- 1Department of Neurosurgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
- 2Wenzhou Medical University, Wenzhou, China
Background: Lower extremity deep vein thrombosis (DVT) is one of the major postoperative complications in patients with ruptured intracranial aneurysms (RIA) who underwent endovascular treatment (EVT). However, patient-specific predictive models are still lacking. This study aimed to construct and validate a nomogram model for estimating the risk of lower extremity DVT for RIA patients who underwent EVT.
Methods: This cohort study enrolled 471 RIA patients who received EVT in our institution between 1 January 2020 to 4 February 2022. Perioperative information on participants is collected to develop and validate a nomogram for predicting lower extremity DVT in RIA patients after EVT. Predictive accuracy, discriminatory capability, and clinical effectiveness were evaluated by concordance index (C-index), calibration curves, and decision curve analysis.
Result: Multivariate logistic regression analysis showed that age, albumin, D-dimer, GCS score, middle cerebral artery aneurysm, and delayed cerebral ischemia were independent predictors for lower extremity DVT. The nomogram for assessing individual risk of lower extremity DVT indicated good predictive accuracy in the primary cohort (c-index, 0.92) and the validation cohort (c-index, 0.85), with a wide threshold probability range (4–82%) and superior net benefit.
Conclusion: The present study provided a reliable and convenient nomogram model developed with six optimal predictors to assess postoperative lower extremity DVT in RIA patients, which may benefit to strengthen the awareness of lower extremity DVT control and supply appropriate resources to forecast patients at high risk of RIA-related lower extremity DVT.
Introduction
A ruptured intracranial aneurysm (RIA) is a severe neurologic emergency with devastating effects and unfavorable outcomes. It is demonstrated that the case fatality of RIA can be as high as 30–50% and at least 20% of those who survive are unable to regain functional independence (1–4). Since the publication of the International Subarachnoid Aneurysm Trial (ISAT) study, there has been a tendency toward endovascular treatment (EVT) of RIA as the first line of therapy for making smaller surgical incisions and giving patients a better health-related quality of life (5–7). The EVT of RIA has made huge strides but postoperative complications, especially lower extremity deep vein thrombosis (DVT), can not only cause swelling, localized pain, and varicose veins but may also lead to pulmonary embolism (PE), and is still a common and serious disease among RIA patients (8, 9). Several recent studies show that the incidence of lower extremity DVT ranges from 5 to 21%, and the mortality rate is between 9 and 19% (9–13).
Therefore, it is a very urgent problem to identify and predict RIA patients at the highest risk for lower extremity DVT as soon as possible. However, many factors that drive thrombosis in veins or affect its resolution remain unclear (14). Studies have indicated that intraparenchymal cerebral hemorrhage, motor deficit, high D-dimer level at hospitalization, and postoperative paralysis are key risk factors. Additionally, some studies found that an increased neutrophil-to-lymphocyte ratio is valuable in predicting lower extremity DVT (1, 11, 15). However, there is still a lack of an accurate and effective tool to predict the postoperative incidence of lower extremity DVT for RIA patients.
A mathematical model capable of predicting the risk of lower extremity DVT for RIA patients may be an approach to address this problem. A nomogram is a graphical depiction that presents a regression model in a friendly manner and simplifies risk assessment, providing medical staff with a user-friendly interface to map the probability of an event to individual patients, which has widely been used in intracranial aneurysms research (16–18). The purpose of this study is to develop and validate a nomogram model, thus providing a promising and facile avenue for predicting the risk of lower extremity DVT after RIA patients underwent EVT and promoting patient recovery.
Methods
Study population
This is a retrospective cohort study. A total of 507 patients newly diagnosed with RIA and undergoing EVT in the First Affiliated Hospital of Wenzhou Medical University from 1 January 2020 to 4 February 2022 were included. Study samples and treatment data were retrieved from the respective surgical department databases. Patients with the following characteristics were excluded: (1) age < 18 years (N = 2), (2) complicated with intracranial vascular malformations or moyamoya disease (N = 16), (3) bleeding during EVT or rebleeding after EVT (N = 12), (4) patients with preoperative unexpected events that could affect the outcome: preoperative cardiac arrest (N = 1) and severe head trauma (N = 1), and (5) missing critical information (N = 3). Finally, a total of 471 cases were enrolled; in the enrolled patients, 377 RIA patients were included in the training cohort, and additional data were included in the validation cohort. Figure 1 shows the flow diagram of the screening process in detail. The data used in this study were approved by the Institutional Review Board of Wenzhou Medical University which waived the requirement for individual patient consent because they did not contain personal identifiers. This study follows the principles of the Declaration of Helsinki (19).
