- Department of Neurology, Charité Universitätsmedizin Berlin, Berlin, Germany
Introduction: Patients with Parkinson’s disease (PD) exhibit deficits in social cognition, particularly with respect to Theory of Mind (ToM) capacities. It is unclear whether they are associated with PD-related dopamine deficiency and modulated by levodopa replacement therapy.
Methods: A total of 15 persons with PD and 13 healthy controls (HC) participated in the study. They performed different neuropsychological tasks, including the Faux Pas Recognition Test (FPRT), assessing different dimensions of cognitive ToM (e.g., detection, inappropriateness, intentions), and the Reading the Mind in the Eyes Test (RMET) as an index of affective ToM. Persons with PD were tested twice, once under their regular treatment and another time after at least 18 h of levodopa withdrawal (MED-ON and MED-OFF, respectively). On either occasion, serum drug levels and motor symptom severity [Unified Parkinson’s Disease Rating Scale (UPDRS)] were measured.
Results: MED-ON and MED-OFF conditions in patients with PD were confirmed by higher serum drug levels in the former than in the latter state and a corresponding amelioration of the motor deficit. In so doing, no performance difference in any ToM-related task was identified as a function of the levodopa therapy. Generally, patients performed worse than controls in both affective and cognitive ToM tests.
Conclusion: Patients with PD have deficits in cognitive and affective ToM. Dopamine replacement, effective for improving the motor condition, does not appear to counteract these dysfunctions.
Introduction
Parkinson’s disease (PD) is a neurological movement disorder. However, apart from typical motor symptoms such as bradykinesia, tremor, and rigidity, it implies numerous non-motor signs. Among the latter, deficits of social cognition (SC) are regularly associated with the disease. They could result from the spread of the neurodegenerative process beyond the typical loss of dopaminergic neurons within the nigrostriatal system, for example, to mesocortical networks.
SC refers to the cognitive operations necessary for socially adapted behaviors (1). A fundamental prerequisite of this capacity is the ability to generate own concepts of the mental states of other people (2), labeled as Theory of mind (ToM) (3). Commonly, ToM is divided into an affective part, underlying rather immediate, empathetic processes, and a cognitive aspect, comprising strategical inferences about given mindsets, e.g., as a model of intentions or motivations for perceived behaviors (4). Cognitive ToM strongly involves executive functions, such as updating, e.g., with respect to the concept of a social situation during its evolution, switching, e.g., between the perspectives of characters therein, and flexible informational retrieval, in order to construe complex behaviors as targeted action plans (5, 6). In contrast to this, affective ToM is considered an automatic process in which sensory input, e.g., from observed facial or bodily expressions, is related to mental representations of the own motor repertoire, conceivable as a process of imagery or reenactment (7, 8). Thus, the mechanisms by which PD compromises ToM could be different with respect to its distinct domains. In particular, a disorder of executive functions could contribute to cognitive ToM deficits, whereas the genuine motor deficit could also influence affective ToM performance if it extended to processes of internal motor simulation. Accordingly, the sensitivity of both aspects of ToM to PD treatment could be different.
In PD, deficits of cognitive and affective ToM have been described (9–11) and seem to grow together with disease progression (12). Several studies point to a particular involvement of dopamine deficiency in this regard. In animal studies, for example, amphetamine-induced dopamine release led to reduced affiliative social behavior, whereas antagonists of D1-type dopamine receptors reversed this effect (13). Furthermore, dopaminergic transmission in the mesocorticolimbic system, compromised in PD (14), modulates reward processing and, in so doing, is likely to exert effects on levels of social functioning (15). Concerning PD specifically, findings from patient studies on genuine disease effects and pharmacological replacement therapies appear heterogeneous. For example, ToM performance was described as both unaffected and abnormal in early PD, and particular effects of the pharmacological treatment were deemed absent (16, 17).
On the other hand, dopaminergic therapy was associated with the ability to interpret facial expressions correctly (18, 19). However, these and further investigations did not contrast the task performances of the same persons with PD in pharmacologically treated and untreated states. Hence, conclusions about real-life SC-related consequences of the disease and dopaminergic replacement therapy are difficult to draw. Accordingly, we tested the cognitive and affective components of ToM (20–22) in the same cohort of patients under their regular dopaminergic medication and after drug withdrawal. The results from these conditions were compared with each other and with the corresponding performances of persons without PD. We hypothesized that patients with PD perform worse than controls in cognitive and affective ToM tasks. Levodopa could, first of all, improve affective ToM deficits, given the mentioned results from animal research and a possible impact of motor system states on the decoding of bodily expressions including mimics. A secondary explorative aim was to further study cognitive task performances in either group and with respect to levodopa replacement in PD, given presumed associations of cognitive ToM with executive functions.
Methods
Study participants
Twenty-three patients with Parkinson’s disease (according to the criteria of the Movement Disorder Society) were enrolled in the study, treated only with levodopa with a decarboxylase inhibitor (Benserazide or Carbidopa) and recruited from the outpatient clinic for movement disorders at the Charité-Universitätsmedizin Berlin. The exclusion criteria were other known major psychiatric or neurological disorders and an unwillingness to undergo levodopa withdrawal. Eight participants did not complete the study protocol, of which six decided not to undergo the second test visit, one patient had a fall resulting in in-patient rehabilitation, and another one discontinued the medication and could, therefore, not be assessed under MED-ON. The data from 15 patients could finally be analyzed. From the pool of accompanying persons, 13 controls without Parkinson’s disease and free of any other neurological condition took part. All participants were over 18 years of age and had given written informed consent to the study protocol approved by the ethics committee of the Charité (EA4/165/17) in accordance with the Declaration of Helsinki.
Cognitive screening
To assess the cognitive profile across different domains, patients under MED-ON and controls performed the Parkinson Neuropsychometric Dementia Assessment for cognition (PANDA-cognition). Similar to other screening tools, but designed for PD patients in particular, the PANDA-cognition comprises five subtests, i.e., (i) a word-learning task, (ii) alternating phonemic verbal fluency task, (iii) a visuospatial task, (iv) a working memory and attention task, and (v) delayed recall of the word list. Scores can range between 0 (worst) and 30 (best) points, with a cutoff for suspected dementia at values below 15. Additionally, the PANDA-mood was determined to assess the affective situation of the participants. The mood questionnaire consists of three questions assessing central aspects of depressive mood (mood, interest, drive) with a maximum score of 9 (23). Since, first of all, we aimed at examining potential intraindividual differences between ToM performances in the MED-ON versus MED-OFF condition within a realistic PD population, the PANDA was used to assess the cognitive profile of the participants without defining a cut-off value for study exclusion. With respect to controls, we sought to reach an acceptable match of the PANDA values between the groups.
