- 1Faculty of Rehabilitation Medicine, Weifang Medical University, Weifang, Shandong, China
- 2Physical Education and Sports School, Soochow University, Suzhou, Jiangsu, China
- 3Department of Rehabilitation Medicine, Weifang People's Hospital, Weifang, Shandong, China
- 4Department of Neurology, Weifang People's Hospital, Weifang, Shandong, China
Background: Hemiplegic shoulder pain (HSP) is a common complication in patients with stroke. The pathogenesis of HSP is complex, and muscle hypertonia, especially the hypertonia of internal rotation muscles of the shoulder, may be one of the important causes of shoulder pain. However, the relationship between muscle stiffness and HSP has not been well studied. The purpose of this study is to explore the correlations between the stiffness of internal rotation muscles and clinical symptoms in patients with HSP.
Methods: A total of 20 HSP patients and 20 healthy controls were recruited for this study. The stiffness of internal rotation muscles was quantified using shear wave elastography, and Young's modulus (YM) of the pectoralis major (PM), anterior deltoid (AD), teres major ™, and latissimus dorsi (LD) were measured. Muscle hypertonia and pain intensity were evaluated using the Modified Ashworth Scale (MAS) and Visual Analog Scale (VAS), respectively. The mobility of the shoulder was evaluated using the Neer score. The correlations between muscle stiffness and the clinical scales were analyzed.
Results: YM of internal rotation muscles on the paretic side was higher than that of the control group in the resting and passive stretching positions (P < 0.05). YM of internal rotation muscles on the paretic side during passive stretching was significantly higher than that at rest (P < 0.05). YM of PM, TM, and LD during passive stretching were correlated with MAS (P < 0.05). In addition, the YM of TM during passive stretching was positively correlated with VAS and negatively correlated with the Neer score (P < 0.05).
Conclusion: Increased stiffness of PM, TM, and LD was observed in patients with HSP. The stiffness of TM was associated with pain intensity of the shoulder and shoulder mobility.
Introduction
Hemiplegic shoulder pain (HSP) is one of the most common complications after stroke, and patients with HSP often have shoulder pain and limited mobility (1). The prevalence of HSP in post-stroke patients has been reported to be 84% and remains elevated throughout recovery (2, 3). The etiology of HSP is multifactorial, and factors including impingement syndrome, rotator cuff dysfunction, and muscle hypertonia may be related to HSP (4). The initial weakness and muscle hypertonia after stroke are mainly due to the injury of upper motor neurons, which change the muscle stiffness and cause pain by pulling the periosteal attachments (5, 6). As the clinical manifestations of HSP are variable, there is no universal treatment method at present. However, untreatable shoulder pain can cause secondary problems and limit upper limb function (7). Therefore, further research is needed to clarify the factors affecting HSP. It has been reported that muscle hypertonia was an important cause of shoulder pain in patients with hemiplegia during spasticity (1). In stroke patients, the shoulder girdle on the paretic side usually shows increased stiffness of the internal rotation muscles during the spastic phase, resulting in an abnormal pattern of internal rotation of the humerus and retraction of the scapula, which affects the normal humeral rhythm and squeezes soft tissues and causes shoulder pain (8). The internal rotation muscles with hypertonia included pectoralis major (PM), deltoid anterior (AD), teres major (TM), latissimus dorsi (LD), and subscapularis. These hypertonic muscles may be the main cause of pain and limited movement in HSP (1). Therefore, evaluation and rehabilitation of shoulder internal rotation muscles may be beneficial for patients with HSP.
Focal and characteristic muscle dysfunction is often observed in HSP patients (9). Investigations have shown that the overactivity of PM and subscapularis are obvious in HSP. With the increase in the activity of TM and LD, HSP patients have pain and limited activity (4). In addition, the incidence of disability in HSP is also high. In the Auckland stroke study, patients who were discharged home from a hospital had an increased risk of shoulder pain. Approximately 20% of patients have persistent shoulder pain for more than 6 months and the pain becomes permanent, affecting their activities of daily living (10). Thus, it is very important to find out the disabling factors and implement preventive intervention before HSP occurs. However, the changes in the stiffness of the shoulder's internal rotation muscles and the impact on HSP are still unclear.
The clinical evaluation of hypertonia mainly includes clinical scale evaluation, biomechanical evaluation, and electrophysiological evaluation. The commonly used clinical scale is the Modified Ashworth Scale (MAS), which can only assess the overall condition of the patient and is highly subjective with limited reliability and validity. Moreover, biomechanical evaluation and electrophysiological evaluation are complicated and expensive. Shear wave elastography (SWE), an emerging technique, has the advantages of non-invasive, simple, and timely detection. Most importantly, it has a certain pathological basis for muscle hypertonia assessment, monitoring the structure and viscoelasticity properties of hypertonic muscle. The principle of SWE is to generate shear waves by creating acoustic radio frequency force impulse, stimulating tissue vibration to quantify muscle stiffness (11). Muscle stiffness can be quantified by Young's modulus (YM) or shear wave velocity (SWV) (12). YM is the ratio of longitudinal stress to strain, which indicates the longitudinal deformation trend of the tissue and can directly reflect the change in muscle stiffness (13). YM increases with the increase in muscle stiffness (14). Currently, SWE is widely used to assess muscle stiffness with excellent reliability (15). In particular, it is possible to quantify the stiffness of individual muscle tissues and observe subtle changes in muscle properties in the early stages of the disease, providing clinicians with an objective indicator. In addition, measuring the stiffness of muscles in different states can help physical therapists design targeted, individualized treatment plans.
