- 1Division of Multiple Sclerosis and Autoimmune Neurology, Department of Neurology, Mayo Clinic, Rochester, MN, United States
- 2Department of Radiology, Mayo Clinic, Rochester, MN, United States
- 3Mayo Clinic College of Medicine and Science, Library-Public Services, Mayo Clinic, Rochester, MN, United States
- 4Department of Neurology, The University of Texas Southwestern Medical Center, Dallas, TX, United States
Those of African American or Latin American descent have been demonstrated to have more severe clinical presentations of multiple sclerosis (MS) than non-Latin American White people with MS. Concurrently, radiological burden of disease on magnetic resonance imaging (MRI) in African Americans with MS has also been described as being more aggressive. Here, we review MRI studies in diverse racial and ethnic groups (adult and pediatric) investigating lesion burden, inflammation, neurodegeneration, and imaging response to disease modifying therapy. We also discuss why such disparities may exist beyond biology, and how future studies may provide greater insights into underlying differences.
1. Introduction
Historically, it was believed that multiple sclerosis (MS) was more common amongst those who are White which has been challenged in recent years, and, in fact, has been demonstrated to be equally as prevalent in those who are Black as it is in the White population in Southern California (1, 2). Despite similar prevalence, MS clinical course descriptors (relapses or progression), and radiological features (new and/or enhancing lesions), in those that are African American and Latin American are more aggressive compared to those who are non-Latin American White (3–6). Furthermore, Black people with MS (pwMS) have the highest mortality under the age of 55 (7). These findings of disparity raise the question of whether discrepancies are due to individual socioeconomical or biological factors such as; 1) general limitation of access to care, including access to MS specialists due to varying levels of insurance coverage and/or simple lack of facilities, 2) general limitation of access to higher efficacy disease modifying therapy (DMT) choices due to the high cost of DMTs, 3) social norms surrounding health issues including cultural differences on the understanding and implications of illness (8), 4) potential for implicit bias leading to different timelines for diagnosis and use of DMT between populations, along with poor encounters with the medical system deterring future engagement, 5) other complicating medical comorbidities, 6) different genetic-epigenetic-immunogenic risk for prognostic determinants of MS (intensity of relapses, location of lesions, recovery potential and neurodegeneration), or 7) other not yet identified factors. It is most likely, however, that some combination of all of these factors plays a role in the differential disease trajectories that are present due to race and ethnicity. To answer the overreaching question of disease variability due to disparity among different races and ethnic backgrounds of MS patients, very large study constructs are needed.
Such additional investigation may guide more personalized treatment strategies, which is important given those of African American descent have been shown to respond less well to interferons (9), but reasonably well to natalizumab (10), dimethyl fumarate (11), and ocrelizumab (12), both clinically and from a neuroimaging perspective. It has also been shown that African American pwMS repopulate their B-cells more quickly than White pwMS, with 33% of African Americans with MS or neuromyelitis optica (NMO) repleting their B-cells, compared to 0% of White patients with MS or NMO between 6 to 12 months after anti-CD20 therapy (13). One potential explanation for this discrepancy is that healthy Black people have a higher baseline CD19 count than White people (14), and so there may be less depletion of B-cells than anticipated because of starting off with a higher B-cell count to begin with (13, 15). Furthermore, plasmablasts are significantly elevated in African Americans and Latin Americans with MS compared to non-Latin American White pwMS (16). Class switching of B-cell populations has been shown to be associated with increased inflammatory activity in MS (16, 17). Taken together, though the data is relatively sparse in the realm of how race and ethnicity can factor into pathophysiological differences in MS, differences in baseline levels of B-cells and class switching to more inflammatory phenotypes could lead to a more robust humoral response, leading to worse disease in African Americans in particular.
The observations of differential responses to DMTs underline that it is paramount to have greater diversity in clinical trials which is currently lacking. A systematic review of MS phase III clinical trials for DMTs from 1995 until 2020 showed that 37.8% of trials did not report on race or ethnicity at all, and that among those that did report on race, 31.1% percent reported on White pwMS only (18, 19).
Magnetic resonance imaging (MRI) is a primary outcome measure for phase II clinical trials and a key secondary outcome measure in phase III clinical trials. MRI is also critical in diagnosis and monitoring of treatment response in MS and has an advanced use in trying to link clinical presentations with specifics of MS pathogenesis as an intermediate biomarker. With the important role of MRI in various facets of MS, we aimed here to systematically review the literature on MRI features in pwMS of different races and ethnicities and speculate on why MRI differences may exist in these populations, as well as how future studies can help address the factors contributing to differences between different racial and ethnic groups with MS.
2. Search strategy
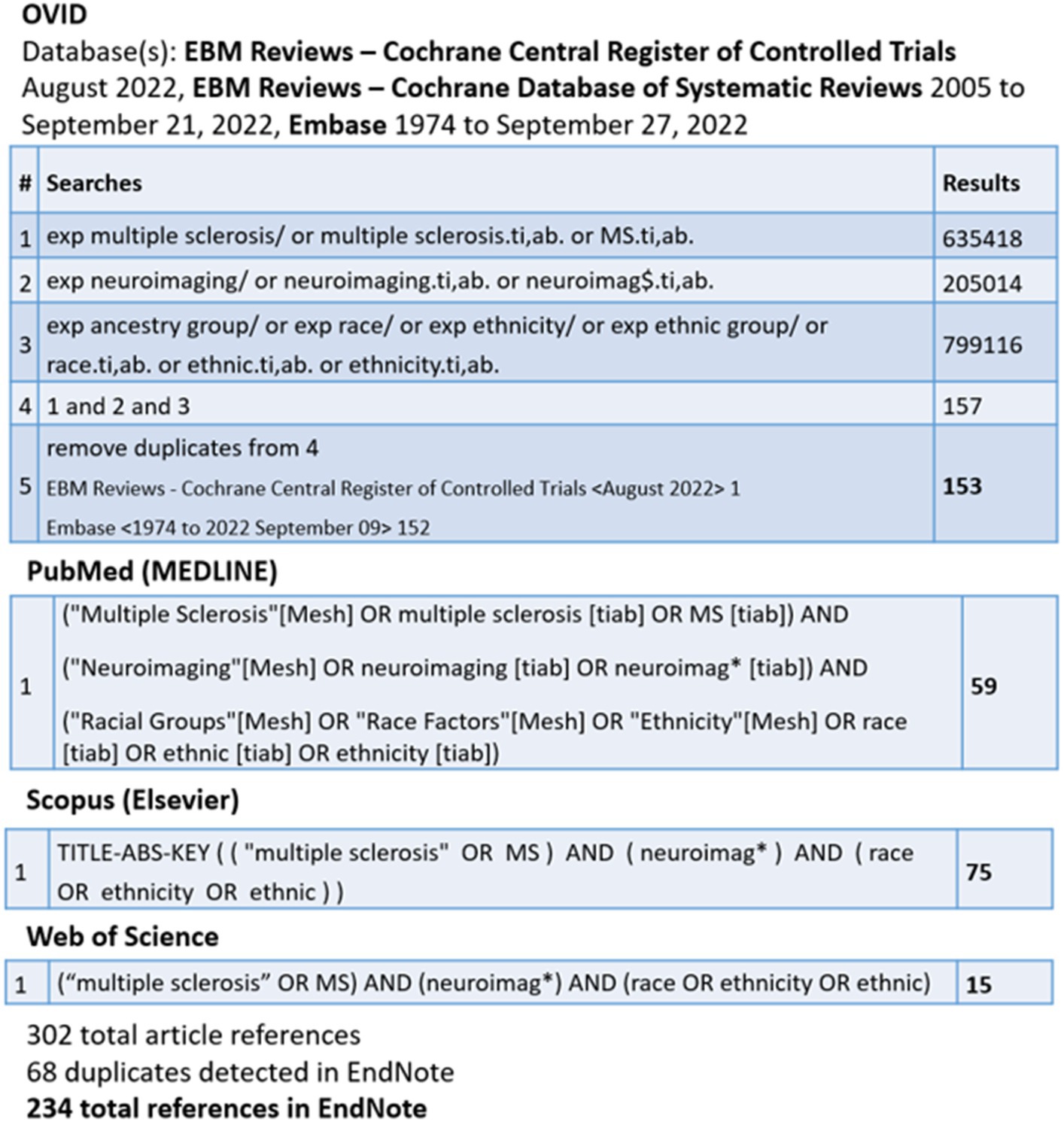
The search strategy (Figure 1) conducted by C.C. yielded 234 results. Only studies published after 2004 were included given others predated the discovery of aquaporin-4 antibody for NMO.
Abstracts were reviewed by N.Na. to determine applicability to the topic of neuroimaging features in pwMS of different races and ethnicities. Pediatric and adult literature were included. Studies looking solely at other demyelinating disorders, such as NMO and myelin oligodendrocyte glycoprotein antibody-associated disease, were excluded. Similarly, papers with a focus on opticospinal MS (in comparison to more classical MS), where some cases of opticospinal MS could be aquaporin-4 negative NMO, were excluded. We did not include papers focused on applicability and reliability of the Barkhof criteria or McDonald criteria for diagnosing MS in different populations, as the focus of this paper was on the use of MRI for pathogenesis and treatment response. Case studies were excluded, though case series were included. Only studies focused on MRI were included; studies using optical coherence tomography were excluded. In the next step, references within the papers included were also reviewed, and any additional applicable papers that were not captured in the search strategy were obtained and included. Relevant abstracts of all languages were reviewed.
