
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 17 July 2023
Sec. Multiple Sclerosis and Neuroimmunology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1201324
Purpose: This study aimed to analyze the severity of the condition and prognosis of patients with anti-gamma-aminobutyric-acid type B receptor (anti-GABABR) encephalitis with tumors.
Methods: Patients with anti-GABABR encephalitis admitted to one of two hospitals from 2020 to 2022 were enrolled and divided into tumor and non-tumor groups. The clinical characteristics, condition severity, treatment options, and prognosis of the two groups of patients were compared and analyzed.
Results: Eighteen patients with anti-GABABR encephalitis were included, ten of whom had tumors. The comparison of clinical characteristics showed that rates of status epilepticus and coma were significantly higher in the group with tumors (P = 0.013 and P = 0.025, respectively); the incidences of pulmonary infection, respiratory failure, hyponatremia, and hypoproteinemia were also substantially more frequent in the tumor group (P = 0.054, P = 0.036, P = 0.015, and P = 0.025, respectively). The laboratory test result comparison showed that serum neuron-specific enolase (NSE) and carcinoembryonic antigen (CEA) were present only in the group with tumors (P = 0.036 and P = 0.092, respectively), but there was no significant difference in the occurrence of elevated CEA between the two groups. Conversely, the percentage of serum systemic autoimmune antibodies was higher in the group without tumors than in the group with tumors (P = 0.043). Patients with tumors tended to have poor outcomes (P = 0.152, OR: 7.000).
Conclusion: Severe brain damage and complications occur in patients with anti-GABABR encephalitis and comorbid tumors. Early screening for serum NSE and CEA helps in the early diagnosis and treatment of tumors. The prognosis is much worse for anti-GABABR encephalitis with tumors.
In the past 10 years, as more neural autoantibodies have been discovered, an increasing number of autoimmune encephalitis (AE) cases have been identified. Anti-gamma-aminobutyric-acid type B receptor (anti-GABABR) encephalitis is an autoimmune disease mediated by antibodies to GABABR and was first reported in 2010 (1). The main clinical manifestations of anti-GABABR encephalitis are seizures, psychiatric behaviors, and cognitive dysfunctions, accounting for 5% of all cases of AE (2). However, the risk of mortality in anti-GABABR encephalitis is higher than that of other AEs, and the presence of a comorbid tumor (49.5%) is presumed to be a key contributor to mortality (3–5). Early identification of the presence of comorbid tumors is important. It was observed that comorbid tumors are mainly small-cell lung cancer and other tumor types with neuroendocrine functions. Neuron-specific enolase (NSE) is specifically located in neurons and neuroendocrine cells, so detection of NSE can be used for early screening of tumors in anti-GABABR encephalitis. However, there are no studies to date on this topic.
Previous studies have been limited to the description of the phenomenon of anti-GABABR encephalitis with or without tumors but have failed to sufficiently analyze the differences in clinical features and prognosis between individuals with and without tumors. Thus, in this study, we compared anti-GABABR-positive patients with or without tumors in their clinical characteristics, treatment responses, and prognosis.
In this retrospective study, eighteen patients with anti-GABABR encephalitis were enrolled at the Department of Neurology of Xuanwu Hospital of Capital Medical University and People's Hospital of Inner Mongolia Autonomous Region between February 2020 to June 2022. The inclusion criteria were as follows. (1) Age ≥18 years. (2) Patients who met the diagnostic standards for anti-GABABR encephalitis as recommended by Graus et al. (6): (a) acute or subacute onset of memory deficits, seizures, or psychiatric symptoms and unilateral or bilateral medial temporal lobe (MTL) abnormalities on T2-weighted fluid-attenuated inversion (FLAIR) MRI or 18F-fluoro-2-deoxy-d-glucose positron emission tomography (18F-FDG-PET). (b) the leukocyte count being >5/mm3 in cerebrospinal fluid (CSF) or the electroencephalogram (EEG) showed seizure/slow wave activity involving the temporal lobe. (c) positive levels of anti-GABABR antibodies being present in the serum or CSF. (d) If one of the first two criteria is not met, the third one must be met. (e) Alternative causes are to be reasonably excluded.
All patients in this retrospective cohort study were divided into tumor and non-tumor groups based on tumor screenings. Demographic information, clinical features, imaging results, neurophysiological examinations, laboratory tests, tumor screenings, treatment options, and prognosis were also compared.