Patients were tested twice at intervals of at least 2 months, on the one hand, under their regular levodopa treatment (MED-ON), and on the other hand, at least 18 h after their last levodopa intake (MED-OFF). The order of the examination days per condition (first MED-ON, second MED-OFF/first MED-OFF, second MED-ON) was balanced between the patients. Patients ran two task versions for further cognitive tests, one under MED-ON and the other under MED-OFF, starting with version 1 or 2 in random order.
Assessment of affective and cognitive theory of mind
The participants engaged in two standard theory of mind (ToM) tasks: the Reading the Mind in the Eyes Task (RMET) and the Faux Pas Recognition Test (FPRT). In the RMET, one has to decide about emotional states based on photos of the periocular eye region of different persons (one photo per person). The decision is made from four predefined options (e.g., angry, sad, friendly, and flirty). Since the task requires the perceptual decoding of facial expressions, the RMET measures empathetic capacities, i.e., affective ToM (24).
For the patients, the original set of 36 pictures was divided into two parts of 18 photos in order to not repeat presentations in either MED-ON or MED-OFF but to have them engage in the entire task over the two sessions. Controls (having one test session only) ran the original task. Scores were expressed as the percentage of correctly evaluated pictures from all the presented pictures. Unlike the RMET, the FPRT is text-based. Although the last question in the FPRT task addresses an aspect of affective ToM, the entire task paradigm demands cognitive ToM capacity, implying strategic thinking and perspective changes (16, 20). The task comprises 20 short stories in which persons communicate with each other. Half of them contain conversations with inappropriateness regarding social rules, and the other half do not. Per story, one has to answer (i) whether the misconduct was present (Faux Pas Detection), and, if this was the case, (ii) why it is inappropriate (Faux Pas Inappropriateness), (iii) which goal it pursued (Faux Pas Intention), (iv) whether it was formulated accidentally or on purpose (Faux Pas Belief), and (v) which emotions it triggered in the interlocutors (Empathy). For correct (incorrect) responses, one (no) point is given; scores are expressed as the percentage ratio of reached to maximally possible points. The stories in the FPRT task were presented to the patients on paper and read aloud by the examiner. The answers were given verbally and noted down by the examiner. There was no time limit for the responses to the questions since the task focus was on accuracy. PD patients engaged in split test versions with 10 stories per MED-ON and MED-OFF, respectively (containing 5 stories with and without a faux pas each), so they ran the entire task over the two test sessions. Controls performed the original task in one session.
Further cognition assessment
The PD patients performed several additional tasks to assess whether potential changes in ToM performance by levodopa intake were associated with modulations of other cognitive functions. Specifically, the Cognitive Subscale of the Alzheimer’s Disease Assessment Scale (ADAScog) was used, comprising subtasks for word recall and recognition, naming objects and fingers, following commands, constructional and ideational praxis, orientation, language capacities, and memory (25) Furthermore, a number of dedicated functions of interest, typically compromised in PD, were tested with the digit span forward and backward tests (26), the clock drawing test (27), the German standard test for verbal fluency (Regensburger Wortflüssigkeitstest, RWT) (28), and the concept shifting test (CST) (29). The digit span forward and backward tests were performed to assess working memory and attention. The examiner verbally presented a span of three to nine digits and captured the participants’ verbal recall. Testing was stopped after two consecutive failures of the same span length. In the RWT, participants were asked to name as many words as possible starting with “S” (set 1) or “K” (set 2; phonematic verbal fluency) or to name animals (set 1) or groceries (set 2; semantic verbal fluency) in 1 min. The naming of erroneous words was not counted. For the CST, paper sheets with 16 small circles arranged in a larger circle were presented. The small circles contained digits, letters, both digits and letters, or were empty. Participants were instructed to cross out as quickly as possible the randomly arranged numbers in numerical order, the letters in alphabetical order, and the numbers and letters alternatingly in numerical and alphabetical order, respectively (e.g., 1 A 2 B 3 C).
Alternative versions were run for all tasks in the MED-ON and MED-OFF conditions.
Assessment of the motor condition and drug levels in patients
To determine the patients’ movement condition as a function of levodopa intake, the motor part of the Unified Parkinson Disease Rating Scale (UPDRS Part III) in the MED-ON and MED-OFF conditions was used. UPDRS scores range from 0 to 108, with lower scores representing less severe motor impairment. Furthermore, serum levels of levodopa and oximethyl-DOPA (metabolite) were assessed in blood samples taken in either condition. As the plasma half-life of levodopa is up to 2 h, the levodopa levels were expected to be hardly detectable under MED-OFF. However, as the effects of levodopa have been described for up to 2 weeks after levodopa withdrawal, we analyzed oximethyl-DOPA, a major metabolite of levodopa, possibly indicating prolonged levodopa effects.
Data analysis
Normal data distribution was analyzed by the Kolmogorov–Smirnov and Shapiro–Wilk tests with further assessment of skewness and kurtosis. For normally distributed variables, between-group and within-group comparisons were performed by corresponding t-tests; otherwise, non-parametric tests for independent samples (Mann–Whitney U-Test) or for related samples (Wilcoxon Signed-Rank Test) were used. To determine whether there was evidence for the absence of the effect, we conducted Bayesian statistical analyses. The significance level was set at p-values < 0.05. For data analysis, the software package SPSS, Version 27.0.0.0, was used (IBM Corp., Armonk, NY, United States).