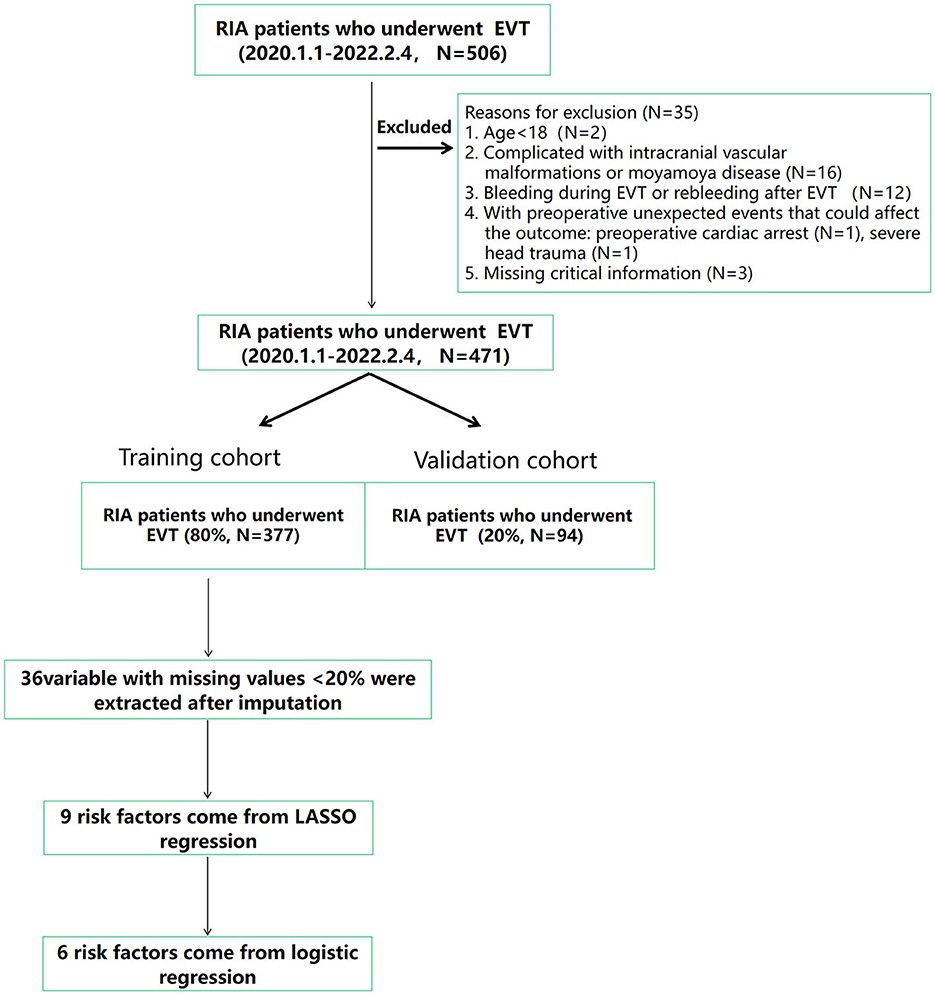
Figure 1. Flow diagram of the study showing the selection of RIA patients who were included in the analysis. DVT, deep venous thrombosis; EVT, endovascular treatment; RIA, ruptured intracranial aneurysms.
Variable definition and data collection
RIA patients were diagnosed by biplanar digital subtraction angiography (DSA) after the diagnosis of subarachnoid hemorrhage by head computed tomography (CT) (20, 21). Our diagnosis criterion for lower extremity DVT was based on the observation of lower limb deep vein intravascular shadows by Doppler ultrasound (DUS) that included B-mode imaging and color Doppler flow imaging with or without probe compression within 2 weeks of EVT treatment (22, 23). Considering the high incidence of lower extremity DVT, each patient needs to be examined by DUS every week after admission to our institution. Other variables include gender, age, height, underlying diseases (hypertension, diabetes, coronary heart disease, and infection), the location of the aneurysm [anterior communicating artery (ACoA), posterior communicating artery (PCoA), internal carotid artery (ICA), vertebrobasilar aneurysm (VBA)], stent-assisted EVT, ventricular drainage, smoking, modified Fisher scale, Hunt-Hess grades, World Federation of Neurosurgical Societies (WFNS) grade, Glasgow Coma Scale (GCS), and presence or absence of motor deficits on admission, laboratory examination on admission (total cholesterol, uric acid, triglyceride, glucose, albumin, D-dimer, C-reactive protein (CRP), mean corpuscular volume (MCV), hemoglobin, lymphocyte count, monocyte count, neutrophil counts, and platelet count), mechanical ventilation time after EVT, and delayed cerebral ischemia (DCI) after EVT.
Statistical analysis
SPSS software version 25.0 and R software version 4.0.2 were used for statistical analysis. Continuous variables with normal distribution were expressed as mean and standard deviation (SD), and differences between groups were compared by an independent sample t-test. Continuous variables that were not normally distributed were represented by median and range, and differences between groups were compared using the Mann–Whitney U-test. Categorical variables were presented as frequencies or percentages, and the chi-square tests were used to compare differences between groups. The least absolute contraction and selection operator (LASSO) regression model was used to deal with the collinearity problem of candidate variables to select the optimal predictor variables. A logistic regression analysis was used to estimate univariate and multivariate odds ratios and 95% confidence intervals. A P-value of <0.05 was statistically significant, and the C-index was used to evaluate the discrimination of the model. Clinical utility and net benefits were determined by decision curve analysis (DCA) (24). In total, 1,000 bootstrap re-samples were used for external validation, and the relative corrected C-index was calculated to ensure the stability of the nomogram in the validation cohort.
