
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 08 January 2024
Sec. Sleep Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1191233
 Lijuan Hao1*
Lijuan Hao1* Kangkang Peng1
Kangkang Peng1 Qi Bian2
Qi Bian2 Suting Guo2
Suting Guo2 Chengmin Duan1
Chengmin Duan1 Lei Feng1
Lei Feng1 Zhenguo Chen1
Zhenguo Chen1 Caiang Renzeng1
Caiang Renzeng1 Huaixia Pang1
Huaixia Pang1 Zhen Ma1
Zhen Ma1Background: Obstructive sleep apnea-hypopnea syndrome (OSAHS) is a common sleep disorder. The lower atmospheric pressure and decreased oxygen levels of high-altitude areas can exacerbate the severity of OSAHS, but research into OSAHS in high-altitude areas remains limited. This study, from June 2015 to January 2020, involved 4,667 patients with suspected OSAHS and 38 healthy volunteers. The non-OSAHS group (AHI <5/h) had 395 patients, while the larger OSAHS group (AHI ≥5/h) comprised 4,272 patients. The significant size difference between the groups emphasized the study’s focus on OSAHS, using the non-OSAHS mainly for comparison.
Methods: Sleep technicians monitored the OSAHS patient group overnight by polysomnography (PSG), the apnea-hypopnea index (AHI), the mean oxygen saturation (MSpO2), lowest oxygen saturation (LSpO2), the oxygen desaturation index (ODI) and the total sleep time with oxygen saturation less than 90% (TST-SpO2 <90%). Healthy volunteers self-monitored sleep patterns at home, using the CONTEC RS01 respiration sleep monitor with a wristwatch sleep apnea screen meter. The RSO1 wristwatch-style device has already been studied for consistency and sensitivity with the Alice-6 standard multi-lead sleep monitor and can be used for OSAHS screening in this region.
Results: LSpO2 recordings from healthy volunteers (86.36 ± 3.57%) and non-OSAHS (AHI <5/h) cohort (78.59 ± 11.99%) were much lower than previously reported normal values. Regression analysis identified no correlations between AHI levels and MSpO2 or TST-SpO2 <90%, weak correlations between AHI levels and LSpO2 or MSpO2, and a strongly significant correlation between AHI levels and the ODI (r = 0.76, p < 0.05). The data also indicated that the appropriate clinical thresholds for OSAHS patients living at mild high altitude are classified as mild, moderate, or severe based on LSpO2 saturation criteria of 0.85–0.90, 0.65–0.84, or <0.65, respectively.
Conclusion: The study findings suggest that individuals with an AHI score below 5 in OSAHS, who reside in high-altitude areas, also require closer monitoring due to the elevated risk of nocturnal hypoxia. Furthermore, the significant correlation between ODI values and the severity of OSAHS emphasizes the importance of considering treatment options. Additionally, the assessment of hypoxemia severity thresholds in OSAHS patients living in high-altitude regions provides valuable insights for refining diagnostic guidelines.
Obstructive sleep apnea-hypopnea syndrome (OSAHS), also referred to as obstructive sleep apnea (OSA), is characterized by repetitive narrowing and collapse of the upper airway that can cause an individual to briefly stop breathing (apnea), or only take shallow breaths (hypopnea) while sleeping. As a result, oxygen levels in the blood are reduced and the sleep cycle is greatly disrupted (1, 2). According to the World Health Organization (WHO), an estimated 100 million people worldwide have OSAHS, with many cases that are undiagnosed and untreated (3). OSAHS is associated with a range of health problems, including cardiovascular disease, stroke, diabetes, and depression, among others. In severe cases, OSAHA contributes to severe comorbidities which increase mortality risk, such as cardiac arrest or respiratory failure (2, 3). The prevalence of OSAHS in the United States of America (USA) is 9%–24% for men and 4%–9% for women who are not obese (body mass index <30 kg/m2) and aged between 30 and 60 years, and it is estimated that approximately 25 million adults have some form of OSAHS, with the prevalence increasing with age (4). European countries have prevalence rates of 17%–30% in men and 5%–15% in women, with higher rates in older age groups (5), and it is estimated that millions of patients suffer from OSAHS in the Middle East and Arab countries (4). OSAHS is also common in Asian countries, particularly in China and India. One study estimated that the prevalence of OSAHS in China is approximately 10%, while another study has recorded that 13.7% of adults in India have OSAHS (3). Overall, OSAHS is a global health problem that affects a significant portion of the adult population, with a higher prevalence in older age groups and in individuals who are overweight or obese (2).
