- 1The Center of Sociomedical Research, Faculty of Applied Social Sciences and Resocialization, University of Warsaw, Warsaw, Poland
- 2Department of Neurology, School of Health Sciences, Medical University of Silesia in Katowice, Katowice, Poland
- 3Department of Neurology, Upper-Silesian Medical Center of the Medical University of Silesia in Katowice, Katowice, Poland
- 4Department and Clinic of Psychiatric Rehabilitation, Faculty of Medical Sciences in Katowice, Medical University of Silesia in Katowice, Katowice, Poland
- 5Department of Clinical Medicine, University of Bergen, Bergen, Norway
- 6Department of Neurology, Haukeland University Hospital, Bergen, Norway
The survey aimed to explore patients’ perspectives with myasthenia gravis (MG) toward the diagnosis made and the therapy used to treat MG. The survey was conducted with a quantitative method, using the CAWI technique. A total of 321 people participated in the survey. More than half of the respondents (56.4%) had suffered from MG for less than 10 years. In three out of 10 cases (30.9%), the diagnosis of MG lasted 3 years or longer. The diagnostic delay was significantly longer in female respondents than in the males (p = 0.029). Cholinergic drugs were used in 92.9% of cases initially, and as maintenance therapy in 84.3% of cases. Corticosteroids were used in initiating therapy (45.8%) and as maintenance therapy (46.4%). One in four respondents (25.5%) reported experiencing very strong and strong side effects after using steroids. The side effects from steroid therapy very strong or strong affected overall physical health in 55.9% of respondents, very strong or strong affected self-acceptance in 52%, to a very large or large extent on mental health in 47.1%, and to a very strong or strong extent influenced the performance of daily activities in 28.2%. More than half of the respondents (57.0%) had had a thymectomy. Seven out of 10 respondents (72.0%) declared that the therapy they were on at the time of the survey allowed them (to varying degrees) to control their course of MG. Low therapy acceptance and less well controlled MG was associated with a preference for non-tablet therapies (p = 0.045). Regular follow-up and cooperation with the specialist health care system should improve MG symptoms, activities of daily living, and quality of life.
Introduction
Myasthenia gravis (MG) is an autoimmune disease with an annual incidence rate of 5–30 people per million (1–4). It is the most common neuromuscular transmission disorder, primarily affecting adults (5–7). Its prevalence has increased in recent decades as detection and survival rates have improved (8, 9). Although the exact cause of MG is unknown, the thymus appears to play a role (10, 11). Viral and bacterial infections have been suggested to initiate the immune response (12, 13). Additionally, thyroid disease is present in 3–8% of MG patients, and tumors are found in the thymus of 10–15% of patients (8, 14). A typical symptom is general muscle weakness affecting the bulbar muscles and those responsible for limb and eye movements (15–17).
Myasthenia gravis is diagnosed based on a detailed history, clinical examination, and laboratory testing (18). Sometimes, however, it can take a long time to diagnose the disease, and the availability of the tests conducted to detect MG can vary depending on regional and institutional conditions (19). MG therapy can take on various forms—more or less invasive from the patient’s perspective (20–22).
Understanding patients’ attitudes toward therapy and diagnosis allows medical personnel to respond appropriately through drug therapy and other therapeutic modalities, improving disease management and patients’ quality of life (23, 24). Factors affecting patients’ attitudes toward the disease include access to new medications, such as biologics, and access to non-medical sources of information.
Our study aimed to explore the perspectives of patients with MG concerning the diagnostic process and the treatment administered at the time of diagnosis, and to assess the impact of the therapy administered on patients’ well-being.
Materials and methods
Study design
The survey was a sociomedical study, combining interdisciplinary thinking on the interaction between social factors, demographics, and MG sufferers’ perspectives on diagnosis and therapy. The survey was conducted among people with MG in November/December 2022 in Poland.
Population and sample size
The number suffering from MG in Poland is estimated to be about 8,000 people (25). Our study included 321 adults with MG, representing 0.05% of the total number in Poland. It was impossible to use a sampling frame for our survey, and the study is unrepresentative from the point of view of random sampling.
The questionnaire
For this study, a survey instrument was created to find answers to the research questions and to achieve our research objective. The survey consisted of 38 questions. Four questions were metrics; they asked about gender, age, education, and place of residence. The remaining questions were pertinent to the research objective. The survey questions consisted of three thematic sections: diagnosis of MG, therapy of MG, and psychosocial aspects of MG. Most of the questions were closed, and a few were open-ended. Closed questions were dichotomous, questions with a scale, or questions with an option to choose one or more answers. Questions with scales were dominated by variants of the Likert scale, arranged on a verbal or numerical axis to explore attitudes toward a given phenomenon.
The survey instrument was prepared due to multi-stage consultations with doctors and people with MG. The tool was validated ad hoc by specialists in the field. A pilot study was conducted including eight people with MG to evaluate the tool methodologically. Data from the pilot study were not included in the actual study.
Data collection procedure
The questionnaire was formally adapted to the requirements of the Computer Assisted Web Interviewing (CAWI) technique, with the help of which the survey was implemented. The questionnaire for the study was hung on Google’s cipher drive so that only the paper’s first author had access to all the data collected. At the same time, IP numbers or any other information identifying a specific person completing the questionnaire was not collected during the survey. The link with the survey questionnaire with instructions for self-completion was forwarded to the Polish Association of Myasthenia Gravis “Gioconda.” Representatives of the association sent the link to all members of their association. Eligibility for the survey required being an adult with MG and giving informed consent to participate in the study. The study included three individuals who completed the questionnaire on behalf of their children with MG; however, these responses were not included in the results pool for methodological and ethical reasons. Survey results were collected on disk.
Statistical analysis
Statistical analyses were performed in IBM SPSS Statistics 28.0.1.0. Data for all outcomes are reported for all participants. The relationship between variables was evaluated by using the Chi-squared test. The Kruskal-Wallis’ test was used to analyze the questions using the Likert scale. Answers to questions are presented with a total number of respondents (n) and frequencies (%). For all analyzes, a p level of <0.05 was considered statistically significant.
Ethical considerations
In implementing the survey, care was taken to ensure that it complied with the ethical rules of conducting social research. Only adults (age > 18 years) participated in the survey, and each gave informed consent to complete the survey. To ensure the respondents’ sense of confidentiality and anonymity in the subsequent analysis, all surveys were coded on the platform collecting the results. Only one person had access to the coded results, so it was impossible to link a person to specific results in the analysis process. The survey results are analyzed collectively, and identification at the data reporting level of a specific surveyed person is also impossible. The study received Silesia Medical University Ethics Committee’s approval (no. BNW/NWN/0052/KB/61/23).
