
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol., 18 July 2023
Sec. Movement Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1213772
This article is part of the Research TopicVoice Analysis in Healthy Subjects and Patients with Neurologic DisordersView all 9 articles
 Francesco Cavallieri1†
Francesco Cavallieri1† Giulia Di Rauso2,3†
Giulia Di Rauso2,3† Annalisa Gessani3
Annalisa Gessani3 Carla Budriesi2,3
Carla Budriesi2,3 Valentina Fioravanti1
Valentina Fioravanti1 Sara Contardi4
Sara Contardi4 Elisa Menozzi5
Elisa Menozzi5 Serge Pinto6
Serge Pinto6 Elena Moro7
Elena Moro7 Francesca Antonelli3*
Francesca Antonelli3* Franco Valzania1
Franco Valzania1Background: Very few studies have assessed the presence of a possible correlation between speech variables and limb bradykinesia in patients with Parkinson's disease (PD). The objective of this study was to find correlations between different speech variables and upper extremity bradykinesia under different medication conditions in advanced PD patients.
Methods: Retrospective data were collected from a cohort of advanced PD patients before and after an acute levodopa challenge. Each patient was assessed with a perceptual-acoustic analysis of speech, which included several quantitative parameters [i.e., maximum phonation time (MPT) and intensity (dB)]; the Unified Parkinson's Disease Rating Scale (UPDRS) (total scores, subscores, and items); and a timed test (a tapping test for 20 s) to quantify upper extremity bradykinesia. Pearson's correlation coefficient was applied to find correlations between the different speech variables and the tapping rate.
Results: A total of 53 PD patients [men: 34; disease duration: 10.66 (SD 4.37) years; age at PD onset: 49.81 years (SD 6.12)] were included. Levodopa intake increased the MPT of sustained phonation (p < 0.01), but it reduced the speech rate (p = 0.05). In the defined-OFF condition, MPT of sustained phonation positively correlated with both bilateral mean (p = 0.044, r-value:0.299) and left (p = 0.033, r-value:0.314) tapping. In the defined-ON condition, the MPT correlated positively with bilateral mean tapping (p = 0.003), left tapping (p = 0.003), and right tapping (p = 0.008).
Conclusion: This study confirms the presence of correlations between speech acoustic variables and upper extremity bradykinesia in advanced PD patients. These findings suggest common pathophysiological mechanisms.
Speech alterations are very common in Parkinson's disease (PD) and are reported in 70–90% of patients (1, 2). Hypokinetic dysarthria is the most frequent manifestation and can emerge at any stage of the disease, but it particularly aggravates in the later stages, causing a progressive loss of communication and leading to social isolation (3). Based on the perceptual analysis, hypokinetic dysarthria is characterized by harsh, breathy voice quality, reduced variability of pitch and loudness, reduced stress, imprecise consonant articulation, and short rushes of speech interrupted by inappropriate periods of silence (4).
In recent years, the acoustic analysis of speech has become an important tool in the study of PD and other movement disorders, allowing for the quantification of the alterations in the different dimensions of speech production (5–9).
Mixed results have been reported regarding the effects of dopaminergic treatment on speech acoustic variables in PD patients (6, 10, 11). This inconsistency in results could be secondary to the complex pathophysiology of speech alterations in PD that involve both dopaminergic and non-dopaminergic (i.e., cholinergic) pathways (10).
Besides speech alterations, PD is mainly characterized by well-known cardinal motor features, including bradykinesia, rigidity, tremor, and postural instability (12). According to the MDS Clinical Diagnostic Criteria for PD, bradykinesia is defined as “slowness of movement and decrement in amplitude or speed (or progressive hesitations/halts) as movements are continued (13).” Bradykinesia may impair the fine motor movements, which are usually demonstrated in PD patients during rapid alternating movements of the fingers, hands, or feet as a progressive reduction of speed and motion amplitude (14). Upper extremity bradykinesia can be clinically evaluated by using finger tapping, hand movements, and pronation-supination movements (13). It has been proposed that bradykinesia may result from a failure of basal ganglia output to reinforce the cortical mechanisms that prepare and execute the commands to move (15). This leads to particular difficulties with self-paced movements, prolonged reaction times, and abnormal pre-movement EEG activity (15). In PD patients, movement amplitude is disproportionally more affected than movement speed in the OFF-medication condition. Levodopa normalizes movement speed to a greater extent than movement amplitude, suggesting that movement speed and amplitude may be associated with partially separate mechanisms (16, 17). To date, prevailing evidence have indicated that hypokinetic dysarthria is related to axial motor symptoms, while only a few studies have documented an association between a speech disorder and limb bradykinesia in PD (4, 18–22). In this setting, very few studies have quantitatively assessed the possible correlations between speech acoustic variables and upper limb bradykinesia, including the presence of possible similarities in terms of response to levodopa (18, 19, 23). In addition, as previously reported, some features of hypokinetic dysarthria may respond to dopaminergic treatment (6) raising the doubt that hypokinetic dysarthria in PD should not be considered tout court an axial symptom but that it should be deconstructed by looking for different aspects either linked or not linked to axial and appendicular PD symptoms.