Result
Population characteristics
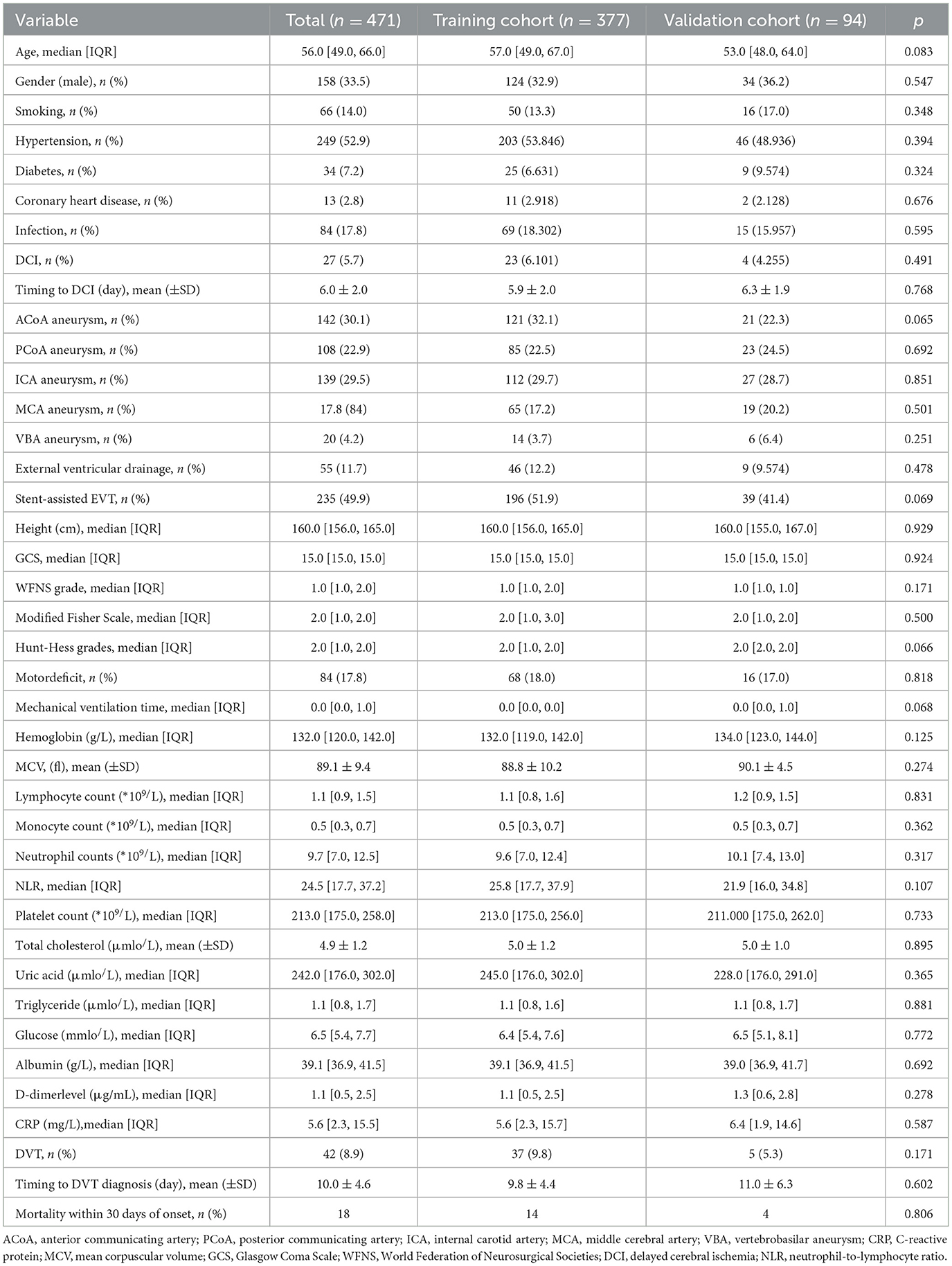
Table 1 shows the clinical characteristics of the study population. A total of 471 RIA patients who underwent EVT were enrolled in this study, separated by the training cohort (377 patients, from 1 January 2020 to 16 November 2020) and validation cohort (94 patients, from 17 August 2021 to 4 February 2022). Patients' age ranged from 18 to 89 years (54.0 ± 15.5). Among the primary cohort patients, 42 patients had lower extremity DVT, leading to an incidence rate of 8.9%. Demographics and clinical and laboratory characteristics for both cohorts are shown in Table 1. No significant differences were noted between the cohorts.
Selected predictors
Of the 36 variables with missing values, <20% was extracted after interpolation, and nine potential predictors were finally screened from the LASSO regression analysis (Figure 2). Inclusion of these nine variables in a logistic regression model resulted in six variables that were independently statistically significant predictors of critical illness and were included in the risk score (Table 2). These variables include age (OR, 1.07; 95% CI, 1.02–1.11; P = 0.003), albumin level (OR, 0.88; 95% CI, 0.81–0.96; P = 0.005), D-dimer level (OR, 1.24; 95% CI, 1.12–1.39; P < 0.001), GCS score (OR, 0.72; 95% CI, 0.63–0.81; P < 0.001), middle cerebral artery (MCA) aneurysm (OR, 5.67; 95% CI, 1.95–16.94; P = 0.001), and DCI (OR, 3.79; 95% CI, 1.03–13.244; P = 0.039).
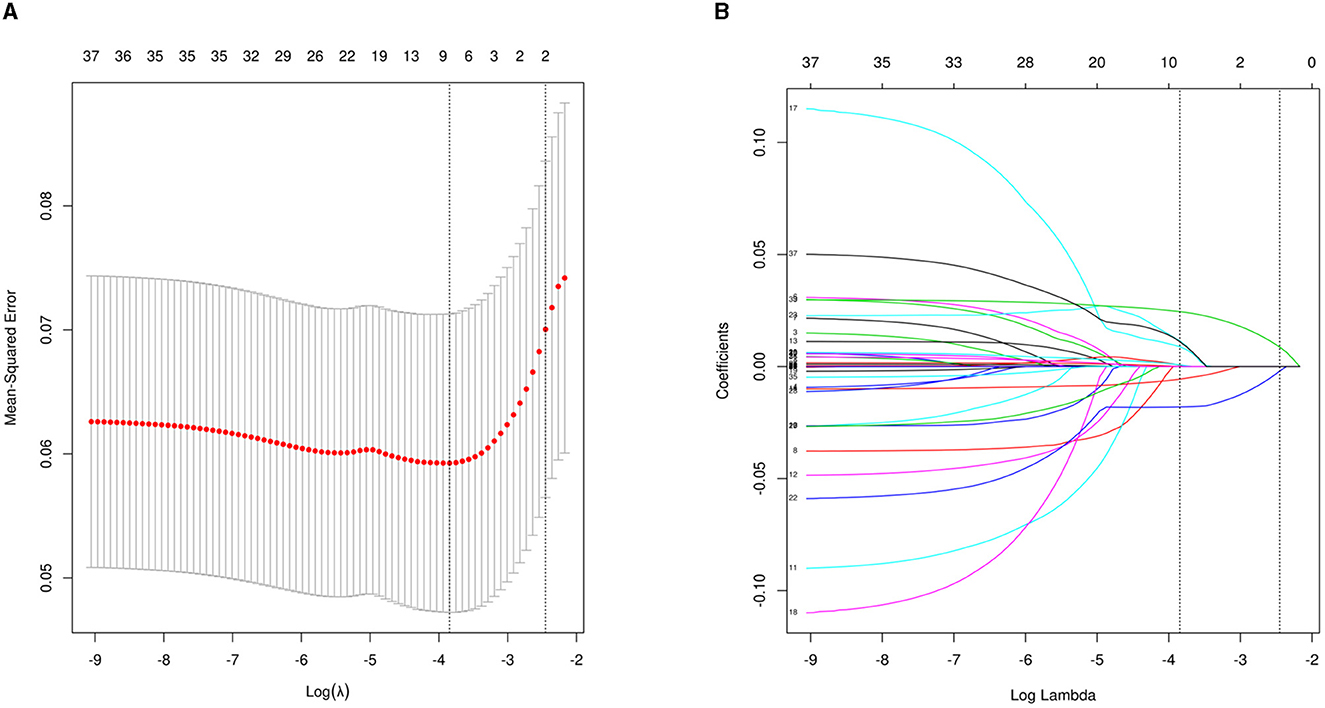
Figure 2. Perioperative variable selection using a LASSO logistic regression model. (A) Dotted vertical lines were depicted at the optimal values by using the minimum criteria (lambda.min) and 1 SE of the minimum criteria (lambda.1se). (B) LASSO coefficient profile of 36 variables. The model achieves optimality at lambda.1SE, where variables with nonzero coefficients are screened out as potential predictors, effectively reducing the number of influencing factors from 36 to 9.