Results
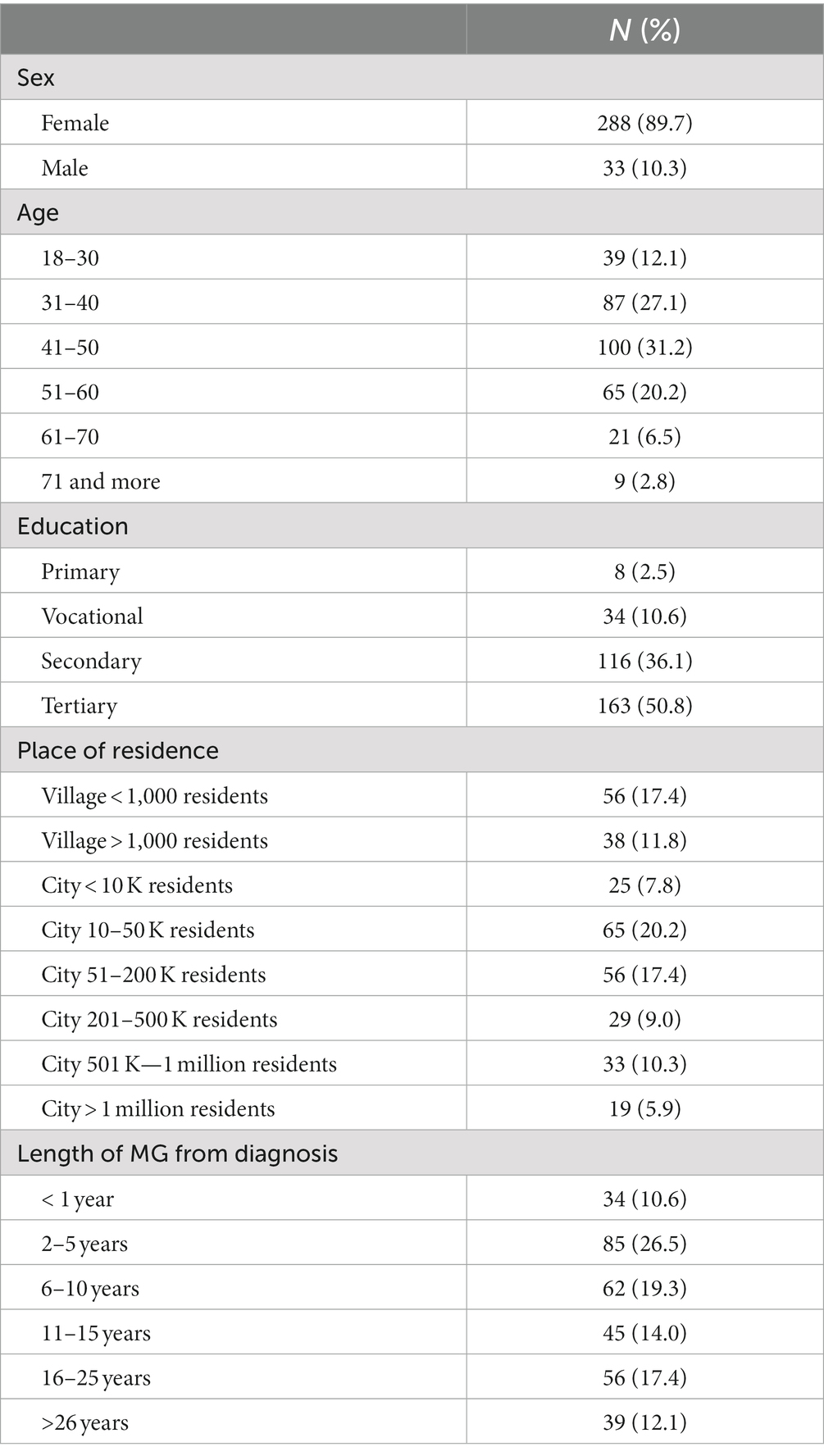
Most of the respondents were women. More than a half of respondents (56.4%) had suffered from MG for less than 10 years (Table 1).
Myasthenia gravis diagnosis
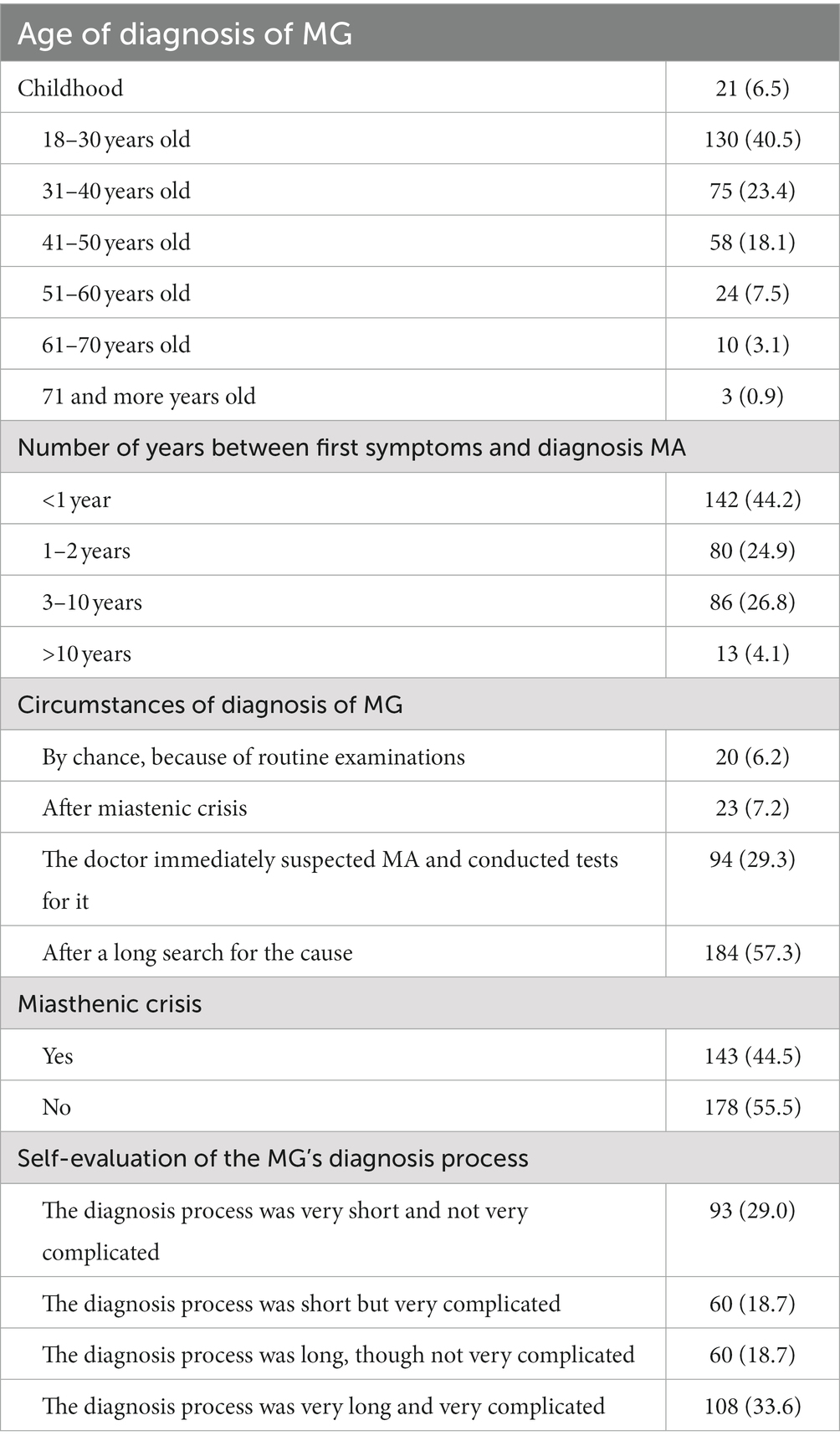
Half of respondents (47%) had been diagnosed with MG before age 30 years, often after a long delay (Table 2).