Based on these premises, the objective of this study was to verify if there are correlations between different speech variables and upper extremity bradykinesia in different medication conditions in advanced PD patients.
This study included retrospective data from a cohort of consecutive advanced PD patients admitted to the Neurology Unit of the OCB Hospital, Italy, for a preoperative evaluation before subthalamic nucleus deep brain stimulation (STN-DBS) surgery from 2012 to 2017.
The criteria of inclusion were PD diagnosis according to the MDS criteria (13), the presence of disabling motor complications (i.e., motor fluctuations or L-dopa-induced dyskinesia) not optimized with anti-PD medication; and age younger than 75 years (24, 25).
Patients with severe cognitive impairment or non-native Italian speakers were excluded from the analysis. This study was approved by the local ethics committee (Protocol number: 0031287/18), and written informed consent was obtained from participants according to the Declaration of Helsinki (26).
Clinical evaluations were performed following the Core Assessment Program for Surgical Interventional Therapies in Parkinson's Disease (CAPSIT-PD) protocol (17).
Each patient was evaluated in the defined-OFF medication condition (after 12-h withdrawal of antiparkinsonian medication) and in the defined-ON medication condition (60 min after the administration of a 30% higher dose of the usual levodopa morning intake). Disease severity was assessed through the four parts of the Unified Parkinson's Disease Rating Scale (UPDRS) (27) and the Hoehn and Yahr (H&Y) scale staging system.
Furthermore, upper extremity bradykinesia was quantitatively assessed through a tapping timed test in accordance with the CAPSIT-PD protocol (17, 28) in the defined-OFF and -ON medication conditions. Each patient tapped alternatingly on two buttons (at a 20 cm distance) with the index finger by using the whole upper extremity for a defined fixed time (20 s). Each hand was tested twice, and the mean value of the tapping rate was reported. All tests were videotaped, and through the retrospective analysis of each video, it was possible to calculate with certainty the correct number of taps for each task. A free editing software (Wondershare Filmora 9) was used to analyze the video in slow motion. The retrospective analysis of the video was performed by a GDR blinded to both defined-ON and -OFF conditions.
The total amount of the dopaminergic treatment was determined using the L-dopa equivalent daily dose (LEDD) (29).
Patients' speech was evaluated in both the defined-OFF and defined-ON medication conditions by two speech and language therapists (CB and AG) with expertise in phonetics and movement disorders related to speech disturbances. Each session of speech evaluation took place immediately at the end of each neurological examination. Evaluations were made in a quiet room. The speech was recorded using a digital voice recorder maintained at 20 cm from the patients' lips. The acoustic speech analysis was performed using the Praat software (30). The perceptual-acoustic analysis was retrospectively performed, with the speech and language therapists blinded to the patient's condition. The speech assessment protocol (6, 7) consisted of various tasks, including sustained production of the phoneme /a/ for as long as possible and performed three times, counting from 1 to 20, and an oral diadochokinesis task in which the participants produced the syllables /pa/, /ta/, /ka/ and the pseudoword /pataka/ as fast as they could with habitual pitch and loudness. The variables considered were maximum phonation time (MPT) [s], intensity [dB], a fraction of locally unvoiced frames, and a number of voice breaks (all these parameters were evaluated during sustained phonation tasks); speech rate [syllables/second] calculated during counting tasks; and irregular rhythm [presence of absence] and uncontrolled acceleration [presence of absence] evaluated during oral diadochokinesis tasks. Single-word intelligibility (calculated as the percentage of words correctly transcribed by the examiner among a set of 25 recorded words) was also calculated.