3. Details of MRI acquisitions for included studies
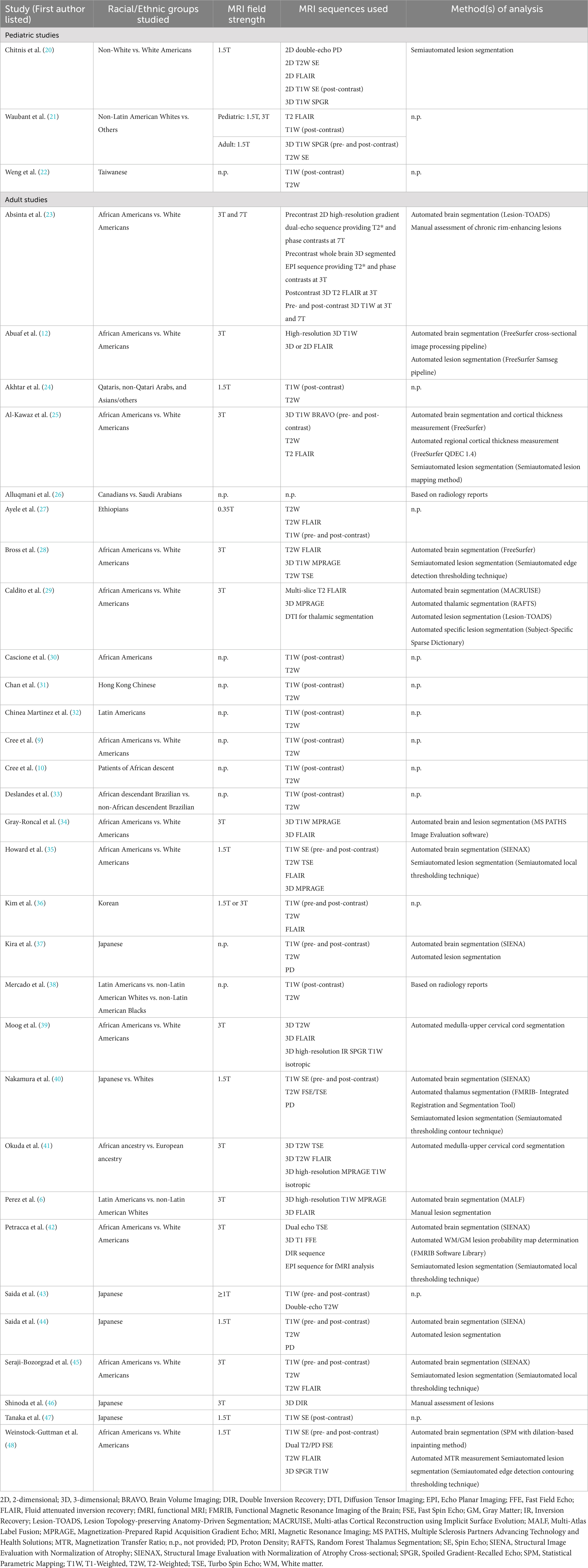
The MRI field strength used, acquisition protocols, and analyses methods used for each study included below are outlined in Table 1. The MRI field strength of studies included ranged from 0.35T to 7T.
4. Pediatric MS studies
4.1. Studies on lesion burden, volume, and location
There is a paucity of studies looking at race and ethnic differences in pediatric MS patients in the realm of neuroimaging. One study found that non-White children with MS defined as any of African American, mixed origin/other, and American Indian, had higher T2 lesion volumes and a larger maximal individual T2 lesion volume than White children with MS (20). They did not find differences in the number of T2 lesions or gadolinium-enhancing lesions (20). Though this study included diverse populations, all non-White children with MS were grouped together, which could be problematic given each population in the non-White group may not necessarily behave similarly clinically, or with respect to neuroimaging. This should be accounted for in the study design which is understandably difficult given the lower numbers of pediatric MS patients that could lead to underpowered studies, but is important, nonetheless.
When looking at lesion locations differing between races and ethnicities, pediatric MS patients in Taiwan had typical involvement of the periventricular and subcortical white matter, but also had considerable thalamic and basal ganglia involvement (22). Another study looking at infratentorial involvement found that there was no independent effect of race or ethnicity when comparing non-Latin American White to others, which was how race and ethnicity were delineated in their study (21). They also did not note an effect of race or ethnicity on initial brain MRI with respect to the number of gadolinium-enhancing lesions, total number of T2 lesions, number of large T2 lesions, or location of T2 lesions. However, they did find that non-Latin American White pediatric MS patients had a significantly higher likelihood of corpus callosum involvement (21).
Taken together, the minimal data to date in pediatric MS suggests that racial and ethnic differences may exist with respect to lesion volume and lesion location. However, there are too few studies conducted in this population to date to come to any real conclusions about trends in MRI findings between racial and ethnic groups in pediatric MS. Notably, no studies have been conducted looking at the differences in atrophy in pediatric MS among those of diverse races and ethnicities. There are also no MRI studies looking at treatment response to DMTs in diverse races and ethnic groups of pediatric MS patients. This is important given it is known that African American pediatric MS patients have a higher relapse rate compared to European-origin White pediatric patients living in the United States (49). Thus, it would be useful to see if there is a relationship between this known increase in relapse rate and MRI findings of more aggressive disease, as this could also help dictate DMT selection, which is critical given there are multiple ongoing clinical trials for DMTs in the pediatric MS population.
5. Adult MS studies
5.1. Studies on lesion burden, volume, and location
Studies looking at lesion burden, volume, and location have primarily included African American and Latin American populations in the United States. Other studies have looked specifically at Japanese MS patients. There are two studies looking at MS in the Middle East. One study was conducted in Ethiopian MS patients.
Across multiple studies, African American patients with MS have been shown to have significantly greater T2 lesion volume compared to White patients with MS (29, 34, 35, 45, 48) with one of these studies determining that this finding remained even after adjusting for age, sex, optic neuritis history, and disease duration (29). In one of these studies, the higher lesion volume in African Americans was due to periventricular and infratentorial lesion volumes being significantly greater than those in White Americans, while juxtacortical and other lesion volumes were not significantly different between groups (34). One study demonstrated that African Americans with MS have a greater number of gadolinium-enhancing lesions compared to White patients with MS (45), indicative of active inflammation. African Americans with MS also have greater T1 lesion volume (35, 48), indicative of severe axonal damage. Few studies have looked specifically at cortical lesions between those of different races and ethnicities, likely in part because other pulse sequences (e.g., double inversion recovery) not part of the standard MS acquisition protocol should be used to optimize detection. One study using 3T MRI that did look at differences in cortical lesion number and volume between African Americans and White Americans with MS found no significant difference between groups with respect to cortical lesion number or volume (42).
With respect to Latin American MS patients, two studies have been conducted. One study found significantly greater T2 lesion volumes in Latin American MS patients compared to non-Latin American White MS patients, and the T2 lesion volumes were correlated with expanded disability status scale (EDSS) scores in Latin American patients (6). Another study looking specifically at Latin American, non-Latin American White, and non-Latin American Black patients in Texas, United States did not find any significant differences between groups with respect to the number of T1, T2, and gadolinium-enhancing lesions at time of diagnosis, nor were there differences between groups in the number of T2 or gadolinium-enhancing lesions involving the spinal cord at diagnosis (38).
Few studies have been conducted in Japanese MS patients. One study compared Japanese MS patients to White MS patients, finding that Japanese patients had significantly larger T2 lesion volume per lesion though no differences in T2 lesion volume in total (40). The T2 lesion volumes had significant correlations with EDSS scores in Japanese patients. With respect to lesion location, Japanese MS patients had lesions in the cerebellum and parietal lobe less frequently than White MS patients (40). Another study looked solely at the number of asymptomatic gadolinium-enhancing lesions in 23 Japanese patients with relapsing–remitting MS (RRMS) due to the presence of gadolinium-enhancing lesions being a pertinent outcome measures in clinical trials; they found that 39% of their cohort had an asymptomatic gadolinium-enhancing lesion with the number of asymptomatic gadolinium-enhancing lesions per scan being 0.37, lower than what has been reported in White MS patients (47). Another study looked specifically at cortical and intracortical lesions in a group of 92 Japanese MS patients using 3T MRI with double inversion recovery (46). This study aimed to relate the presence of cortical lesions with risk alleles, finding that carriers of the HLA-DRB1*1501 had a higher likelihood of and higher number of intracortical lesions than those who were not carriers (46). Interestingly, that study found that those with the HLA-DRB1*0405 allele were less likely to have intracortical lesions (46).