Results
Clinical and demographic characteristics
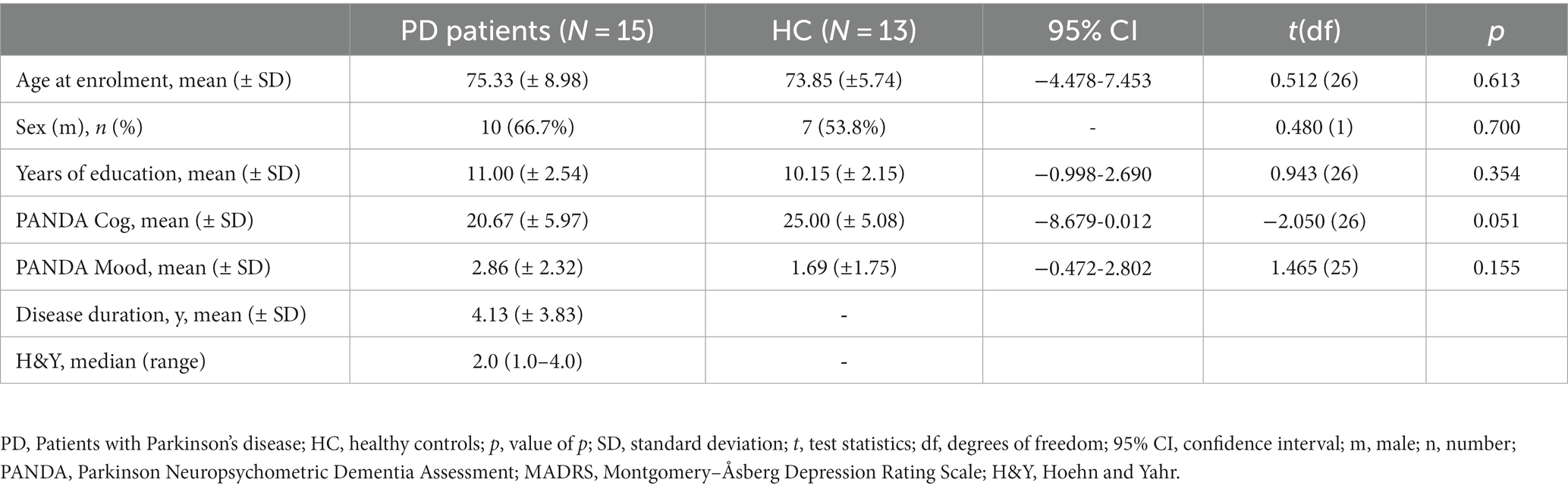
PD patients and controls did not significantly differ with respect to age, sex, and duration of school education. PANDA scores did not statistically differ between the groups. Still, the group contrast of PANDA-cog values almost reached significance. PD patients showed lower scores than healthy controls in line with known, foremost executive deficits compared to the age-matched persons. PD patients were treated by levodopa with a dopamine decarboxylase inhibitor (Benserazide or Carbidopa). The mean interval between the test sessions in the MED-ON and MED-OFF conditions was 72 days (± 66 days). Clinical and demographic characteristics are displayed in Table 1.
Patients’ motor condition and drug levels in MED-ON and MED-OFF conditions
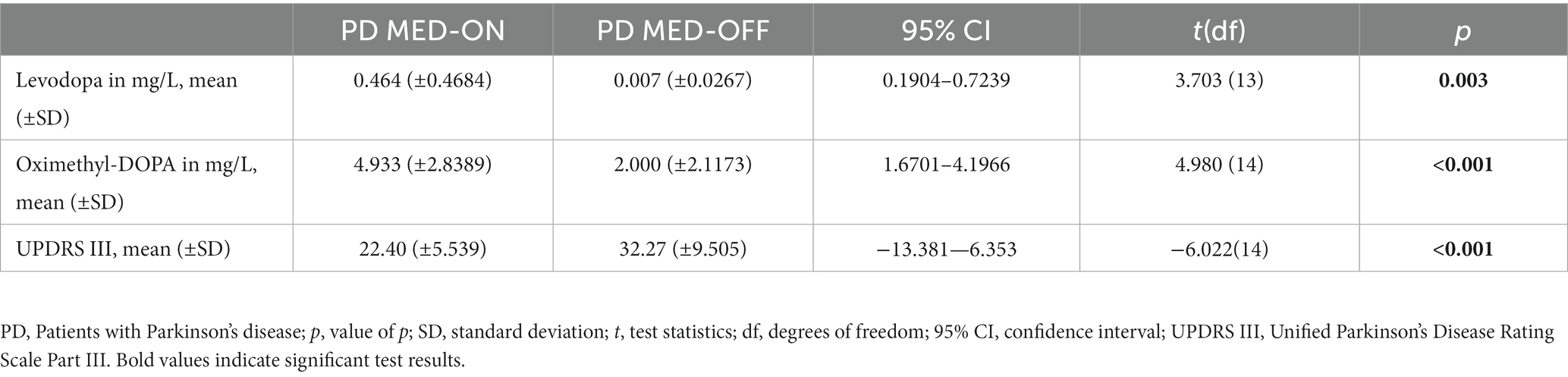
Levodopa [t(df) = 3.703 (13); p = 0.003] and oximethyl-DOPA [t(df) = 4.980 (14); p < 0.001] levels differed significantly between the MED-ON and MED-OFF conditions of the patients. Whereas levodopa was almost absent in the MED-OFF condition, oximethyl-DOPA was still detectable in the MED-OFF condition but significantly lower than in the MED-ON condition. Accordingly, the motor condition was significantly worse in the MED-OFF than in the MED-ON condition, as indicated by higher scores in the UPDRS Part III [t(df) = −6.022 (14); p < 0.001; see Table 2].
