
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 10 August 2023
Sec. Dementia and Neurodegenerative Diseases
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1220473
This article is part of the Research Topic Hydrocephalus View all 7 articles
 Hanlin Cai1†
Hanlin Cai1† Feng Yang1†
Feng Yang1† Hui Gao1
Hui Gao1 Keru Huang2
Keru Huang2 Linyuan Qin1
Linyuan Qin1 Ruihan Wang1
Ruihan Wang1 Yi Liu2
Yi Liu2 Liangxue Zhou2
Liangxue Zhou2 Zilong Hao1
Zilong Hao1 Dong Zhou1
Dong Zhou1 Qin Chen1*
Qin Chen1*Objective: Idiopathic normal-pressure hydrocephalus (iNPH) is a treatable cause of dementia; however, its etiology and pathogenesis remain poorly understood. The objective of this study was to investigate the prevalence and impact of vascular risk factors in patients with iNPH compared to a control cohort to better understand the potential mechanisms and preventive measures.
Methods: We systematically searched PubMed, Web of Science, Embase, and the Cochrane Library (from inception to December 20, 2022) for studies reporting vascular risk factors for the development of iNPH. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using random-effects models.
Results: After screening 1,462 articles, 11 case-control studies comprising 1,048 patients with iNPH and 79,668 cognitively unimpaired controls were included in the meta-analysis. Our data showed that hypertension (N = 991, OR = 2.30, 95% CI 1.64 to 3.23, I2= 64.0%), diabetes mellitus (DM) (N = 985, OR = 3.12, 95% CI 2.29 to 4.27, I2= 44.0%), coronary heart disease (CHD; N = 880, OR = 2.34, 95% CI 1.33 to 4.12, I2= 83.1%), and peripheral vascular disease (N = 172, OR = 2.77, 95% CI 1.50 to 5.13, I2= 0.0%) increased the risk for iNPH, while overweight was a possible factor (N = 225, OR = 2.01, 95% CI 1.34 to 3.04, I2= 0.0%) based on the sensitivity analysis. Smoking and alcohol consumption were not associated with iNPH.
Conclusions: Our study suggested that hypertension, DM, CHD, peripheral vascular disease, and overweight were associated with iNPH. These factors might be involved in the pathophysiological mechanisms promoting iNPH. These findings require further investigation in future studies.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, CRD42022383004.
Idiopathic normal-pressure hydrocephalus (iNPH) is a clinical syndrome characterized by cognitive decline, gait disturbance, and urinary incontinence, with ventricular enlargement apparent on brain imaging (1). A recent epidemiological study revealed a prevalence of 0.2% in population aged 70–79 years and ~6% in those 80 years and older (2). As expected, with an aging population, the number of patients with iNPH has been steadily increasing. Currently, iNPH is a treatable cause of dementia; however, it is often underdiagnosed and undertreated (3). In addition, the etiology and pathogenesis of this disease remain poorly understood.
Some observational studies have indicated that about one in four patients with iNPH have vascular risk factors (4). At present, several vascular risk factors for iNPH have been reported, including hypertension (2, 4–15), diabetes mellitus (DM) (4, 6–10, 12–14, 16), hyperlipidemia (4, 7, 13), smoking (4, 6, 7, 10, 13, 14), alcohol use (6, 14), overweight (2, 4, 6, 7), coronary heart disease (CHD) (4, 6, 8–10, 12–14), and peripheral vascular disease (4, 13); some may play an important role in the development of iNPH. However, these findings have been inconsistent (4–6, 8, 10, 13, 14). In addition, most of those studies involved relatively small sample sizes. Therefore, we conducted a systematic review and meta-analysis to investigate the association between vascular risk factors and iNPH for understanding the potential mechanisms and preventive measures.
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (17). We have registered this meta-analysis on PROSPERO (CRD42022383004). We systematically searched the PubMed, Embase, Web of Science, and Cochrane Library databases (from inception to December 20, 2022) for observational studies (cohort, cross-sectional, and case-control studies) to evaluate the association between vascular risk factors and iNPH. The following search strategy was used for these databases, with appropriate modifications, by two investigators (HL. C and F. Y): (“Hydrocephalus, Normal Pressure” OR “Normal Pressure Hydrocephalus” OR “Hakim Syndrome”) AND (“Risk Factors” OR “Hypertension” OR “High blood pressure” OR “Diabetes Mellitus” OR “Diabetes” OR “Hyperlipidemias” OR “Overweight” OR “Obesity” OR “Smoking” OR “Alcohol Drinking”). The reference lists of the included studies were also reviewed.