Our MG group included more females in the younger group and more males in the elderly group (p < 0.001). MG was diagnosed more often due to myasthenic crisis in the youngest age group (p = 0.002; Supplementary Table S1).
The diagnostic delay was significantly longer in female respondents than in the males (p = 0.029). The physician’s suspicion of MG right away and conducting specific tests for it was significantly more common in males than in females (p = 0.029; Table 3).
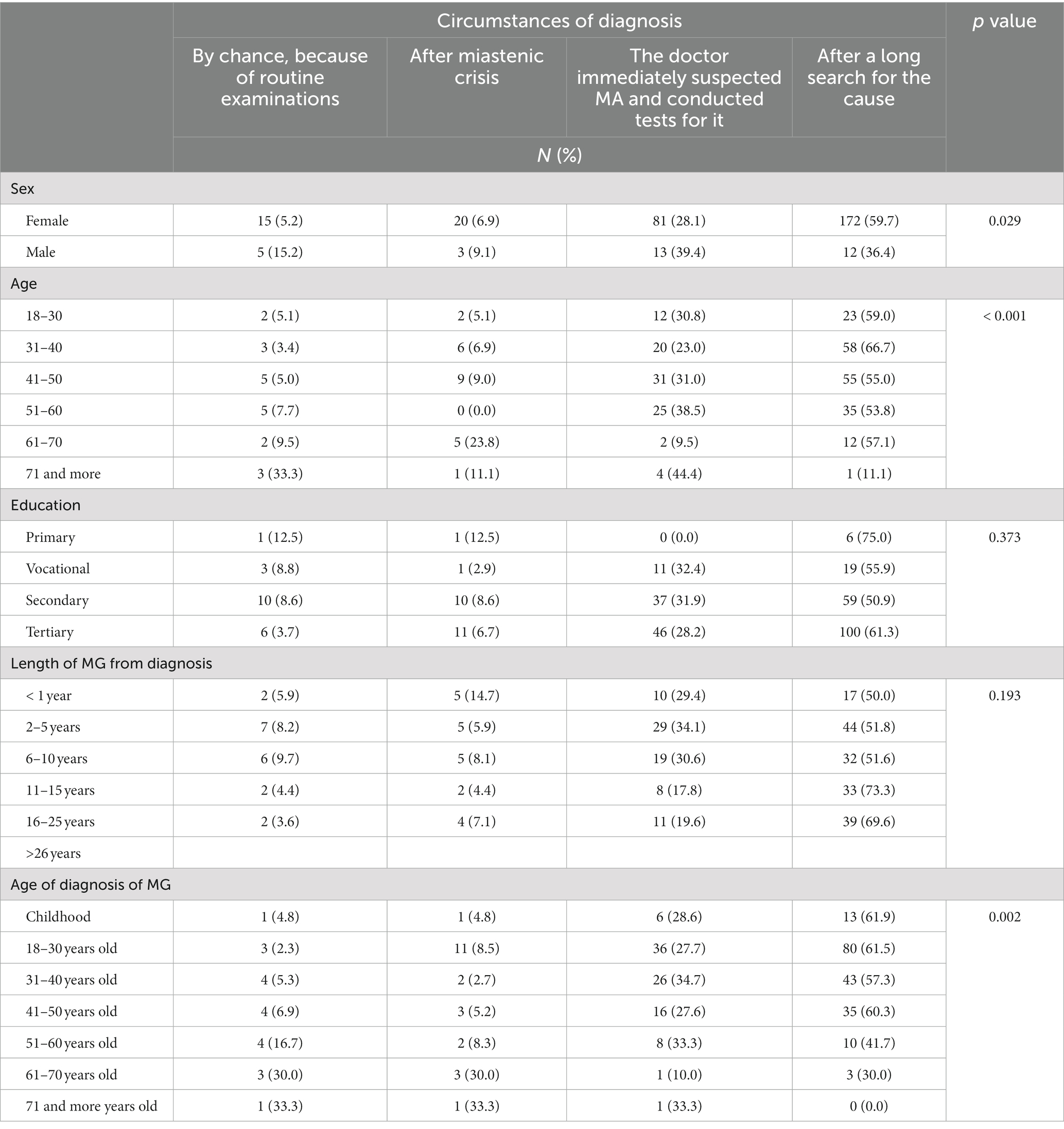
Table 3. Circumstance of diagnosis of MG vs. sociodemographic characteristics of respondents (N = 321).
Myasthenia gravis therapy
Corticosteroids and cholinergic drugs were the most frequently used drugs in initiating and maintaining therapy. The same percentage of patients stated that corticosteroids were used in initiating therapy (45.8%) and as maintenance therapy (46.4%). Cholinergic drugs were used in 92.9% of cases initially, and as maintenance therapy in 84.3% of cases. An overview of all therapeutic combination and monotherapies is shown in Figure 1.
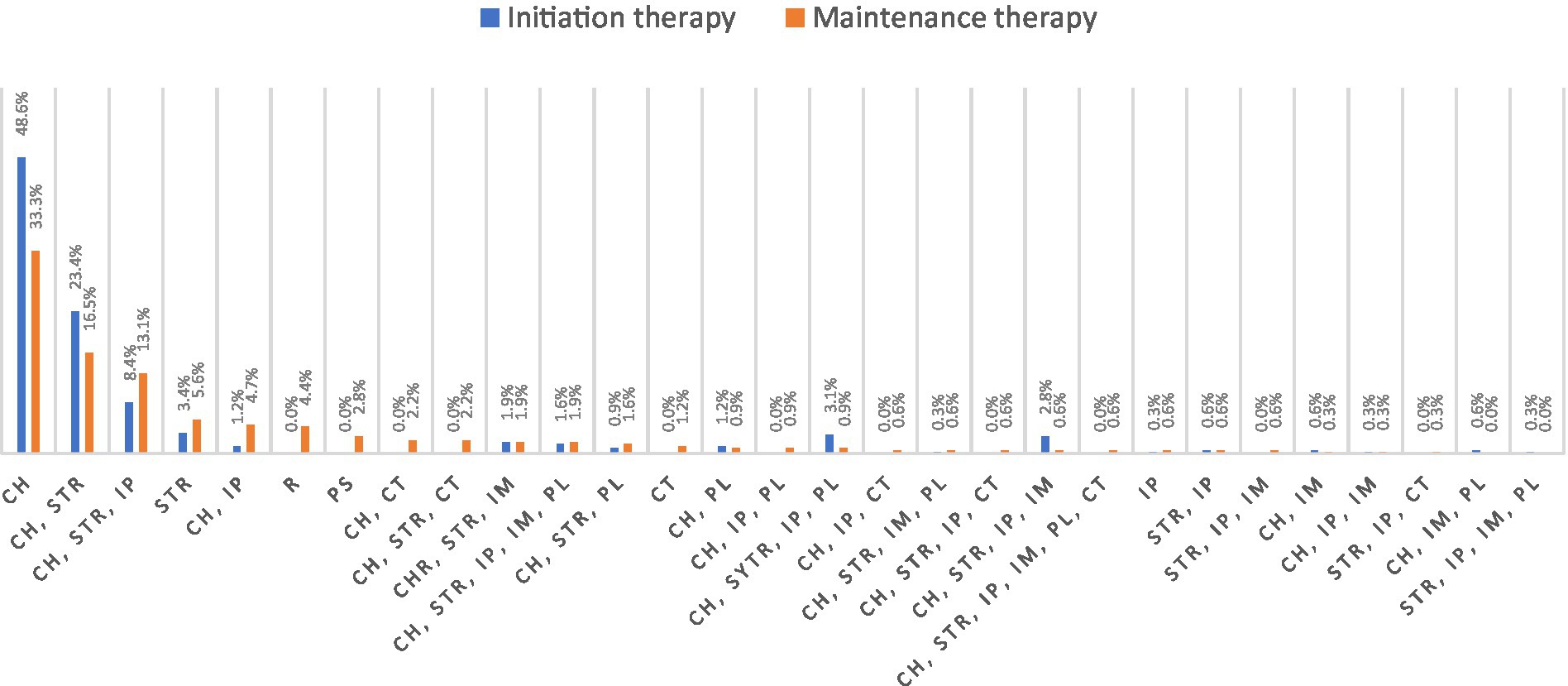
Figure 1. Distribution of initiating therapy and maintenance therapy in all included patients (N = 321). CH, cholinergic; STR, steroid therapy; IP, immunosuppressants; IM, immunoglobulins; PL, plasmapheresis; CT, clinical trial; PS, parasympathomimetics; and R, remission.