In this prospective observational study, we hypothesized that the stiffness of internal rotation muscles was related to the clinical symptoms of HSP. Using the SWE technique, we quantitatively measured the YM of internal rotation muscles and analyzed the relationship between muscle stiffness and hypertonia, pain intensity, and shoulder dysfunction.
Materials and methods
Subjects
A total of 20 stroke patients with HSP and 20 healthy controls participated in this study. The inclusion criteria for the stroke patients with HSP were as follows: (1) patients with cerebral hemorrhage or cerebral infarction confirmed by computer tomography or magnetic resonance imaging (16), (2) patients with first onset of stroke with unilateral involvement, (3) patients with the duration of stroke < 6 months, (4) patients with shoulder pain and muscle hypertonia on the paretic side, (5) patients with the paretic side had no history of shoulder pain before the stroke, (6) patients who could cooperate with the examination and assessment, (7) patients with sitting balance ≥1 grade, (8) patients with the visual analog scale (VAS) scored >0, (9) patients with MAS graded 1–3, and (10) patients with shoulder pain lasting for 2 weeks or more (17). The exclusion criteria were as follows: patients with (1) impairment of consciousness, cognition, or language, (2) severe muscle or bone joint disease affecting upper limbs, (3) a history of shoulder surgery, and (4) VAS score = 10 or MAS grade = 0. The inclusion criteria for the control group were as follows: (1) patients with no history of stroke, (2) patients with normal range of motion of shoulder joint (18), (3) patients who could cooperate with inspection and evaluation, (4) patients who could tolerate ultrasound examination, and (5) patients who matched with HSP group for age, sex, weight, height, and BMI (19). The exclusion criteria for the control group were as follows: patients with (1) a history of shoulder trauma and surgery and (2) a history of shoulder disease.
The participants were recruited for this study from October 2021 to April 2022. This study was approved by the Ethics Committee of Weifang Medical College, and all participants provided written informed consent before participation.
Clinical evaluation
Before the ultrasonic measurement, all patients were evaluated by experienced physiotherapists using the MAS, VAS, and Neer scores for the evaluation of shoulder muscle hypertonia, pain, and function, respectively. MAS is one of the most commonly used scales to evaluate muscle hypertonia in clinics. The clinician passively moved the patient's upper limb in the 0° to 90° range of external rotation and rated the resistance from 0 (no increase in muscle tone) to 4 (the joint is rigid) (20), and the patients were asked to rate their sense of pain numerically (VAS) on a scale from 0 to 10, with 0 representing “no pain” and 10 indicating “very severe pain.” The Neer score consists of four aspects, namely, numerical ratings of pain, function, range of motion, and anatomy, with a total score of 100 points. A higher score indicates a better shoulder function (21).
Experimental equipment
Ultrasound images were captured using the SWE ultrasound system (Supersonic Imagine, Aix-en-Provence, France) with a 2–10 MHz linear transducer array (Super Linear, 10-2, Vermon, France). All subjects were seated in a neutral position with the torso, shoulder abduction 0°, and upper limbs naturally relaxed on the legs. The probe was applied perpendicularly to the skin, paralleling the muscle bundle. First, the measurement position was determined by the B-mode grayscale. Thereafter, SWE was switched to establish the region of interest (ROI), set to an ROI of 1 mm diameter, and a depth of 0–2 cm (Figure 1 and Supplementary Figure S1). YM was measured three times at the same location, and the average value was taken for analysis. Participants were asked to hold their breath during the measurement to avoid muscle elongation by chest movement. The patient's upper limb was then passively stretched to shoulder abduction at 45° and maximum tolerable external rotation (4, 22, 23), which could induce muscle hypertonia and pain through this position, and YM of the muscle in the passive stretching position was measured.

Figure 1. SWE images of the shoulder internal rotation muscles at rest (A–D) in patients with HSP. The dark blue area on the left is the elastic sampling frame. Three white circles are regions of interest. PM, pectoralis major; AD, anterior deltoid; TM, teres major; LD, latissimus dorsi.
The measurement sites were as follows: PM was measured at the midpoint between the greater tubercle of the humerus and sternoclavicular joint; anterior deltoid (AD) was measured at a site 2 to 3 cm below 1/3 lateral to the midpoint of the clavicle. The probe is perpendicular to the line between the inferior angle of the scapula and the acromion to measure TM; LD was measured at the position of the eighth thoracic vertebra parallel to the lower part of the inferior angle of the scapular.