The classification of obstructive sleep apnea-hypopnea syndrome (OSAHS) uses the Apnea-Hypopnea Index (AHI), which counts apneic and hypopneic events per hour of sleep. OSAHS severity is based on AHI values: mild (5–14/h), moderate (15–30/h), and severe (>30/h) (6). Other key measures include mean and lowest oxygen saturation (MSaO2, LSaO2), oxygen desaturation index (ODI), and time with oxygen saturation below 90% (TST-SpO2 <90%). These assess oxygen desaturation severity during sleep and correlate with AHI (7–9). Higher AHI often means lower MSaO2 and LSaO2, higher TST-SpO2 <90%, and higher ODI, indicating more severe OSAHS (7, 10). However, there are currently few studies exploring these metrics in high-altitude areas, especially in mainland China. This study aims to investigate this and provide updated information.
Evidence indicates that the incidence of OSAHS is higher in high-altitude areas than in non-plateau areas (11, 12), which may be explained by several factors, such as lower oxygen levels, colder temperatures, and in response to ventilatory acclimatization to hypoxia at mild high altitude. Moreover, at mild high altitude, the reduced atmospheric pressure and lower oxygen levels can exacerbate the symptoms of OSAHS (11), Decreased oxygen saturation during sleep may increase the workload on respiratory muscles, potentially complicating the ability to keep the airway open, which is a central issue of OSAHS (13). Notably, some high-altitude areas may have limited medical resources, which could lead to the under-diagnosis and under-treatment of OSAHS (14, 15). The aim of this paper is to investigate the impact of high-altitude living on obstructive sleep apnea-hypopnea syndrome (OSAHS) and provide valuable insights for refining diagnostic guidelines.
Between June 2015 and January 2020, this study included 4,667 patients admitted to the Qinghai Red Cross Hospital (Qinghai, China) with suspected OSAHS. A substantial number of these patients presented clinical symptoms, often brought to their attention by family members or dormitory mates who observed severe snoring. Additionally, the study involved 38 healthy volunteers, primarily comprising hospital staff and their family members who did not exhibit clinical symptoms of OSAHS. The diagnosis and severity of OSAHS was based upon the AHI as either non-OSAHS (AHI <5/h; n = 395) or OSAHS (AHI >5/h; n = 4,272). The inclusion criteria were: (a) age between 12 and 70 years; (b) treatment-naïve, newly diagnosed patients; (c) no serious cardiovascular or cerebrovascular diseases; (d) no history of mental or neurological disorders. Exclusion criteria included: pregnancy or lactation, previous malignancy, mental illness, and any sleep disorder other than OSAHS. At the time of enrolment, each study participant had been living in the Qinghai province (with an average elevation of over 2,500 m) for over 1 year. The study was approved by the Qinghai Red Cross Hospital Institutional Review Board and it was conducted in compliance with national legislation and the Declaration of Helsinki guidelines. Neither the patients nor the general public were involved in the design or conduct of the study.