All autoimmune antibodies against neuronal cell surface antigens or neurologic paraneoplastic antibodies against intracellular neuronal antigens were measured using indirect immunofluorescence tests (IIFT) (Euroimmun, Luebeck, Germany). The antigens included GABABR, N-methyl-D-aspartate (NMDA) receptors, a-amino-3-hydroxy-5-methyl-4-isoxazol-propionic acid (AMPA) receptors, contactin-associated protein 2 (CASPR2), leucine-rich glioma-inactivated 1 (LGI-1), dipeptidyl-peptidase-like protein 6 (DPPX), IgLON family member 5 (IgLON5), Hu, Ri, Yo, CV2, amphiphysin, paraneoplastic antigen Ma2 (PNMA2), glutamic acid decarboxylase 65 (GAD65), and sry-related box genes (SOX1). Anti-nuclear antibodies (ANAs), anti-SSA, anti-SSB, anti-Ro-52, and anti-Scl-70 antibodies were also separately tested by IIFT and immunoblotting assays. Serum thyroglobulin antibody (Tg-Ab) and thyroid peroxidase antibody (TPO-Ab) were detected by electrochemiluminescence immunoassay.
All patients underwent tumor screening, which included chest/abdominal CT, abdominal ultrasonography, 18F-fluoro-2-deoxy-d-glucose positron emission temography (18F-FDG-PET), and tumor biomarkers. Tumor marker tests, including those for carbohydrate antigen 724, alpha-fetoprotein (AFP), NSE, serum cytokeratin 19 fragment antigen, carbohydrate antigen 125, carbohydrate antigen 199, carbohydrate antigen 153, and carcinoembryonic antigen (CEA), were measured by a commercial electrochemiluminescence assay (Roche Diagnostics, Mannheim, Germany).
All patients were assessed for condition severity before treatment using GCS and APACHE-2 scores. Treatment options included immunotherapy, antitumor therapy, and complication management. Immunotherapy treatments included first-line immunotherapy [corticosteroids and intravenous immunoglobulins (IVIg), plasma exchange (PLEX)], second-line immunotherapy [rituximab (RTX) and cyclophosphamide], and long-term immunotherapy [mycophenolate mofetil (MMF), RTX, azathioprine]. Antineoplastic therapies included tumor resection and chemotherapy. We interviewed participants over the telephone using the modified Rankin Scale (mRS) to estimate the prognosis; mRS of 0–2 was classified as a good prognosis, and mRS of 3–6 was classified as a poor prognosis (7).
Statistical analyses were performed with the statistical software SPSS 22.0 (IBM Corporation, Armonk, NY, USA). Clinical data were expressed as descriptive statistics, and count data were expressed as frequencies, composition ratios, and rates. The test of variability was performed using a four-compartment or a columnar table (R × C) χ2, and Fisher's exact probability method was used when more than 20% of the theoretical frequencies in the four tables or columns were < 5. All statistical tests were bilateral tests, and differences were considered statistically significant at a P-value of < 0.05.
Eighteen patients were enrolled, with ten cases having associated tumors and eight cases without tumors. The comorbid tumors included seven cases of pulmonary malignancy, including six cases with small-cell lung cancer and one case with adenocarcinoma, one case of abnormal growth of mediastinal and supraclavicular lymph nodes of unknown location, one case of pancreatic cancer, and one case of primary fallopian tube cancer who had known tumors at the time of presentation with encephalitis. There were no significant differences in age, sex, medial temporal lobe T2/FLAIR high signal intensity, antibody titers, or EEG abnormalities between the two groups.
Clinical manifestations were compared between the tumor group and the non-tumor group (Table 1). Epilepsy, cognitive dysfunction, and psychiatric abnormalities were the most common nervous system symptoms at 94, 88, and 72%, respectively. However, the incidences of status epilepticus and decline in consciousness (GCS score ≤ 8) were significantly higher in the group with tumors than in the group without tumors (60 vs. 0%, P = 0.013; 50 vs. 0%, P = 0.036, respectively).