Table 2. Multivariable logistic regression model for predicting lower extremity DVT in RIA patients.
Construction and validation of the nomogram
The nomogram for predicting lower extremity DVT probability in RIA patients who underwent EVT was shown in Figure 3. In this model, the D-dimer level and GCS score exhibited the greatest influence on lower extremity DVT, followed by age, albumin level, MCA aneurysm, and DCI, respectively. Quantified by the C-index, model discrimination was 0.92, demonstrating that the prognostic model effectively predicted lower extremity DVT (Figure 4A). The calibration plot (Figure 4B) indicated good consistency between the predictive risk of lower extremity DVT and the observed real risk. Based on the net benefit and threshold probabilities, the clinical value of the nomogram was evaluated using a decision curve analysis. As for lower extremity DVT of RIA patients, the graph (Figure 4C) indicated that the nomogram threshold probability analysis had a preferable net benefit (with a wide range of 4–82%). We externally verified the nomogram generated in the training cohort with the aim of confirming the stability of the model. The validation cohort was made up of 94 RIA patents from 17 November 2020 to 4 February 2022 when applied to a validation cohort with a C-index of 0.85 (Figure 4D).
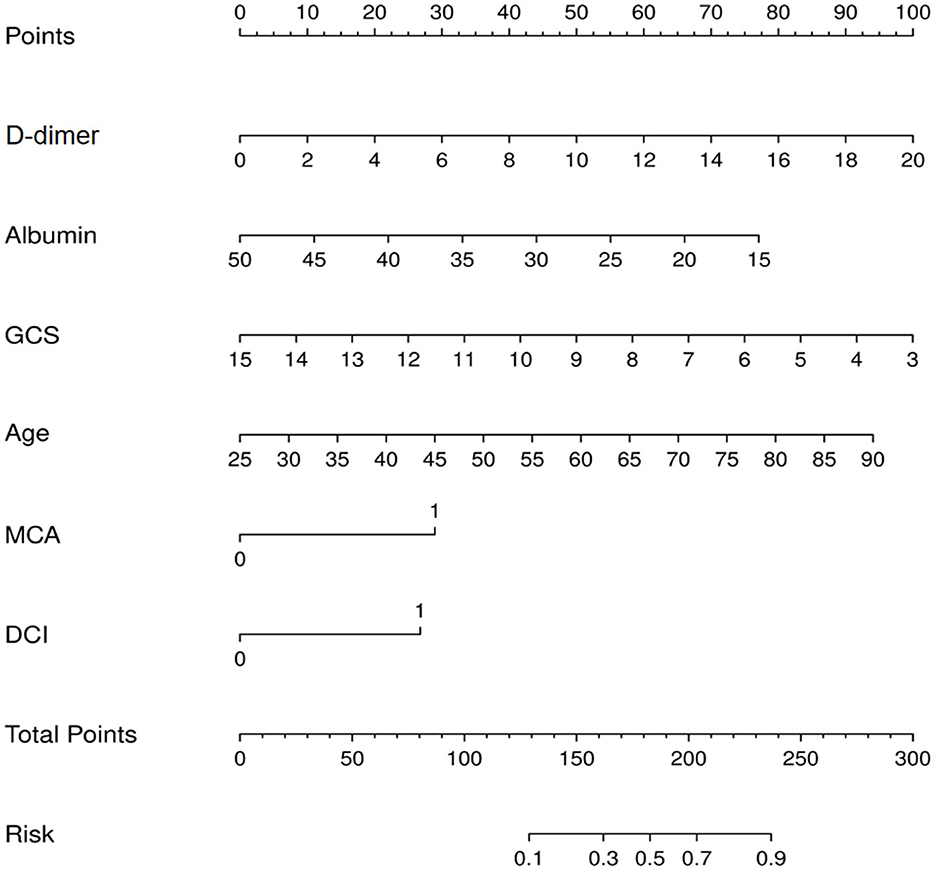
Figure 3. Nomogram for prediction of lower extremity deep vein thrombosis (DVT) in ruptured intracranial aneurysms (RIA) patients who underwent endovascular treatment (EVT).