Two studies have looked at MS patients in the Middle East. One was a comparative study, looking at characteristics of RRMS patients in Medina, Saudi Arabia and Edmonton, Canada. More White MS patients in Canada had posterior fossa and spinal cord lesions than Bedouin Arabic MS patients from Saudi Arabia (26). The authors note that the higher propensity for cord lesions in White MS patients in Canada could be confounded by the fact that more spinal cord imaging occurs in Canada (26). The other key factor not addressed in this study was the potential difference in the type and frequency of DMT use in the cohorts studied, which is important because despite the White Canadian MS patients having more infratentorial and spinal cord lesions, EDSS scores were higher in the Saudi Arabian population (26). A different study looked at newly diagnosed MS patients presenting to a tertiary hospital in Doha, Qatar (24). Over half the patients (58%) were Qataris, while 31% were non-Qatari Arabs, with the rest being Asian and classified as “other” (24). Within the Qatari population with follow-up over 36 months, 20% had worsening of EDSS score while radiological disease progression (did not define what progression refers to) was noted in 55% (24). The subgroups of Qataris, non-Qatari Arabs and Asian/other were not compared to know how clinical and radiological activity differed between groups.
There has been one MRI study originating from Africa, in Addis Ababa, Ethiopia. With a 0.35 T MRI scanner, 30 persons with MS were studied, finding classic MRI features of MS with white matter lesions involving the corpus callosum, juxtacortical region, and spinal cord (cervical and thoracic) (27).
Altogether, the clearest trend is that African Americans with MS have greater T2 and gadolinium-enhancing lesion burden compared to White pwMS. Latin Americans with MS have more variable MRI data, which may relate to the fact that they are cited to have a lower incidence of MS compared to Whites with MS (50, 51), making studies potentially underpowered to detect significant differences.
5.2. Studies looking at atrophy
Measures of brain atrophy have been shown to correlate better with clinical disability than T2 lesion burden (52–54). Accordingly, studies have been conducted comparing degree of atrophy among those with MS of different races and ethnicities.
Multiple studies have shown that African American pwMS have lower brain volumes than White pwMS with respect to total grey matter volume (34, 45) and cortical thickness (25, 28, 29). One of these studies found an association between reduced grey matter volume and elevated CSF IgG index in African Americans (45). Some studies have also looked at region-specific differences and found that there is greater cerebellar atrophy (42), thalamic atrophy (29, 34) and deep grey matter atrophy (34) in African Americans compared to White Americans. However, one study demonstrated that White Americans had significantly lower thalamic volume compared to African Americans with MS. Notably, this was measured relatively early in the disease course, where patients had an average disease duration of less than 7 years (25). One study also did not find any differences between groups looking at global brain volume (35). Two studies to date have looked at spinal cord volumes. One of these studies found that African American pwMS had greater total spinal cord atrophy, and more specifically, also within the ventral and dorsal regions, compared to White pwMS (39). The other study demonstrated that Europeans with MS had greater volume loss at the medulla-upper cervical spinal cord region than African Americans, but that African Americans had greater baseline pontine volume loss, which continued to worsen at one-year follow-up (41).
Similar relationships have been seen when comparing Latin American pwMS to non-Latin American White pwMS, with significantly lower total brain volume, cortex volume, basal ganglia volume, and lower white matter volumes in Latin American pwMS compared to White pwMS (6). Lower baseline thalamic volume portended a higher risk for progression in the Latin American group (6). Importantly, there were no significant differences between the two groups with respect to demographics (smoking, body mass index, comorbidities, baseline vitamin D levels, educational attainment), age of onset, diagnostic lag, or treatment duration, suggesting that disease is inherently worse in Latin Americans (6). Conversely, a study from Texas, United States comparing non-Latin American White, Latin American, and non-Latin American Black pwMS did not find a significant difference in brain atrophy or spinal cord atrophy between groups (38). However, the number of non-Latin American White pwMS included in this study was low at 11, and thus could have been underpowered to detect differences between groups.
One study compared brain volumes in Japanese MS patients to White MS patients, finding that the Japanese MS patients had significantly lower total brain volume, white matter volume, deep grey matter volume, and thalamic volume compared to White MS patients (40). However, despite the lower volumes, Japanese MS patients had significantly less ambulatory impairment with lower EDSS scores than White MS patients (40). Cognitive and higher cortical function associations were not studied. Medical comorbidities were also unaccounted for. This raises the speculation of how differential reserves of function or spinal cord involvement pattern relate to clinical disability, questions that remain mostly unanswered in studies to date.
The abovementioned study from Ethiopia also looked at atrophy, finding global cortical and corpus callosum atrophy in a minority of persons with MS (27).
5.3. Studies using non-conventional MRI metrics
Few studies have used non-conventional “advanced” structural MRI methods. One study using magnetization transfer ratio demonstrated that African Americans with MS had lower magnetization transfer ratio values in lesions, normal-appearing grey matter, and normal-appearing white matter (all in the range of 10–15%) compared to White Americans with MS (48). This would suggest potentially different myelin structure or content between African Americans and White Americans with MS. Another study used both 3 T and 7 T MRI to obtain susceptibility-based MRI sequences including T2* and phase contrast to look at paramagnetic rim lesions in both White Americans and African Americans (23). African Americans were not found to have a statistically greater number of rim lesions compared to White Americans (23). Only one known study to date has been conducted with functional MRI (42). That study used a simple motor task of flexion and extension of the last 4 digits of the right hand, finding that African Americans had decreased activation of the prefrontal cortex compared to White Americans with MS (42). This could be indicative of impaired compensatory activity in African Americans (42).
5.4. Studies using MRI as an outcome measure for treatment response to DMTs
Studies have been conducted looking at the effects of various MS DMTs on MRI outcomes in those of diverse racial and ethnic backgrounds (Table 2).
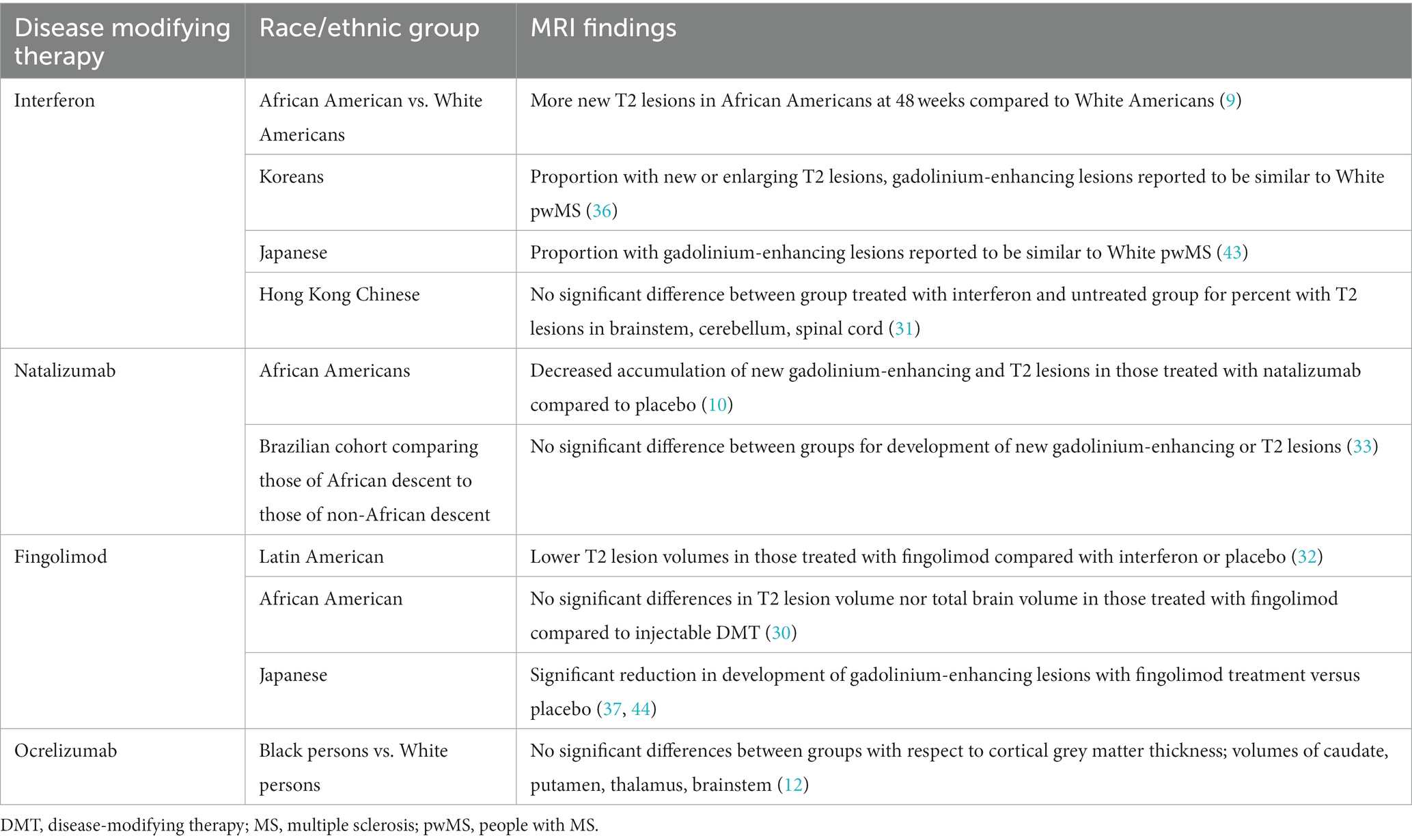
Table 2. Effects of various disease modifying therapies for multiple sclerosis on MRIs in those of diverse racial and ethnic groups.