Descriptive statistics were performed for clinical and acoustic variables; continuous variables were expressed as mean [standard deviation (SD)] and median (range), while frequencies and percentages were calculated for categorical variables. The variables were tested for normal distribution using the Kolmogorov-Smirnov test of normality. A p-value of < 0.05 was considered significant.
The primary outcome of the study was the possible correlation between the different speech variables and upper extremity bradykinesia quantified through the tapping test in different medication conditions. Concerning the tapping test, the following variables were selected for both defined-OFF and defined-ON medication conditions: mean value of the left hand; mean value of the right hand; and mean value of both hands ([mean value of the left hand + mean value of the right hand]/2).
The secondary outcome was the possible correlation between the levodopa-induced variation of speech variables and upper extremity bradykinesia, both calculated as follows: [(defined-ON value minus defined-OFF value)/defined-ON value] × 100. Positive scores denote an increase in speech variables or tapping rate. The correlation between speech variables and UPDRS motor scores and subscores was not included in the analysis because it was already performed in a previous study by our group (6). Considering that most variables were normally distributed, the Pearson correlation coefficient was applied. The correlation analyses, including speech and motor variables, were performed for one of the single conditions tested (defined-OFF, defined-ON). Statistical analyses were performed using the IBM SPSS Statistics software for Windows version 20.0 (IBM, Armonk, NY, USA).
From a total of 66 consecutive advanced PD patients, 13 patients were excluded from the analyses for the following reasons: non-native Italian speakers (five patients), missing data (four patients), and severe cognitive impairment (four patients). The clinical and demographic characteristics of the remaining 53 patients are reported in Table 1.
In the defined-ON medication condition, all motor scores, subscores, and tapping rates significantly improved, whereas only speech rate (p = 0.005) and MPT (p = 0.001) were influenced (Table 2).
The correlations between speech variables and upper extremity bradykinesia are reported in Table 3.
In the defined-OFF medication condition, the MPT of sustained phonation correlated positively with bilateral mean tapping (p = 0.044, r-value:0.299). Analyzing the test for the single limb, the correlation remained significant for the left (p = 0.033, r-value:0.314), which presented a worse performance, while only a trend was detected on the right (p = 0.067, r-value:0.272).
In the defined-ON medication condition, the MPT correlated positively with bilateral mean tapping (p = 0.003, r-value:0.429); in this case, a significant correlation was maintained for both left (p = 0.003, r-value:0.438) and right tapping (p = 0.008, r-value:0.391).