Studies that met the following criteria were included: (1) patients with iNPH with at least one of the features of the Hakim's triad (gait disturbance, cognitive impairment, and urinary problems) and radiologically confirmed ventricular enlargement according to the existing diagnostic criteria (18); (2) study evaluating at least one of the predefined vascular risk factors for iNPH [adapting from INTERHEART (19) and INTERSTROKE (20) study], including hypertension, DM, hyperlipidemia, smoking, alcohol use, overweight, CHD, and peripheral vascular diseases (4); and (3) control group consisted of cognitively unimpaired individuals.
Studies were excluded if they: (1) were reviews, meeting abstracts, editorial materials, or articles not published in English; (2) the odds ratios (ORs) and 95% confidence intervals (CIs) were unextractable; (3) included participants with symptomatic or secondary NPH, including cerebrovascular diseases, head trauma, brain tumors, or infections; and (4) only evaluated patients with asymptomatic ventricular enlargement.
The titles and abstracts were independently screened by HL. C and F. Y, who read the full text and extracted the data. Any discrepancies were resolved through consensus. The following information was extracted from each study using a predesigned data extraction form: name of the first author, publication year, country, sample size, age, sex, study design, matched factors, diagnostic criteria for iNPH, and risk factors investigated. Adjusted data were recorded when studies reported both crude and adjusted ORs. The calculation of the crude OR and interval estimation were based on previously published methods when a specific OR was not provided in the original articles (21). Quality assessments were performed using the Newcastle–Ottawa Scale (NOS) (https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). A “star system” was devised to evaluate studies, with a focus on three aspects: the selection of the study groups, comparability of the groups, and ascertainment of either the exposure or outcome of interest in the context of case-control or cohort studies, respectively. Two investigators (HL. C and F. Y) independently assessed all eligible studies, and disagreements were resolved through consensus. Articles with 8–9 stars were rated as high quality, 5–7 stars as moderate quality, and 4 stars as low quality (22).
A pooled OR with a 95% CI was calculated for patients with iNPH based on possible vascular risk factors. The I2 test was used to quantify heterogeneity, and an I2 value >50% was considered to indicate substantial heterogeneity (23). Since clinical heterogeneity between studies was significant, all meta-analyses were conducted using random-effect models (Dersimonian-Laird method). Meta-regression analysis was also conducted based on the mean age, sex, region, year of publication, and sample sizes of certain studies. Sensitivity analysis for potential factors (N ≥ 3) was performed by eliminating one study at a time to evaluate the stability of the results and explain the possible sources of heterogeneity. Publication bias was assessed by visually inspecting the funnel plot and statistically examining the results using the Begg's and Egger's tests if more than five studies were synthesized for each factor. P < 0.05 was considered statistically significant. All statistical analyses were performed using Stata SE 16.0 (StataCorp., T.X., USA).
In total, 1,462 publications were retrieved after the initial search. The titles and abstracts of 1,043 studies were screened after removing duplicates, leaving 69 articles that were assessed for eligibility by reading the full text. Fifty-eight records were excluded for several reasons. Finally, 11 studies were included in the meta-analysis (Figure 1).
Detailed characteristics of the included studies are presented in Table 1. The included studies consisted of 11 case-control studies, which were conducted in the United States, Italy, Germany, Norway, Sweden, Finland, and Japan. Among these studies, 1,048 patients with iNPH (sample size ranging from 12 to 440 with a median of 29) and 79,668 controls were included. For the diagnosis of iNPH, seven studies used the American-European Guideline criteria (18), three used self-defined criteria, and one used the Japanese criteria (24) (Supplementary Table S1). Most patients with iNPH were over 70 years of age. For vascular risk factors, eight vascular risk factors were evaluated, including hypertension, hyperlipidemia, DM, overweight, smoking, alcohol use, CHD, and peripheral vascular disease. The definitions of the vascular risk factors for each included study are summarized in Supplementary Table S2. Ten studies included hypertension, nine studies mentioned DM, seven studies mentioned CHD, and seven studies included these three risk factors. All the included studies were of moderate to high quality. The NOS scores ranged from 6 to 9, with a median NOS score of 8 (Supplementary Table S4).
Hypertension was the most widely studied risk factor for iNPH, with 10 included studies (N = 991). The studies defined hypertension based on previous medical history, and in most (80%), the cut-off value was set at 140/90 mmHg, except for two early studies that used 160/95 mmHg and 160/90 mmHg (6, 7). The median proportion of hypertension was 65% (range: 40.91–85.71%). The results of meta-analysis showed that hypertension was associated with the diagnosis of iNPH (OR = 2.30, 95% CI 1.64 to 3.23, I2= 64.0%, P = 0.003) (Figure 2). Sensitivity analysis showed reliable and stable results (Supplementary Table S5A). The meta-regression analysis indicated that the year of publication might be the main source of heterogeneity in the results (Supplementary Table S6).