Performance on theory of mind tests
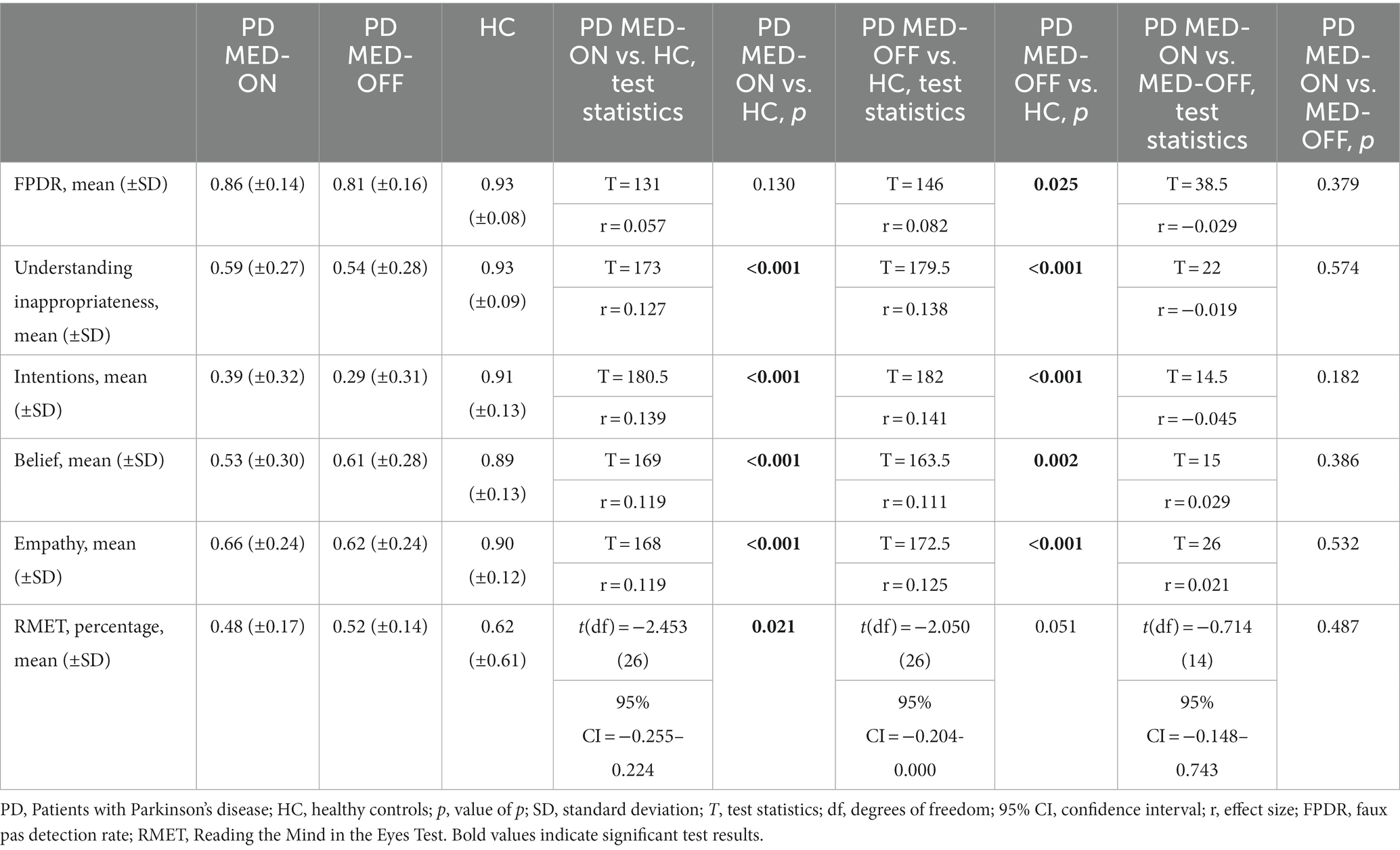
The results of the FPRT were not normally distributed. The faux pas detection rate was lower in patients under MED-OFF than in controls (T = 146; r = 0.082; p = 0.025). This statistical group difference vanished when patients were in the MED-ON condition. Concerning all other cognitive theory of mind dimensions (understanding inappropriateness, intentions, belief, and empathy), patients, be they under MED-ON or MED-OFF, performed worse than controls. Within patients, no performance differences were identified between the MED-ON and MED-OFF conditions (Figures 1 2).
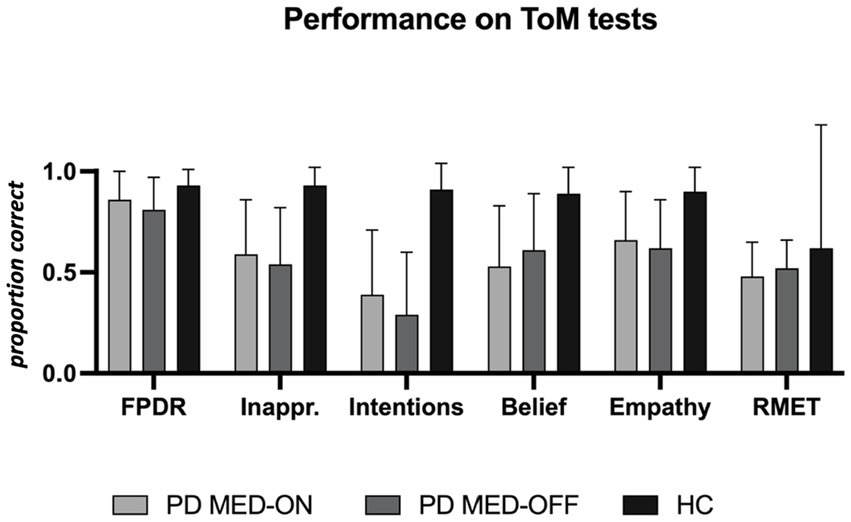
Figure 1. Performance on theory of mind tests. PD MED-ON, Patients with Parkinson’s disease on levodopa; PD MED-OFF, Patients with Parkinson’s disease off levodopa; HC, healthy controls; FPDR, Faux pas discovery rate; Inappr., Understanding inappropriateness; RMET, Reading the mind in the eyes test.