5.4.1. Interferons
Variable responses to DMTs between racial and ethnic groups were first demonstrated in an exploratory post-hoc analysis of the European North American Comparative Efficacy (EVIDENCE) study, which compared different formulations of interferon beta-1a (Avonex vs. Rebif). This post-hoc analysis demonstrated that compared to White Americans, African Americans were less likely to remain relapse-free and developed significantly more new T2 lesions on MRI at 48 weeks (9). However, whether the patients who had relapses and new MRI lesion development had neutralizing antibodies to interferons was unknown in this study.
The remainder of studies looking at the use of interferons in non-White pwMS have been conducted in Asian countries. A retrospective study looking at five centers in Korea found that 55% of their study population was free of new or enlarging T2 lesions, and gadolinium-enhancing lesions, reportedly similar to White pwMS. However, a comparative percentage in White pwMS or number of new or enlarging T2 lesions in the Korean population was not provided in this study (36). Similar results were seen in a study of Japanese RRMS patients with those on interferon-beta 1a treated for 6 months, with a significant reduction in the number of gadolinium-enhancing lesions in the pre-treatment versus post-treatment periods with values indeed similar to what has been seen in White pwMS (43). Conversely, there was a study looking at Hong Kong Chinese pwMS where no significant differences were seen in the percent with T2 lesions in the brainstem, cerebellum, and spinal cord between those treated with interferon beta-1a and not, which corresponded clinically with no significant differences in EDSS between groups (31). However, exclusion of NMO was done via aquaporin-4 antibody testing using indirect immunofluorescence, not the contemporary cell-based assay, which begs the question of whether cases of NMO were inadvertently included, which does not respond well to interferons. Testing for neutralizing antibodies to interferons was also not undertaken in this study.
Overall, given the known mechanism of action of interferon in MS, these results would suggest potentially higher disease activity potential in African American MS patients where the relatively lower efficacy DMTs (compared to higher efficacy drugs discussed later) may be insufficiently effective. Alternative explanations as related to immune activation patterns that may be determined by ethnicity can also be more obvious than with higher efficacy DMTs. The results may highlight the reason to consider higher efficacy DMTs as first-line treatment in African American patients. This is further suggested by the studies below.
5.4.2. Natalizumab
Post-hoc analysis was undertaken looking at the African Americans included in the Natalizumab Safety and Efficacy in RRMS (AFFIRM) and Safety and Efficacy of Natalizumab in Combination With Interferon Beta-1a in Patients with RRMS (SENTINEL) studies (10). When comparing those that were treated with natalizumab versus placebo, there was reduced annualized relapse rate (ARR) and accumulation of new MRI lesions over 2 years including both gadolinium-enhancing lesions and T2 lesions in those treated with natalizumab (10). A different study looked at a Brazilian cohort, comparing African descendants to non-African descendants with MS, finding that there was no significant difference between the two groups with respect to development of new gadolinium-enhancing or T2 lesions with natalizumab treatment, with both groups responding well to treatment (33). Altogether, though studies have been minimal, Black pwMS seem to benefit from treatment with natalizumab. The reasons why this is the case are unclear; it could be that natalizumab is a high efficacy medication and would be beneficial, irrespective of race or ethnicity.
5.4.3. Fingolimod
Post-hoc analysis was undertaken for Latin American pwMS for the FTY720 Research Evaluating Effects of Daily Oral therapy in MS (FREEDOMS, FREEDOMS II) studies comparing fingolimod to placebo and Trial Assessing Injectable Interferon vs. FTY720 Oral in RRMS (TRANSFORMS) which compared fingolimod to interferon beta-1a (32). Latin Americans with MS treated with fingolimod had lower T2 lesion volumes compared to those treated with interferon beta-1a or placebo along with reduced ARR for 2 years (32).
Post-hoc analysis of the 141 African Americans with MS included in the Prospective, Randomized, active-controlled, open-label study to Evaluate patient retention on Fingolimod versus approved first-line disease-modifying therapies in adults with Relapsing–remitting MS (PREFERMS) comparing fingolimod to an injectable DMT showed no significant differences between groups with respect to T2 lesion volume or normalized brain volume (30).
A randomized-controlled trial (RCT) was undertaken in Japanese people with RRMS comparing fingolimod at a dose of 0.5 mg or 1.25 mg versus placebo for 6 months, with the primary endpoint being percentages of patients without development of gadolinium-enhancing lesions at follow-up at three and 6 months (44). Fingolimod treatment at either dose was significantly more effective than placebo, with 70–86% not developing gadolinium-enhancing lesions at three and 6 months compared to 40% in the placebo group (44). This work was then extended to 12 months with all participants being put on fingolimod 0.5 mg with maintenance of a high percentage being free of gadolinium-enhancing lesions at months 7–12 (all greater than 80%) (37). Similar proportions were free of new or enlarging T2 lesions (37). There was also a higher proportion of subjects with reduced ARR at months 7–12 compared to months 0–6 (37).
Although clearly effective in the Latin American and Japanese populations with MS, the relative efficacy of fingolimod with respect to MRI outcomes specifically in the African American population is less clear. This is pertinent given the use of not just fingolimod, but the other S1P inhibitors as a DMT with efficacy that is in the middle of the spectrum, which could provide a viable oral option; this would be critical in situations where access to an infusion center is limited.
5.4.4. Ocrelizumab
A retrospective study was conducted on Black and White pwMS on ocrelizumab with MRIs being obtained in a proportion of the study subjects at baseline and year two (12). No significant differences were present between groups comparing cortical grey matter thickness, along with volumes of the thalamus, caudate, putamen, and brainstem (12).
6. Discussion
Although the number of studies conducted in the realm of neuroimaging features among diverse racial and ethnic groups with MS remains low, certain trends are apparent. First, African American pwMS consistently have worse MRI findings (higher lesion burden, substantial atrophy) compared to White pwMS. Second, data in Latin American pwMS is more variable which may relate to their lower cited incidence of MS compared to non-Latin American White pwMS (50, 51) as mentioned above, but may also relate to underdiagnosis in certain Latin American countries (50). Third, though studies have been minimal in Asian populations, it appears that Japanese pwMS may have higher MRI burden though can have lower EDSS and respond well to DMTs. Part of this could pertain to the relatively lower number of lesions in the cerebellum compared to those who are White in one study (40), but otherwise why this is the case is unclear, and if this relationship will hold true when higher numbers of patients are studied is uncertain. Comparing the presence of lesions specifically in the brainstem and spinal cord in Japanese versus White pwMS could also be useful as these tend to lead to greater clinical disability which could translate to a higher EDSS.
6.1. DMTs: response to treatment, discontinuation of treatment, and access to treatment
In the realm of treatment, there is a pressing need for studies using DMTs in diverse racial and ethnic groups with MS with MRI as an outcome measure, given different responses to treatment, particularly in the African American population. As we regularly factor the presence of medical comorbidities into our DMT selection for MS patients, so too should racial or ethnic group based on data we have to date (e.g., perhaps avoiding interferons in African Americans and instead opt for dimethyl fumarate, natalizumab, or ocrelizumab). Given the increasing number of anti-CD20 therapies coming down the pipeline and their high frequency of use, we need more data on how responses to treatment varies between racially and ethnically diverse pwMS. There has been speculation that elevated CSF IgG index in African Americans with MS could signal that use of anti-CD20 therapies is indicated, given its effect on the humoral immune system (55). Notably, there are no known studies using certain oral options including teriflunomide, siponimod, ozanimod, or cladribine. Dimethyl fumarate has shown clinical efficacy in African Americans with MS though MRI data was unavailable in that study (11).
Along with looking at treatment efficacy, data is needed on the safety profile of these agents in diverse populations. Although higher efficacy treatments seem to be beneficial in African American pwMS, painting all patients of a particular racial or ethnic group with the same brush can be dangerous, as higher efficacy treatments come with more potentially severe adverse events, including death, albeit rarely.
Furthermore, discontinuation of DMTs is an important factor to consider and reasons for this are diverse. One study showed that African Americans or other Black pwMS changed DMT due to injection fatigue (with desire for an oral medication) and subjective perceived lack of efficacy more frequently than White pwMS, but that they were less likely to switch DMT based on side effects (56). These are pertinent issues to keep in mind when pondering different clinical MS trajectories which could, in part, relate to persistence of staying on a specific DMT and the reasons why some pwMS may not stay on a prescribed DMT. Furthermore, the magnitude of DMT waste in this study was over one million dollars in 1 month, with the majority switching therapies for reasons other than inadequate disease control and intolerability (56). This raises the question of whether these DMTs being wasted could be used in populations who have inadequate access to DMTs due to difficulties with insurance coverage. All of this comes around to potentially impacting MRI outcomes from an access issue rather than direct biological differences in various ethnic groups.
Lastly, access to physicians and DMTs vary by region and not by ethnicity alone. Not all DMTs are readily available in every country, with studies from South Africa (57), Sudan (58), and Zambia (59) citing this issue, yet there is increasing knowledge that MS is present in populations in most countries worldwide. It is essential to find accessible and effective treatments that can be utilized in the international population with MS. Otherwise, comparisons are difficult to make between different populations. One way to get around this is in migrant populations who have moved from low access to higher access areas and get diagnosed with MS afterwards. In the absence of elapsed “generational” time for exposure, most differences will be driven by the genetic background. These types of migration studies were done in MS originally, but not necessarily with race and ethnicity stratification.