In both the defined-OFF and -ON medication conditions, speech rate did not show a correlation with bradykinesia, with the exception of a weak significance (p = 0.038, r-value:0.307) for the right tapping in the defined-OFF condition. Nevertheless, these data were insufficient to support an unequivocal relationship between the two findings.
Although both tapping tests, speech rate, and MPT significantly changed after levodopa intake, no significant correlations were found, which means that the effects of levodopa on these variables were not uniform (Table 4).
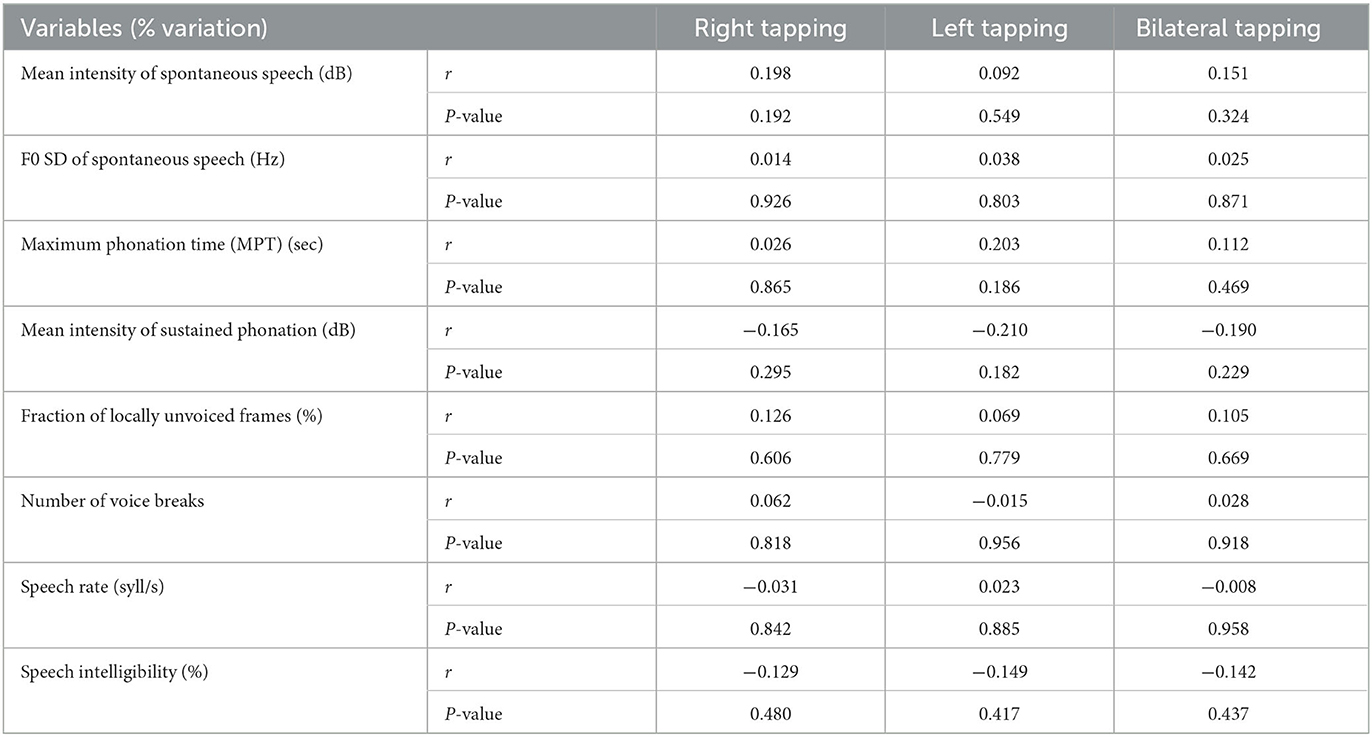
Table 4. Correlation between levodopa-induced changes of speech variables and upper extremity bradykinesia.
The main objective of this study was to determine a relationship between speech and upper extremity bradykinesia in advanced PD patients. We found different correlations between speech acoustic variables and tapping rate in both the defined-OFF and defined-ON medication conditions. However, it is important to keep in mind that the correlation tests, either Pearson or Spearman, do not prove causality but only strength of association. In particular, in our study, neither the methodology employed nor the statistical analysis was designed to infer causation. In addition, curiously, we found a positive correlation between speech impairment and left bradykinesia during the defined-OFF condition and with both left and right bradykinesia during the defined-ON condition, which could be surprising. However, in the defined-OFF condition, the correlation between speech acoustic variables and right tapping showed a trend toward significance. Thus, we may assume that, with a larger cohort, the correlation might become significant even with the right tapping.
In our cohort, the MPT correlated with upper limb bradykinesia in both pharmacological conditions, meaning that patients with a longer MPT performed better on the tapping test. Phonatory alterations are quite common in PD, including insufficient breath support, a reduction in phonation time, increased acoustic noise, instability of the articulatory organs, microperturbations of frequency/amplitude, and a harsh, breathy voice quality (31). The physiological and anatomical correlates of these alterations have been investigated through laryngoscopy, stroboscopy, photoglottography, laryngeal electromyography, computed-tomography, pulmonary function testing, and aerodynamic assessments (32). These correlates have revealed numerous abnormalities, including incomplete glottic closure and vocal fold hypoadduction/bowing to account for these voice changes. Many of these phenomena are likely related to rigidity or bradykinesia of the laryngeal muscles (32).
The clinical feature of hypokinetic dysarthria reflects the effects on the speech of aberrations in the control of proper background tone and the supportive neuromuscular activity on which the quick, discrete, phasic movements of speech are superimposed. Hypokinetic dysarthria prominently affects aspects of speech motor control such as the preparation, maintenance, and switching of motor programs with movements that are attenuated in range and amplitude and restricted in their flexibility and speed, allowing inferences about the role of the basal ganglia control circuit in speech motor control (33).