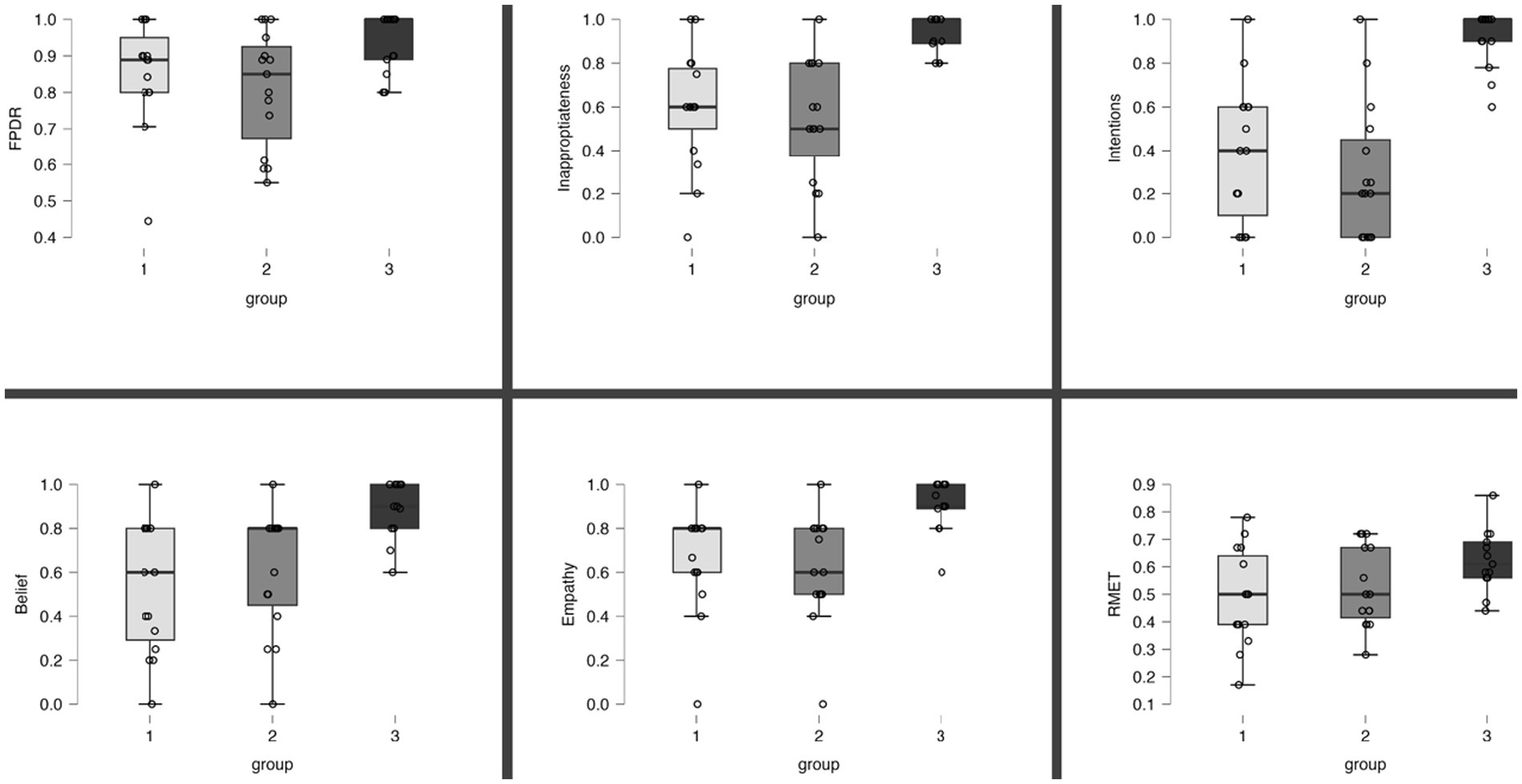
Figure 2. Overview of individual results per ToM task. Proportion of correct responses in the ToM tests. Boxes represent interquartile range and the median. Group 1: PD, MED-ON; Group 2: PD, MED-OFF; Group 3, Healthy controls; FPDR, faux pas detection rate; RMET, Reading the mind in the eyes test.
The RMET results were lower in the patients under MED-ON than in controls [t(df) = 2.453 (26); p = 0.021]. However, under MED-OFF, this group difference failed to be significant, with the difference between MED-ON and MED-OFF performances being marginal (Figures 1 2; Table 3).
In Bayesian statistics, the likelihood of performance differences between MED-ON and MED-OFF being absent was three to four times higher than the likelihood of these differences being present [FPDR (BF01 = 3.209; p = 0.343), Understanding inappropriateness (BF01 = 4.018; p = 0.481), Intentions (BF01 = 3.099, p = 0.313), Belief (BF01 = 3.786; p = 0.433), Empathy (BF01 = 4.331; p = 0.557), RMET (BF01 = 4.046, p = 0.487)].
Neuropsychological assessment
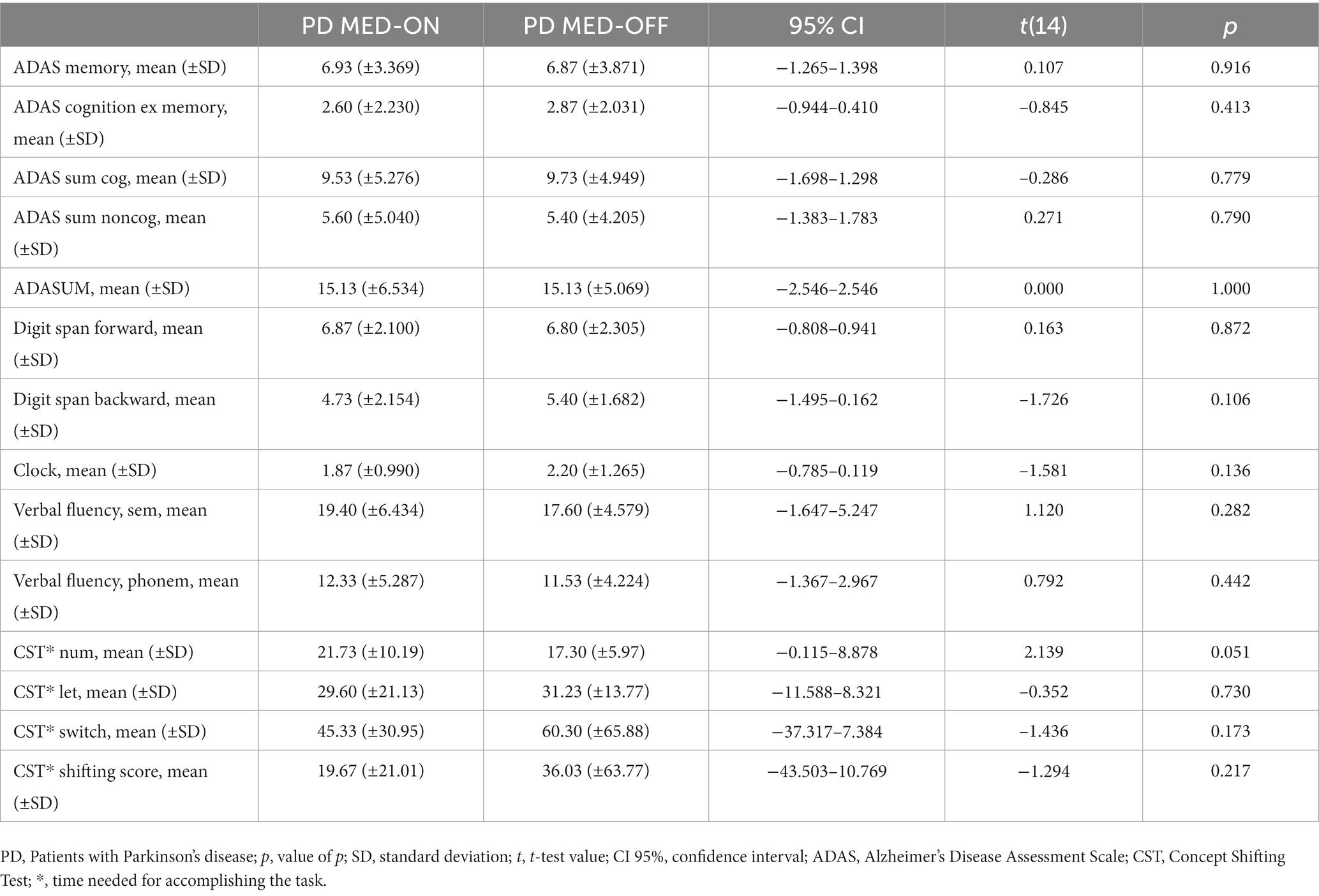
The results from the further neuropsychological assessments (ADAScog, the digit span forward and backward, the clock drawing test, semantic and phonematic verbal fluency tests, and concept shifting tests) were not significantly different between the MED-ON and MED-OFF conditions in PD patients (Table 4).