One in four respondents (25.5%) reported experiencing very strong or strong side effects after using steroids. In 4.4% of cases, side effects were severe but passed quickly. In three out of 10 cases (30.2%), side symptoms after using steroids were minor but constant, and in 7.8%, they were minor and passed quickly. One in seven (13.1%) said they did not experience any side effects from steroid use. One in five patients (19%) had never taken steroids for MG treatment.
The side effects from steroid therapy very strong or strong affected overall physical health in 55.9% of respondents, very strong or strong affected self-acceptance in 52%, to a very large or large extent on mental health in 47.1%, and to a very strong or strong extent influenced the performance of daily activities in 28.2% (Table 4).
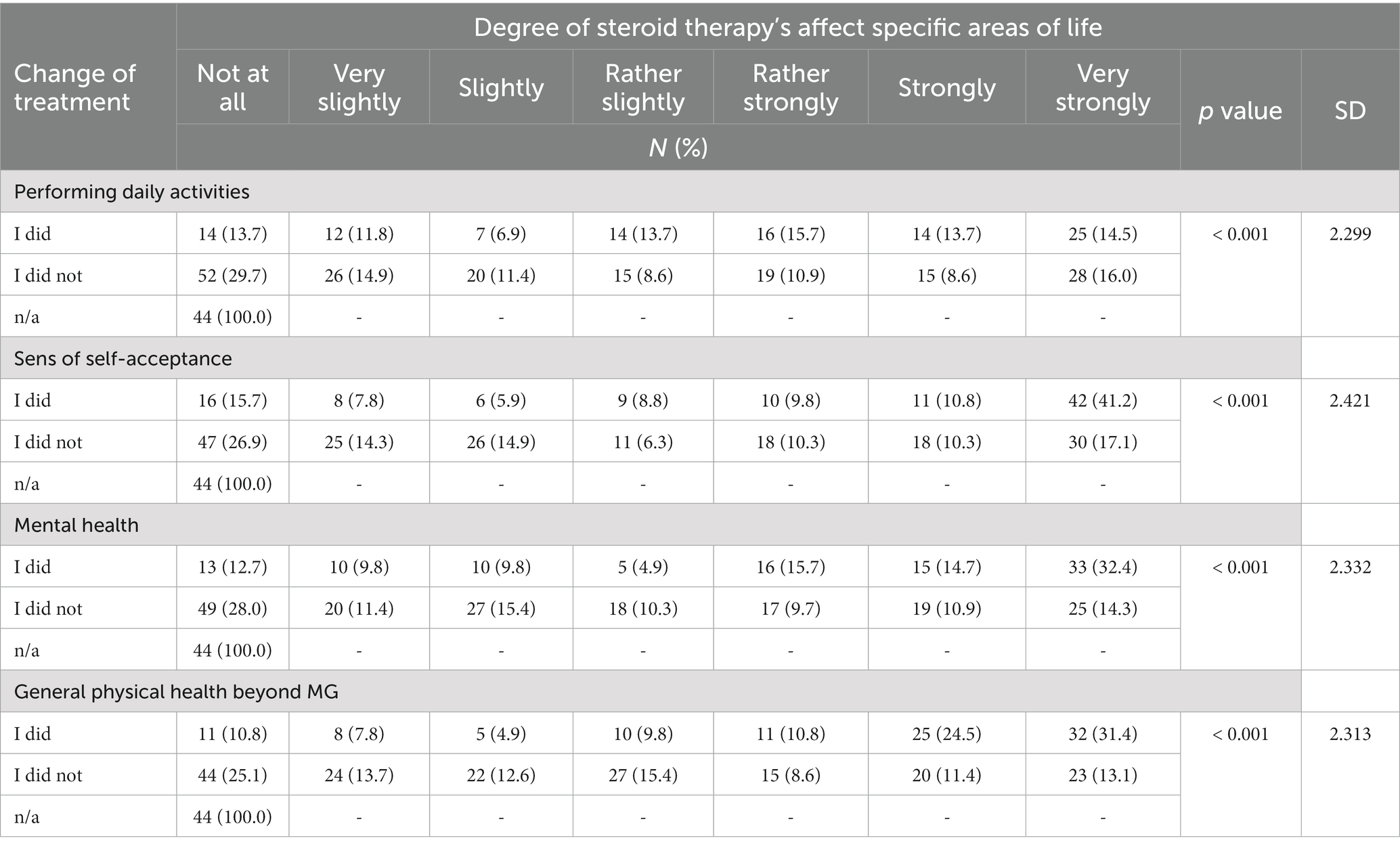
Table 4. Summary of answers to the questions: did steroid side effects cause a change in treatment? vs. did the use of steroid therapy affect specific areas of life? (N = 321).
One in eight respondents (12.5%) had experienced immunoglobulin therapy once or twice, and one in nine (11.5%) three or more times. Three-quarters of the patients (76.0%) had not had immunoglobulin therapy.
Plasmapheresis once or twice had been given to 6.9% of respondents, and 12.8% had plasmapheresis three or more times. Eight out of 10 respondents (80.4%) said they had never received plasmapheresis.
More than half of the respondents (57.0%) had had a thymectomy.