Patients suspected of having OSAHS underwent overnight PSG using the Philips Alice-6 LDx system for at least 7 h. Simultaneous monitoring of respiratory parameters [AHI, oxygen desaturation index (ODI), mean oxygen saturation (MSpO2), lowest oxygen saturation (LSpO2), and the total sleep time with oxygen saturation less than 90% (TST-SpO2 <90%)] was conducted by sleep technicians. All participants arrived at the designated time to the sleep monitoring room at Qinghai Red Cross Hospital, where specialized sleep technicians attended to them. After a half-hour adaptation period, professional sleep technicians, working in two shifts, monitored the participants throughout the night. Machine data were reviewed the next morning, and the same specialized sleep technician manually assessed the images, exported the data, and recorded the results. Exclusion criteria for volunteers included no history of afternoon coffee consumption, heavy drinking, or the use of sedative, hypnotic, or muscle relaxant drugs, as well as the absence of a family history of sleep-related diseases. Healthy volunteers self-monitored sleep patterns at home, using the CONTEC RS01 Respiration Sleep Monitor with a wrist watch sleep apnea screen meter (Contec Medical Systems Co., Ltd.). RS01 monitoring results were collected and analyzed by the same group of sleep technicians on the second day. The sensitivity and accuracy of respiratory monitoring by RS01 is reportedly consistent with that of Alice-6 (16). All sleep monitoring results were analyzed and interpreted by two experienced professional sleep technicians.
Statistical analysis was conducted using the SPSS Statistics 19.0 software package (IBM Corporation, NY, United States). Receiver operating characteristic curve (ROC) analysis was performed using R (R 3.4.0 for Windows). Descriptive statistics for the data were presented as mean ± standard deviation (SD). Between-group differences were assessed using student’s t-tests for variance, while one-way analysis of variance (ANOVA) was employed to compare means among two or more groups. Partial correlation and linear regression models were utilized to control for covariates. The p-values were calculated to determine the significance of these relationships. It is important to note that we have followed a convention to underline the statistically significant values in our tables, denoting those with p-values less than 0.05.
As shown in Table 1, the healthy volunteer group had a mean daytime SpO2 of 94.03 ± 1.66, MSpO2 of 92.19 ± 1.49 and LSpO2 of 86.36 ± 3.57, indicating that the average blood oxygen saturation during sleep at night was significantly lower than that during the daytime (p < 0.05) (Figure 1A). The much lower LSpO2 value compared with daytime SpO2 and MSpO2 values in the healthy volunteer group (p < 0.001) (Figure 1A) indicates that hypoxic sleep at night can occur even among healthy persons living at mild high altitudes. Previous research has reported that a normal reading for daytime SpO2 is typically between 96% and 99% (17, 18). At 1,600 meters altitude, oxygen saturation should be above 92% (19). In this study, the MSpO2 of the healthy volunteer group was significantly lower than people living at sea level (p < 0.001) (Figure 1B). Importantly, we found the daytime SpO2 values of non-OSAHS were 91.01 ± 6.10, which was lower than the expected 92% saturation at an altitude of 1,600 meters (Table 1 and Figure 1B). The normal values of LSpO2 have been known as 90.4 ± 3.1%, and MSpO2 have been known as 96.5 ± 1.5% (20). This study identified much lower LSpO2 values recorded from the healthy volunteers (86.36 ± 3.57%) and non-OHSAS (78.59 ± 11.99%) groups compared with normal values in previous reports (p < 0.001) (Figure 1B) (20). We also found significantly lower LSpO2 values in the non-OSAHS group (78.59 ± 11.99) compared with those of the healthy volunteers (86.36 ± 3.57) (p < 0.001) (Table 1 and Figure 1B), with the non-OSAHS group exhibiting excessive daytime sleepiness, poor concentration, memory problems and mood changes (data not shown).