6.2. Possible reasons for variable MRI findings in those of diverse races and ethnicities with MS: pathophysiology
The reasons underlying the findings of different MRI features in those of different races and ethnicities of pwMS remain understudied. Moving forward, rather than simply outlining the MRI features seen between populations, future studies should also aim to elucidate what factors could be contributing to these differences, which few studies have done. Such factors include access to care, social determinants of health, cultural factors, systemic racism and implicit bias, genetic risk variants, presence of cardiovascular comorbidities, smoking status, Epstein–Barr virus (EBV) exposure, and vitamin D deficiency, amongst others (Figure 2). With respect to causal factors for MS, there is a differential effect of EBV on MS in diverse populations. Black and Latin American people have higher Epstein–Barr nuclear antigen-1 (EBNA-1) titers and frequency of seropositivity compared to White people (60), which is relevant given the greatly increased risk of developing MS in those with EBV infection (61). Having a history of infectious mononucleosis due to EBV in Black people leads to an odds ratio (OR) of 4.43 of developing MS or clinically isolated syndrome (CIS) compared to Latin Americans with an OR of 3.36, both greater than in White people with an OR of 2.24 for developing MS (60). EBV infection causes long-term changes in the host cytokine response and prolongs the survival of memory B-cells which could drive inflammation (62). Interestingly, having cytomegalovirus (CMV) positivity was associated with a lower risk of CIS and MS in Latin Americans, even when accounting for HLA-DRB*1501 status and EBNA-1 positivity (60).
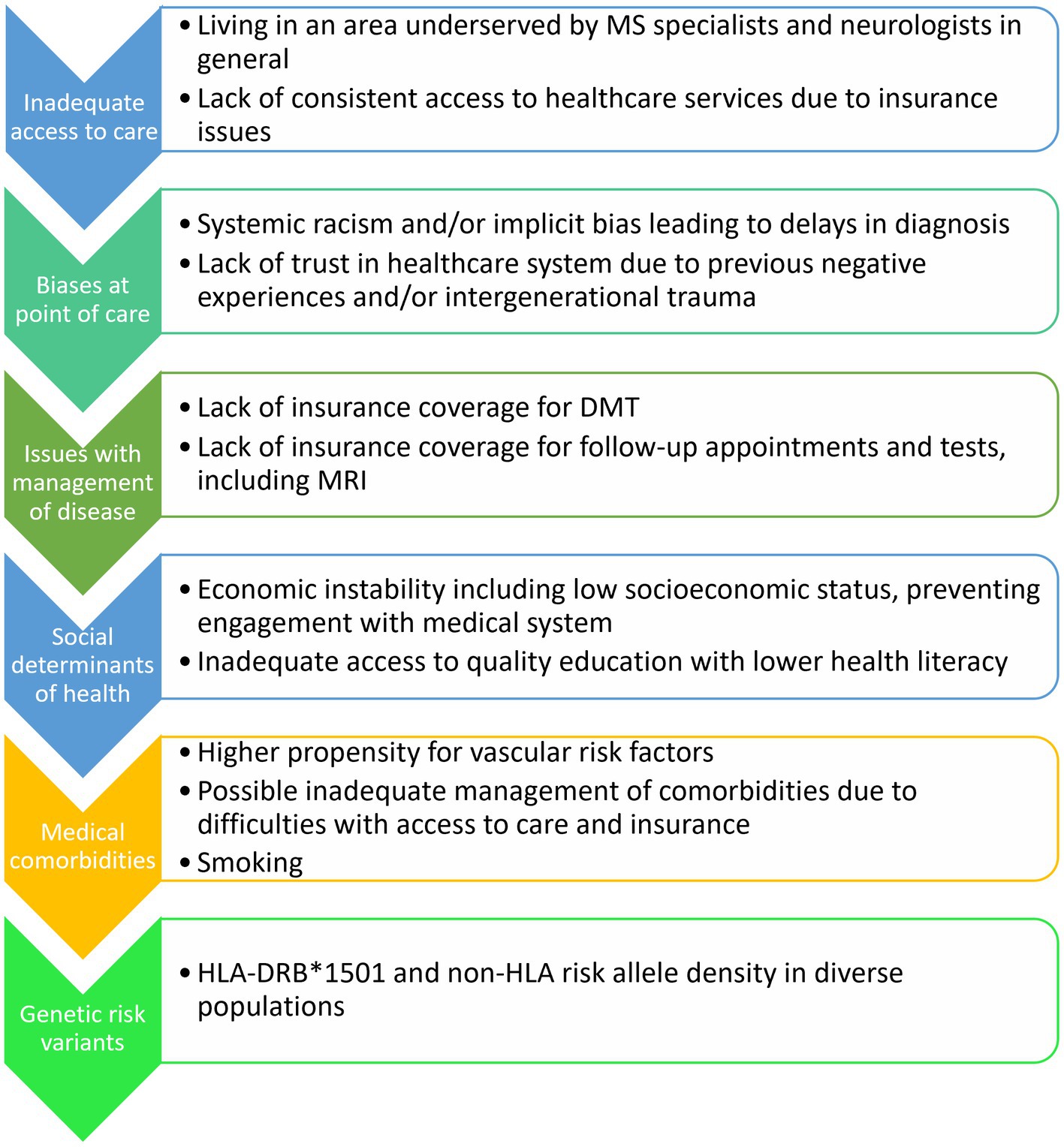
Figure 2. Potential factors contributing to MRI and clinical differences in diverse racial and ethnic groups with MS.
Another risk factor associated with development of MS is vitamin D deficiency. African Americans have lower vitamin D levels than non-Latin American Whites and Mexican Americans in the general population (63). This is partially due to differences in melanin levels in the skin, with darker pigmented skin limiting cutaneous vitamin D synthesis, and requiring longer UV exposure to produce the same amount of vitamin D. African American pwMS have lower vitamin D levels than controls based on differences in climate and geography (64). One study showed that having lower vitamin D levels is associated with attack severity with optic neuritis in pwMS (65) while another did not find a relationship between vitamin D deficiency and disease severity (64). Taken together, the predisposition of African Americans to vitamin D deficiency could lead to development of MS and more severe clinical disease. The relationship between vitamin D deficiency and MRI outcomes in diverse races and ethnic populations with MS warrants further exploration.
6.3. Possible reasons for variable MRI findings in those of diverse races and ethnicities with MS: social determinants of health
Social determinants of health include both individual and structural factors encompassing five domains: economic stability, education, health and health care, neighborhood and built environment, and social and community context (66, 67). A review has demonstrated that Black pwMS and Latin American pwMS have issues in all of these areas compared to non-Latin American White pwMS including lower income, lower educational attainment, lower health literacy, and higher unemployment rates, all of which could contribute to a worse MS prognosis in Black and Latin American pwMS (66). Being of a lower socioeconomic status can preclude access to higher efficacy DMT (67). These inequities in social determinants of health can also stem from systemic racism and other forms of discrimination, which invariably impact Black and Latin American pwMS a disproportionate amount and can lead to increased mortality (66, 68). Along with this comes added complexity of previous poor encounters with the medical system with potential for intergenerational trauma, which can deter accessing care.
The creation of a National African Americans with MS Registry (NAAMSR) in September 2020 is aimed at determining the impact of social determinants of health on access to care, initiation of DMT, and long-term health outcomes in 20,000–30,000 African Americans with MS (8); this will be an invaluable addition to our knowledge on the impacts of social determinants of health on African Americans with MS at a much larger scale than any data we have at present. It would be of interest to look at MRIs of some of those in this registry to see how T2 lesion burden, gadolinium-enhancing lesion burden, and brain and spinal cord volumes change over time, and how this relates to clinical trajectory and DMT use.
6.4. Possible reasons for variable MRI findings in those of diverse races and ethnicities with MS: access to care
Issues with access to care have been well-described in certain populations with MS. African Americans are less likely to seek MS care from a neurologist, though were also more likely to be disabled, which was a risk factor for not seeking care from a neurologist (69). The specific reasons other than disability as to why African Americans were less likely to seek care from a neurologist were not discussed further. One could speculate that the known high cost of DMTs, need to access an infusion center (if using an anti-CD20 therapy like ocrelizumab), and perhaps general lack of connection with a provider of a different racial and ethnic background could factor in.
6.5. Possible reasons for variable MRI findings in those of diverse races and ethnicities with MS: genetic risk
With respect to genetic risk, African Americans with the HLA-DRB*1501 risk allele are three times more likely to develop MS than those with the African haplotype (70) and Japanese MS patients with the HLA-DRB*1501 risk allele are more likely to have intracortical lesions associated with more severe disease (46).