Statistical analyses
The Mann–Whitney test or independent samples t-test was used for the comparisons as appropriate, and the Shapiro–Wilk test was used to check whether the data conformed to normal distribution. The gender distribution between HSP and healthy controls was compared using the chi-square test. The correlations between muscle stiffness and the MAS, VAS, and Neer score were analyzed using Spearman's rank test. The p-values of multiple comparisons and correlation analysis were corrected by controlling the false discovery rate (FDR) at a level of 0.05 (24). All calculations were performed in SPSS Statistics 26 (SPSS Inc, Chicago, IL, USA).
Results
Subject characteristics
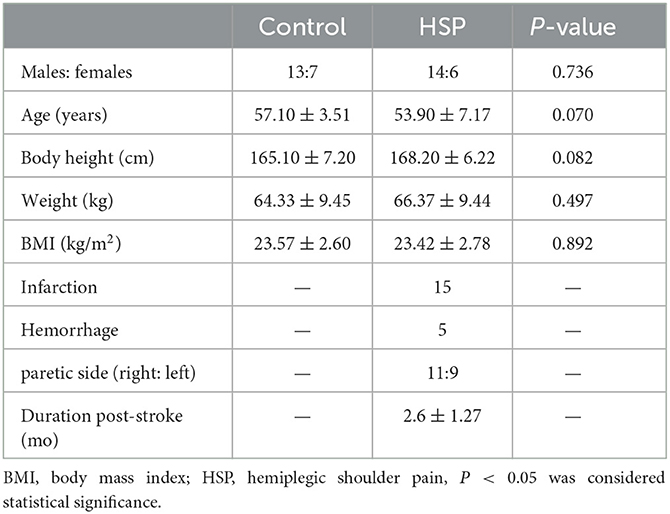
The demographic data are summarized in Table 1. A total of 20 HSP patients (14 male and 6 female patients) and 20 healthy controls (13 male and 7 female patients) were included for further analysis. The average ages of HSP patients and the control group were 53.90 ± 7.17 years and 57.10 ± 3.51 years, respectively. The average body weight of HSP patients and control group were 66.37 ± 9.44 kg and 64.33 ± 9.45 kg, respectively. The mean height of HSP patients and the control group were 168.20 ± 6.22 cm and 165.10 ± 7.20 cm, respectively. The mean BMI of HSP patients and control group were 23.42 ± 2.78 kg/m2 and 23.57 ± 2.60 kg/m2, respectively. There was no statistical difference in age, height, weight, and BMI between the two groups (all P > 0.05).
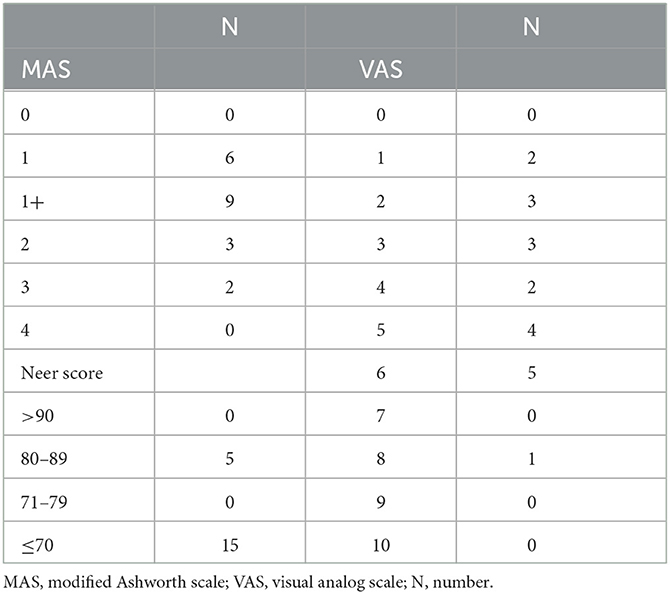
The results of the clinical evaluation for the HSP group are summarized in Table 2. According to the MAS evaluation, six patients were in level 1, nine in level 1+, three in level 2, and two in level 3. On the VAS scale, the distribution of patients ranged from 1 to 8. In the Neer score, 15 patients scored below 70, and five scored 80–89.
SWE evaluation
There was no significant difference in the stiffness of shoulder internal rotation muscles between the two sides of the control group in the resting and passive stretching positions (all P > 0.05) (Supplementary Table 1 and Supplementary Figure S2). The dominant side of the control group was used to compare with the HSP group. Compared with the control group, YM of PM, AD, TM, and LD on the paretic side of HSP patients were significantly increased in the resting and passive stretching positions (all P < 0.001) (Table 3 and Figure 2). Then, the YM of the paretic side was measured at shoulder abduction 45° and maximum tolerable external rotation, concluding that the YM of the paretic side under passive stretching was significantly higher than that at rest (all P < 0.001) (Table 3 and Figure 3).

Table 3. Comparison of the paretic side of the HSP group with the dominant side of the control group at rest and stretching position.