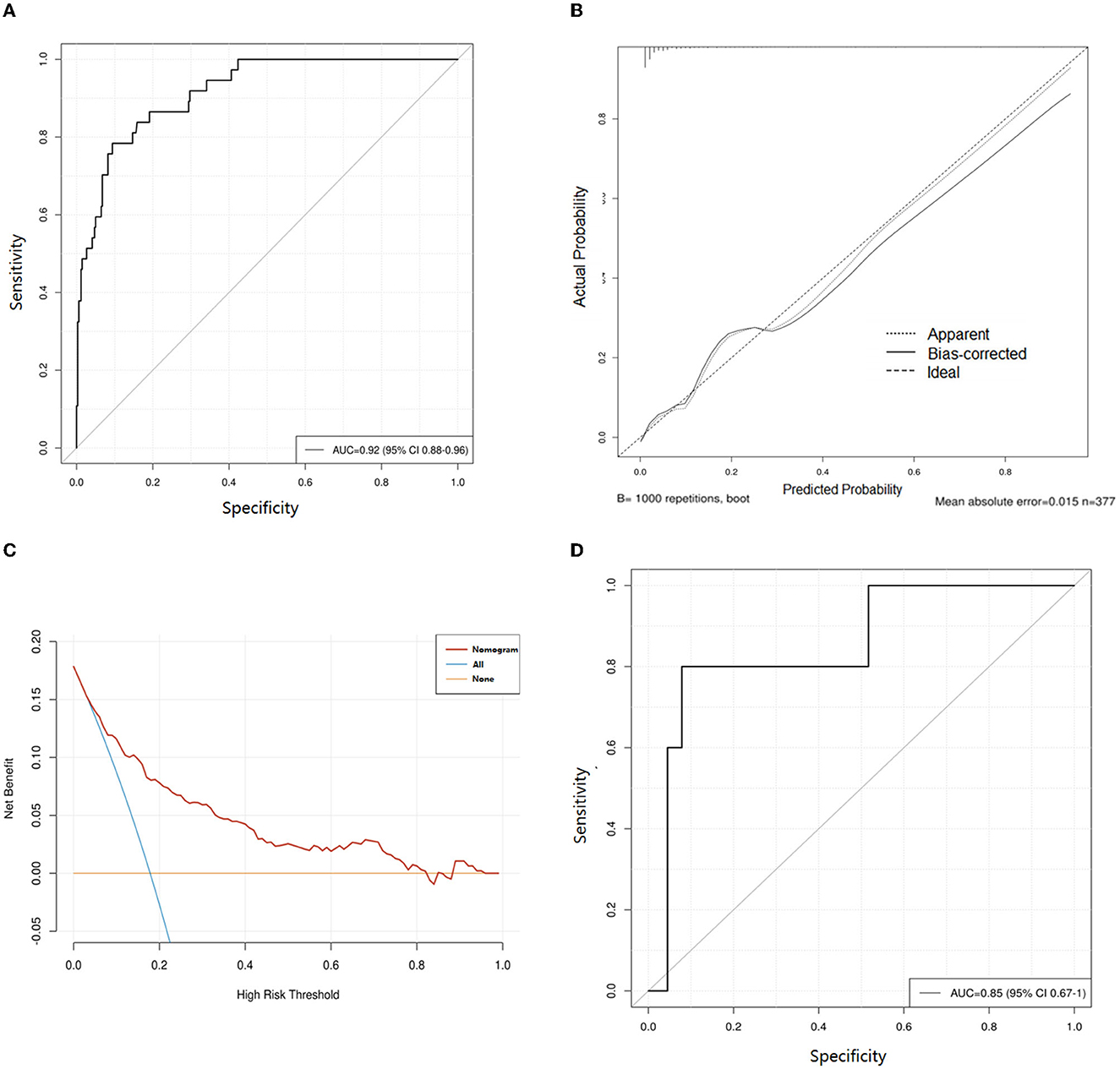
Figure 4. Evaluation of the nomogram for prediction of lower extremity deep vein thrombosis (DVT) in ruptured intracranial aneurysms (RIA) patients who underwent endovascular treatment (EVT). (A) Receiver operating characteristic curves of the training cohort. (B) Calibration curves of the DVT predictive nomogram in the training cohort. (C) Decision curve analysis for assessing the clinical usefulness of the low extremity DVT nomogram. (D) Receiver operating characteristic curves of validation cohort. AUC, area under the receiver operating characteristic curve.
Discussion
In this retrospective study, we developed and validated a clinical risk score and a nomogram to predict the development of lower extremity DVT among RIA patients who underwent EVT treatment. The performance of this risk score was satisfactory with accuracy based on C-indexes in the development cohorts of 0.92 and validation cohorts of 0.85, which showed that it was discriminatory and well-calibrated, and external validation showed satisfactory accuracy and generalizability. Moreover, the logistic regression analysis demonstrated that age, GCS score, MCA aneurysm, DCI, albumin, and D-dimer were the best predictors of low extremity DVT.
Due to the current advances in antithrombotic therapy and thrombus monitoring, the death of aSAH patients caused by DVT has not occurred in our center since 2020. DVT mainly leads to the length of hospital stay and increases the medical cost of patients, and anticoagulant therapy may increase the risk of bleeding in patients without surgical treatment (25, 26). This suggests that we still need to recognize the risk factors of DVT and actively intervene in the treatment.
It is almost universally accepted that there is an association between age and lower extremity DVT. The older the patient was, the worse his/her functional status became, and the more likely he/she was to present in poor condition following aSAH, such as the activation of coagulation and decreased muscle strength (26–29). Moreover, our research provides several new insights. First of all, our study revealed that a low GCS score, MCA aneurysm, and DCI were important predictors for lower extremity DVT. The risk factor of a low GCS score has been reported in many studies (30, 31), but MCA aneurysm and DCI have not been reported as independent risk factors for lower extremity DVT. This may be because a ruptured MCA aneurysm had the highest rate of parenchymatous hematoma, which reaches 40–50% in all kinds of intracranial aneurysms (32, 33). Hematomas destroy brain parenchyma and lead to limb disability (34, 35). Moreover, when embolizing an MCA aneurysm, the coil may affect the parent artery blood flow, which may result in the ischemia of the motor cortex and also lead to limb disability (36). DCI is a common postoperative complication affecting 20–30% of patients with RIA, which aggravates the patient's disturbance of consciousness and low limb disability (37). Above all, patients with advanced age, low GCS score, MCA aneurysm, and DCI often have poor limb function, especially the reduction of lower limb activity. Those all lead to reduced muscle contraction in the lower extremities and slower venous blood flow, which increases the likelihood of low extremity DVT (30).