The prevalence of respiratory failure, hyponatremia, and hypoproteinemia was significantly more frequent in the group with tumors than in the group without tumors (50 vs. 0%, P = 0.036; 80 vs. 12.5%, P = 0.015; 70 vs. 12.5%, P = 0.025, respectively). Patients with tumors were more likely to have a pulmonary infection and were admitted to the ICU, with a statistical trend (80 vs. 25%, P = 0.054; 60 vs. 12.5%, P = 0.066, respectively). The disease was more severe (APACHE-2 score ≥ 15) in those with concomitant tumors compared to those without (60% vs. 0%, P = 0.013) (Table 1).
Eighteen cases showed positive results for GABABR antibodies in the serum, and seventeen cases had positive levels in the CSF. There was no significant difference in either high or low antibody titers when compared between groups. Serum NSE and CEA were both present in the group with tumors, and the levels were significantly different (50 vs. 0%, P = 0.036) or approached significance (40 vs. 0%, P = 0.092), respectively, when compared with the group without tumors. There was one patient who had superimposed anti-CV2 and anti-GAD65 antibodies, and one patient had anti-SOX1 antibodies in both their serum and CSF. The percentage of patients with positive serum systemic autoimmune antibodies was significantly higher in the group without tumors than in the group with tumors (62.5 vs. 10%, P = 0.043) (Table 2).
All patients received first-line treatment in the acute phase. Of these patients, 3/18 (16.7%) received a single corticosteroid, 3/18 (16.7%) received a single IVIg, 11/18 (61.1%) received a corticosteroid combined with IVIg, and 2/18 (11.1%) received PLEX after receiving a corticosteroid or corticosteroid combined with IVIg. The two groups did not receive significantly different first-line treatments from each other. A total of 8/18 (44.4%) patients received second-line treatment in the acute phase. Two patients received RTX, and six patients received MMF, and second-line treatments were not significantly different between the two groups. Of the patients in the concomitant tumor group, three were treated with tumor resection, two with chemotherapy, four were under conservative observation, and one abandoned treatment and was discharged.
With a follow-up interval of 1–24 months (median 8 months), there was no significant difference in the prevalence of poor outcomes (mRS score of 3–6) compared to that of good outcomes (mRS score of 0–2) but showed an increasing trend (50 vs. 12.5%, p = 0.152). The prognosis was poorer in the group with tumors than in the group without tumors (OR 7.000, 95% CI 0.613–79.871) (Figure 1). There were four deaths among all the patients, including three patients with tumors and one without a tumor; of the patients with tumors, one died of refractory status epilepticus and two died of lung malignancy; the cause of death was unknown for the patient in the group without tumors.
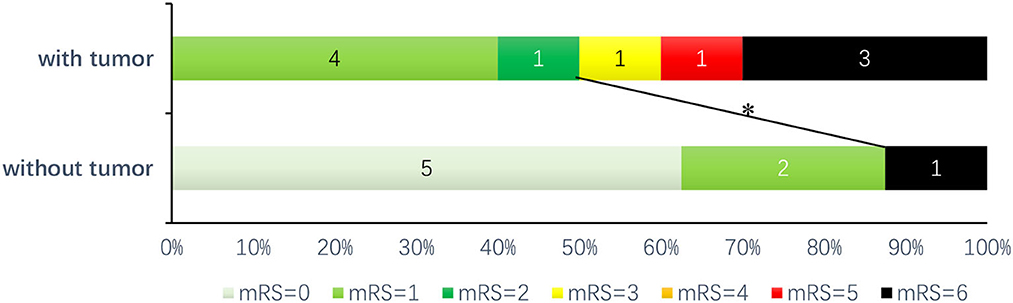
Figure 1. Prognosis of patients with anti-GABABR encephalitis: after follow-up (1–24 months, median 8 months), *there was a trend toward the good prognosis group (mRS score of 0–2) compared with the poor prognosis group (mRS score of 3–6) (P = 0.152, OR = 7.000, 95% CI: 0.613–79.871).
The main findings of this study were as follows: (1) There was a high rate of comorbid tumors in anti-GABABR encephalitis (55.6%), including one case of primary fallopian tube cancer detected for the first time. (2) Elevated serum NSE and CEA levels suggested anti-GABABR encephalitis with potential tumors; a positive level of serum systemic autoimmune antibodies was more common in anti-GABABR encephalitis without associated tumors. (3) Anti-GABABR encephalitis in the presence of a tumor had a rapid onset and was severe, mainly manifesting as status epilepticus and a decline in consciousness. In addition, it also showed a high rate of complications (72.2%), such as pulmonary infections, respiratory failure, hypoproteinemia, and hyponatremia, which aggravated the severity of anti-GABABR encephalitis in patients with tumors. (4) The risk of a poor prognosis was significantly increased in anti-GABABR encephalitis with comorbid tumors even though patients received first- and second-line therapy.