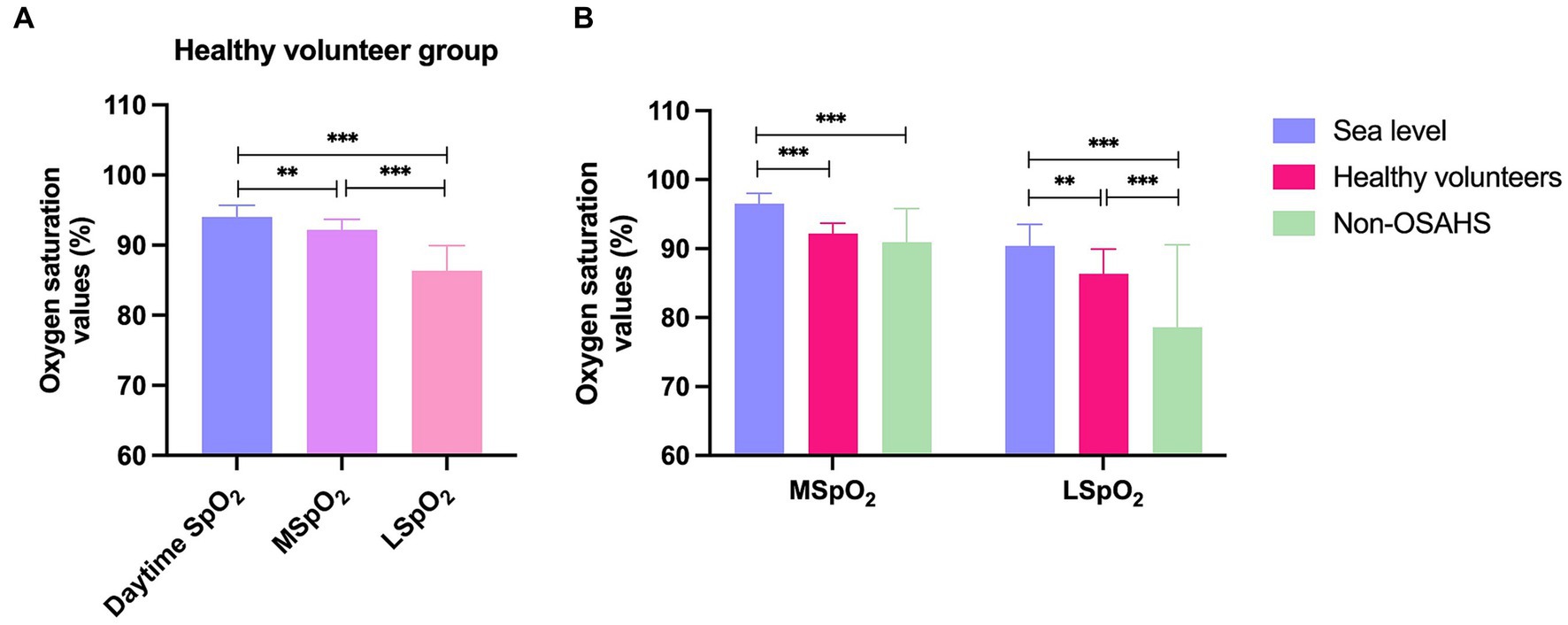
Figure 1. The oxygen saturation values defined as normal in previous publications, with the means for the healthy volunteers and non-OSAHS group in this study. (A) The oxygen saturation values of the healthy volunteer group. (B) The mean and lowest oxygen saturation values among the sea level, healthy volunteer, and non-OSAHS groups. SpO2 = oxygen saturation, how much oxygen the blood is carrying as a percentage of the maximum it can carry. MSpO2 = mean oxygen saturation, the average level of oxygen saturation during a specified period; LSpO2 = lowest oxygen saturation, the lowest recorded oxygen saturation level during the observation period, indicating the severity of respiratory disruptions. Descriptive statistics for the data were presented as mean ± standard deviation (SD) and the SD values were visually represented using error bars. Statistically significant results are denoted with an asterisk(*), with *p < 0.05, **p < 0.01, and ***p < 0.001 indicating the significance levels.