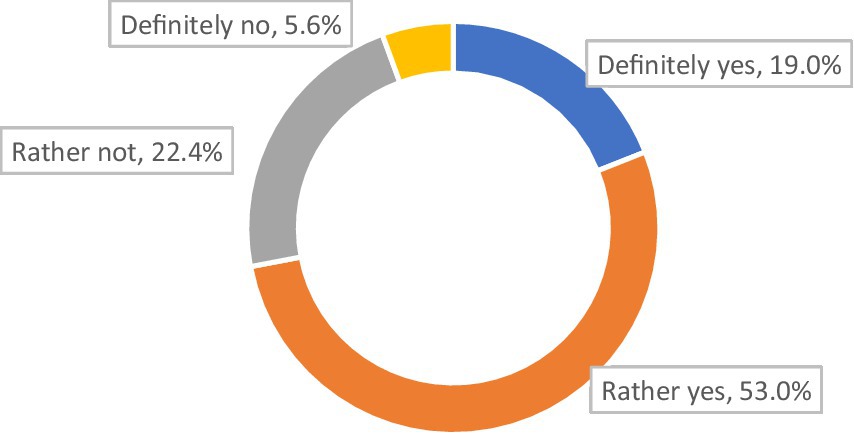
Seven out of 10 respondents (72.0%) declared that the therapy they were on at the time of the survey allowed them (to varying degrees) to control their course of MG (Figure 2).
Among those with no plasmapheresis, three-quarters (77.2%) stated that their present therapy [definitely (19.4%) or in part (57.8%)] allowed them to control the symptoms of their disease (p < 0.001). Among those who had plasmapheresis once or twice, four in 10 (40.9%) stated that their present therapy [definitely (22.7%) or in part (18.2%)] allowed them to control the disease (p < 0.001). For the group who had plasmapheresis three times or more, more than half (56.1%) stated that their therapy [definitely (14.6%) or in part (41.5%)] allowed them to control (to varying degrees) their disease (p < 0.001).
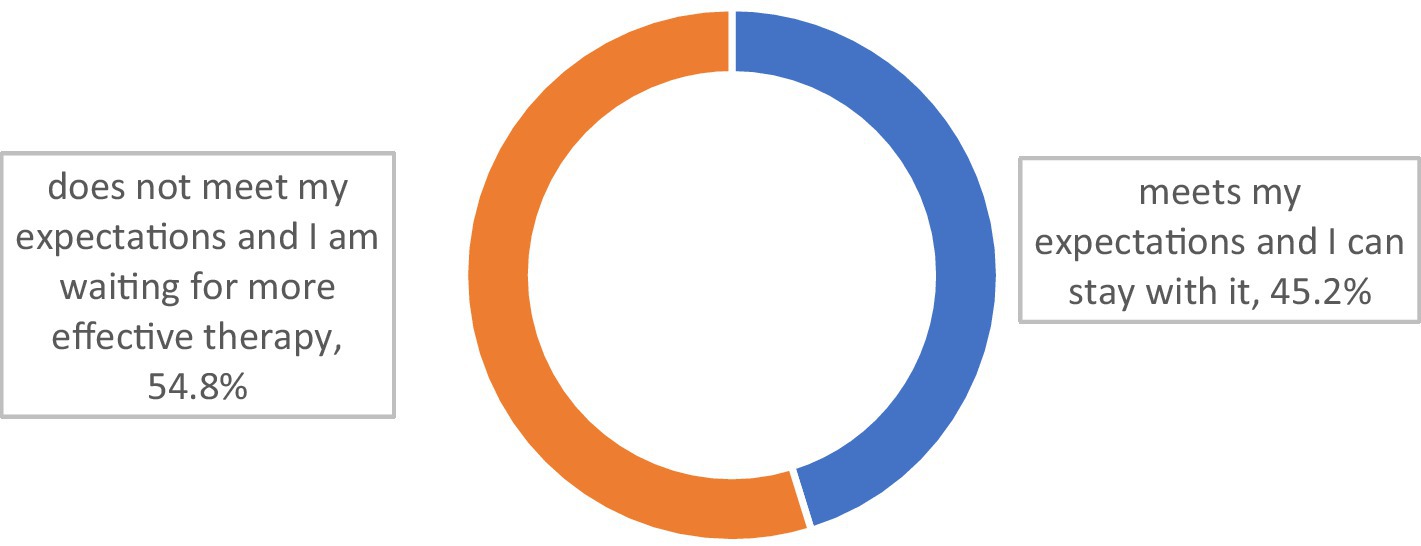
More than half of the respondents (54.8%) declared that their present MG treatment did not meet their expectations, and they expected it to be modified or escalated (Figure 3).
Those who had immunoglobulin therapy were more likely to declare that their present therapy did not meet their expectations (p = 0.007). The trend was the same for those who had received plasmapheresis (p = 0.051; Table 5).
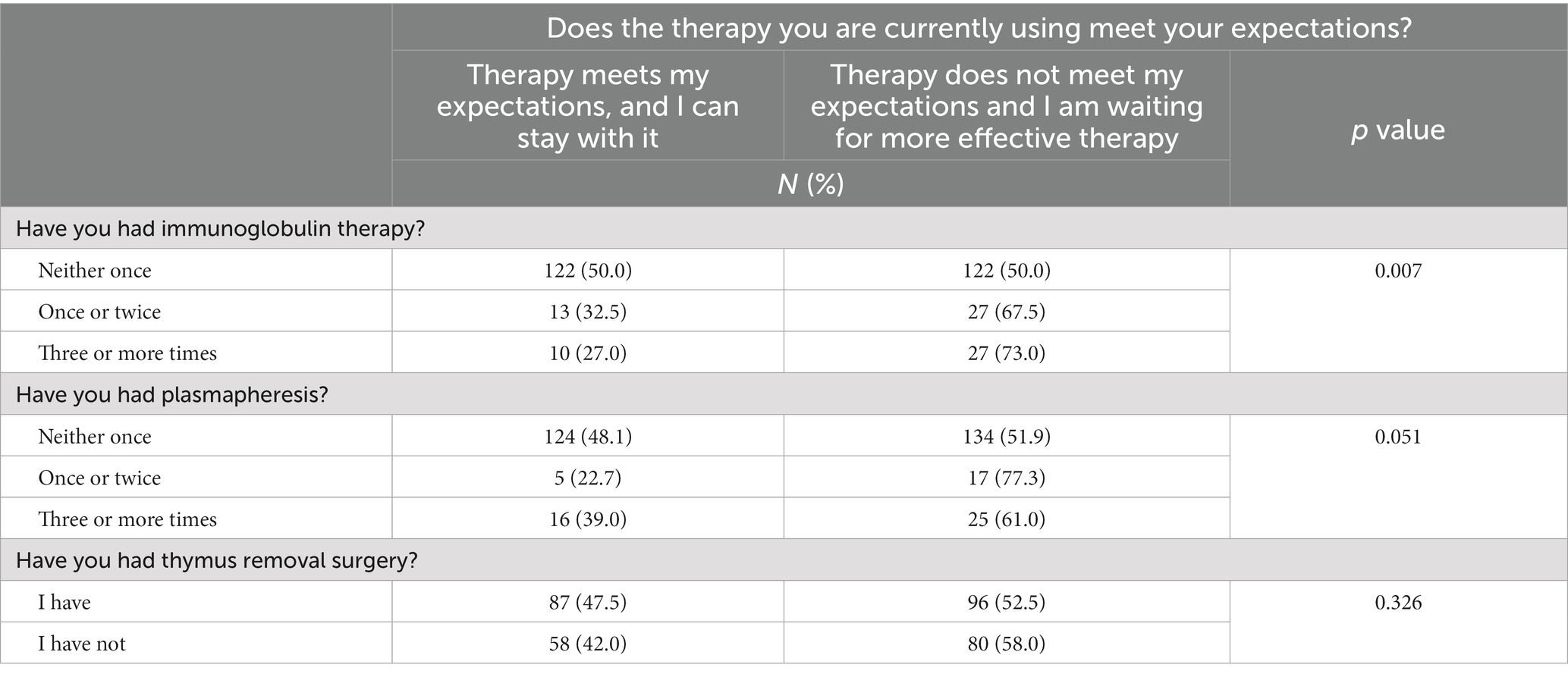
Table 5. Summary of responses to the question: does the current MG treatment therapy meet your expectations? vs. immunoglobulin treatment and interventional treatment (N = 321).