6.6. How to increase MRI research in diverse racial and ethnic groups with MS
An additional noteworthy issue is access to MRI scanners. There are an average of 0.7 MRI scanners per million people in Africa compared to 40 MRI scanners per million people in the United States (71). In areas where studies are being undertaken that have poor access to MRIs (including both in North America and beyond), low-field portable MRI could be considered, which can detect white matter lesions in MS (72, 73), though this would not necessarily help address the issue of cost. Low-field MRI (0.35T) has been used to aid in diagnosis and management of MS in Ethiopia (27). Upon procuring or building low-field MRIs in low- and middle-income countries, additional considerations come to mind. The first is that the MRI system needs to be functional; in nearly 40% of cases, low-field MRI scanners in Africa were obsolete systems (74). A second aspect is the need for software that is open source to enable better understanding of how to use and repair the MRI (71). A third consideration is how to carry out image analysis which has been proposed to be helped with the use of artificial intelligence (71). In the field of MS, this could relate to automated detection of white matter lesions and measuring brain volume. Lastly, with MRI scanners, trained personnel are needed to operate the MRI scanner, perform necessary maintenance, and to analyze the MRIs. In 2019, the Consortium for Advancement of MRI Education and Research in Africa (CAMERA) was created and assessed MRI use in sub-Saharan Africa, identifying gaps not just in acquisition of MRI scanners, but also the abovementioned issues about maintenance and personnel as well (71, 74). In order to build capacity and sustainable use of MRI in low- and middle-income countries worldwide, high-income countries will need to invest in this, which can also be aided by international imaging societies.
Even if access to MRIs is obtained, though low field can be used, many novel imaging biomarkers require more advanced imaging techniques (e.g., central vein sign, which requires a field strength >1.5T). Therefore, development of practical, easy to apply, and affordable imaging metrics to limit misdiagnosis and monitor treatment efficacy and disability is important to overcome race diversity differences. One example would be the use of manual C5 level spinal cord area measurement as a practical biomarker of disease progression (75). Increasing awareness of and access to such imaging methods should be prioritized.
7. Conclusions and future directions
The current review highlights that differences in MRI findings in diverse racial and ethnic groups with MS may be offering clues in this area, may be offering clues to not only the biology of MS but also providing a potential roadmap of personalizing treatment of MS patients with ethnicity being a consideration in addition to age, sex, efficacy, safety profile, access to health care and cost effectiveness. Even with race being a social construct, the reality is that there are differences present between these groups which need to be addressed to provide more equitable care. Ultimately, we should aim to diagnose and manage all of our MS patients well, regardless of race or ethnicity, with a personalized approach and aim for equity in MS care, which necessitates further inquiry into why differences between those of diverse races and ethnicities with MS exist, with MRI providing a key tool for investigation. To help increase MRI studies in those of diverse racial and ethnic groups with MS, there needs to be better access to MRI scanners worldwide, and use of scanners, ideally with software and analysis methods that are open access. In addition to international imaging societies, international MS interest committees (e.g., Latin American Committee for Treatment and Research in MS [LACTRIMS] and Middle East North Africa Committee for Treatment and Research in MS [MENACRTRIMS]) can be part of the effort to acquire and operationalize regular use of MRI scanners to acquire data on diverse populations, given seemingly poorer outcomes in non-White persons with MS, both clinically and radiologically. With improved access and use of MRIs, studies can look at the relationship between imaging findings and the relative contributions of medical comorbidities, inadequate access to care, social determinants of health, cultural factors, systemic racism, and genetic risk variants, all of which warrant further exploration in future studies to help fill in the gaps of why such imaging differences exist.
Author contributions
NNa, BZ, DO, and OK contributed to the conception and design of the study. NNa wrote the first draft of the manuscript. NNe contributed to the generation of content for figures and tables. CC aided in acquisition of the data and generation of content for figures. All authors contributed to the article and approved the submitted version.
Conflict of interest
DO received personal compensation for consulting and advisory services from Alexion, Biogen, Celgene/Bristol Myers Squibb, EMD Serono, Genentech, Genzyme, Janssen Pharmaceuticals, Novartis, Osmotica Pharmaceuticals, RVL Pharmaceuticals, Inc., TG Therapeutics, and research support from Biogen, Novartis, and EMD Serono/Merck. DO has issued national and international patents along with pending patents related to other developed technologies. DO received royalties for intellectual property licensed by The Board of Regents of The University of Texas System. BZ receives research funding from the NIH (U54 AG044170) and is supported by the Mayo Clinic Eugene and Marcia Applebaum Award.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Langer-Gould, A, Brara, SM, Beaber, BE, and Zhang, JL. Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology. (2013) 80:1734–9. doi: 10.1212/WNL.0b013e3182918cc2
2. Langer-Gould, AM, Gonzales, EG, Smith, JB, Li, BH, and Nelson, LM. Racial and ethnic disparities in multiple sclerosis prevalence. Neurology. (2022) 98:e1818–27. doi: 10.1212/WNL.0000000000200151
3. Amezcua, L, and McCauley, JL. Race and ethnicity on MS presentation and disease course. Mult Scler. (2020) 26:561–7. doi: 10.1177/1352458519887328
4. Cree, BA, Khan, O, Bourdette, D, Goodin, DS, Cohen, JA, Marrie, RA, et al. Clinical characteristics of African Americans vs Caucasian Americans with multiple sclerosis. Neurology. (2004) 63:2039–45. doi: 10.1212/01.WNL.0000145762.60562.5D
5. Mowry, EM, Pesic, M, Grimes, B, Deen, SR, Bacchetti, P, and Waubant, E. Clinical predictors of early second event in patients with clinically isolated syndrome. J Neurol. (2009) 256:1061–6. doi: 10.1007/s00415-009-5063-0
6. Perez, CA, Salehbeiki, A, Zhu, L, Wolinsky, JS, and Lincoln, JA. Assessment of racial/ethnic disparities in volumetric MRI correlates of clinical disability in multiple sclerosis: a preliminary study. J Neuroimaging. (2021) 31:115–23. doi: 10.1111/jon.12788
7. Amezcua, L, Rivas, E, Joseph, S, Zhang, J, and Liu, L. Multiple sclerosis mortality by race/ethnicity, age, sex, and time period in the United States, 1999-2015. Neuroepidemiology. (2018) 50:35–40. doi: 10.1159/000484213
8. Okai, AF, Howard, AM, Williams, MJ, Brink, JD, Chen, C, Stuchiner, TL, et al. Advancing care and outcomes for African American patients with multiple sclerosis. Neurology. (2022) 98:1015–20. doi: 10.1212/WNL.0000000000200791
9. Cree, BA, Al-Sabbagh, A, Bennett, R, and Goodin, D. Response to interferon beta-1a treatment in African American multiple sclerosis patients. Arch Neurol. (2005) 62:1681–3. doi: 10.1001/archneur.62.11.1681
10. Cree, BA, Stuart, WH, Tornatore, CS, Jeffery, DR, Pace, AL, and Cha, CH. Efficacy of natalizumab therapy in patients of African descent with relapsing multiple sclerosis: analysis of AFFIRM and SENTINEL data. Arch Neurol. (2011) 68:464–8. doi: 10.1001/archneurol.2011.45
11. Williams, MJ, Amezcua, L, Okai, A, Okuda, DT, Cohan, S, Su, R, et al. Real-world safety and effectiveness of dimethyl fumarate in black or African American patients with multiple sclerosis: 3-year results from ESTEEM. Neurol Ther. (2020) 9:483–93. doi: 10.1007/s40120-020-00193-5
12. Abuaf, AF, Javed, A, Bunting, SR, Carroll, TJ, Reder, AT, and Cipriani, VP. Effectiveness of ocrelizumab on clinical and MRI outcome measures in multiple sclerosis across black and white cohorts: a single-center retrospective study. Mult Scler Relat Disord. (2023) 71:104523. doi: 10.1016/j.msard.2023.104523
13. Saidenberg, L, Arbini, AA, Silverman, GJ, Lotan, I, Cutter, G, and Kister, I. Faster B-cell repletion after anti-CD20 infusion in black patients compared to white patients with neurologic diseases. Mult Scler Relat Disord. (2022) 63:103830. doi: 10.1016/j.msard.2022.103830
14. Tollerud, DJ, Clark, JW, Brown, LM, Neuland, CY, Pankiw-Trost, LK, Blattner, WA, et al. The influence of age, race, and gender on peripheral blood mononuclear-cell subsets in healthy nonsmokers. J Clin Immunol. (1989) 9:214–22. doi: 10.1007/BF00916817
15. Abbadessa, G, Miele, G, Cavalla, P, Valentino, P, Marfia, GA, Signoriello, E, et al. CD19 cell count at baseline predicts B cell repopulation at 6 and 12 months in multiple sclerosis patients treated with Ocrelizumab. Int J Environ Res Public Health. (2021) 18:8163. doi: 10.3390/ijerph18158163
16. Telesford, KM, Kaunzner, UW, Perumal, J, Gauthier, SA, Wu, X, Diaz, I, et al. Black African and Latino/a identity correlates with increased plasmablasts in MS. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e634. doi: 10.1212/NXI.0000000000000634
17. Eggers, EL, Michel, BA, Wu, H, Wang, SZ, Bevan, CJ, Abounasr, A, et al. Clonal relationships of CSF B cells in treatment-naive multiple sclerosis patients. JCI Insight. (2017) 2:e92724. doi: 10.1172/jci.insight.92724