After dividing the OSAHS cohort into 4 groups (AHI <5/h; AHI ≥5 to <15/h; AHI ≥15 to <30/h; and AHI ≥30/h), associations were analyzed between the AHI groups and LSpO2, MSpO2, TST-SpO2 <90%, and ODI values. Among people residing in high-altitude areas, LSpO2 levels were negatively associated with AHI values (p < 0.01), whereas the TST-SpO2 <90% and ODI values were positively associated with AHI values (p < 0.01) (Table 2). No associations were observed between MSpO2 and overall AHI values (p = 0.65), but MSpO2 was significantly decreased in the AHI ≥30/h group (Table 2). In a ROC analysis, the ODI was associated with the highest specificity and sensitivity values (Figure 2). The sensitivity and specificity of the ODI scores were 0.831 and 0.895, 0.810 and 0.887, and 0.825 and 0.857 for the AHI <5/h, AHI ≥5 to <15/h, AHI ≥15 to <30/h and AHI ≥30/h groups, respectively (Table 3). In linear regression analysis, a strongly significant correlation was identified between AHI levels and ODI values (r = 0.76, p < 0.01) (Figure 3A), weak correlations were observed between AHI levels and LSpO2 or MSpO2 values (r = 0.53 and 0.42, p < 0.01) (Figures 3B,C), while no correlation was observed between AHI levels and TST-SpO2 <90% values (r = 0.32, p < 0.01) (Figure 3D).

Figure 2. Receiver operating characteristic curve (ROC) analysis of MSpO2, LSpO2, TST-SpO2 <90% and ODI values at (A) AHI ≥ 5 to < 15/h, (B) AHI ≥ 15 to < 30/h, and (C) AHI ≥ 30/h.