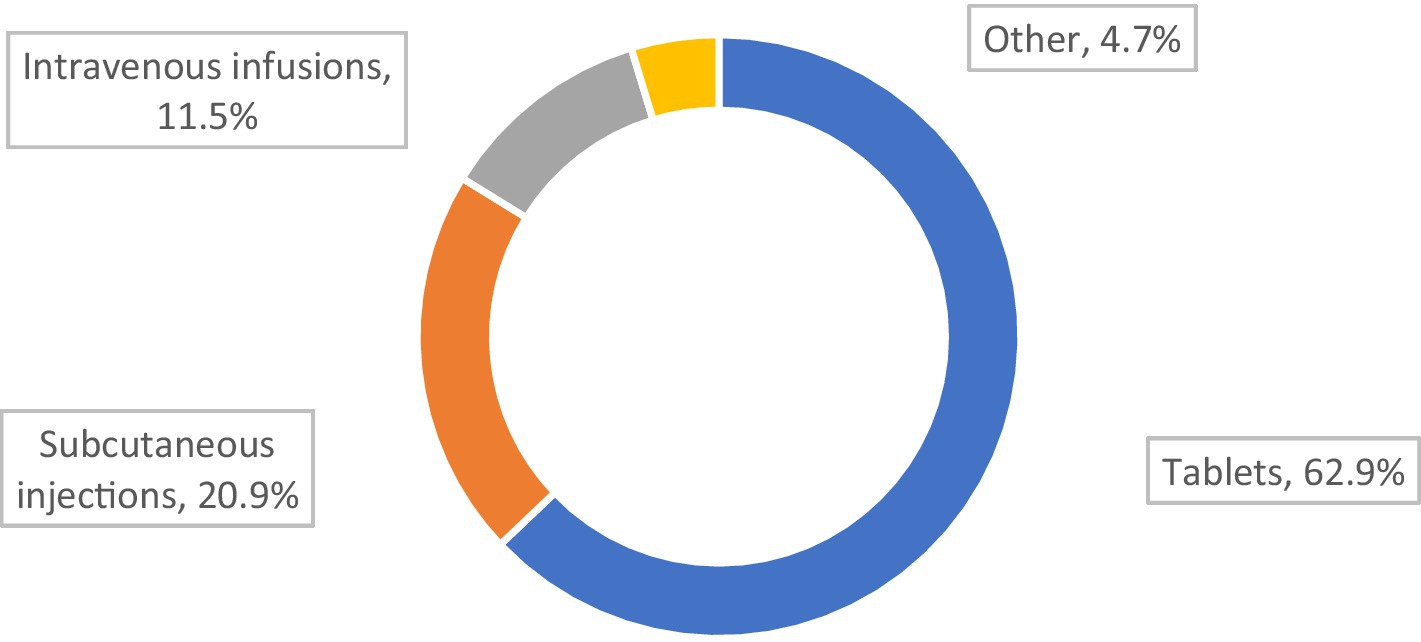
The most desirable form of drug administration for our MG respondents was tablets, and the least desirable was intravenous infusions (Figure 4).
Low therapy acceptance and less well controlled MG was associated with a preference for non-tablet therapies (p = 0.045; Table 6).
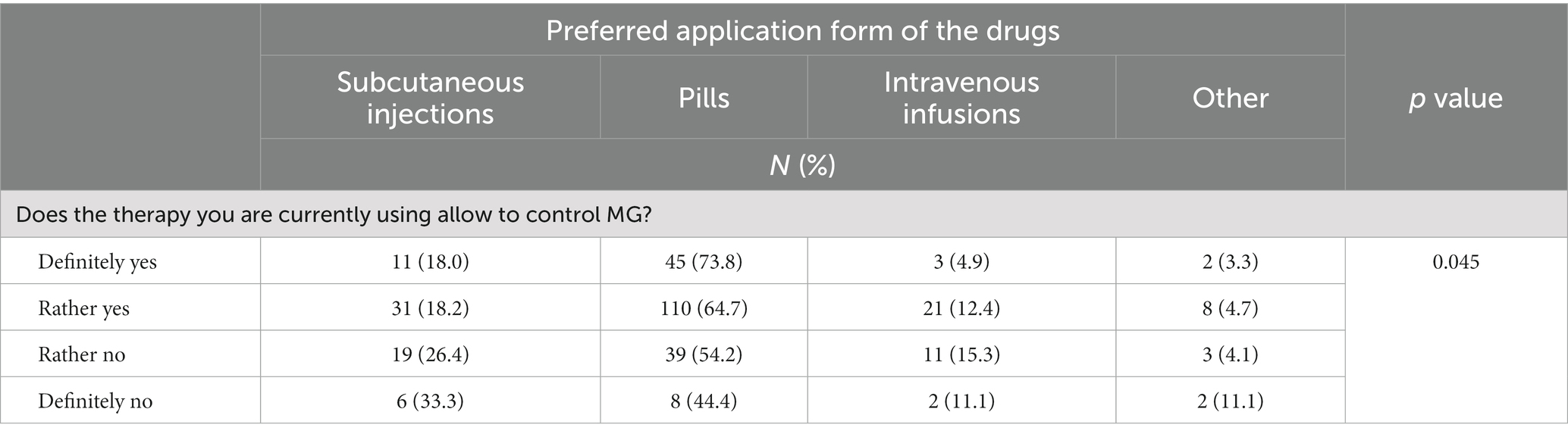
Table 6. Summary of responses to the preferred application form of the drugs vs. question: does the therapy you are currently using allow to control MG? (N = 321).
A trend indicated that tablets represent the preferred form of drug administration by those who declared that their therapy met their expectations (p = 0.053) The patients who stated that the therapy did not meet their expectations were twice as likely to prefer subcutaneous for drug administration (p = 0.053).
Eight in 10 patients (81.2%) reported an additional burden of non-MG diseases; 16.8% reported one comorbidity, whereas 61.7% had two or more comorbidities. The most common comorbidities were depression, diabetes, hypertension, Hashimoto’s thyroiditis, and hypothyroidism.
Discussion
In clinical practice, a neurologist periodically assesses the condition of MG patients by evaluating their clinical and functional status. Available scales make it possible to estimate the patient’s neurological status and severity of symptoms but usually do not consider the impact of the disease on the patients’ sociopsychological condition (23).
In the present study, patients completed questionnaires without the presence of their neurologist. To achieve optimal collaboration with the patient, it is necessary to determine their level of knowledge about their disease, its therapy, and their quality of life.