Once AE is combined with an associated tumor, it is typically called paraneoplastic AE (8). The neurological symptoms of anti-GABABR encephalitis are mainly short-term memory impairment, abnormal psychiatric behavior, and seizures, and EEG and imaging results show MTL involvement. Therefore, anti-GABABR encephalitis with associated tumors is also called paraneoplastic limbic encephalitis. Status epilepticus (60%) and a decline in consciousness (GCS score ≤ 8) (50%) were more prominent in paraneoplastic anti-GABABR encephalitis and were the main reasons for admission to the ICU (60%). It has been reported in the literature that the rate of status epilepticus in anti-GABABR encephalitis is as high as 62%, of which 68% of cases are refractory status epilepticus; the overall rate of declining consciousness in this previous study was 51%, but no comparative analysis was performed on patients with and without tumors (9, 10). In addition, paraneoplastic anti-GABABR encephalitis showed a high complication rate (72.2%) dominated by pulmonary infections (80% of complications), which was significantly higher than that of previous reports (2/3 of patients with pulmonary infection) (3). It is hypothesized that the high incidence of pulmonary infections may be related to obstructive pneumonia caused by pulmonary malignancies, hypostatic pneumonia resulting from status epilepticus and consciousness disorder, and increased risk of pneumonia opportunistic infections due to immunotherapy. Undoubtedly, severe pulmonary infections exacerbate and accelerate respiratory failure due to central hypoventilation in paraneoplastic anti-GABABR encephalitis. The high occurrence of respiratory failure (50%) is another major reason for critical illness and admission to the ICU. Tumor cells have been shown to secrete antidiuretic hormones in vivo, leading to hypo-osmolar hyponatremia, which not only aggravates the condition but also predicts the possibility of tumors (11). The incidence of hyponatremia in paraneoplastic anti-GABABR encephalitis was as high as 80%. In fact, under the double effect of tumor malignancy and lung infection, protein catabolism is greater than anabolism, leading to a 70% prevalence of hypoproteinemia. In conclusion, anti-GABABR encephalitis with tumors is typically very severe and requires high levels of attention. In the acute stage, in addition to aggressive and effective immunotherapy, screening for tumors is needed.
One finding of this study was that paraneoplastic anti-GABABR encephalitis had a poor prognosis with a mortality rate of 30%, similar to the previously reported mortality rate (23.2 or 41.7%), despite standardized first-line immunotherapy and antitumor therapy in the acute phase (10, 12). The reasons for this high mortality rate may be related to the severity and complications associated with the condition, poor responses to immunotherapy, and neoplasms with less differentiation. In contrast, patients who had anti-GABABR encephalitis without tumors had a high prevalence of positive levels of serum systemic autoimmune antibodies (5/8), such as Tg-Ab and anti-Ro-52 antibodies (3/5). Other autoimmune diseases (ADs), such as Hashimoto's thyroiditis, Sjogren's syndrome, and systemic lupus erythematosus (SLE), in combination with anti-GABABR encephalitis, have been reported (13). SLE had the highest percentage of positive anti-GABABR antibodies (20.5%) (14). Although the mechanism for the coexistence of two or more antibodies is not clear, it may be related to ADs having common genetic loci that increase the risk of one AD causing another (15). This study also found that the prognosis of anti-GABABR encephalitis without a tumor with two or more immune antibodies was better than that of paraneoplastic anti-GABABR encephalitis; however, this finding has yet to be confirmed by a large sample and scientifically explained.