Discussion
Persons with PD performed worse than controls in cognitive and affective ToM tasks, in line with previous reports on social cognitive deficits in this condition. Dopamine replacement therapy did not exert a relevant impact on these dysfunctions.
Whether a therapy partially compensating for the central neurotransmitter deficit in PD and robustly reducing motor symptoms also improves relevant non-motor dysfunctions is an obvious question, which, with respect to ToM, has not been studied in the same patients in the MED-ON and MED-OFF conditions. Absent levodopa effects on ToM dysfunctions in PD fall in line with the observation that cognitive changes of PD are largely unresponsive to dopaminergic treatment (30). This is worthwhile to note since ToM may, at least partly, reflect processes relatively independent of other cognitive functions (31), but also because different data and concepts suggest its susceptibility to PD medication. For example, dopamine-releasing drugs weakened affiliative behaviors in animal studies, and this effect vanished after pharmacological dopamine receptor blockade (13). Furthermore, in PD, dopamine transmission is not only deficient in the nigrostriatal system, associated primarily with motor function, but also in mesocorticolimbic networks (14), presumably involved in processing social cognitive functions (15, 32). Previously, subtle differences in the recognition of negative facial expressions were reported with respect to unmedicated versus medicated PD patients (18, 19), whereby it has to be noted that these groups differed largely with respect to disease parameters (e.g., the medicated group was in a more advanced disease stage). A theoretical basis for the assumption of levodopa-related effects on ToM dysfunctions in PD comes from cognitive embodiment concepts. In this view, internal simulation or imagery is conceived to be instrumental in the semantic decoding of, e.g., observed gestures, postures, or facial expressions, providing a quasi-experience of the perceived (33, 34). In this vein, a human mirror neuron network implying primary motor regions was proposed based on brain activations during mere movement observation (35–37). From this perspective, levodopa-induced improvement of ToM via enhancing motor system state functions in PD seems conceivable.
However, the current data suggest that levodopa has no relevant impact on ToM dysfunctions under realistic therapeutic conditions. This analysis is supported by the Bayesian statistics finding the likelihood of absent differences in the MED-ON versus MED-OFF conditions three to four times higher than the likelihood of their presence. Nevertheless, considering several study limitations, minor effects cannot be ruled out. Due to the fact that affected persons are mostly under different PD drugs, candidates on a monotherapy able to undergo the test protocol were altogether rare, so the group size remained small with 15 patients. Given that in trials with comparable research questions similar numbers were reported (16–19), further studies on related topics might therefore consider multicenter recruitment strategies from the beginning to overcome this shortcoming. Furthermore, levodopa was reported to unfold verifiable motor effects even weeks after drug withdrawal, suggesting that its serum levels do not necessarily represent central drug actions (38). Therefore, we additionally assessed oximethyl-dopa as an active metabolite, decreased in the MED-OFF compared to the MED-ON condition, but naturally, it is theoretically possible that levodopa effects on ToM could become evident only after longer drug withdrawal phases. However, longer intake pauses would not have been feasible since one-third of the recruited patients dropped out for excessive strain caused by the demanded drug withdrawal.
Since patients in the PD group underwent two test sessions, they only performed one-half of the ToM task trials per measurement, so they altogether went through the same tasks as the controls did in one session. Thus, the shorter test series in the PD patients than in the controls could theoretically have influenced the results, e.g., in that learning occurs throughout task performance. However, no difference between the ToM performances in the first compared to the second session of ToM task performances was identified in the PD patients. Therefore, we think the disparity of ToM testing per session did not relevantly influence the current results. However, the exact behavioral meaning of the FPRT and RMET results remains to be settled. For example, cognitive ToM functions, such as first- and second-order beliefs, are not differentiated by the FPRT, and the narration of social situations or the presentation of static pictures differs from dynamic and polymodal event perception in real life. In this regard, the development of dedicated task designs and the comparison of test outcomes with indices of natural social conduct are a need that should be addressed in future studies.
Finally, concerning potential subtle effects, it deserves mention that, compared to the controls, the patients’ performance in the RMET was only statistically abnormal under MED-ON and in one dimension of the FPRT only under MED-OFF (the faux pas detection rate, FPDR). This is theoretically compatible with task-specific levodopa effects. According to the overdose hypothesis, levodopa replacement compensates for the overall dopamine deficit in dorsal striatal networks but may flood better-preserved ventral striatal regions (39). The latter form part of networks implying the ventromedial prefrontal and orbitofrontal regions, which are involved in affective ToM processing (40, 41), whereas the dorsal striatum projects to dorsolateral prefrontal areas with a role in cognitive ToM (10, 21). From this perspective, levodopa-induced worsening of RMET performance seems conclusive, just as a (cognitive ToM-related) FPDR improvement. However, given their almost negligible size, corresponding intraindividual differences need to be corroborated in further studies and are, for the time being, not deemed clinically relevant.