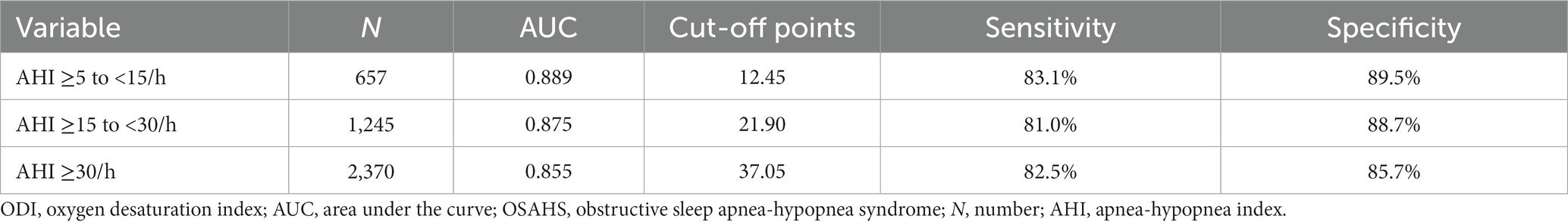
Table 3. ODI performance in ROC analysis: sensitivity and specificity across different AHI categories.
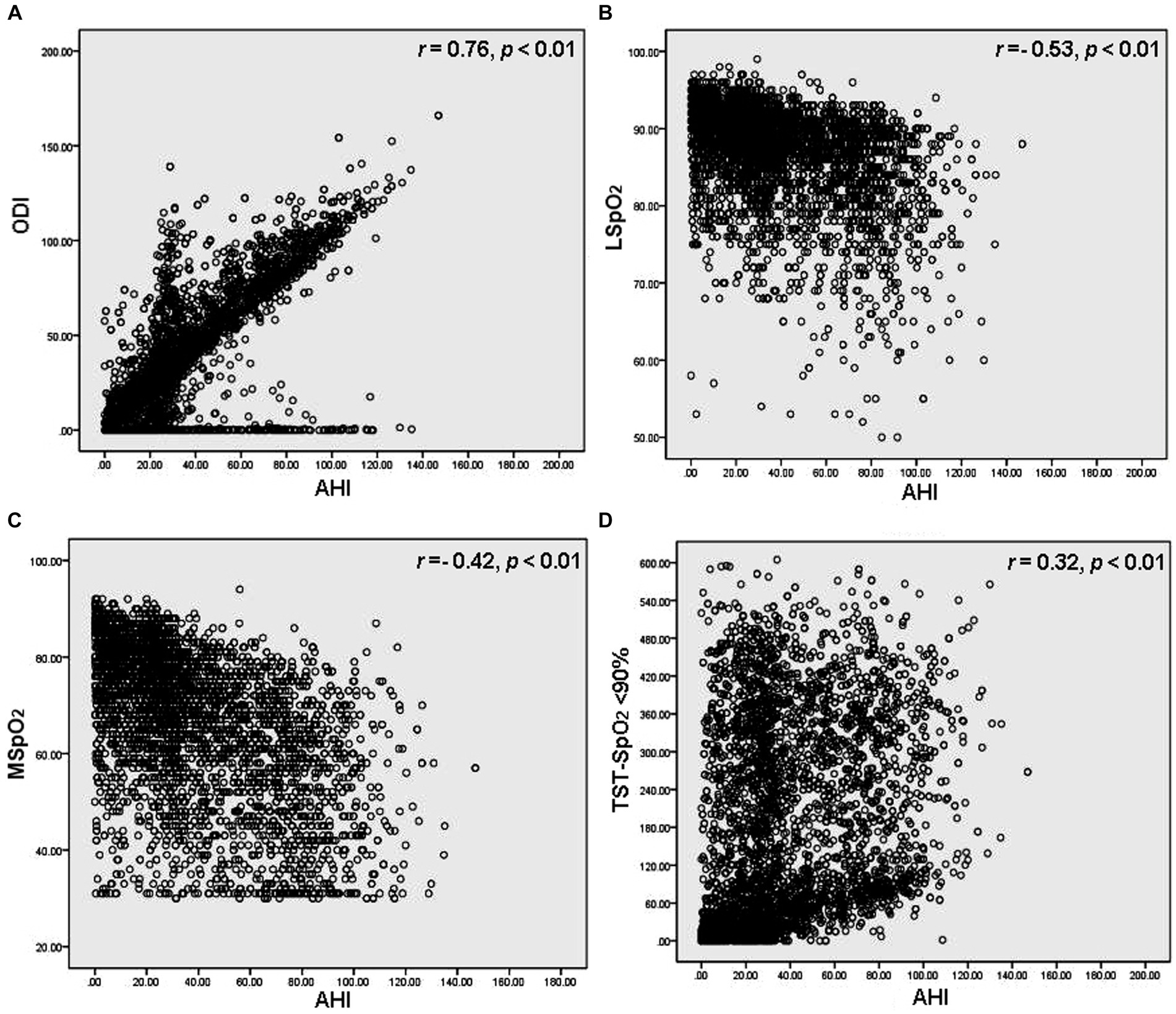
Figure 3. Regression analysis of correlations between AHI levels and (A) ODI, (B) LSpO2, (C) MSpO2 and (D) TST-SpO2 <90% values. Every data point corresponds to an individual participant.
Average oxygen saturation differs during rapid eye movement (REM) and non-REM (NREM) sleep, according to the severity of sleep breathing disorders (21). In this study, we found that in the AHI <5/h cohort, MSpO2 values (90.41 ± 4.27) differed between wakefulness (WK) (91.18 ± 4.61) and REM states, respectively, (90.36 ± 5.11) (p < 0.01), but did not differ significantly between REM and NREM sleep (p = 0.16) (Table 4), whereas for all OSAHS patients, MSpO2 values gradually decreased from WK to NREM and then to REM sleep (p < 0.01) (Table 4). Previous research has reported that oxygen saturation in patients with mild or moderate OSAHS does not differ significantly between REM and NREM states (21). Our study identified significantly higher MSpO2 values in patients with mild or moderate OSAHS during REM sleep than during NREM sleep (p < 0.01 for both comparisons) (Table 4), these results were different with OSAHS patients not living at mild high altitude.