More than 60% of patients stated that the process preceding the diagnosis of MG was long and complicated. This may be surprising given that, in most cases, the first symptoms of MG are quite distinctive and include typical eye movement disturbances. However, such a diagnostic delay has been reported also in previous studies and reflects that MG is a rare disease. Most general practitioners have not seen the disease preciously. Within 3 years, about 90% of patients develop symptoms of generalized muscle weakness and fatigue (26), which can affect the daily acts of swallowing, chewing, breathing, and speaking (27). Interestingly, most of our study patients resided in large cities with relatively easy access to medical care specialists, but still they experienced diagnostic delay.
The chronic character of the disease and the variation of MG symptoms over time with a risk of severe exacerbations worsen the patients’ quality of life. Corticosteroid therapy may cause mental side-effects (28). Interestingly, in the present study, over 40% of patients believed they had experienced a myasthenic crisis. Many patients have probably mistaken it for a severe disease exacerbation, as epidemiological data do not indicate that myasthenic crisis is so common among MG patients (29). Crisis with a transient need of intensive care and respiratory support usually occurs in 15–20% of all MG patients and can be precipitated by infection, a stressful situation, a surgical procedure, or as a reaction to medication (8, 30). Modifiable risk factors for disease exacerbation should be identified, discussed with the patient, and treated.
According to the results of the present study, one fourth of the patients poorly tolerate steroids, and only 13% are free from adverse effects. Physical effects were reported by over half of the study population, and mental effects were reported by nearly one half. Many expressed having problems with self-acceptance.
Most patients did not undergo human immunoglobulin or plasmapheresis treatment, but every 10 underwent at least three courses of such therapies. The beneficial effects of such therapies last only for 3–4 months, and this may be the main reason why so many of our study patients found that this form of therapy failed their expectations. It is worth noting that immunosuppressants and their side effects can affect patients’ perspective (31) However, over half of those treated with plasmapheresis reported good disease management. The present study shows that most patients prefer taking tablets, despite potential swallowing problems as part of the disease. 20% of the study patients preferred subcutaneous drug administration, which may be relevant when planning future pharmacological policies.
Over half of the study patients had their thymus resected. Anxiety about a potential thymus tumor can affect patients’ well-being, as the incidence of thymoma is 10–15% of all MG patients (8, 32, 33). Surgical removal of the tumor and the good prognosis may reduce the prevalence of anxiety and depression among MG patients with thymoma (34, 35). The median 5-year survival rate is 69% for patients with advanced thymoma or thymic carcinoma (36).
The results presented in this article illustrate the need for further patient education. Patients’ adequate interpretation of disease symptoms improves their understanding and reduces their associated anxiety or stress. If a patient’s knowledge is inaccurate, they may want to intensify unnecessary therapies and thus become exposed to the adverse effects of drugs. They may also underreport MG-associated symptoms and thus miss useful therapy.
A strength of our study is that most of the patients have had MG for over 10 years. It illustrates long-term management and disease knowledge acquired by experience. Knowing patients’ views and attitudes toward the disease aids in therapy management and is an integral part of health technology assessment.
Limitations of the study
The patients in our study were unequally distributed in terms of education, with a large proportion having higher education levels. Although our patients’ sample was large, simple random sampling was not used. Patients surveyed made self-assessments in many aspects of the study, which many factors could have influenced at the time of completion. Underrepresented in the survey are the oldest people, over 60. It was because the survey was conducted over the Internet, which for many older people is a significant barrier or a result of digital exclusion. Nevertheless, the results allow us to capture the main cognitive categories in diagnosing and treating MG patients in Poland.
Conclusion and recommendations
Diagnostic delays and misconceptions about the myasthenic crisis were observed, indicating the necessity for improved patient education. A significant proportion of patients reported adverse effects of therapies, particularly corticosteroids, emphasizing the importance of personalized treatment approaches.
Considering the above research results, we recommend that healthcare professionals focus on enhancing patient education, helping patients better understand MG symptoms, interpret them accurately, and manage the condition effectively. It could reduce unnecessary therapies and improve treatment outcomes. It is essential to raise awareness among general practitioners about the distinct symptoms of MG, aiming to minimize diagnostic delays. Early referral to specialists can ensure prompt diagnosis and timely initiation of treatment. Treatment approaches should be optimized by considering patient preferences. Factors such as the mode of drug administration (e.g., tablets or subcutaneous delivery) should be considered. Personalized approaches can minimize adverse effects and enhance treatment adherence. We think there is a need to educate patients about myasthenic crises, providing accurate information about their nature and prevalence. It will help prevent unnecessary anxiety and inappropriate treatment-seeking behaviors.
Implementing these recommendations will support a patient-centered approach to managing MG, considering patients’ overall well-being. It will improve treatment outcomes and an enhanced quality of life for individuals with MG.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Bioethic Commitee of Medical University of Silesia. The patients/participants provided their informed consent to participate in this study.
Author contributions
TS designed the study, conceptualized the methodology, and performed data curation, formal analysis, and statistical analysis. TS, AL-B, and NG wrote original draft and edited and revised the manuscript. NG and MK supervised the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors wanted to thank all the patients with MG who participated in the study and The Polish Association of Myasthenia Gravis “Gioconda.”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1214041/full#supplementary-material
References
1. Somnier, FE, and Engel, PJ. The occurrence of anti-titin antibodies and thymomas: a population survey of MG 1970-1999. Neurology. (2002) 59:92–8. doi: 10.1212/wnl.59.1.92