The MPT, a marker of reduced phonation time, depends on many factors, including phonation volume (which varies with age, sex, and stature), mean airflow rate, comprehension of the task, and maximal effort (34). Reduced MPT has been well documented mainly in PD hypokinetic dysarthria (9, 35–39), probably as a consequence of laryngeal dysfunction or decreased respiratory volume, leading to the development of short phrases and short rushes of speech (6). This hyporespiratory pattern may result from the rigidity and bradykinesia in the respiratory muscles, particularly the intercostal ones (38). In PD patients, reduced respiratory excursions, reduced vital capacity, paradoxical respiratory movements, rapid breathing cycles, and difficulty altering vegetative breathing for speech breathing seem consistent with rigidity, hypokinesia, and difficulty in initiating movements (33). These factors could significantly contribute to reduced physiologic support for speech and some of the phonatory disorders and prosodic abnormalities, including short phrases and short rushes of speech (33).
The short-term improvement of MPT with levodopa was demonstrated previously, supporting the hypothesis that levodopa might improve thoracic mobility in PD patients (38). This finding was also confirmed by a recent study from our group, which showed that MPT was the only speech acoustic variable responsive to levodopa in the acute setting (6). The correlation between phonatory alterations and limb bradykinesia has also been confirmed (based on clinical scales) in de novo PD patients (22), suggesting that dopaminergic deficiency may be involved in voice dysfunction in PD, presumably through bradykinesia and/or rigidity of the laryngeal musculature (22).
Based on these premises, we suppose that the correlation found between MPT and upper extremity tapping rate in our cohort might reflect a common pathophysiological basis (i.e., bradykinesia of appendicular, laryngeal, and respiratory muscles) with the involvement of prevalent dopaminergic pathways responsive to levodopa. In fact, the degree of response to levodopa is different between these two parameters, as confirmed in our cohort. Indeed, no correlations were found between levodopa-induced percentage changes in MPT and upper extremity bradykinesia, which means that, even if levodopa improves both parameters, this improvement is not uniform. Moreover, it must be considered that the tapping test and MPT are only two specific findings of two more complex functions such as movement and speech.
We found a weak correlation between speech rate and right tapping in the defined-OFF medication condition. Nonetheless, the absence of correlations in the other defined-OFF and -ON medication conditions would exclude an unequivocal relationship between the two findings.
Speech rate is generally expressed as the number of syllables pronounced during a defined time period. It is affected by different factors such as segment duration, variability between the duration of utterances, and the pause time between the different utterances (40). In PD patients, speech rate alterations have been found in both directions (i.e., slower and faster), and the mean rate differences between PD and control speakers were not found to be significant due to a highly heterogeneous overall group performance (3). Previous studies have also shown little or no effect of levodopa administration or bilateral STN-DBS on speech rate in PD patients (41–43). This was not confirmed in our cohort; indeed, speech rate was significantly reduced after levodopa intake. These contradictory results about the short-term effects of levodopa on speech rate and rhythm indicate a considerable impact of the nondopaminergic mechanism, which are implicated in the impairment of time perception, motor planning, and dysfunctional feedback mechanisms (40).
Our study has several limitations, including the retrospective origin of the data and the lack of a control group of healthy subjects to compare with the PD cohort. In addition, we assessed only advanced PD patients, so further studies are needed to test the relationship between upper limb bradykinesia and speech variables in early PD patients.
Furthermore, we did not collect several demographic and anthropometric variables of the participants, including weight, height, and BMI. In addition, voice features relevant to the acoustic analysis of hypokinetic dysarthria in PD have not been included in the study. It is known that f0, jitter, and shimmer, among other voice features, can be severely impaired in hypokinetic dysarthria, especially in advanced-stage PD. Also, these features are relevant for discriminating early- and advanced-stage PD, so they should always be considered in speech analysis in movement disorders, as nicely reported in Rusz et al. (8) and Suppa et al. (44).
To the best of our knowledge, only one study has compared speech acoustic variables and upper limb motor dysfunction with a quantitative approach, supporting the hypothesis that pathophysiological processes leading to limb motor dysfunction in PD may play a role, at least partially, also in a more complex function such as speech (18). In particular, significant relationships were observed between the quality of voice assessed by jitter and amplitude decrement of finger tapping, consonant articulation evaluated using voice onset time and expert rating of finger tapping, and the number of pauses and Purdue Pegboard Test score (18). Based on their results, Rusz et al. in their study assumed that vocal fold vibration irregularities appeared to be influenced by mechanisms similar to amplitude decrement during repetitive limb movements, while consonant articulation deficits were associated with decreased manual dexterity and movement speed, likely reflecting fine motor control involvement in PD (18). Furthermore, MPT was not included among the different speech variables, and no correlation was found between diadochokinetic rate and markers of upper limb motor dysfunction, as opposed to our cohort (18). This could be explained both by the different tasks used to quantify speech rate (oral diadochokinesis vs. counting from 1 to 20) and the different pharmacological conditions tested in the two cohorts. In fact, our patients were evaluated both in the defined-OFF and defined-ON medication conditions, while in the study by Rusz et al. the patients were evaluated only in chronic pharmacological treatment (18).