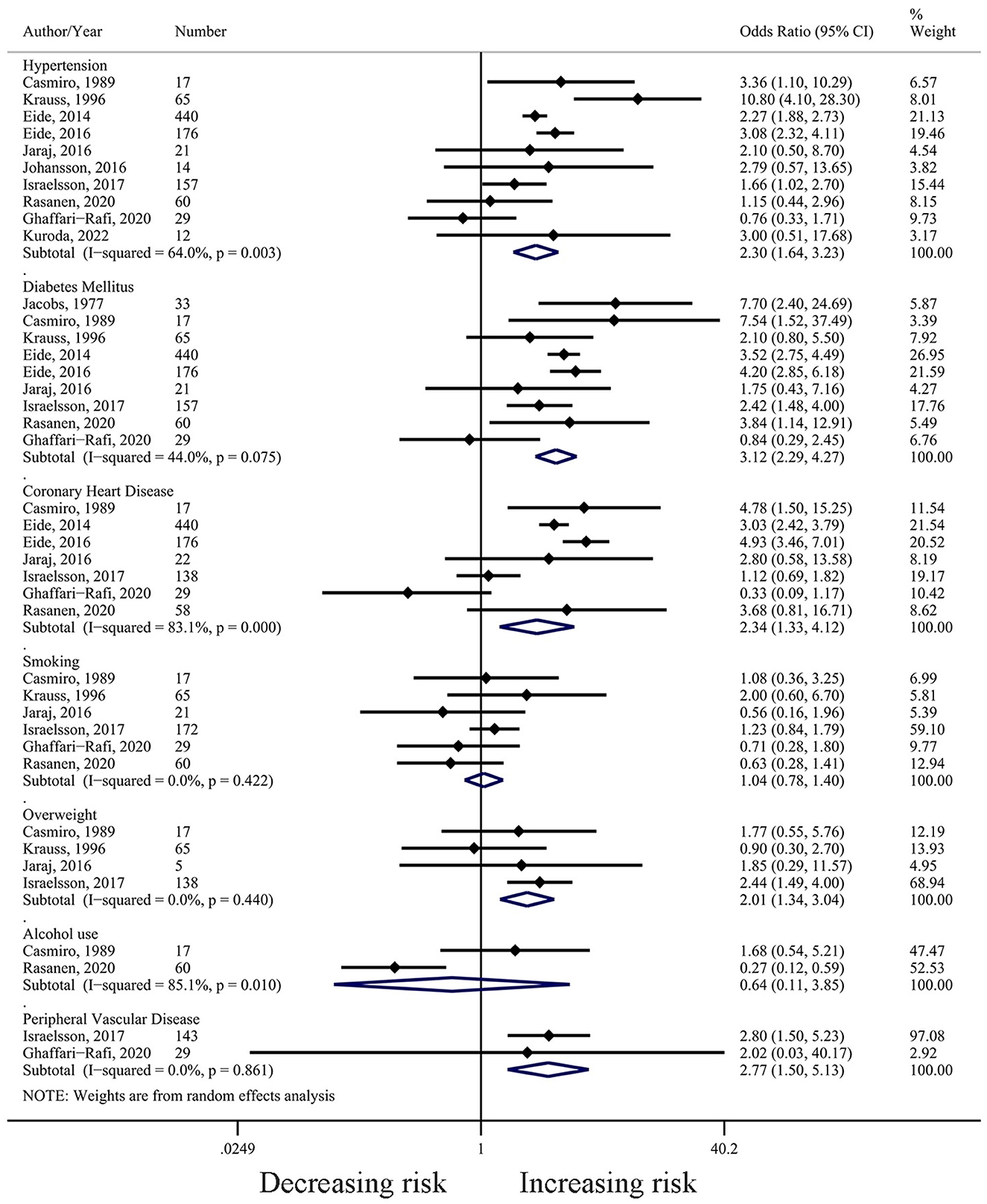
Figure 2. Forest plot (based on random-effect model) of the associations between hypertension, diabetes mellitus, smoking, alcohol use, overweight, coronary heart disease, and peripheral vascular disease with iNPH.
Nine studies (N = 985) reported a relationship between DM and iNPH development. DM was mostly defined (89%) based on medical history or medication use, with only one study using the results of a glucose tolerance test (16). The median proportion of DM was 25.15% (Range: 13.04–51.52%). The combined result showed that DM was associated with the increasing risk for iNPH (OR = 3.12, 95% CI 2.29 to 4.27, I2= 44.0%, P = 0.075) (Figure 2). Sensitivity analysis revealed no effect on the stability of the results after using the leave-one-out method (Supplementary Table S4B). Meta-regression analysis showed that the age of the patients included in the study was the main source of heterogeneity in the results (Supplementary Table S5).