Improving the prognosis of paraneoplastic anti-GABABR encephalitis is apparently challenging as it is difficult to detect tumors at an early stage. It has been reported in the literature that anti-GABABR encephalitis with tumors accounts for 50% of anti-GABABR encephalitis (1, 16–18). Tumors are predominantly small-cell lung cancers (SCLCs) (91.3%) as well as others such as thymoma, melanoma, rectal cancer, gastric cancer, and pancreatic cancer (4, 10, 19). Therefore, imaging and screening for tumor biomarkers in cases of anti-GABABR encephalitis have become important steps for early diagnosis. This study and previous studies have found that paraneoplastic anti-GABABR encephalitis has a high rate of positive serum levels of CEA and NSE (40–50%), and a positive test result motivates the further search for tumors in vivo (5). NSE is a macromolecular protein. CEA is an acidic glycoprotein of the human embryonic antigen-specific determinant cluster that is highly expressed in tumor and embryonic tissues. Most tumors associated with anti-GABABR encephalitis, such as SCLC, have neuroendocrine attributes. SCLC usually has high expression of NSE (68.3%) or CEA (45.5%); the sensitivities of NSE and CEA in diagnosing SCLC alone are 68.3 and 45.5%, respectively, while the sensitivity of their combination increases to 81.2%; therefore, it can provide a basis for tumor screening and early diagnosis (20).
Another focus for a better prognosis in paraneoplastic anti-GABABR encephalitis is to improve immunotherapy protocols. Immunotherapy regimens were similar in both groups, and PLEX was rarely used (20% of the cases included in this study), especially when the response to immunotherapy was poor. Whether enhanced immunotherapy or PLEX is preferred is not known.
In this study, for the first time, we found a patient (60 years old) exhibiting a case of anti-GABABR encephalitis with primary fallopian tube cancer who had undergone total hysterectomy and bilateral adnexal resection 3 months before the disease and received regular chemotherapy with paclitaxel in conjunction with carboplatin after the surgery.
The pathological staging of primary tubal carcinoma is endometrioid adenocarcinoma with no carcinoma of the uterus, ovaries, abdominal lymph nodes, or omental tissue.
With status epilepticus as the initial symptom, T2/FLAIR showed a high signal in the hippocampus bilaterally and positive anti-GABABR antibodies in serum and CSF tests. Death from acute pulpitis and septic shock occurred after 8 months of treatment with methylprednisolone combined with IVIg. Primary tubal cancer is a rare gynecologic malignancy, with an incidence rate of 1.0–2.0% among them (21). There have been no reports of anti-GABABR encephalitis with primary tubal cancer. In theory, endometrioid adenocarcinoma with a neuroendocrine nature can express GABA receptors, and immunosurveillance against tumors may induce anti-GABABR encephalitis.
In summary, there were differences in condition severity and prognosis between patients with anti-GABABR encephalitis with tumors and without tumors. Hope for reducing severity lies in intensive immunotherapy and effective treatment of comorbidities. Improvements in prognosis are likely to depend on enhanced tumor screening, including biomarker testing and imaging, to provide opportunities for early diagnosis and treatment of tumors. In addition, screening for serum systemic autoimmune antibodies is important for predicting an accurate prognosis and outcome. The main limitation of this study is that the sample size was small, and the single-factor analysis alone was far from sufficient. It is expected that data will be accumulated from more cases and analyzed in a progressive stratified manner.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
CG designed and administrated the study, analyzed data, and drafted paper. ZC and HH participated within the investigation, data curation, and analysis. RZ took part in investigation. YS provided the resources, supervised the study, and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. (2010) 9:67–76. doi: 10.1016/S1474-4422(09)70324-2
2. Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. (2011) 77:179–89. doi: 10.1212/WNL.0b013e318224afde