Our study also confirmed a significant correlation between the level of D-dimer at admission and the development of lower extremity DVT. Abnormally elevated D-dimer levels often indicate a high likelihood of thrombosis (38). However, any process that increases fibrin production or breakdown also increases D-dimer levels, including pregnancy, inflammation, cancer, and surgery. This results in a high false-positive probability and low specificity of D-dimer in predicting. Therefore, we should dynamically detect and evaluate those patients' D-dimer levels to monitor the occurrence of DVT at an earlier stage (30).
Finally, our study also found that hypoalbuminemia was significantly associated with the occurrence of lower extremity DVT, which has been reported in the prediction of lower extremity DVT in other diseases (39, 40). This may be due to the decrease of plasma colloid osmotic pressure in patients with hypoalbuminemia, which easily leads to brain edema (41) and neurological dysfunction, thus leading to the reduction of lower limb activity and being more prone to lower extremity DVT. In addition, patients with hypoalbuminemia are more likely to have various infections after surgery (42). Postoperative infection often leads to a prolonged hospital stay and decreased activity willingness of patients, thus leading to an increased risk of lower extremity DVT (30).
In conclusion, our model can be used to assess the risk of lower extremity DVT during hospitalization for RIA patients who underwent EVT. Previous research had identified a wide heterogeneity of prophylactic protocols in neurosurgery, and any prophylaxis for DVT and PE should be individualized with careful evaluation of the benefits vs. risks (25). Our model not only incorporated age, GCS, and D-dimer, which were widely reported predictors of DVT but also identified the unique and indispensable role of DCI, albumin level, and MCA aneurysm in predicting DVT in patients with RIA. We need to pay more attention to patients at high risk of lower extremity DVT according to the score and take corresponding measures to reduce the braking time and encourage patients to get out of bed early while ensuring their safety. In addition, Chibbaro et al. (43) provided evidence supporting the safe and effective implementation of prophylactic protocols (mechanical +/- pharmacological) during the preoperative period and continued perioperatively and postoperatively (depending on the risk profile of the individual patient) with a remarkable reduction in the incidence of DVT and PE and without any increase in the risk of postoperative intracranial bleeding. Therefore, pharmacological prevention should be prioritized for RIA patients at high risk of DVT. In patients with temporary immobilization or loss of motor ability, lower limbs should be elevated, leg massage should be performed, pharmacological prevention or mechanical thrombus prevention should be performed using progressive pressure socks, intermittent pneumatic compression (IPC), etc. (44, 45). At the same time, the frequency of lower limb venous ultrasound screening should be increased to discover lower extremity DVT earlier (46).
This study has some limitations. First, the sample of participants was limited, and the study was limited by a lack of information on sufficient variables. In addition, our retrospective study is a single-center study with an unrepresentative sample, which provide only Level of Evidence 4 according to the Oxford Centre for Evidence-Based Medicine and may not be generalizable to other centers. Moreover, our data are missing intracranial hematoma volume and postoperative anticoagulation in patients with RIA. These data are critical for DVT prediction. However, these data were not included in the analysis because of the difficulties in obtaining them in our hospital and the differences in the type of prophylactic protocol and its duration, which need to be further improved in follow-up studies. Finally, due to inconsistent methodological descriptions of risk factors in included studies, we performed only systematic reviews rather than meta-analyses, so the dimensions of association of these risk factors with RIA-related lower extremity DVT are largely unclear. Therefore, these results may be interpreted with caution, and further research on these important risk factors should be warranted, which could help to ameliorate or control key risk factors for RIA-related low extremity DVT.
Conclusion
Our study found that D-dimer level, age, albumin, and GCS score were reliable predictors of DVT. The discrimination, accuracy, clinical effectiveness, and external verification of the prognostic nomogram developed in this study led to satisfactory performance for predicting lower extremity DVT. The finding can help medical staff identify patients at high risk of lower extremity DVT for RIA patients who underwent EVT, so targeted interventions can be administered to decrease the occurrence of lower extremity DVT. Further studies that take other clinically relevant variables into account will refine the nomogram.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JZ, XY, and CZ: conception, design, and revision of the article. XY, MZ, ZY, CY, and XW: data collection. HJ, XY, JZ, CZ, and MZ: analysis and drafting of the article. JZ, CZ, MZ, ZY, CY, XW, HJ, and XY: contributed to the article and approved the final version.