In the included studies, CHD was defined as a previous diagnosis of CHD, angina pectoris, or myocardial infarction. The median proportion of CHD in the included studies was 19.32% (range: 13.8–47.1%). Pooled results from seven studies (N = 880) suggest that CHD may be a risk factor for the development of iNPH (OR = 2.34, 95% CI 1.33 to 4.12, I2= 83.1%, P = 0.000) (Figure 2). Sensitivity analysis confirmed the stability of the results (Supplementary Table S4C).
The combined results of the six studies (N = 364) suggest that smoking was not a risk factor for the development of iNPH (OR = 1.04, 95% CI 0.78 to 1.40, I2= 0.0%, P = 0.422) (Figure 2). Sensitivity analysis confirmed the stability of the results (Supplementary Table S4D).
A total of four studies (N = 225) discussed the relationship between overweight and the development of iNPH, and the results suggested that overweight was a risk factor for iNPH (OR = 2.01, 95% CI 1.34 to 3.04, I2= 0.0%, P = 0.440) (Figure 2). Two studies defined overweight as body mass index (BMI) ≧ 27 kg/m2 (6, 7), while another two defined it as BMI ≧ 25 kg/m2 (4, 10). Sensitivity analysis showed a significant change in the combined results of the remaining three papers after removing the study by Israelsson et al. (OR = 1.32, 95% CI 0.63 to 2.75, I2= 0.0%, P = 0.659) (Supplementary Table S4E).
Three studies (N = 234) investigated the relationship between hyperlipidemia and iNPH and defined hyperlipidemia using different criteria. One study found that elevated Apolipoprotein B/A1 ratio was relevant with iNPH (OR = 2.51, 95% CI 1.61 to 3.9, P < 0.001) (4), while another two studies did not find a causal relationship between fasting triglyceride level (OR = 0.6, 95% CI 0.2 to 2.0, P = 0.37) or history of hyperlipidemia (OR = 0.90, 95% CI 0.40 to 2.04, P = 0.97) and iNPH (7, 13).
Only two studies (N = 77) examined the relationship between alcohol use and iNPH development, and the combined results showed that alcohol consumption was not a risk factor for iNPH (OR = 0.64, 95% CI 0.11 to 3.85, I2= 85.1%, P = 0.01) (Figure 2).
Two studies (N = 172) investigated the relationship between self-reported peripheral vascular disease and iNPH development and found that it was associated with the iNPH development (OR = 2.77, 95% CI 1.50 to 5.13, I2= 0.0%, P = 0.861) (Figure 2).
We visually inspected funnel plots and statistically used the Begg's and Egger's tests to evaluate publication bias for hypertension, DM, CHD, and smoking. As shown in funnel plots, no significant publication bias was detected (Supplementary Table S6, Supplementary Figure S1).
In this study, we comprehensively reviewed the vascular risk factors for iNPH and identified five modifiable factors associated with iNPH. Hypertension was the most common vascular comorbidity, followed by DM, CHD, and peripheral vascular disease. Although overweight was also considered a potential vascular risk factor, its association with iNPH remained inconclusive owing to unstable sensitivity analysis results. In contrast, based on a few studies, smoking, alcohol consumption, and hyperlipidemia were not associated with iNPH.
The relationship between hypertension and iNPH has been reported in previous studies (4–8, 10, 12). Consistent with the previous literature, our study found that hypertension was the most common vascular risk factor in patients with iNPH. Meta-regression analysis found that the year of publication may be a source of heterogeneity, which could be explained by changes in the diagnostic criteria for hypertension (25). The association between arterial hypertension and iNPH was first described in 1987 (5), and a subsequent study found that only systolic blood pressure (BP) and pulse pressure, but not diastolic BP, were related to ventricular enlargement (26), which was in accordance with previous animal studies (27, 28). Hydrodynamic theory, a classic hypothesis of iNPH pathogenesis, may explain the association between hypertension and iNPH. Aging and hypertension can impair the elastic arteries' “Windkessel effect” (i.e., the ability of elastic arteries to distend during cardiac systole) (29). Consequently, a high pulse pressure is transmitted to the brain capillaries, leading to an increase in the pressure gradient within and outside the ventricles, eventually resulting in ventricular dilation (26, 30). Another animal study also demonstrated that arterial hypertension caused alterations in vessel dynamics that led to a decrease in perivascular pumping, subsequently reducing the overall cerebrospinal fluid flow within the perivascular spaces and further impacting the glymphatic system, a brain clearance pathway known to participate in the removal of amyloid-β (31, 32).