Our study confirms the presence of some correlations between speech acoustic variables and upper extremity bradykinesia in advanced PD patients. This may be due to common pathophysiological mechanisms and the possible involvement of dopaminergic pathways, as assumed for MPT. This confirms the need to take into account independently every single speech parameter altered in PD. As a consequence, speech alterations should not be considered anymore as a solely axial manifestation of the disease unresponsive to dopaminergic treatment and without a relationship with PD cardinal motor symptoms. Future studies will be needed to confirm these data on larger samples and on early-stage PD patients.
The datasets for this article are not publicly available due to concerns regarding participant/patient anonymity. Requests to access the datasets should be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Comitato Etico dell'Area Vasta Emilia Nord (Protocol Number: 0031287/18), and written informed consent was obtained from participants according to the Declaration of Helsinki.
FC, GDR, AG, CB, VF, SC, EMe, FA, and FV were responsible for writing the manuscript, data collection, and analysis. FC, GDR, CB, AG, SC, VF, EMe, SP, EMo, FA, and FV were responsible for manuscript drafting. CB, SP, EMe, FA, and FV were responsible for manuscript revision. All authors have read and approved the final manuscript.
This study was partially supported by Italian Ministry of Health – Ricerca Corrente Annual Program 2023.
EMo has received honoraria from Medtronic for consulting and lecturing and has received research grants from Ipsen and Abbott.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chenausky K, MacAuslan J, Goldhor R. Acoustic analysis of PD speech. Parkinson's Disease. (2011) 2011:1–13. doi: 10.4061/2011/435232
2. Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson's disease. Behav Neurol. (1999) 11:131–7. doi: 10.1155/1999/327643
3. Skodda S. Aspects of speech rate and regularity in Parkinson's disease. J Neurol Sci. (2011) 310:231–6. doi: 10.1016/j.jns.2011.07.020
4. Skodda S, Grönheit W, Schlegel U. Intonation and speech rate in Parkinson's disease: general and dynamic aspects and responsiveness to levodopa admission. J Voice. (2011) 25:e199–205. doi: 10.1016/j.jvoice.2010.04.007
5. Rossi J, Cavallieri F, Giovannini G, Budriesi C, Gessani A, Carecchio M, et al. Spasmodic dysphonia as a presenting symptom of spinocerebellar ataxia type 12. Neurogenetics. (2019) 20:161–4. doi: 10.1007/s10048-019-00580-7
6. Cavallieri F, Budriesi C, Gessani A, Contardi S, Fioravanti V, Menozzi E, et al. Dopaminergic treatment effects on dysarthric speech: acoustic analysis in a cohort of patients with advanced Parkinson's disease. Front Neurol. (2020) 11:616062. doi: 10.3389/fneur.2020.616062
7. Gessani A, Cavallieri F, Budriesi C, Zucchi E, Malagoli M, Contardi S, et al. Pearls & Oy-sters: Paroxysmal dysarthria-ataxia syndrome: acoustic analysis in a case of antiphospholipid syndrome. Neurology. (2019) 92:e2727–31. doi: 10.1212/WNL.0000000000007619
8. Rusz J, Tykalova T, Ramig LO, Tripoliti E. Guidelines for speech recording and acoustic analyses in dysarthrias of movement disorders. Movement Disorders. (2021) 36:803–14. doi: 10.1002/mds.28465
9. Rusz J, Bonnet C, Klempír J, Tykalová T, Baborová E, Novotný M, et al. Speech disorders reflect differing pathophysiology in Parkinson's disease, progressive supranuclear palsy and multiple system atrophy. J Neurol. (2015) 262:992–1001. doi: 10.1007/s00415-015-7671-1
10. Brabenec L, Mekyska J, Galaz Z, Rektorova I. Speech disorders in Parkinson's disease: early diagnostics and effects of medication and brain stimulation. J Neural Transm. (2017) 124:303–34. doi: 10.1007/s00702-017-1676-0