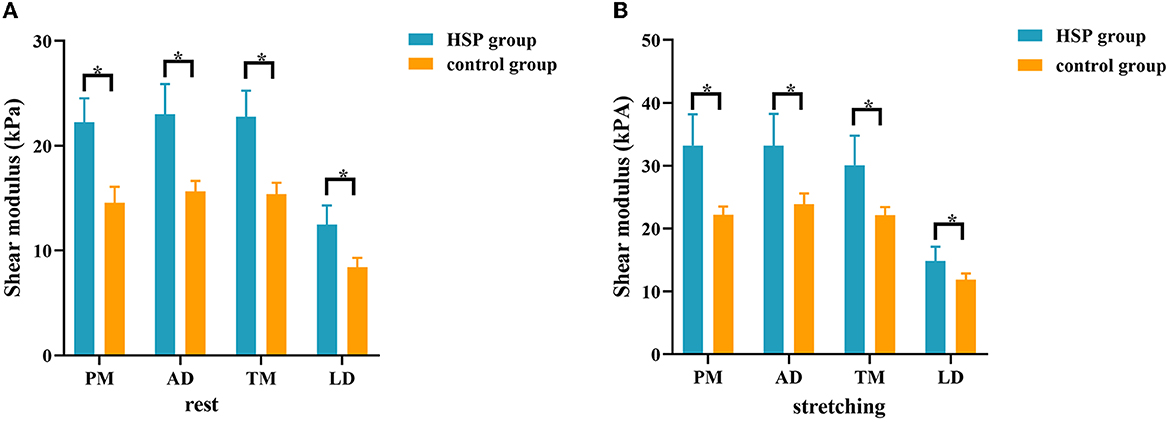
Figure 2. Comparison of the stiffness of shoulder internal rotation muscles between patients with HSP and healthy controls in the resting and passive stretching states. The stiffness of internal rotation muscles was higher in HSP patients than in healthy controls in both the resting (A) and passive stretching (B) positions. *p < 0.05. PM, pectoralis major; AD, anterior deltoid; TM, teres major; LD, latissimus dorsi.
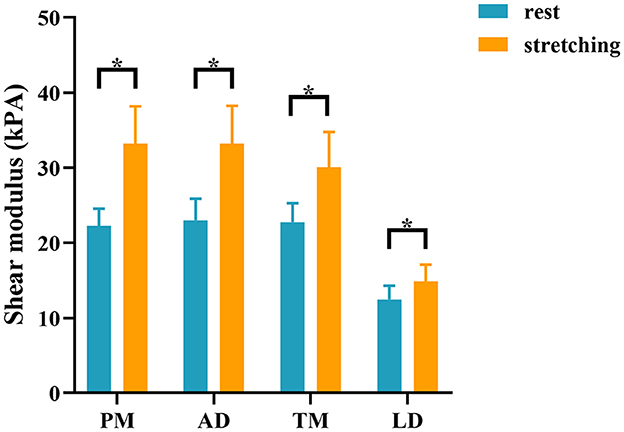
Figure 3. Comparison of the stiffness of shoulder internal rotation muscles in the resting and passive stretching positions in patients with HSP. The stiffness of internal rotation muscles in the HSP group was higher in the stretching position than in the resting position. *p < 0.05. PM, pectoralis major; AD, anterior deltoid; TM, teres major; LD, latissimus dorsi.
Correlation between muscle stiffness and the MAS, VAS, and Neer scores
No correlation was found between stiffness and MAS in PM (r = 0.083, P = 0.727), AD (r = −0.051, P = 0.832), TM (r = −0.039, P = 0.869), and LD (r = 0.223, P = 0.344) at rest. However, YM of PM (r = 0.839, P = 0.000), TM (r = 0.491, P = 0.044), and LD (r = 0.478, P = 0.044) were correlated with MAS during passive stretching (Figures 4A–C). YM of AD (r = −0,030, P = 0.901) did not correlate with MAS. YM of TM was positively correlated with VAS (r = 0.562, P = 0.039) (Figure 4D). In addition, the YM of TM was also negatively correlated with the Neer score (r = −0.553, P = 0.046) (Figure 4E).