In China, two guidelines have been issued for OSAHS diagnosis and treatment: (guideline 1) diagnosis and treatment of obstructive sleep apnea hypopnea syndrome (22) and (guideline 2) the guideline for primary care of adult obstructive sleep apnea: practice version (2018) (23). One difference between these guidelines is the thresholds of LSpO2, which are given as 0.85–0.90, 0.65–0.85 or <0.65 as mild, moderate, or severe in guideline 1; and 0.85–0.90, 0.80–0.85 or <0.80 as mild, moderate, or severe in guideline 2 (Table 5). LSpO2 values have been used to assess hypoxemia severity (mild, moderate or severe hypoxemia) (24), with the different LSpO2 values affecting the treatment strategy. We therefore analyzed the clinical thresholds of AHI and LSpO2 by following these two guidelines. As shown in Table 5, when we classified our OSAHS cohort by guideline 2, more than 50% of the patients were found to have severe hypoxemia, even in the mild OSAHS cohort. When we used Spearman rank correlation analysis to measure the degrees of association between AHI and LSpO2 values in each guideline, the correlation coefficient Rs values were 0.495 and 0.361 for guidelines 1 and 2, respectively (Table 6). These data indicate that the appropriate clinical thresholds for assessing hypoxemia severity for OSAHS patients residing in high-altitude areas are values of 0.85–0.90, 0.65–0.85 or <0.65 graded as mild, moderate or severe hypoxemia, respectively.
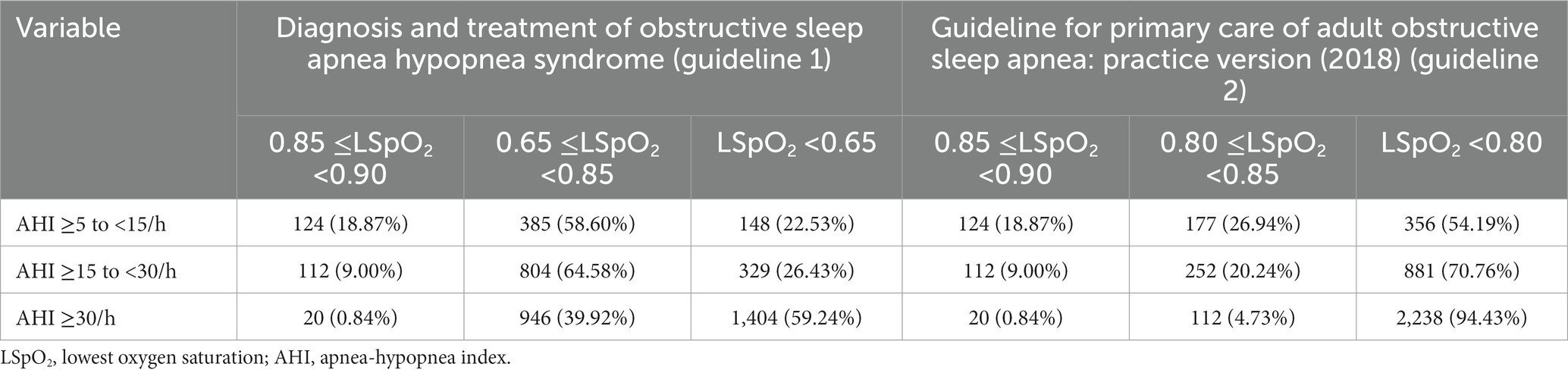
Table 5. Comparisons of correlations between AHI grades and LSpO2 values for classifying patients by OSAHS severity by two different clinical guidelines issued in China.

Table 6. Spearman rank correlation coefficients (Rs) and p-values of two OSAHS clinical guidelines issued in China.
OSAHS can impact significantly upon health and quality of life (1, 2). In high-altitude environments, the reduced atmospheric pressure and lower oxygen levels can exacerbate OSAHS symptoms, as the reduced oxygen levels can increase the workload on the respiratory muscles and make it more difficult to maintain an open airway during sleep (11, 12). In this research, we discovered a significant difference in LSpO2 (86.36 ± 3.57) compared to both daytime SpO2 (94.03 ± 1.66) and MSpO2 (92.19 ± 1.49) values within the healthy volunteer group (p < 0.001). This study highlights that even healthy persons residing in a high-attitude environment can experience night-time episodes of hypoxic sleep.