Funding
This work was supported by the Wenzhou Science and Technology Bureau (no. Y20220120) and Major Science and Technology Special Project of Wenzhou Science and Technology Bureau (no. ZY2021006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cai L, Zeng H, Tan X, Wu X, Qian C, Chen G. The role of the blood neutrophil-to-lymphocyte ratio in aneurysmal subarachnoid hemorrhage. Front Neurol. (2021) 12:671098. doi: 10.3389/fneur.2021.671098
2. Sharma D. Perioperative management of aneurysmal subarachnoid hemorrhage. Anesthesiology. (2020) 133:1283–305. doi: 10.1097/ALN.0000000000003558
3. Jaja B, Saposnik G, Lingsma H, Macdonald E, Thorpe K, Mamdani M, et al. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: the SAHIT multinational cohort study. BMJ. (2018) 360:j5745. doi: 10.1136/bmj.j5745
4. van Gijn J, Kerr R, Rinkel G. Subarachnoid haemorrhage. Lancet. (2007) 369:306–18. doi: 10.1016/S0140-6736(07)60153-6
5. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. (2002) 360:1267–74. doi: 10.1016/S0140-6736(02)11314-6
6. Hua X, Gray A, Wolstenholme J, Clarke P, Molyneux A, Kerr R, et al. Survival, dependency, and health-related quality of life in patients with ruptured intracranial aneurysm: 10-year follow-up of the United Kingdom cohort of the international subarachnoid aneurysm trial. Neurosurgery. (2021) 88:252–60. doi: 10.1093/neuros/nyaa454
7. Lindgren A, Vergouwen M, van der Schaaf I, Algra A, Wermer M, Clarke M, et al. Endovascular coiling versus neurosurgical clipping for people with aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. (2018) 8:CD003085. doi: 10.1002/14651858.CD003085.pub3
8. Etminan N, Chang H, Hackenberg K, de Rooij N, Vergouwen M, Rinkel G, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. (2019) 76:588–97. doi: 10.1001/jamaneurol.2019.0006
9. Liang C, Su K, Liu J, Dogan A, Hinson H. Timing of deep vein thrombosis formation after aneurysmal subarachnoid hemorrhage. J Neurosurg. (2015) 123:891–6. doi: 10.3171/2014.12.JNS141288
10. Kshettry V, Rosenbaum B, Seicean A, Kelly M, Schiltz N, Weil R. Incidence and risk factors associated with in-hospital venous thromboembolism after aneurysmal subarachnoid hemorrhage. J Clin Neurosci. (2014) 21:282–6. doi: 10.1016/j.jocn.2013.07.003
11. Ray W, Strom R, Blackburn S, Ashley W, Sicard G, Rich K. Incidence of deep venous thrombosis after subarachnoid hemorrhage. J Neurosurg. (2009) 110:1010–4. doi: 10.3171/2008.9.JNS08107
12. Moussouttas M, Bhatnager M, Huynh T, Lai E, Khoury J, Dombrowski K, et al. Association between sympathetic response, neurogenic cardiomyopathy, and venous thromboembolization in patients with primary subarachnoid hemorrhage. Acta Neurochir. (2013) 155:1501–10. doi: 10.1007/s00701-013-1725-x
13. Zhang Z, Lei J, Shao X, Dong F, Wang J, Wang D, et al. Trends in hospitalization and in-hospital mortality from VTE, 2007 to 2016, in China. Chest. (2019) 155:342–53. doi: 10.1016/j.chest.2018.10.040
14. Budnik I, Brill A. Immune factors in deep vein thrombosis initiation. Trends Immunol. (2018) 39:610–23. doi: 10.1016/j.it.2018.04.010
15. Geraldini F, De Cassai A, Correale C, Andreatta G, Grandis M, Navalesi P, et al. Predictors of deep-vein thrombosis in subarachnoid hemorrhage: a retrospective analysis. Acta Neurochir. (2020) 162:2295–301. doi: 10.1007/s00701-020-04455-x
16. Grimes D. The nomogram epidemic: resurgence of a medical relic. Ann Intern Med. (2008) 149:273–5. doi: 10.7326/0003-4819-149-4-200808190-00010
17. Zhao L, Chen T, Yan H, Liu C, Cao Y, Zhang Y, et al. Development and validation of an early predictive nomogram for delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Ann Transl Med. (2021) 9:1664. doi: 10.21037/atm-21-5200
18. Chen X, Chen Y, Lin N, Chen J, Ding C, Kang D, et al. A nomogram for predicting the need of postoperative tracheostomy in patients with aneurysmal subarachnoid hemorrhage. Front Neurol. (2021) 12:711468. doi: 10.3389/fneur.2021.711468
19. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
20. Beck J, Rohde S, el Beltagy M, Zimmermann M, Berkefeld J, Seifert V, et al. Difference in configuration of ruptured and unruptured intracranial aneurysms determined by biplanar digital subtraction angiography. Acta Neurochir. (2003) 145:861–5. doi: 10.1007/s00701-003-0124-0
21. Petridis A, Kamp M, Cornelius J, Beez T, Beseoglu K, Turowski B, et al. Aneurysmal subarachnoid hemorrhage. Dtsch Arztebl Int. (2017) 114:226–36. doi: 10.3238/arztebl.2017.0226
22. Anand S, Wells P, Hunt D, Brill-Edwards P, Cook D, Ginsberg J. Does this patient have deep vein thrombosis? JAMA. (1998) 279:1094–9. doi: 10.1001/jama.279.14.1094
23. Kearon C, Ginsberg J, Hirsh J. The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism. Ann Intern Med. (1998) 129:1044–9. doi: 10.7326/0003-4819-129-12-199812150-00009
24. Hijazi Z, Oldgren J, Lindbäck J, Alexander J, Connolly S, Eikelboom J, et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. (2016) 387:2302–11. doi: 10.1016/S0140-6736(16)00741-8
25. Ganau M, Prisco L, Cebula H, Todeschi J, Abid H, Ligarotti G, et al. Risk of deep vein thrombosis in neurosurgery: state of the art on prophylaxis protocols and best clinical practices. J Clin Neurosci. (2017) 45:60–6. doi: 10.1016/j.jocn.2017.08.008
26. Stienen MN, Germans M, Burkhardt J-K, Neidert MC, Fung C, Bervini D, et al. Predictors of in-hospital death after aneurysmal subarachnoid hemorrhage: analysis of a nationwide database (Swiss SOS [Swiss study on aneurysmal subarachnoid hemorrhage]). Stroke. (2018) 49:333–40. doi: 10.1161/STROKEAHA.117.01932