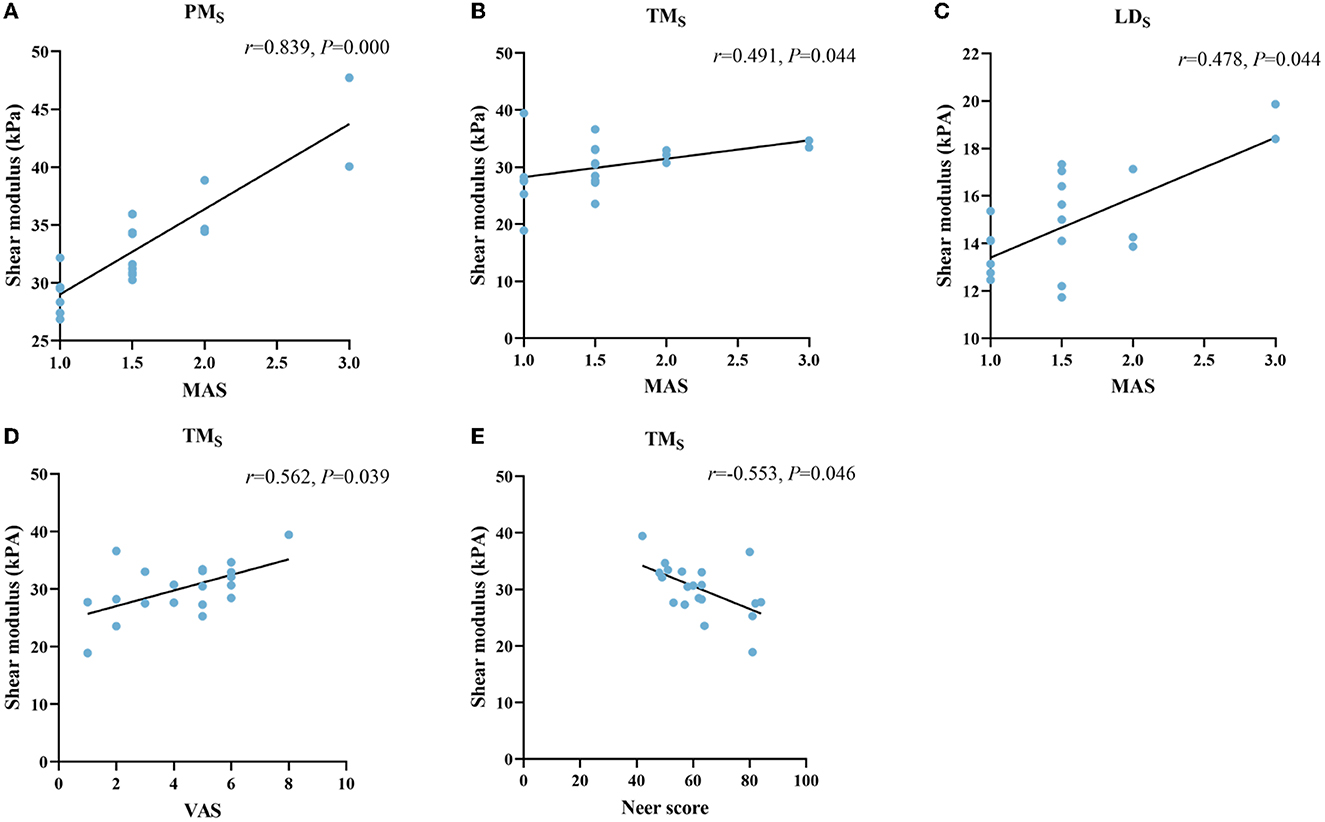
Figure 4. Correlation between the stiffness of shoulder internal rotation muscles with the MAS, VAS, and Neer score in patients with HSP. The stiffness of PM (A), TM (B), and LD (C) at stretched (S subscript) was positively correlated with MAS. The stiffness of TM (D, E) was positively correlated with the VAS and negatively correlated with the Neer score. PM, pectoralis major; TM, teres major; LD, latissimus dorsi.
Discussion
Using ultrasonic SWE, we found that the stiffness of shoulder internal rotation muscles was significantly increased on the paretic side of patients with HSP. Moreover, as to paretic side muscles, the muscle stiffness was significantly higher during passive stretching than at rest. The stiffness of PM, TM, and LD during passive stretching was correlated with MAS. We also found that the stiffness of TM was positively correlated with the VAS and negatively correlated with the Neer score. These results suggested that the increased stiffness of the shoulder's internal rotation muscles may be related to the severity of HSP.
In this study, SWE was used to evaluate the internal rotation muscles of the shoulder. The results of our study were consistent with the study of Lee et al. (19). They reported that stroke patients had higher SWV in the biceps on the paretic side than controls. This may be attributed to the change in muscle segment length. Fridén et al. (25) found that resting muscle segment length in spastic patients was shorter than that in normal ones. In addition, after nerve injury, the muscle structure was changed, with stiffer fibers (26), increased muscle collagen, and abnormal aggregation of extracellular matrix (27). These studies suggested that changes in the composition and structure of paretic muscles may cause increased passive stiffness (28). We also observed the stiffness of the paretic side muscles at shoulder abduction 45°, and maximum tolerable external rotation was significantly higher than that at rest position. As the muscle is stretched, the length of the muscle increases, creating greater passive resistance and increases in stiffness. Lee et al. (29) found that SWV was positively correlated with muscle length, and the changes in SWV were associated with an increase in passive tension. Moreover, passive muscle tension is related to titin (30), which bears the major load when the muscle is passively stretched (31). However, the role of titin in passive tension may vary considerably in different muscles (30), and whether titin isoform size was altered in stroke patients with hypertonia was still controversial (27, 32).
In stroke patients, it has been reported that shoulder pain was related to muscle hypertonia, motor, sensory disturbance, and musculoskeletal system problems (33). Previous studies using the SWE technique have reported a correlation between the stiffness of the upper limb hypertonic muscle and MAS in stroke patients (18, 34, 35). However, these studies have focused on the flexors of the upper extremity, and few studies have involved the girdle of the shoulder. In this study, we also found that the stiffness of PM, TM, and LD during passive stretching was correlated with MAS, and the stiffness of internal rotation muscles was not related to MAS at rest. This may be because the stiffness of the muscles in the stretched state better reflects the differences between pathological muscles (36). Our results showed that the stiffness of PM was highly correlated with MAS, and the stiffness of TM and LD was weakly correlated with MAS. This indicates that the stiffness changes of internal rotation muscles under passive stretching, especially PM, were consistent with MAS. The muscle hypertonia of PM was more predominant in the shoulder than in other muscles. With the muscle hypertonia of TM and LD, shoulder movement is inhibited. These results are consistent with clinical features. Many patients' families reported that muscle tension was increased only when the shoulder joint was passively moved. In chronic stroke patients, it has been reported that patients with higher MAS scores have longer shoulder pain duration, which often leads to secondary complications (7).
