoya disease. Chin Neurosurg J. (2023) 9:10. doi: 10.1186/s41016-023-00321-8
Keywords: ischemic MMD, superficial temporal artery-middle cerebral artery anastomosis, regional cerebral oxygen saturation (rSO2), anesthesia management, mean arterial pressure
Citation: Chen X, Qin X, Wang J, Wang R, Guo X and Yao L (2023) Effect of cerebral oxygen saturation monitoring in patients undergoing superficial temporal anterior-middle cerebral artery anastomosis for ischemic Moyamoya disease: a prospective cohort study. Front. Neurol. 14:1226455. doi: 10.3389/fneur.2023.1226455
Received: 30 May 2023; Accepted: 24 August 2023;
Published: 21 September 2023.
Edited by:
Teodor Svedung Wettervik, Uppsala University, SwedenReviewed by:
Yu Lei, Fudan University, ChinaCopyright © 2023 Chen, Qin, Wang, Wang, Guo and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lan Yao, eWFvbGFuQHBrdWloLmVkdS5jbg==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.