Several factors may explain the association between DM and iNPH. In mouse models, DM has been linked to neuroinflammation, waste accumulation, and reduced aquaporin 4 (AQP4) density, resulting in impairment of the glymphatic system (33, 34). This system was named after its similarity to the lymphatic system. AQP4 is the primary protein that facilitates material exchange (31). Recent studies have implicated reduced glymphatic clearance in iNPH development (35–37). Additionally, metabolic disturbances and microvascular damage resulting from DM may contribute to iNPH pathogenesis (11, 38). Another plausible explanation for the high proportion of DM comorbidities in patients with iNPH is that ventricular enlargement can cause mechanical stress-induced dysregulation of the hypothalamic-pituitary axis, resulting in dysregulation of hormonal secretion, as evidenced by decreased levels of growth hormone and insulin-like growth factor 1 in previous studies (39). However, age might act as a confounding factor in the relationship between DM and iNPH according to the meta-regression result. We cannot therefore exclude the possibility of spurious correlation between DM and iNPH in our study, given the inherent limitations of the included original studies. Larger cohort studies are warranted in the future to mitigate the influence of age on the conclusions.
Our meta-analysis suggested that overweight may be a risk factor for iNPH. Although no obvious heterogeneity was detected, instability in the results was found on omitting a large prospective case—control study (4). This may have resulted from multivariate adjustment of the effect size and different definitions of overweight across studies. Therefore, more consistent studies are needed to confirm the relationship between overweight and iNPH. There is limited knowledge regarding the underlying mechanism linking overweight to the development of iNPH, although a previous study revealed that a high BMI was associated with higher lumbar puncture opening pressure in patients with iNPH (40). Other research indicated that obesity was linked to decreased cerebral blood flow, alterations in gray matter, and microangiopathy (as observed by white matter hyperintensity and lacunar infarcts on magnetic resonance imaging) in healthy individuals (41–43). These findings may be due to the relationship between obesity and several pathophysiological changes, including neuroinflammation, mitochondrial dysfunction, and hormone alterations, which can exacerbate the process of neurodegeneration and cognitive decline (43–45). Further investigation is required to assess the potential risks of overweight in patients with iNPH.
In addition, we found that both CHD and peripheral vascular disease were risk factors for iNPH. However, heterogeneity may arise from variations in the diagnostic criteria across studies. Because these diseases are mainly atherosclerotic in nature, atherosclerosis may play a crucial role in the pathogenesis of iNPH (46). A previous autopsy-based study confirmed that severe hypertensive and arteriosclerotic vasculopathy with multiple lacunar infarcts was found in a patient with iNPH (47). Atherosclerosis causes ischemic-hypoxic damage to brain vessels and parenchyma, resulting in extensive changes in metabolism, blood-brain barrier function, and cerebrospinal fluid hydrodynamics (48). This contributes to demyelination and ventriculomegaly in patients with iNPH, as seen using magnetic resonance imaging (15). Further investigations are required to explore the pathophysiological mechanisms underlying these factors.
Given the high prevalence of vascular comorbidities among individuals with iNPH, it becomes imperative for clinicians to understand the potential impact of these factors on the surgical outcome. Vascular risk factors, such as hypertension, diabetes mellitus, coronary heart disease, peripheral vascular disease, cerebrovascular disease, and smoking, tend to exert a detrimental influence on the prognosis of iNPH patients (49–54). However, their effect on long-term outcomes appears to be relatively minor (51), albeit the lack of studies with extended follow-up periods. Consequently, the option of shunt surgery should not be denied, as nearly half of iNPH patients with cerebrovascular diseases still derive substantial benefits from shunt surgery over an extended duration (53, 54).
Our study had several limitations. First, most of the included studies were case-control studies, and establishing causality was challenging. Therefore, long-term follow-up studies are required. Second, some ORs were calculated from raw data without adjusting for potential confounders; we could not exclude unknown variables that affected the results. Third, publications written in languages other than English and conference proceedings were excluded, which might have resulted in a publication bias.
Further research is required to better understand the etiology and pathogenesis of iNPH. First, the current study had a cross-sectional design; therefore, we could not explore a causal relationship between these vascular risk factors and the development of iNPH. In addition, it is unclear whether these modifiable risk factors affect the prognosis of patients with iNPH. Long-term follow-up studies are needed to clarify these issues. Second, novel modifiable vascular risk factors, such as chronic kidney disease, physical inactivity, obstructive sleep apnea, inflammatory markers, and cerebral small vessel disease, also need to be explored to better understand disease mechanisms. Third, an internationally unified algorithm for diagnosing iNPH is required to compare different studies. Finally, despite conducting a systematic search, most studies were conducted in Western countries, and the current research lacks data from low- and middle-income countries; therefore, epidemiological characteristics and differences among these regions are urgently needed.