In this study, we analyzed the correlation between the stiffness of internal rotation muscles of the shoulder and VAS and Neer scores, finding that only the stiffness of TM was correlated with them. This is probably because TM is the main internal rotation muscle, and when TM hypertonia occurs, it leads to limited external rotation and shoulder abduction and causes pain. The results indicated that HSP patients with higher muscle stiffness may have more severe pain, and treatment to reduce muscle stiffness may relieve pain. This finding was consistent with the results of Itoigawa et al. (37). They also found a significant correlation between muscle stiffness and shoulder pain during exercise. In addition, the TM, as a deep muscle, may have a more significant dominant effect on the internal rotation of the shoulder joint. This result provides a new intervention idea for clinical practitioners that deep muscles may play an essential role in the development of HSP and focusing interventions on deep muscles of HSP patients may better relieve their shoulder pain. Currently, botulinum toxin-A (BoNT-A) combined with rehabilitation treatment, such as passive muscle stretching and exercise therapy, is an effective method of treating HSP to relieve pain and increase joint mobility (38). Ashford et al. (39) conducted the intervention of BoNT-A combined with rehabilitation therapy on hypertonic muscles of 16 patients with shoulder pain, and the results showed that hypertonia and pain of patients were significantly improved. However, BoNT-A is rarely used in TM alone. Aksoy et al. (6) compared the efficacy of BoNT-A injections in PM and TM (Group 1) with a suprascapular nerve block (Group 2). They found that the two groups had the same effect in the short term (2 weeks), while the improvement in pain and range of motion was more significant in the medium term (6 weeks) in group 1. Many previous studies have chosen PM combined with TM for treatment possibly because the majority of patients treated with BoNT-A had moderate-severe muscle hypertonia. In this study, our results initially suggested that the stiffness of TM was associated with pain and impaired movement in patients with mild-moderate muscle hypertonia. However, the results still need to be confirmed in a larger cohort.
There are several limitations to this study. First, SWE cannot fully observe the stiffness of the subscapular due to the special anatomical position, which may affect the judgment of the author. Second, we only assessed the pain intensity of HSP patients using the VAS scale, but not the frequency and duration of pain, and the VAS had a large range, which could have influenced the results. At present, self-reporting is an effective method to measure pain. However, VAS can still be used as a method to evaluate pain due to the special population. Third, the SWE has some limitations. For example, transducer placement, muscle position, and the lack of a uniform measure have limited the development of SWE in the musculoskeletal field. Fourth, although we measured multiple times in order to reduce the error, we did not calculate intraclass correlation coefficients to evaluate the reliability of SWE in the HSP group. In addition, the relationship between MAS grade 4 and muscle stiffness could not yet be determined because patients with MAS grade 4 were unable to cooperate with passive stretching movement. Finally, due to the small sample size, the results of this study need to be validated in a larger cohort.
In conclusion, our study found increased stiffness of the internal rotation muscles in patients with HSP. The stiffness of the TM was associated with the pain intensity and function of the shoulder. The stiffness of internal rotation muscles can be used as a quantitative indicator for HSP evaluation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Weifang Medical College. The patients/participants provided their written informed consent to participate in this study.
Author contributions
FJ, X-RZ, L-YK, and X-ZY developed the study concept and design. FJ and X-RZ collected clinical and imaging data and interpreted the data. S-YZ and X-ZY designed and revised this manuscript. J-CF, Z-JZ, and L-ZL analyzed the data. FJ wrote the first draft. All authors critically reviewed this manuscript, contributed to this article, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2023.1195915/full#supplementary-material
References
1. Yelnik AP, Colle FM, Bonan IV. Treatment of pain and limited movement of the shoulder in hemiplegic patients with botulinum toxin a in the subscapular muscle. Eur Neurol. (2003) 50:91–3. doi: 10.1159/000072505
2. Najenson T, Yacubovich E, Pikielni SS. Rotator cuff injury in shoulder joints of hemiplegic patients. Scand J Rehabil Med. (1971)3:131–7.