We conclude that levodopa replacement does not unfold relevant effects on ToM dysfunctions in PD. This inertness of social cognitive deficits to the pharmacological mainstay of PD treatment is unfortunate but is in parallel with the behavior of further cognitive dysfunctions characteristic of the condition.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Charité Universitätsmedizin Berlin. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TU: research project: conception, organization, and execution, statistical analysis: design and execution, and manuscript: writing of the first draft. EK: research project: organization and execution, statistical analysis: review and critique, and manuscript: review and critique. FK: research project: conception, organization, and execution, statistical analysis: design and review and critique, manuscript: writing and review and critique. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the transverse research project (S2020649). We acknowledge financial support from the Open Access Publication Fund of Charité – Universitätsmedizin Berlin and the German Research Foundation (DFG).
Conflict of interest
FK received honoraria for lecturing and advisory activities, unrelated to the presented research, from Abbvie, Stadapharm, Esteve, and CSL Behring.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Adolphs, R. Social cognition and the human brain. Trends Cogn Sci. (1999) 3:469–79. doi: 10.1016/S1364-6613(99)01399-6
2. Premack, D, and Woodruff, G. Premack and Woodruff: chimpanzee theory of mind. Behav Brain Sci. (1978) 4:515–26.
3. Henry, JD, Von Hippel, W, Molenberghs, P, Lee, T, and Sachdev, PS. Clinical assessment of social cognitive function in neurological disorders. Nat Rev Neurol. (2016) 12:28–39. doi: 10.1038/nrneurol.2015.229
4. Shamay-Tsoory, SG, Tibi-Elhanany, Y, and Aharon-Peretz, J. The ventromedial prefrontal cortex is involved in understanding affective but not cognitive theory of mind stories. Soc Neurosci. (2006) 1:149–66. doi: 10.1080/17470910600985589
5. Shamay-Tsoory, SG, and Aharon-Peretz, J. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia. (2007) 45:3054–67. doi: 10.1016/j.neuropsychologia.2007.05.021
6. Thoma, P, Winter, N, Juckel, G, and Roser, P. Mental state decoding and mental state reasoning in recently detoxified alcohol-dependent individuals. Psychiatry Res. (2013) 205:232–40. doi: 10.1016/j.psychres.2012.08.042
7. Hynes, CA, Baird, AA, and Grafton, ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. (2006) 44:374–83. doi: 10.1016/j.neuropsychologia.2005.06.011
8. Völlm, BA, Taylor, ANW, Richardson, P, Corcoran, R, Stirling, J, McKie, S, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage. (2006) 29:90–8. doi: 10.1016/j.neuroimage.2005.07.022
9. Kosutzka, Z, Kralova, M, Kusnirova, A, Papayova, M, Valkovic, P, Csefalvay, Z, et al. Neurocognitive predictors of understanding of intentions in Parkinson disease. J Geriatr Psychiatry Neurol. (2019) 32:178–85. doi: 10.1177/0891988719841727
10. Bodden, ME, Mollenhauer, B, Trenkwalder, C, Cabanel, N, Eggert, KM, Unger, MM, et al. Affective and cognitive theory of mind in patients with Parkinson’s disease. Parkinsonism Relat Disord. (2010) 16:466–70. doi: 10.1016/j.parkreldis.2010.04.014
11. Roca, M, Torralva, T, Gleichgerrcht, E, Chade, A, Arévalo, GG, Gershanik, O, et al. Impairments in social cognition in early medicated and unmedicated Parkinson disease. Cogn Behav Neurol. (2010) 23:152–8. doi: 10.1097/WNN.0b013e3181e078de
12. Poletti, M, Vergallo, A, Ulivi, M, Sonnoli, A, and Bonuccelli, U. Affective theory of mind in patients with Parkinson’s disease. Psychiatry Clin Neurosci. (2013) 67:273–6. doi: 10.1111/pcn.12045
13. Homberg, JR, Olivier, JDA, VandenBroeke, M, Youn, J, Ellenbroek, AK, Karel, P, et al. The role of the dopamine D1 receptor in social cognition: studies using a novel genetic rat model. Dis Model Mech. (2016) 9:1147–58. doi: 10.1242/dmm.024752
14. Castrioto, A, Thobois, S, Carnicella, S, Maillet, A, and Krack, P. Emotional manifestations of PD: neurobiological basis. Mov Disord. (2016) 31:1103–13. doi: 10.1002/mds.26587
15. Skuse, DH, and Gallagher, L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. (2009) 13:27–35. doi: 10.1016/j.tics.2008.09.007
16. Péron, J, Vicente, S, Leray, E, Drapier, S, Drapier, D, Cohen, R, et al. Are dopaminergic pathways involved in theory of mind? A study in Parkinson’s disease. Neuropsychologia. (2009) 47:406–14. doi: 10.1016/j.neuropsychologia.2008.09.008
17. Del Prete, E, Turcano, P, Unti, E, Palermo, G, Pagni, C, Frosini, D, et al. Theory of mind in Parkinson’s disease: evidences in drug-naïve patients and longitudinal effects of dopaminergic therapy. Neurol Sci. (2020) 41:2761–6. doi: 10.1007/s10072-020-04374-w
18. de Letter, M, Santens, P, Estercam, I, van Maele, G, de Bodt, M, Boon, P, et al. Levodopa-induced modifications of prosody and comprehensibility in advanced Parkinson’s disease as perceived by professional listeners. J Multiling Commun Disord. (2007) 21:783–91. doi: 10.1080/02699200701538181
19. Sprengelmeyer, R, Young, AW, Mahn, K, Schroeder, U, Woitalla, D, Büttner, T, et al. Facial expression recognition in people with medicated and unmedicated Parkinson’s disease. Neuropsychologia. (2003) 41:1047–57. doi: 10.1016/S0028-3932(02)00295-6