2. Fang, W, Li, Y, Mo, R, Wang, J, Qiu, L, Ou, C, et al. Hospital and healthcare insurance system record-based epidemiological study of myasthenia gravis in southern and northern China. Neurol Sci. (2020) 41:1211–23. doi: 10.1007/s10072-019-04146-1
3. Bettini, M, Chaves, M, Cristiano, E, Pagotto, V, Perez, L, Giunta, D, et al. Incidence of autoimmune myasthenia gravis in a health maintenance Organization in Buenos Aires. Argentina Neuroepidemiol. (2017) 48:119–23. doi: 10.1159/000477733
4. Carr, AS, Cardwell, CR, McCarron, PO, and McConville, J. A systematic review of population based epidemiological studies in myasthenia gravis. BMC Neurol. (2010) 10:46. doi: 10.1186/1471-2377-10-46
5. Gilhus, NE, Tzartos, S, Evoli, A, Palace, J, Burns, TM, and Verschuuren, JJGM. Myasthenia gravis. Nat Rev Dis Primers. (2019) 5:30. doi: 10.1038/s41572-019-0079-y
6. Dresser, L, Wlodarski, R, Rezania, K, and Soliven, B. Myasthenia gravis: epidemiology, pathophysiology and clinical manifestations. J Clin Med. (2021) 10:2235. doi: 10.3390/jcm10112235
7. Spillane, J, Beeson, DJ, and Kullmann, DM. Myasthenia and related disorders of the neuromuscular junction. J Neurol Neurosurg Psychiatry. (2010) 81:850–7. doi: 10.1136/jnnp.2008.169367
8. Rahman, MM, Islam, MR, and Dhar, PS. Myasthenia gravis in current status: epidemiology, types, etiology, pathophysiology, symptoms, diagnostic tests, prevention, treatment, and complications—correspondence. Int J Surg. (2023) 109:178–80. doi: 10.1097/JS9.0000000000000164
9. Bubuioc, AM, Kudebayeva, A, Turuspekova, S, Lisnic, V, and Leone, MA. The epidemiology of myasthenia gravis. J Med Life. (2021) 14:7–16. doi: 10.25122/jml-2020-0145
10. Goldstein, G. Myasthenia gravis and the thymus. Annu Rev Med. (1971) 22:119–24. doi: 10.1146/annurev.me.22.020171.001003
11. Weiss, JM, Cufi, P, Le Panse, R, and Berrih-Aknin, S. The thymus in autoimmune myasthenia gravis: paradigm for a tertiary lymphoid organ. Rev Neurol (Paris). (2013) 169:640–9. doi: 10.1016/j.neurol.2013.02.005
12. Gummi, RR, Kukulka, NA, Deroche, CB, and Govindarajan, R. Factors associated with acute exacerbations of myasthenia gravis. Muscle Nerve. (2019) 60:693–9. doi: 10.1002/mus.26689
13. Hehir, MK, and Silvestri, NJ. Generalized myasthenia gravis: classification, clinical presentation, natural history, and epidemiology. Neurol Clin. (2018) 36:253–60. doi: 10.1016/j.ncl.2018.01.002
14. Bojikian, KD, and Francis, CE. Thyroid eye disease and myasthenia gravis. Int Ophthalmol Clin. (2019) 59:113–24. doi: 10.1097/IIO.0000000000000277
15. Gwathmey, KG, and Burns, TM. Myasthenia gravis. Semin Neurol. (2015) 35:327–39. doi: 10.1055/s-0035-1558975
16. Keesey, JC. Clinical evaluation and management of myasthenia gravis. Muscle Nerve. (2004) 29:484–505. doi: 10.1002/mus.20030
17. Shuey, NH. Ocular myasthenia gravis: a review and practical guide for clinicians. Clin Exp Optom. (2022) 105:205–13. doi: 10.1080/08164622.2022.2029683
18. Lazaridis, K, and Tzartos, SJ. Autoantibody specificities in myasthenia gravis; implications for improved diagnostics and therapeutics. Front Immunol. (2020) 11:212. doi: 10.3389/fimmu.2020.00212
19. Rousseff, RT. Diagnosis of myasthenia gravis. J Clin Med. (2021) 10:1736. doi: 10.3390/jcm10081736
20. Gilhus, NE, and Verschuuren, JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. (2015) 14:1023–36. doi: 10.1016/S1474-4422(15)00145-3
21. Lascano, AM, and Lalive, PH. Update in immunosuppressive therapy of myasthenia gravis. Autoimmun Rev. (2021) 20:102712. doi: 10.1016/j.autrev.2020.102712
22. Mantegazza, R, and Cavalcante, P. Diagnosis and treatment of myasthenia gravis. Curr Opin Rheumatol. (2019) 31:623–33. doi: 10.1097/BOR.0000000000000647
23. Gilhus, NE, Verschuuren, JJGM, Hovland, SIB, Simmonds, H, Groot, F, and Palace, J. Myasthenia gravis: do not forget the patient perspective. Neuromuscul Disord. (2021) 31:1287–95. doi: 10.1016/j.nmd.2021.07.396
24. Lehnerer, S, Jacobi, J, Schilling, R, Grittner, U, Marbin, D, Gerischer, L, et al. Burden of disease in myasthenia gravis: taking the patient's perspective. J Neurol. (2022) 269:5688–9. doi: 10.1007/s00415-022-11290-w
25. Sobieszczuk, E, Napiórkowski, Ł, Szczudlik, P, and Kostera-Pruszczyk, A. Myasthenia gravis in Poland: National Healthcare Database Epidemiological Study. Neuroepidemiology. (2021) 55:62–9. doi: 10.1159/000512973
26. Yang, Y, Zhang, M, Guo, J, Ma, S, Fan, L, Wang, X, et al. Quality of life in 188 patients with myasthenia gravis in China. Int J Neurosci. (2016) 126:455–62. doi: 10.3109/00207454.2015.1038712
27. Chu, HT, Tseng, CC, Liang, CS, Yeh, TC, Hu, LY, Yang, AC, et al. Risk of depressive disorders following myasthenia gravis: a Nationwide population-based retrospective cohort study. Front Psychol. (2019) 10:481. doi: 10.3389/fpsyt.2019.00481
28. Yamamoto, A, Kimura, T, Watanabe, S, and Yoshikawa, H. Clinical characteristics of patients with myasthenia gravis accompanied by psychiatric disorders. Neurology and Clinical Neuroscience. (2019) 2:65–70. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/ncn3.12267 (Accessed January 12, 2023).
29. Wendell, LC, and Levine, JM. Myasthenic crisis. Neurohospitalist. (2011) 1:16–22. doi: 10.1177/1941875210382918
30. Rabinstein, AA, and Mueller-Kronast, N. Risk of extubation failure in patients with myasthenic crisis. Neurocrit Care. (2005) 3:213–5. doi: 10.1385/NCC:3:3:213
31. Mantegazza, R, and Antozzi, C. From traditional to targeted immunotherapy in myasthenia gravis: prospects for research. Front Neurol. (2020) 11:981. doi: 10.3389/fneur.2020.00981
32. Romi, F, Skeie, GO, Aarli, JA, and Gilhus, NE. Muscle autoantibodies in subgroups of myasthenia gravis patients. J Neurol. (2000) 247:369–75. doi: 10.1007/s004150050604
33. Aydemir, B. The effect of myasthenia gravis as a prognostic factor in thymoma treatment. North Clin Istanb. (2017) 3:194–200. doi: 10.14744/nci.2016.60352
34. Zhang, Z, Cui, Y, Jia, R, Xue, L, and Liang, H. Myasthenia gravis in patients with thymoma affects survival rate following extended thymectomy. Oncol Lett. (2016) 11:4177–82. doi: 10.3892/ol.2016.4528
35. Tian, W, Li, X, Tong, H, Weng, W, Yang, F, Jiang, G, et al. Surgical effect and prognostic factors of myasthenia gravis with thymomas. Thorac Cancer. (2020) 11:1288–96. doi: 10.1111/1759-7714.13396
Keywords: corticosteroids, thymoma, physical health, mental health, Poland
Citation: Sobierajski T, Lasek-Bal A, Krzystanek M and Gilhus NE (2023) Diagnosis and therapy of myasthenia gravis—the patients’ perspective: a cross-sectional study. Front. Neurol. 14:1214041. doi: 10.3389/fneur.2023.1214041
Edited by:
Carlo Provenzano, Catholic University of the Sacred Heart, Rome, ItalyReviewed by:
Paolo Emilio Alboini, Home for Relief of Suffering (IRCCS), ItalyClaudia Vinciguerra, University of Salerno, Italy
Copyright © 2023 Sobierajski, Lasek-Bal, Krzystanek and Gilhus. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marek Krzystanek, krzystanekmarek@gmail.com
†ORCID: Tomasz Sobierajski, https://orcid.org/0000-0002-6853-9358
Anetta Lasek-Bal, https://orcid.org/0000-0003-2481-7528
Marek Krzystanek, https://orcid.org/0000-0002-1665-7344
 Tomasz Sobierajski
Tomasz Sobierajski