18. Khan, O, Williams, MJ, Amezcua, L, Javed, A, Larsen, KE, and Smrtka, JM. Multiple sclerosis in US minority populations: clinical practice insights. Neurol Clin Pract. (2015) 5:132–42. doi: 10.1212/CPJ.0000000000000112
19. Onuorah, HM, Charron, O, Meltzer, E, Montague, A, Crispino, A, Largent, A, et al. Enrollment of non-white participants and reporting of race and ethnicity in phase III trials of multiple sclerosis DMTs: a systematic review. Neurology. (2022) 98:e880–92. doi: 10.1212/WNL.0000000000013230
20. Chitnis, T, Guttmann, CR, Zaitsev, A, Musallam, A, Weinstock-Guttman, B, Yeh, A, et al. Quantitative MRI analysis in children with multiple sclerosis: a multicenter feasibility pilot study. BMC Neurol. (2013) 13:173. doi: 10.1186/1471-2377-13-173
21. Waubant, E, Chabas, D, Okuda, DT, Glenn, O, Mowry, E, Henry, RG, et al. Difference in disease burden and activity in pediatric patients on brain magnetic resonance imaging at time of multiple sclerosis onset vs adults. Arch Neurol. (2009) 66:967–71. doi: 10.1001/archneurol.2009.135
22. Weng, WC, Yang, CC, Yu, TW, Shen, YZ, and Lee, WT. Multiple sclerosis with childhood onset: report of 21 cases in Taiwan. Pediatr Neurol. (2006) 35:327–34. doi: 10.1016/j.pediatrneurol.2006.05.002
23. Absinta, M, Sati, P, Masuzzo, F, Nair, G, Sethi, V, Kolb, H, et al. Association of Chronic Active Multiple Sclerosis Lesions with Disability in Vivo. JAMA Neurol. (2019) 76:1474–83. doi: 10.1001/jamaneurol.2019.2399
24. Akhtar, N, Elsetouhy, A, Deleu, D, Kamran, S, AlHail, H, Elalamy, O, et al. Newly diagnosed multiple sclerosis in state of Qatar. Clin Neurol Neurosurg. (2013) 115:1333–7. doi: 10.1016/j.clineuro.2012.12.035
25. Al-Kawaz, M, Monohan, E, Morris, E, Perumal, JS, Nealon, N, Vartanian, T, et al. Differential impact of multiple sclerosis on cortical and deep Gray matter structures in African Americans and Caucasian Americans. J Neuroimaging. (2017) 27:333–8. doi: 10.1111/jon.12393
26. Alluqmani, M, Roda, W, Qqrmli, M, Blevins, G, Giuliani, F, and Power, C. Differential disease phenotypes and progression in relapsing-remitting multiple sclerosis: comparative analyses of single Canadian and Saudi Arabian clinics. BMC Neurol. (2021) 21:295. doi: 10.1186/s12883-021-02317-2
27. Ayele, BA, Wuhib, MZ, Zenebe, BG, Zewde, YZ, Wolde, YT, and Metaferia, GZ. Neuroimaging features and associated factors in multiple sclerosis patients: a perspective from a private Care Center in Addis Ababa, Ethiopia. Ethiop J Health Sci. (2021) 31:1043–52. doi: 10.4314/ejhs.v31i5.17
28. Bross, M, Hackett, M, Bernitsas, MM, Bao, F, Carla Santiago, M, and Bernitsas, E. Cortical surface thickness, subcortical volumes and disability between races in relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. (2021) 53:103025. doi: 10.1016/j.msard.2021.103025
29. Caldito, NG, Saidha, S, Sotirchos, ES, Dewey, BE, Cowley, NJ, Glaister, J, et al. Brain and retinal atrophy in African-Americans versus Caucasian-Americans with multiple sclerosis: a longitudinal study. Brain. (2018) 141:3115–29. doi: 10.1093/brain/awy245
30. Cascione, M, Tenenbaum, N, Wendt, J, Meng, X, Schofield, L, Cree, BAC, et al. Treatment retention on fingolimod compared with injectable multiple sclerosis therapies in African-American patients: a subgroup analysis of a randomized phase 4 study. Mult Scler Relat Disord. (2018) 25:50–6. doi: 10.1016/j.msard.2018.07.014
31. Chan, KH, Tsang, KL, Ho, PW, Tse, CT, Kwan, JS, Ho, JW, et al. Clinical outcome of relapsing remitting multiple sclerosis among Hong Kong Chinese. Clin Neurol Neurosurg. (2011) 113:617–22. doi: 10.1016/j.clineuro.2011.04.013
32. Chinea Martinez, AR, Correale, J, Coyle, PK, Meng, X, and Tenenbaum, N. Efficacy and safety of fingolimod in Hispanic patients with multiple sclerosis: pooled clinical trial analyses. Adv Ther. (2014) 31:1072–81. doi: 10.1007/s12325-014-0154-4
33. Deslandes, MQ, Alves, PT, Alvarenga, MP, Lessa, VCC, Camargo, S, Alvarenga, RMP, et al. Effectiveness and adverse events of use of Natalizumab in a Brazilian cohort of patients with multiple sclerosis. Clin Ther. (2020) 42:1292–301. doi: 10.1016/j.clinthera.2020.05.007
34. Gray-Roncal, K, Fitzgerald, KC, Ryerson, LZ, Charvet, L, Cassard, SD, Naismith, R, et al. Association of Disease Severity and Socioeconomic Status in black and white Americans with multiple sclerosis. Neurology. (2021) 97:e881–9. doi: 10.1212/WNL.0000000000012362
35. Howard, J, Battaglini, M, Babb, JS, Arienzo, D, Holst, B, Omari, M, et al. MRI correlates of disability in African-Americans with multiple sclerosis. PLoS One. (2012) 7:e43061. doi: 10.1371/journal.pone.0043061
36. Kim, SH, Huh, SY, Kim, W, Park, MS, Ahn, SW, Cho, JY, et al. Clinical characteristics and outcome of multiple sclerosis in Korea: does multiple sclerosis in Korea really differ from that in the Caucasian populations? Mult Scler. (2013) 19:1493–8. doi: 10.1177/1352458513477712
37. Kira, J, Itoyama, Y, Kikuchi, S, Hao, Q, Kurosawa, T, Nagato, K, et al. Fingolimod (FTY720) therapy in Japanese patients with relapsing multiple sclerosis over 12 months: results of a phase 2 observational extension. BMC Neurol. (2014) 14:21. doi: 10.1186/1471-2377-14-21
38. Mercado, V, Dongarwar, D, Fisher, K, Salihu, HM, Hutton, GJ, and Cuascut, FX. Multiple sclerosis in a multi-ethnic population in Houston, Texas: a retrospective analysis. Biomedicine. (2020) 8:534. doi: 10.3390/biomedicines8120534
39. Moog, TM, McCreary, M, Stanley, T, Wilson, A, Santoyo, J, Wright, K, et al. African Americans experience disproportionate neurodegenerative changes in the medulla and upper cervical spinal cord in early multiple sclerosis. Mult Scler Relat Disord. (2020) 45:102429. doi: 10.1016/j.msard.2020.102429
40. Nakamura, Y, Gaetano, L, Matsushita, T, Anna, A, Sprenger, T, Radue, EW, et al. A comparison of brain magnetic resonance imaging lesions in multiple sclerosis by race with reference to disability progression. J Neuroinflammation. (2018) 15:255. doi: 10.1186/s12974-018-1295-1
41. Okuda, DT, Stanley, T, McCreary, M, Smith, A, Wilson, A, Pinho, MC, et al. Selective vulnerability of brainstem and cervical spinal cord regions in people with non-progressive multiple sclerosis of black or African American and European ancestry. Mult Scler. (2022) 29:691–701. doi: 10.1177/13524585221139575
42. Petracca, M, Zaaraoui, W, Cocozza, S, Vancea, R, Howard, J, Heinig, MM, et al. An MRI evaluation of grey matter damage in African Americans with MS. Mult Scler Relat Disord. (2018) 25:29–36. doi: 10.1016/j.msard.2018.06.007
43. Saida, T, Itoyama, Y, Tashiro, K, Kira, J, and Hao, Q, Interferon Beta-1a Multiple Sclerosis Study Group of Japan. Intramuscular interferon beta-1a is effective in Japanese patients with relapsing-remitting multiple sclerosis: a pre-treatment versus treatment comparison study of gadolinium-enhanced MRI brain lesions. Mult Scler. (2012) 18:1782–90. doi: 10.1177/1352458512442261
44. Saida, T, Kikuchi, S, Itoyama, Y, Hao, Q, Kurosawa, T, Nagato, K, et al. A randomized, controlled trial of fingolimod (FTY720) in Japanese patients with multiple sclerosis. Mult Scler. (2012) 18:1269–77. doi: 10.1177/1352458511435984
45. Seraji-Bozorgzad, N, Khan, O, Cree, BAC, Bao, F, Caon, C, Zak, I, et al. Cerebral Gray matter atrophy is associated with the CSF IgG index in African American with multiple sclerosis. J Neuroimaging. (2017) 27:476–80. doi: 10.1111/jon.12435
46. Shinoda, K, Matsushita, T, Nakamura, Y, Masaki, K, Yamasaki, R, Yamaguchi, H, et al. HLA-DRB1*04:05 allele is associated with intracortical lesions on three-dimensional double inversion recovery images in Japanese patients with multiple sclerosis. Mult Scler. (2018) 24:710–20. doi: 10.1177/1352458517707067
47. Tanaka, M, Matsui, M, Tahara, M, and Tanaka, K. Low incidence of asymptomatic contrast-enhancing brain lesions in Japanese patients with multiple sclerosis. Eur Neurol. (2011) 65:119–22. doi: 10.1159/000324151
48. Weinstock-Guttman, B, Ramanathan, M, Hashmi, K, Abdelrahman, N, Hojnacki, D, Dwyer, MG, et al. Increased tissue damage and lesion volumes in African Americans with multiple sclerosis. Neurology. (2010) 74:538–44. doi: 10.1212/WNL.0b013e3181cff6fb
49. Boster, AL, Endress, CF, Hreha, SA, Caon, C, Perumal, JS, and Khan, OA. Pediatric-onset multiple sclerosis in African-American black and European-origin white patients. Pediatr Neurol. (2009) 40:31–3. doi: 10.1016/j.pediatrneurol.2008.09.004
50. Cristiano, E, Rojas, J, Romano, M, Frider, N, Machnicki, G, Giunta, D, et al. The epidemiology of multiple sclerosis in Latin America and the Caribbean: a systematic review. Mult Scler. (2013) 19:844–54. doi: 10.1177/1352458512462918
51. Cristiano, E, and Rojas, JI. Multiple sclerosis epidemiology in Latin America: an updated survey. Mult Scler J Exp Transl Clin. (2017) 3:2055217317715050. doi: 10.1177/2055217317715050
52. Calabrese, M, Atzori, M, Bernardi, V, Morra, A, Romualdi, C, Rinaldi, L, et al. Cortical atrophy is relevant in multiple sclerosis at clinical onset. J Neurol. (2007) 254:1212–20. doi: 10.1007/s00415-006-0503-6
53. Fisher, E, Lee, JC, Nakamura, K, and Rudick, RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. (2008) 64:255–65. doi: 10.1002/ana.21436
54. Jacobsen, C, Hagemeier, J, Myhr, KM, Nyland, H, Lode, K, Bergsland, N, et al. Brain atrophy and disability progression in multiple sclerosis patients: a 10-year follow-up study. J Neurol Neurosurg Psychiatry. (2014) 85:1109–15. doi: 10.1136/jnnp-2013-306906
55. Brayo, P, and Kimbrough, D. Multiple sclerosis in the black population in the United States. Pract Neurol. (2021) 4, 31–34.