20. Stone, VE, Baron-Cohen, S, and Knight, RT. Frontal lobe contributions to theory of mind. Sci Ment Health. (2013) 2:226–42. doi: 10.1162/089892998562942
21. Gregory, C, Lough, S, Stone, V, Erzinclioglu, S, Martin, L, Baron-Cohen, S, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: theoretical and practical implications. Brain. (2002) 125:752–64. doi: 10.1093/brain/awf079
22. Baron-Cohen, S, Wheelwright, S, Hill, J, Raste, Y, and Plumb, I. The “Reading the mind in the eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry Allied Discip. (2001) 42:241–51. doi: 10.1111/1469-7610.00715
23. Kalbe, E, Calabrese, P, Kohn, N, Hilker, R, Riedel, O, Wittchen, HU, et al. Screening for cognitive deficits in Parkinson’s disease with the Parkinson neuropsychometric dementia assessment (PANDA) instrument. Parkinsonism Relat Disord. (2008) 14:93–101. doi: 10.1016/j.parkreldis.2007.06.008
24. Baron-Cohen, S, O’Riordan, M, Stone, V, Jones, R, and Plaisted, K. Recognition of faux pas by normally developing children and children with asperger syndrome or high-functioning autism. J Autism Dev Disord. (1999) 29:407–18. doi: 10.1023/A:1023035012436
25. Kueper, JK, Speechley, M, and Montero-Odasso, M. The Alzheimer’s disease assessment scale-cognitive subscale (ADAS-cog): modifications and responsiveness in pre-dementia populations. A Narrative Review. J Alzheimers Dis. (2018) 63:423–44. doi: 10.3233/JAD-170991
26. Grogan, JP, Knight, LE, Smith, L, Irigoras Izagirre, N, Howat, A, Brogan, L, et al. Effects of Parkinson’s disease and dopamine on digit span measures of working memory. Psychopharmacology. (2018) 235:3443–50. doi: 10.1007/s00213-018-5058-6
27. Shulman, KI. Clock-drawing: is it the ideal cognitive screening test? Int J Geriatr Psychiatry. (2000) 15:548–61. doi: 10.1002/1099-1166(200006)15:6<548::AID-GPS242>3.0.CO;2-U
28. Aschenbrenner, S., Tucha, O., and Lange, K. W. Regensburger Wortflüssigkeits-Test RWT. Hogrefe, Verlag für Psychologie. (2000) Available at: https://www.worldcat.org/title/53428620 (Accessed 8 July 2023)
29. Van Der Elst, W, Van Boxtel, MPJ, Van Breukelen, GJP, and Jolles, J. The concept shifting test: adult normative data. Psychol Assess. (2006) 18:424–32. doi: 10.1037/1040-3590.18.4.424
30. Rukavina, K, Batzu, L, Boogers, A, Abundes-Corona, A, Bruno, V, and Chaudhuri, KR. Non-motor complications in late stage Parkinson’s disease: recognition, management and unmet needs. Expert Rev Neurother. (2021) 21:335–52. doi: 10.1080/14737175.2021.1883428
31. Aboulafia-Brakha, T, Christe, B, Martory, MD, and Annoni, JM. Theory of mind tasks and executive functions: a systematic review of group studies in neurology. J Neuropsychol. (2011) 5:39–55. doi: 10.1348/174866410X533660
32. Van’t Hooft, JJ, YAL, P, SAM, S, Scheltens, P, Spikman, JM, Jaschke, AC, et al. Frontotemporal dementia, music perception and social cognition share neurobiological circuits: a meta-analysis. Brain Cogn. (2021) 148:105660. doi: 10.1016/j.bandc.2020.105660
33. Gallese, V. Before and below “theory of mind”: embodied simulation and the neural correlates of social cognition. Philos Trans R Soc Lond. (2007) 362:659–69. doi: 10.1098/rstb.2006.2002
34. Mier, D, Haddad, L, Diers, K, Dressing, H, Meyer-Lindenberg, A, and Kirsch, P. Reduced embodied simulation in psychopathy. World J Biol Psychiatry. (2014) 15:479–87. doi: 10.3109/15622975.2014.902541
35. Dreyer, AM, Michalke, L, Perry, A, Chang, EF, Lin, JJ, Knight, RT, et al. Grasp-specific high-frequency broadband mirror neuron activity during reach-and-grasp movements in humans. Cereb Cortex. (2022) 33:bhac504. doi: 10.1093/cercor/bhac504
36. Dreyer, AM, and Rieger, JW. High-gamma mirror activity patterns in the human brain during reach-to-grasp movement observation, retention, and execution—an MEG study. PLoS One. (2021) 16:1–19. doi: 10.1371/journal.pone.0260304
37. Perry, A, Stiso, J, Chang, EF, Lin, JJ, Parvizi, J, and Knight, RT. Mirroring in the human brain: deciphering the spatial-temporal patterns of the human mirror neuron system. Cereb Cortex. (2018) 28:1039–48. doi: 10.1093/cercor/bhx013
38. Hauser, RA, Koller, WC, Hubble, JP, Malapira, T, Busenbark, K, and Olanow, CW. Time course of loss of clinical benefit following withdrawal of levodopa/carbidopa and bromocriptine in early Parkinson’ s disease. Mov Disord. (2000) 15:485–9. doi: 10.1002/1531-8257(200005)15:3<485::AID-MDS1010>3.0.CO;2-F
39. Cools, R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. (2006) 30:1–23. doi: 10.1016/j.neubiorev.2005.03.024
40. Shamay-Tsoory, SG, Aharon-Peretz, J, and Perry, D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. (2009) 132:617–27. doi: 10.1093/brain/awn279
Keywords: Parkinson’s disease, ToM, social cognition, levodopa, nonmotor symptoms
Citation: Usnich T, Krasivskaya E and Klostermann F (2023) Theory of mind deficits in Parkinson’s disease are not modulated by dopaminergic medication. Front. Neurol. 14:1208638. doi: 10.3389/fneur.2023.1208638
Edited by:
Alexandre Gironell, Universitat Autònoma de Barcelona, SpainReviewed by:
Marit Ruitenberg, Leiden University, NetherlandsJacky Ganguly, Institute of Neurosciences, Kolkata (I-NK), India
Copyright © 2023 Usnich, Krasivskaya and Klostermann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fabian Klostermann, ZmFiaWFuLmtsb3N0ZXJtYW5uQGNoYXJpdGUuZGU=
 Tatiana Usnich
Tatiana Usnich Elena Krasivskaya
Elena Krasivskaya Fabian Klostermann
Fabian Klostermann