11. Lechien JR, Blecic S, Huet K, Delvaux V, Piccaluga M, Roland V, et al. Voice quality outcomes of idiopathic Parkinson's disease medical treatment: a systematic review. Clin Otolaryngol. (2018) 43:882–903. doi: 10.1111/coa.13082
12. Goubault E, Nguyen HP, Bogard S, Blanchet PJ, Bézard E, Vincent C, et al. Cardinal motor features of Parkinson's disease coexist with peak-dose choreic-type drug-induced dyskinesia. J Parkinsons Dis. (2018) 8:323–31. doi: 10.3233/JPD-181312
13. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson's disease: MDS-PD clinical diagnostic criteria. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
14. Magrinelli F, Picelli A, Tocco P, Federico A, Roncari L, Smania N, et al. Pathophysiology of motor dysfunction in Parkinson's disease as the rationale for drug treatment and rehabilitation. Parkinsons Dis. (2016) 2016:9832839. doi: 10.1155/2016/9832839
15. Berardelli A. Pathophysiology of bradykinesia in Parkinson's disease. Brain. (2001) 124:2131–46. doi: 10.1093/brain/124.11.2131
16. Espay AJ, Beaton DE, Morgante F, Gunraj CA, Lang AE, Chen R. Impairments of speed and amplitude of movement in Parkinson's disease: a pilot study: bradykinesia in Parkinson's disease. Mov Disord. (2009) 24:1001–8. doi: 10.1002/mds.22480
17. Defer GL, Widner H, Marié RM, Rémy P, Levivier M. Core assessment program for surgical interventional therapies in Parkinson's disease (CAPSIT-PD). Mov Disord. (1999) 14:572–84.
18. Rusz J, Tykalová T, Krupička R, Zárubová K, Novotný M, Jech R, et al. Comparative analysis of speech impairment and upper limb motor dysfunction in Parkinson's disease. J Neural Transm (Vienna). (2017) 124:463–70. doi: 10.1007/s00702-016-1662-y
19. Skodda S, Visser W, Schlegel U. Gender-related patterns of dysprosody in Parkinson disease and correlation between speech variables and motor symptoms. J Voice. (2011) 25:76–82. doi: 10.1016/j.jvoice.2009.07.005
20. Midi I, Dogan M, Koseoglu M, Can G, Sehitoglu MA, Gunal DI. Voice abnormalities and their relation with motor dysfunction in Parkinson's disease. Acta Neurol Scand. (2008) 117:26–34. doi: 10.1111/j.1600-0404.2007.00965.x
21. Zarzur AP, Duprat AC, Shinzato G, Eckley CA. Laryngeal electromyography in adults with Parkinson's disease and voice complaints. Laryngoscope. (2007) 117:831–4. doi: 10.1097/MLG.0b013e3180333145
22. Polychronis S, Niccolini F, Pagano G, Yousaf T, Politis M. Speech difficulties in early de novo patients with Parkinson's disease. Parkinsonism Relat Disord. (2019) 64:256–61. doi: 10.1016/j.parkreldis.2019.04.026
23. Goberman AM. Correlation between acoustic speech characteristics and non-speech motor performance in Parkinson disease. Med Sci Monit. (2005) 11:CR109–116.
24. Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, et al. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. (2003) 349:1925–34. doi: 10.1056/NEJMoa035275
25. Cavallieri F, Fraix V, Bove F, Mulas D, Tondelli M, Castrioto A, et al. Predictors of long-term outcome of subthalamic stimulation in Parkinson disease. Ann Neurol. (2021) 89:587–97. doi: 10.1002/ana.25994
26. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
27. Fahn S, Marsden CD, Calne DB, Goldstein M. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Health Care Information (1987).
28. Pötter-Nerger M, Wenzelburger R, Deuschl G, Volkmann J. Impact of subthalamic stimulation and medication on proximal and distal bradykinesia in Parkinson's disease. Eur Neurol. (2009) 62:114–9. doi: 10.1159/000222783
29. Jost ST, Kaldenbach M-A, Antonini A, Martinez-Martin P, Timmermann L, Odin P, et al. Levodopa dose equivalency in Parkinson's disease: updated systematic review and proposals. Mov Disord. (2023) mds.29410. doi: 10.1002/mds.29410
30. Boersma P, Weenink D. Praat: Doing Phonetics by Computer [Computer Program]. Version 5.3.51. (2013). Available online at: http://www.praat.org/ (accessed June 2, 2013).