3. Wilson RD, Chae J. Hemiplegic shoulder pain. Phys Med Rehabil Clin N Am. (2015) 26:641–55. doi: 10.1016/j.pmr.2015.06.007
4. Vasudevan JM, Browne BJ. Hemiplegic shoulder pain: an approach to diagnosis and management. Phys Med Rehabil Clin N Am. (2014) 25:411–37. doi: 10.1016/j.pmr.2014.01.010
5. Niessen M, Janssen T, Meskers C, Koppe P, Konijnenbelt M, Veeger D. Kinematics of the contralateral and ipsilateral shoulder: a possible relationship with post-stroke shoulder pain. J Rehabilit Med. (2008) 40:482–6. doi: 10.2340/16501977-0201
6. Kasapoglu-Aksoy M, Aykurt-Karlibel I, Altan L. Comparison of the efficacy of intramuscular botulinum toxin type-a injection into the pectoralis major and the teres major muscles and suprascapular nerve block for hemiplegic shoulder pain: a prospective, double-blind, randomized, controlled trial. Neurol Sci. (2020) 41:2225–30. doi: 10.1007/s10072-020-04334-4
7. Lundström E, Terént A, Borg J. Prevalence of disabling spasticity 1 year after first-ever stroke. Eur J Neurol. (2008) 15:533–9. doi: 10.1111/j.1468-1331.2008.02114.x
8. Chuang LL, Chen YL, Chen CC, Li YC, Wong AM, Hsu AL, et al. Effect of EMG-triggered neuromuscular electrical stimulation with bilateral arm training on hemiplegic shoulder pain and arm function after stroke: a randomized controlled trial. J Neuroeng Rehabil. (2017) 14:122. doi: 10.1186/s12984-017-0332-0
9. Karaahmet OZ, Eksioglu E, Gurcay E, Karsli PB, Tamkan U, Bal A, et al. Hemiplegic shoulder pain: associated factors and rehabilitation outcomes of hemiplegic patients with and without shoulder pain. Top Stroke Rehabil. (2014) 21:237–45. doi: 10.1310/tsr2103-237
10. Sommerfeld DK, Eek EU, Svensson AK, Holmqvist LW, von Arbin MH. Spasticity after stroke: its occurrence and association with motor impairments and activity limitations. Stroke. (2004) 35:134–9. doi: 10.1161/01.STR.0000105386.05173.5E
11. Davis LC, Baumer TG, Bey MJ, Holsbeeck MV. Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography. (2019) 38:2–12. doi: 10.14366/usg.18039
12. Gennisson JL, Deffieux T, Fink M, Tanter M. Ultrasound elastography: principles and techniques. Diagn Interv Imaging. (2013) 94:487–95. doi: 10.1016/j.diii.2013.01.022
13. Bastijns S, De Cock AM, Vandewoude M, Perkisas S. Usability and pitfalls of shear-wave elastography for evaluation of muscle quality and its potential in assessing sarcopenia: a review. Ultrasound Med Biol. (2020) 46:2891–907. doi: 10.1016/j.ultrasmedbio.2020.06.023
14. Boulard C, Mathevon L, Arnaudeau LF, Gautheron V, Calmels P. Reliability of shear wave elastography and ultrasound measurement in children with unilateral spastic cerebral palsy. Ultrasound Med Biol. (2021) 47:1204–11. doi: 10.1016/j.ultrasmedbio.2021.01.013
15. Gao J, He W, Du LJ, Chen J, Park D, Wells M, et al. Quantitative ultrasound imaging to assess the biceps brachii muscle in chronic post-stroke spasticity: preliminary observation. Ultrasound Med Biol. (2018) 44:1931–40. doi: 10.1016/j.ultrasmedbio.2017.12.012
16. Huang YC, Liang PJ, Pong YP, Leong CP, Tseng CH. Physical findings and sonography of hemiplegic shoulder in patients after acute stroke during rehabilitation. J Rehabilit Med. (2010) 42:21–6. doi: 10.2340/16501977-0488
17. Uzdu A, Kirazli Y, Karapolat H, Unlu B, Tanigör G, Çaliş FA. Efficacy of platelet-rich plasma in the treatment of hemiplegic shoulder pain. Neurol Sci. (2021) 42:1977–86. doi: 10.1007/s10072-020-04710-0
18. Wu CH, Ho YC, Hsiao MY, Chen WS, Wang TG. Evaluation of post-stroke spastic muscle stiffness using shear wave ultrasound elastography. Ultrasound Med Biol. (2017) 43:1105–11. doi: 10.1016/j.ultrasmedbio.2016.12.008
19. Lee SSM, Jakubowski KL, Spear SC, Rymer WZ. Muscle material properties in passive and active stroke-impaired muscle. J Biomech. (2019) 83:197–204. doi: 10.1016/j.jbiomech.2018.11.043
20. Eby S, Zhao H, Song P, Vareberg BJ, Kinnick R, Greenleaf JF, et al. Quantitative evaluation of passive muscle stiffness in chronic stroke. Am J Phys Med Rehabil. (2016) 95:899–910. doi: 10.1097/PHM.0000000000000516
21. Brien H, Noftall F, MacMaster S, Cummings T, Landells C, Rockwood P. Neer's classification system: a critical appraisal. J Trauma. (1995) 38:257–60. doi: 10.1097/00005373-199502000-00022
22. Francisco-Martínez C, Padilla-Medina JA, Prado-Olivarez J, Pérez-Pinal FJ, Barranco-Gutiérrez AI, Martínez-Nolasco JJ. Kinect V2-assisted semi-automated method to assess upper limb motor performance in children. Sensors. (2022) 22:2258. doi: 10.3390/s22062258