56. Okuda, DT, Burgess, KW, Cook, K, McCreary, M, Winkler, MD, and Moog, TM. Hiding in plain sight: the magnitude of unused disease modifying therapies in multiple sclerosis and strategies for reducing the economic burden of care. Mult Scler Relat Disord. (2022) 63:103920. doi: 10.1016/j.msard.2022.103920
57. Dean, G, Bhigjee, AI, Bill, PL, Fritz, V, Chikanza, IC, Thomas, JE, et al. Multiple sclerosis in black south Africans and Zimbabweans. J Neurol Neurosurg Psychiatry. (1994) 57:1064–9. doi: 10.1136/jnnp.57.9.1064
58. Idris, MN, Sokrab, TE, Ibrahim, EA, Ali, HE, Elzibair, MA, Abadalatif, M, et al. Multiple sclerosis in Sudan: a prospective study of clinical presentation and outcome. Mult Scler. (2009) 15:1537–8. doi: 10.1177/1352458509345913
59. Miskin, DP, Saadi, A, Chikoya, L, Sloane, JA, Koralnik, IJ, and Siddiqi, OK. Challenges in the diagnosis and treatment of CNS demyelinating disorders in Zambia. Mult Scler J Exp Transl Clin. (2016) 2:205521731665711. doi: 10.1177/2055217316657117
60. Langer-Gould, A, Wu, J, Lucas, R, Smith, J, Gonzales, E, Amezcua, L, et al. Epstein-Barr virus, cytomegalovirus, and multiple sclerosis susceptibility: a multiethnic study. Neurology. (2017) 89:1330–7. doi: 10.1212/WNL.0000000000004412
61. Bjornevik, K, Cortese, M, Healy, BC, Kuhle, J, Mina, MJ, Leng, Y, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. (2022) 375:296–301. doi: 10.1126/science.abj8222
62. Soldan, SS, and Lieberman, PM. Epstein-Barr virus and multiple sclerosis. Nat Rev Microbiol. (2023) 21:51–64. doi: 10.1038/s41579-022-00770-5
63. Ginde, AA, Liu, MC, and Camargo, CA Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. (2009) 169:626–32. doi: 10.1001/archinternmed.2008.604
64. Gelfand, JM, Cree, BA, McElroy, J, Oksenberg, J, Green, R, Mowry, EM, et al. Vitamin D in African Americans with multiple sclerosis. Neurology. (2011) 76:1824–30. doi: 10.1212/WNL.0b013e31821cccf5
65. Malik, MT, Healy, BC, Benson, LA, Kivisakk, P, Musallam, A, Weiner, HL, et al. Factors associated with recovery from acute optic neuritis in patients with multiple sclerosis. Neurology. (2014) 82:2173–9. doi: 10.1212/WNL.0000000000000524
66. Amezcua, L, Rivera, VM, Vazquez, TC, Baezconde-Garbanati, L, and Langer-Gould, A. Health disparities, inequities, and social determinants of health in multiple sclerosis and related disorders in the US: a review. JAMA Neurol. (2021) 78:1515–24. doi: 10.1001/jamaneurol.2021.3416
67. Dobson, R, Rice, DR, D'Hooghe, M, Horne, R, Learmonth, Y, Mateen, FJ, et al. Social determinants of health in multiple sclerosis. Nat Rev Neurol. (2022) 18:723–34. doi: 10.1038/s41582-022-00735-5
68. Galea, S, Tracy, M, Hoggatt, KJ, Dimaggio, C, and Karpati, A. Estimated deaths attributable to social factors in the United States. Am J Public Health. (2011) 101:1456–65. doi: 10.2105/AJPH.2010.300086
69. Minden, SL, Hoaglin, DC, Hadden, L, Frankel, D, Robbins, T, and Perloff, J. Access to and utilization of neurologists by people with multiple sclerosis. Neurology. (2008) 70:1141–9. doi: 10.1212/01.wnl.0000306411.46934.ef
70. Chi, C, Shao, X, Rhead, B, Gonzales, E, Smith, JB, Xiang, AH, et al. Admixture mapping reveals evidence of differential multiple sclerosis risk by genetic ancestry. PLoS Genet. (2019) 15:e1007808. doi: 10.1371/journal.pgen.1007808
71. Webb, A, and Obungoloch, J. Five steps to make MRI scanners more affordable to the world. Nature. (2023) 615:391–3. doi: 10.1038/d41586-023-00759-x
72. Arnold, TC, Tu, D, Okar, SV, Nair, G, By, S, Kawatra, KD, et al. Sensitivity of portable low-field magnetic resonance imaging for multiple sclerosis lesions. Neuroimage Clin. (2022) 35:103101. doi: 10.1016/j.nicl.2022.103101
73. Mateen, FJ, Cooley, CZ, Stockmann, JP, Rice, DR, Vogel, AC, and Wald, LL. Low-field portable brain MRI in CNS demyelinating disease. Mult Scler Relat Disord. (2021) 51:102903. doi: 10.1016/j.msard.2021.102903
74. Anazodo, UC, Ng, JJ, Ehiogu, B, Obungoloch, J, Fatade, A, Mutsaerts, H, et al. A framework for advancing sustainable magnetic resonance imaging access in Africa. NMR Biomed. (2023) 36:e4846. doi: 10.1002/nbm.4846
75. Zeydan, B, Ucem, S, Dubuche, T, Azodi, S, Bhagavatheeshwaran, G, Tillema, JM, et al. C5 Level Area Can Replace Whole Cervical Spinal Cord Area Measurements in Multiple Sclerosis as a Practical Biomarker of Progression. Concord, CA: International Society for Magnetic Resonance in Medicine (2020).
Keywords: African American, ethnicity, Latin American, magnetic resonance imaging, multiple sclerosis, neuroimaging, race
Citation: Nathoo N, Zeydan B, Neyal N, Chelf C, Okuda DT and Kantarci OH (2023) Do magnetic resonance imaging features differ between persons with multiple sclerosis of various races and ethnicities? Front. Neurol. 14:1215774. doi: 10.3389/fneur.2023.1215774
Edited by:
Robert Zivadinov, University at Buffalo, United StatesReviewed by:
Niels Bergsland, University at Buffalo, United StatesLorena Lorefice, ATS Sardegna, Italy
Copyright © 2023 Nathoo, Zeydan, Neyal, Chelf, Okuda and Kantarci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Orhun H. Kantarci, a2FudGFyY2kub3JodW5AbWF5by5lZHU=
 Nabeela Nathoo
Nabeela Nathoo Burcu Zeydan
Burcu Zeydan Nur Neyal1,2
Nur Neyal1,2 Cynthia Chelf
Cynthia Chelf Darin T. Okuda
Darin T. Okuda Orhun H. Kantarci
Orhun H. Kantarci