3. Lin J, Li C, Li A, Liu X, Wang R, Chen C, et al. Encephalitis with antibodies against the GABA(B) receptor: high mortality and risk factors. Front Neurol. (2019) 10:1030. doi: 10.3389/fneur.2019.01030
4. McKay JH, Dimberg EL, Lopez Chiriboga AS. A systematic review of Gamma-aminobutyric Acid Receptor Type B autoimmunity. Neurol Neurochir Pol. (2019) 53:1–7. doi: 10.5603/PJNNS.a2018.0005
5. Chen W, Wang Y, Guo X, Gao L, Huang Z, Lin Y, et al. A prognostic analysis of the outcomes in patients with anti-gamma-aminobutyric acid B receptor encephalitis. Front Immunol. (2022) 13:847494. doi: 10.3389/fimmu.2022.847494
6. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
7. Bamford JM, Sandercock PA, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. (1989) 20:828. doi: 10.1161/01.STR.20.6.828
8. Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. (2004) 75:1135–40. doi: 10.1136/jnnp.2003.034447
9. de Bruijn M, van Sonderen A, van Coevorden-Hameete MH, Bastiaansen AEM, Schreurs MWJ, Rouhl RPW, et al. Evaluation of seizure treatment in anti-LGI1, anti-NMDAR, and anti-GABA(B) R encephalitis. Neurology. (2019) 92:e2185–96. doi: 10.1212/WNL.0000000000007475
10. Ghimire P, Khanal UP, Gajurel BP, Karn R, Rajbhandari R, Paudel S, et al. Anti-LGI1, anti-GABABR, and Anti-CASPR2 encephalitides in Asia: a systematic review. Brain Behav. (2020) 10:e01793. doi: 10.1002/brb3.1793
11. Onyema MC, Drakou EE, Dimitriadis GK. Endocrine abnormality in paraneoplastic syndrome. Best Pract Res Clin Endocrinol Metab. (2022) 36:101621. doi: 10.1016/j.beem.2022.101621
12. Zhong R, Chen Q, Zhang X, Zhang H, Lin W. Risk factors for mortality in anti-NMDAR, anti-LGI1, and anti-GABABR encephalitis. Front Immunol. (2022) 13:845365. doi: 10.3389/fimmu.2022.845365
13. Zhao J, Wang C, Xu X, Zhang Y, Ren H, Ren Z, et al. Coexistence of autoimmune encephalitis and other systemic autoimmune diseases. Front Neurol. (2019) 10:1142. doi: 10.3389/fneur.2019.01142
14. Tsuchiya H, Haga S, Takahashi Y, Kano T, Ishizaka Y, Mimori A. Identification of novel autoantibodies to GABA(B) receptors in patients with neuropsychiatric systemic lupus erythematosus. Rheumatology. (2014) 53:1219–28. doi: 10.1093/rheumatology/ket481
15. Zhernakova A, Withoff S, Wijmenga C. Clinical implications of shared genetics and pathogenesis in autoimmune diseases. Nat Rev Endocrinol. (2013) 9:646–59. doi: 10.1038/nrendo.2013.161
16. Höftberger R, Titulaer MJ, Sabater L, Dome B, Rózsás A, Hegedus B, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. (2013) 81:1500–6. doi: 10.1212/WNL.0b013e3182a9585f
17. Zhao XH, Yang X, Liu XW, Wang SJ. Clinical features and outcomes of Chinese patients with anti-gamma-aminobutyric acid B receptor encephalitis. Exp Ther Med. (2020) 20:617–22. doi: 10.3892/etm.2020.8684
18. Wu H, Wang Y, Wei K, Qiao S, Liu L, Zhang R, et al. Clinical characteristics and elevated ProGRP and positive oligoclonal bands of 13 Chinese cases with anti-GABABR encephalitis. Int J Dev Neurosci. (2021) 81:492–501. doi: 10.1002/jdn.10121
19. Jia XT, Pan Y, Di Z, Gu N, Liu Z, Kang YM. Anti-GABA(B) receptor encephalitis in a patient with gastric adenocarcinoma. Neurol Sci. (2018) 39:1981–4. doi: 10.1007/s10072-018-3536-6
20. Chen Z, Liu X, Shang X, Qi K, Zhang S. The diagnostic value of the combination of carcinoembryonic antigen, squamous cell carcinoma-related antigen, CYFRA 21-1, neuron-specific enolase, tissue polypeptide antigen, and progastrin-releasing peptide in small cell lung cancer discrimination. Int J Biol Markers. (2021) 36:36–44. doi: 10.1177/17246008211049446
Keywords: anti-gamma-aminobutyric-acid type B receptor (anti-GABABR) encephalitis, tumor, clinical characteristics, prognosis, disease severity
Citation: Gao C, Chen Z, Huang H, Zhu R and Su Y (2023) Assessment of disease severity and patient prognosis in anti-GABABR encephalitis with and without comorbid tumors. Front. Neurol. 14:1201324. doi: 10.3389/fneur.2023.1201324
Received: 06 April 2023; Accepted: 26 June 2023;
Published: 17 July 2023.
Edited by:
Fumitaka Shimizu, Yamaguchi University School of Medicine, JapanReviewed by:
Siyuan Fan, Peking Union Medical College Hospital (CAMS), ChinaCopyright © 2023 Gao, Chen, Huang, Zhu and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingying Su, c3V5aW5neWluZ0B4d2guY2NtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.