In this systematic review, we identified five modifiable vascular risk factors in patients with iNPH: Management of hypertension, DM, CHD, overweight, and peripheral vascular disease. These factors may be involved in the pathophysiological mechanisms promoting iNPH. Further studies are required to confirm these findings.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
HC and FY conceived this study and drafted the manuscript. HC, FY, HG, KH, LQ, and RW collected the information and relevant materials. QC designed the study and revised the manuscript. All coauthors revised the manuscript and approved the final version.
This work was supported by the STI2030-Major Projects Youth Scientist Program (No. 2022ZD0213600), National Natural Science Foundation of China (No. 82071203), Natural Science Foundation of Sichuan (No. 2022NSFSC1325), Chengdu Science and Technology Bureau Program (2019-YF09-00215-SN), and Young Scientists Fund (No. 82201608).
We would like to thank all the authors of the original studies included in this meta-analysis for their contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1220473/full#supplementary-material
1. Adams RD, Fisher CM, Hakim S, Ojemann RG, Sweet WH. Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure. A treatable syndrome. N Engl J Med. (1965) 273:117–26. doi: 10.1056/NEJM196507152730301
2. Jaraj D, Rabiei K, Marlow T, Jensen C, Skoog I, Wikkelso C. Prevalence of idiopathic normal-pressure hydrocephalus. Neurology. (2014) 82:1449–54. doi: 10.1212/WNL.0000000000000342
3. Kazui H, Miyajima M, Mori E, Ishikawa M. Lumboperitoneal shunt surgery for idiopathic normal pressure hydrocephalus (SINPHONI-2): an open-label randomised trial. Lancet Neurol. (2015) 14:585–94. doi: 10.1016/S1474-4422(15)00046-0
4. Israelsson H, Carlberg B, Wikkelso C, Laurell K, Kahlon B, Leijon G, et al. Vascular risk factors in INPH: a prospective case-control study (the INPH-CRasH study). Neurology. (2017) 88:577–85. doi: 10.1212/WNL.0000000000003583
5. Graff-Radford NR, Godersky JC. Idiopathic normal pressure hydrocephalus and systemic hypertension. Neurology. (1987) 37:868–71. doi: 10.1212/WNL.37.5.868
6. Casmiro M, D'Alessandro R, Cacciatore FM, Daidone R, Calbucci F, Lugaresi E. Risk factors for the syndrome of ventricular enlargement with gait apraxia (idiopathic normal pressure hydrocephalus): a case-control study. J Neurol Neurosurg Psychiatry. (1989) 52:847–52. doi: 10.1136/jnnp.52.7.847
7. Krauss JK, Regel JP, Vach W, Droste DW, Borremans JJ, Mergner T. Vascular risk factors and arteriosclerotic disease in idiopathic normal-pressure hydrocephalus of the elderly. Stroke. (1996) 27:24–9. doi: 10.1161/01.STR.27.1.24
8. Eide PK, Pripp AH. Increased prevalence of cardiovascular disease in idiopathic normal pressure hydrocephalus patients compared to a population-based cohort from the HUNT3 survey. Fluids Barriers CNS. (2014) 11:19. doi: 10.1186/2045-8118-11-19
9. Eide PK, Pripp AH. The prevalence of cardiovascular disease in non-communicating hydrocephalus. Clin Neurol Neurosurg. (2016) 149:33–8. doi: 10.1016/j.clineuro.2016.07.024
10. Jaraj D, Agerskov S, Rabiei K, Marlow T, Jensen C, Guo X, et al. Vascular factors in suspected normal pressure hydrocephalus: a population-based study. Neurology. (2016) 86:592–9. doi: 10.1212/WNL.0000000000002369
11. Johansson E, Ambarki K, Birgander R, Bahrami N, Eklund A, Malm J. Cerebral microbleeds in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS. (2016) 13:4. doi: 10.1186/s12987-016-0028-z
12. Pyykko OT, Nerg O, Niskasaari HM, Niskasaari T, Koivisto AM, Hiltunen M, et al. Incidence, comorbidities, and mortality in idiopathic normal pressure hydrocephalus. World Neurosurg. (2018) 112:e624–e31. doi: 10.1016/j.wneu.2018.01.107
13. Ghaffari-Rafi A, Gorenflo R, Hu H, Viereck J, Liow K. Role of psychiatric, cardiovascular, socioeconomic, and demographic risk factors on idiopathic normal pressure hydrocephalus: a retrospective case-control study. Clin Neurol Neurosurg. (2020) 193:105836. doi: 10.1016/j.clineuro.2020.105836