31. Galaz Z, Mekyska J, Zvoncak V, Mucha J, Kiska T, Smekal Z, et al. Changes in phonation and their relations with progress of Parkinson's disease. Appl Sci. (2018) 8:2339. doi: 10.3390/app8122339
32. Ma A, Lau KK, Thyagarajan D. Voice changes in Parkinson's disease: what are they telling us? J Clin Neurosci. (2020) 72:1–7. doi: 10.1016/j.jocn.2019.12.029
33. Duffy JR. Motor Speech Disorders: Substrates, Differential Diagnosis, and Management. Third edition. St Louis, Mo: Elsevier Mosby (2013).
34. Maslan J, Leng X, Rees C, Blalock D, Butler SG. Maximum phonation time in healthy older adults. J Voice. (2011) 25:709–13. doi: 10.1016/j.jvoice.2010.10.002
35. de Angelis EC, Mourão LF, Ferraz HB, Behlau MS, Pontes PA, Andrade LA. Effect of voice rehabilitation on oral communication of Parkinson's disease patients. Acta Neurol Scand. (1997) 96:199–205. doi: 10.1111/j.1600-0404.1997.tb00269.x
36. Gamboa J, Jiménez-Jiménez FJ, Nieto A, Montojo J, Ortí-Pareja M, Molina JA, et al. Acoustic voice analysis in patients with Parkinson's disease treated with dopaminergic drugs. J Voice. (1997) 11:314–20. doi: 10.1016/S0892-1997(97)80010-0
37. Yücetürk A, Yilmaz H, Egrilmez M, Karaca S. Voice analysis and videolaryngostroboscopy in patients with Parkinson's disease. Eur Arch Otorhinolaryngol. (2002) 259:290–3. doi: 10.1007/s00405-002-0462-1
38. De Letter M, Santens P, De Bodt M, Van Maele G, Van Borsel J, Boon P. The effect of levodopa on respiration and word intelligibility in people with advanced Parkinson's disease. Clin Neurol Neurosurg. (2007) 109:495–500. doi: 10.1016/j.clineuro.2007.04.003
39. Sachin S, Shukla G, Goyal V, Singh S, Aggarwal V, Behari M. Clinical speech impairment in Parkinsonís disease, progressive supranuclear palsy, and multiple system atrophy. Neurol India. (2008) 56:5. doi: 10.4103/0028-3886.41987
40. Skodda S, Schlegel U. Speech rate and rhythm in Parkinson's disease. Mov Disord. (2008) 23:985–92. doi: 10.1002/mds.21996
41. Skodda S, Visser W, Schlegel U. Short- and long-term dopaminergic effects on dysarthria in early Parkinson's disease. J Neural Transm. (2010) 117:197–205. doi: 10.1007/s00702-009-0351-5
42. Skodda S, Flasskamp A, Schlegel U. Instability of syllable repetition as a model for impaired motor processing: is Parkinson's disease a “rhythm disorder”? J Neural Transm. (2010) 117:605–12. doi: 10.1007/s00702-010-0390-y
43. Cantiniaux S, Vaugoyeau M, Robert D, Horrelou-Pitek C, Mancini J, Witjas T, et al. Comparative analysis of gait and speech in Parkinson's disease: hypokinetic or dysrhythmic disorders? J Neurol Neurosurg Psychiatry. (2010) 81:177–84. doi: 10.1136/jnnp.2009.174375
Keywords: acoustic, bradykinesia, dysarthria, Parkinson's disease, speech
Citation: Cavallieri F, Di Rauso G, Gessani A, Budriesi C, Fioravanti V, Contardi S, Menozzi E, Pinto S, Moro E, Antonelli F and Valzania F (2023) A study on the correlations between acoustic speech variables and bradykinesia in advanced Parkinson's disease. Front. Neurol. 14:1213772. doi: 10.3389/fneur.2023.1213772
Received: 28 April 2023; Accepted: 15 June 2023;
Published: 18 July 2023.
Edited by:
Luigi M. Romito, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Carlos Zúñiga-Ramírez, Civil Hospital of Guadalajara, MexicoCopyright © 2023 Cavallieri, Di Rauso, Gessani, Budriesi, Fioravanti, Contardi, Menozzi, Pinto, Moro, Antonelli and Valzania. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Antonelli, YW50b25lbGxpLmZAZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.