23. Dean CM, Mackey FH, Katrak P. Examination of shoulder positioning after stroke: a randomised controlled pilot trial. Aust J Physiother. (2000) 46:35–40. doi: 10.1016/S0004-9514(14)60312-3
24. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B—Methodol. (1995) 57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x
25. Fridén J, Lieber RL. Spastic muscle cells are shorter and stiffer than normal cells. Muscle Nerve. (2003) 27:157–64. doi: 10.1002/mus.10247
26. Lieber RL, Runesson E, Einarsson F, Fridén J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve. (2003) 28:464–71. doi: 10.1002/mus.10446
27. Smith LR, Lee KS, Ward SR, Chambers HG, Lieber RL. Hamstring contractures in children with spastic cerebral palsy result from a stiffer extracellular matrix and increased in vivo sarcomere length. J Physiol. (2011) 589:2625–39. doi: 10.1113/jphysiol.2010.203364
28. Lieber RL, Ward SR. Cellular Mechanisms of Tissue Fibrosis. 4 Structural and Functional Consequences of Skeletal Muscle Fibrosis. Am J Physiol Cell Physiol. (2013) 305:C241–52. doi: 10.1152/ajpcell.00173.2013
29. Lee SS, Gaebler-Spira D, Zhang LQ, Rymer WZ, Steele KM. Use of shear wave ultrasound elastography to quantify muscle properties in cerebral palsy. Clin Biomech. (2016) 31:20–8. doi: 10.1016/j.clinbiomech.2015.10.006
30. Prado LG, Makarenko I, Andresen C, Krüger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol. (2005) 126:461–80. doi: 10.1085/jgp.200509364
31. Magid A, Law DJ. Myofibrils bear most of the resting tension in frog skeletal muscle. Science (New York, NY). (1985) 230:1280–2. doi: 10.1126/science.4071053
32. Olsson MC, Krüger M, Meyer LH, Ahnlund L, Gransberg L, Linke WA, et al. Fibre type-specific increase in passive muscle tension in spinal cord-injured subjects with spasticity. J Physiol. (2006) 577:339–52. doi: 10.1113/jphysiol.2006.116749
33. Roosink M, Renzenbrink GJ, Buitenweg JR, Van Dongen RT, Geurts AC MJ IJ. Persistent shoulder pain in the first 6 months after stroke: results of a prospective cohort study. Arch Phys Med Rehabil. (2011) 92:1139–45. doi: 10.1016/j.apmr.2011.02.016
34. Galvão S, de Oliveira LF, de Lima R, Xerez D, Menegaldo LL. Shear wave elastography of the brachioradialis spastic muscle and its correlations with biceps brachialis and clinical scales. Clin Biomech. (2022) 97:105687. doi: 10.1016/j.clinbiomech.2022.105687
35. Liu J, Pan H, Bao Y, Zhao Y, Huang L, Zhan W. The value of real-time shear wave elastography before and after rehabilitation of upper limb spasm in stroke patients. Biomed Res Int. (2020) 2020:6472456. doi: 10.1155/2020/6472456
36. Dubois G, Kheireddine W, Vergari C, Bonneau D, Thoreux P, Rouch P, et al. Reliable protocol for shear wave elastography of lower limb muscles at rest and during passive stretching. Ultrasound Med Biol. (2015) 41:2284–91. doi: 10.1016/j.ultrasmedbio.2015.04.020
37. Itoigawa Y, Koga A, Morikawa D, Kubota A, Uehara H, Maruyama Y, et al. Posterior shoulder stiffness was associated with shoulder pain during throwing in college baseball players: assessment of shear wave elastography. Eur J Orthop Surg Traumatol. (2022) 33:1237–1244. doi: 10.1007/s00590-022-03286-z
38. Franceschini M, Iocco M, Molteni F, Santamato A, Smania N. Management of stroke patients submitted to botulinum toxin type a therapy: a delphi survey of an italian expert panel of specialist injectors. Eur J Phys Rehabil Med. (2014) 50:525–33.
Keywords: hemiplegic shoulder pain, stroke, shear wave elastography, muscle stiffness, ultrasound
Citation: Jia F, Zhu X-R, Kong L-Y, Fan J-C, Zhu Z-J, Lin L-Z, Zhang S-Y and Yuan X-Z (2023) Stiffness changes in internal rotation muscles of the shoulder and its influence on hemiplegic shoulder pain. Front. Neurol. 14:1195915. doi: 10.3389/fneur.2023.1195915
Received: 29 March 2023; Accepted: 10 May 2023;
Published: 02 June 2023.
Edited by:
Haiqing Zheng, Third Affiliated Hospital of Sun Yat-sen University, ChinaReviewed by:
Carlo Trompetto, University of Genoa, ItalyMassimiliano Mangone, Sapienza University of Rome, Italy
Copyright © 2023 Jia, Zhu, Kong, Fan, Zhu, Lin, Zhang and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-Yun Zhang, Sm9obi1sd0Bzb2h1LmNvbQ==; Xiang-Zhen Yuan, eXVhbnhpYW5nemhlbkAxNjMuY29t
 Fan Jia1
Fan Jia1 Xin-Rui Zhu
Xin-Rui Zhu Ling-Yu Kong
Ling-Yu Kong Li-Zhen Lin
Li-Zhen Lin Xiang-Zhen Yuan
Xiang-Zhen Yuan