14. Rasanen J, Huovinen J, Korhonen VE, Junkkari A, Kastinen S, Komulainen S, et al. Diabetes is associated with familial idiopathic normal pressure hydrocephalus: a case-control comparison with family members. Fluids Barriers CNS. (2020) 17:57. doi: 10.1186/s12987-020-00217-0
15. Kuroda T, Honma M, Mori Y, Futamura A, Sugimoto A, Kasai H, et al. White matter lesions may aid in differentiating idiopathic normal pressure hydrocephalus and alzheimer's disease. J Alzheimers Dis. (2022) 85:851–62. doi: 10.3233/JAD-215187
16. Jacobs L. Diabetes mellitus in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. (1977) 40:331–5. doi: 10.1136/jnnp.40.4.331
17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
18. Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery. (2005) 57(3 Suppl):S4–16. doi: 10.1227/01.NEU.0000168185.29659.C5
19. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. (2004) 364:937–52. doi: 10.1016/S0140-6736(04)17018-9
20. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. (2010) 376:112–23. doi: 10.1016/S0140-6736(10)60834-3
21. Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. (1985) 4:213–26. doi: 10.1002/sim.4780040211
22. Kadhim K, Middeldorp ME, Elliott AD, Agbaedeng T, Gallagher C, Malik V, et al. Prevalence and assessment of sleep-disordered breathing in patients with atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol. (2021) 37:1846–56. doi: 10.1016/j.cjca.2021.09.026
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
24. Mori E, Ishikawa M, Kato T, Kazui H, Miyake H, Miyajima M, et al. Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir. (2012) 52:775–809. doi: 10.2176/nmc.52.775
25. Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. (2021) 18:785–802. doi: 10.1038/s41569-021-00559-8
26. Graff-Radford NR, Knopman DS, Penman AD, Coker LH, Mosley TH. Do systolic BP and pulse pressure relate to ventricular enlargement? Eur J Neurol. (2013) 20:720–4. doi: 10.1111/ene.12067
27. Di Rocco C, Pettorossi VE, Caldarelli M, Mancinelli R, Velardi F. Experimental hydrocephalus following mechanical increment of intraventricular pulse pressure. Experientia. (1977) 33:1470–2. doi: 10.1007/BF01918814
28. Bering EA Jr. Circulation of the cerebrospinal fluid demonstration of the choroid plexuses as the generator of the force for flow of fluid and ventricular enlargement. J Neurosurg. (1962) 19:405–13. doi: 10.3171/jns.1962.19.5.0405
29. Belz GG. Elastic properties and windkessel function of the human aorta. Cardiovasc Drugs Ther. (1995) 9:73–83. doi: 10.1007/BF00877747
30. Greitz D. Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev. (2004) 27:145–65. doi: 10.1007/s10143-004-0326-9
31. Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. (2012) 4:147ra11. doi: 10.1126/scitranslmed.3003748
32. Mestre H, Tithof J, Du T, Song W, Peng W, Sweeney AM, et al. Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun. (2018) 9:4878. doi: 10.1038/s41467-018-07318-3
33. Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, et al. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. (2017) 37:1326–37. doi: 10.1177/0271678X16654702
34. Rom S, Zuluaga-Ramirez V, Gajghate S, Seliga A, Winfield M, Heldt NA, et al. Hyperglycemia-driven neuroinflammation compromises bbb leading to memory loss in both diabetes mellitus (DM) type 1 and type 2 mouse models. Mol Neurobiol. (2019) 56:1883–96. doi: 10.1007/s12035-018-1195-5
35. Ringstad G, Vatnehol SAS Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain. (2017) 140:2691–705. doi: 10.1093/brain/awx191
36. Eide PK, Ringstad G. Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: a glymphatic magnetic resonance imaging study. J Cereb Blood Flow Metab. (2019) 39:1355–68. doi: 10.1177/0271678X18760974
37. Reeves BC, Karimy JK, Kundishora AJ, Mestre H, Cerci HM, Matouk C, et al. Glymphatic system impairment in alzheimer's disease and idiopathic normal pressure hydrocephalus. Trends Mol Med. (2020) 26:285–95. doi: 10.1016/j.molmed.2019.11.008
38. Lundin F, Tisell A, Dahlqvist Leinhard O, Tullberg M, Wikkelso C, Lundberg P, et al. Reduced thalamic N-acetylaspartate in idiopathic normal pressure hydrocephalus: a controlled 1H-magnetic resonance spectroscopy study of frontal deep white matter and the thalamus using absolute quantification. J Neurol Neurosurg Psychiatry. (2011) 82:772–8. doi: 10.1136/jnnp.2010.223529