27. Yang X, Li H, Yu L, Yu T, Li F, Yu Z, et al. [Analysis of Influencing Factors for Postoperative Venous Thromboembolism of Thymic Malignancies]. Zhongguo Fei Ai Za Zhi. (2021) 24:497–502. doi: 10.3779/j.issn.1009-3419.2021.101.22
28. Guo F, Shashikiran T, Chen X, Yang L, Liu X, Song L. Clinical features and risk factor analysis for lower extremity deep venous thrombosis in Chinese neurosurgical patients. J Neurosci Rural Pract. (2015) 6:471–6. doi: 10.4103/0976-3147.169801
29. Buchanan I, Lin M, Donoho D, Patel A, Ding L, Amar A, et al. Predictors of venous thromboembolism after nonemergent craniotomy: a nationwide readmission database analysis. World Neurosurg. (2019) 122:e1102–10. doi: 10.1016/j.wneu.2018.10.237
30. Li R, Jiang J, Song Y, Zhang J, Wu Y, Wu L, et al. Prognostic nomogram for predicting lower extremity deep venous thrombosis in neurointensive care unit patients: a prospective observational study. Front Neurol. (2021) 12:761029. doi: 10.3389/fneur.2021.761029
31. Ekeh A, Dominguez K, Markert R, McCarthy M. Incidence and risk factors for deep venous thrombosis after moderate and severe brain injury. J Trauma. (2010) 68:912–5. doi: 10.1097/TA.0b013e3181b21cad
32. Gerner S, Hülsbrink R, Reichl J, Mrochen A, Eyüpoglu I, Brandner S, et al. Parenchymatous hematoma in patients with atraumatic subarachnoid hemorrhage: Characteristics, treatment, and clinical outcomes. Int J Stroke. (2021) 16:648–59. doi: 10.1177/1747493020971878
33. Darkwah Oppong M, Skowronek V, Pierscianek D, Gembruch O, Herten A, Saban D, et al. Aneurysmal intracerebral hematoma: risk factors and surgical treatment decisions. Clin Neurol Neurosurg. (2018) 173:1–7. doi: 10.1016/j.clineuro.2018.07.014
34. Navratil O, Duris K, Juran V, Neuman E, Svoboda K, Smrcka M. Middle cerebral artery aneurysms with intracerebral hematoma-the impact of side and volume on final outcome. Acta Neurochir. (2017) 159:543–7. doi: 10.1007/s00701-016-3070-3
35. Ji R, Wang L, Liu X, Liu Y, Wang D, Wang W, et al. A novel risk score to predict deep vein thrombosis after spontaneous intracerebral hemorrhage. Front Neurol. (2022) 13:930500. doi: 10.3389/fneur.2022.930500
36. Zhou Y, Yang P, Fang Y, Xu Y, Hong B, Zhao W, et al. Endovascular treatment for saccular aneurysms of the proximal (M1) segment of the middle cerebral artery. Acta Neurochir. (2012) 154:1835–43. doi: 10.1007/s00701-012-1453-7
37. Savarraj J, Hergenroeder G, Zhu L, Chang T, Park S, Megjhani M, et al. Machine learning to predict delayed cerebral ischemia and outcomes in subarachnoid hemorrhage. Neurology. (2021) 96:e553–62. doi: 10.1212/WNL.0000000000011211
38. Chen Q, Zhang Z, Dong H, Miao J, Li H. [Perioperative venous thromboembolism (VTE) prophylaxis in thoracic cancer patients: Chinese experts consensus - interpretation of clinical significance of D-dimer]. Zhongguo Fei Ai Za Zhi. (2019) 22:761–6. doi: 10.3779/j.issn.1009-3419.2019.12.05
39. Lee J, Ro Y, Cho J, Park Y, Lee J, Hwang J, et al. Characteristics of venous thromboembolism in pancreatic adenocarcinoma in east Asian ethnics: a large population-based observational study. Medicine. (2016) 95:e3472. doi: 10.1097/MD.0000000000003472
40. Mori T, Yoshioka K, Tanno Y. Frequency of deep vein thrombosis at admission for acute stroke and associated factors: a cross-sectional study. Thromb J. (2021) 19:62. doi: 10.1186/s12959-021-00315-5
41. Unterberg A, Stover J, Kress B, Kiening K. Edema and brain trauma. Neuroscience. (2004) 129:1021–9. doi: 10.1016/j.neuroscience.2004.06.046
42. Zhang F, Liu X, Tan Z, Li J, Fu D, Zhu L. Effect of postoperative hypoalbuminemia and supplement of human serum albumin on the development of surgical site infection following spinal fusion surgery: a retrospective study. Eur Spine J. (2020) 29:1483–9. doi: 10.1007/s00586-020-06306-w
43. Chibbaro S, Cebula H, Todeschi J, Fricia M, Vigouroux D, Abid H, et al. Evolution of prophylaxis protocols for venous thromboembolism in neurosurgery: results from a prospective comparative study on low-molecular-weight heparin, elastic stockings, and intermittent pneumatic compression devices. World Neurosurg. (2018) 109:e510–6. doi: 10.1016/j.wneu.2017.10.012
44. Jeraq M, Cote D, Smith T. Venous thromboembolism in brain tumor patients. Adv Exp Med Biol. (2017) 906:215–28. doi: 10.1007/5584_2016_117
45. Kahn S, Lim W, Dunn A, Cushman M, Dentali F, Akl E, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: american college of chest physicians evidence-based clinical practice guidelines. Chest. (2012) 141:e195S–226S. doi: 10.1378/chest.11-2296
Keywords: aneurysmal subarachnoid hemorrhage, deep venous thrombosis, nomogram, endovascular treatment, prediction
Citation: Zhang C, Zhu J, Zhang M, Yuan Z, Wang X, Ye C, Jiang H and Ye X (2023) Prognostic nomogram for predicting lower extremity deep venous thrombosis in ruptured intracranial aneurysm patients who underwent endovascular treatment. Front. Neurol. 14:1202076. doi: 10.3389/fneur.2023.1202076
Received: 07 April 2023; Accepted: 30 June 2023;
Published: 07 August 2023.
Edited by:
Johannes Boltze, University of Warwick, United KingdomReviewed by:
Alicia Zha, The Ohio State University, United StatesMario Ganau, Oxford University Hospitals NHS Trust, United Kingdom
Michel Roethlisberger, University Hospital of Basel, Switzerland
Copyright © 2023 Zhang, Zhu, Zhang, Yuan, Wang, Ye, Jiang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiong Ye, eGlvbmd5ZTIzMTBAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Chengwei Zhang1,2†
Chengwei Zhang1,2† Xiong Ye
Xiong Ye