39. Hudson M, Nowak C, Garling RJ, Harris C. Comorbidity of diabetes mellitus in idiopathic normal pressure hydrocephalus: a systematic literature review. Fluids Barriers CNS. (2019) 16:5. doi: 10.1186/s12987-019-0125-x
40. Khan QU, Wharen RE, Grewal SS, Thomas CS, Deen HG Jr, Reimer R, et al. Overdrainage shunt complications in idiopathic normal-pressure hydrocephalus and lumbar puncture opening pressure. J Neurosurg. (2013) 119:1498–502. doi: 10.3171/2013.7.JNS13484
41. Kim KW, Seo H, Kwak MS, Kim D. Visceral obesity is associated with white matter hyperintensity and lacunar infarct. Int J Obes. (2017) 41:683–8. doi: 10.1038/ijo.2017.13
42. Knight SP, Laird E, Williamson W, O'Connor J, Newman L, Carey D, et al. Obesity is associated with reduced cerebral blood flow-modified by physical activity. Neurobiol Aging. (2021) 105:35–47. doi: 10.1016/j.neurobiolaging.2021.04.008
43. Gómez-Apo E, Mondragón-Maya A, Ferrari-Díaz M, Silva-Pereyra J. Structural brain changes associated with overweight and obesity. J Obes. (2021) 2021:6613385. doi: 10.1155/2021/6613385
44. Debette S, Beiser A, Hoffmann U, Decarli C, O'Donnell CJ, Massaro JM, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol. (2010) 68:136–44. doi: 10.1002/ana.22062
45. Schmitt LO, Gaspar JM. Obesity-induced brain neuroinflammatory and mitochondrial changes. Metabolites. (2023) 13:86. doi: 10.3390/metabo13010086
46. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the american heart association. Circulation. (2022) 145:e153–639. doi: 10.1161/CIR.0000000000001052
47. Koto A, Rosenberg G, Zingesser LH, Horoupian D, Katzman R. Syndrome of normal pressure hydrocephalus: possible relation to hypertensive and arteriosclerotic vasculopathy. J Neurol Neurosurg Psychiatry. (1977) 40:73–9. doi: 10.1136/jnnp.40.1.73
48. Ammar A, Abbas F, Al Issawi W, Fakhro F, Batarfi L, Hendam A, et al. Idiopathic normal-pressure hydrocephalus syndrome: is it understood? The comprehensive idiopathic normal-pressure hydrocephalus theory (CiNPHT). In:Ammar A, , editor. Hydrocephalus: What do we know? And what do we still not know? Cham: Springer International Publishing (2017). p. 67–82. doi: 10.1007/978-3-319-61304-8_5
49. Boon AJ, Tans JT, Delwel EJ, Egeler-Peerdeman SM, Hanlo PW, Wurzer HA, et al. Dutch normal-pressure hydrocephalus study: the role of cerebrovascular disease. J Neurosurg. (1999) 90:221–6. doi: 10.3171/jns.1999.90.2.0221
50. Kiefer M, Eymann R, Steudel WI. Outcome predictors for normal-pressure hydrocephalus. Acta Neurochir Suppl. (2006) 96:364–7. doi: 10.1007/3-211-30714-1_75
51. Andren K, Wikkelso C, Sundstrom N, Agerskov S, Israelsson H, Laurell K, et al. Long-term effects of complications and vascular comorbidity in idiopathic normal pressure hydrocephalus: a quality registry study. J Neurol. (2018) 265:178–86. doi: 10.1007/s00415-017-8680-z
52. Badagard H, Braun M, Nilsson D, Stridh L, Virhammar J. Negative predictors of shunt surgery outcome in normal pressure hydrocephalus. Acta Neurol Scand. (2020) 141:219–25. doi: 10.1111/ane.13200
53. Uchigami H, Sato K, Samejima N, Watanabe A, Kuwana N, Tsuchida T, et al. Preoperative factors associated with shunt responsiveness in patients with idiopathic normal-pressure hydrocephalus. Clin Neurol Neurosurg. (2022) 222:107425. doi: 10.1016/j.clineuro.2022.107425
Keywords: idiopathic normal pressure hydrocephalus, vascular risk factors, hypertension, diabetes mellitus, meta-analysis
Citation: Cai H, Yang F, Gao H, Huang K, Qin L, Wang R, Liu Y, Zhou L, Hao Z, Zhou D and Chen Q (2023) Vascular risk factors for idiopathic normal pressure hydrocephalus: a systematic review and meta-analysis. Front. Neurol. 14:1220473. doi: 10.3389/fneur.2023.1220473
Received: 24 May 2023; Accepted: 25 July 2023;
Published: 10 August 2023.
Edited by:
Hao Xu, USTC Life Sciences and Medicine, ChinaReviewed by:
Laura Mori, University of Genoa, ItalyCopyright © 2023 Cai, Yang, Gao, Huang, Qin, Wang, Liu, Zhou, Hao, Zhou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Chen, Y2hlbi5xaW5Ac2N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.