The AHI is a measure of the severity of sleep apnea, calculated as the number of apneic or hypopneic events per hour of sleep (6). An AHI value of less than 5 means that a person experiences fewer than 5 episodes of apnea (complete cessation of breathing) or hypopnea (partial obstruction of the airway) per hour of sleep (6). This is considered within the normal range and suggests that the person does not have significant sleep breathing disorders such as OSA. However, this study we identified much lower LSpO2 values recorded from the non-OHSAS (78.59 ± 11.99%) cohort (AHI <5/h) compared with normal values (90.4 ± 3.1%) in previous reports (p < 0.001) (20). We also found significantly lower LSpO2 values in the non-OSAHS group (78.59 ± 11.99) compared with those of the healthy volunteers (86.36 ± 3.57) (p < 0.001), with the non-OSAHS cohort presenting with excessive daytime sleepiness, poor concentration, memory problems, and mood changes. Notably, low LSpO2 levels are associated with an increased risk of cardiovascular and respiratory diseases. We therefore strongly suggest that for people living in high-altitude environments, AHI values of <5/h do not necessarily mean that the patient, who reside in high-altitude areas, should be considered “non-urgent” for treatment. This group should have medical care under the guidance of a healthcare professional.
High-altitude areas are typically associated with difficult terrains, extreme weather conditions and remote locations, making it challenging to access and transport medical supplies, equipment, and personnel. Moreover, the mild high altitude itself can pose health risks, such as the development of OSAHS (11–13). Several other measures, including SpO2 and ODI values, are important when evaluating the impact of OSAHS on a patient’s health (7–10). In this study, we analyzed associations between AHI levels and ODI, LSpO2, MSpO2, and TST-SpO2 <90% values by regression analysis. We found a strongly significant correlation between AHI levels and ODI (r = 0.76, p < 0.01), with the ODI differentiating levels of OSAHS severity with relatively high accuracy in patients residing at mild high altitude. The ODI reflects different clinical characteristics associated with OSAHS from a new perspective. Combining ODI monitoring with simple sleep screening equipment can provide a more accurate prediction and evaluation of OSAHS for medical institutions with limited resources in high-altitude areas.
Apnea can occur during both REM and NREM sleep. Average oxygen saturation during REM and NREM sleep differ according to the severity of sleep breathing disorders (21). Apnea is more common in NREM sleep and more severe in REM sleep (21). Previous research has reported that oxygen saturation does not differ significantly between patients with mild or moderate OSAHS living at sea level during REM and NREM sleep (21). Here, we discovered notable variations in MSpO2 values within the AHI <5/h group. Specifically, MSpO2 levels averaged at 90.41 ± 4.27, showing a significant difference between wakefulness (91.18 ± 4.61) and REM sleep (90.36 ± 5.11) (p < 0.01). However, the difference between REM and NREM sleep stages was not statistically significant (p = 0.16). Conversely, in the overall population of OSAHS patients, a progressive decrease in MSpO2 was observed from wakefulness to NREM sleep, and further into REM sleep (p < 0.01). Interestingly, patients with mild to moderate OSAHS exhibited significantly higher MSpO2 levels during REM sleep compared to NREM sleep (p < 0.01 for both groups). This trend was distinct from that observed in OSAHS patients who do not reside at mild high altitudes. Our findings therefore provide further evidence for differences in average oxygen saturation during REM and NREM sleep in patients with OSAHS and demonstrate that the severity of the disease is linked to sleep stages.
This study highlights the unique challenges and considerations of high-altitude environments. The impact of mild high altitude upon OSAHS severity and nocturnal hypoxia underscores the importance of tailored treatment approaches for patients in these regions. The evaluation of hypoxemia severity thresholds in high-altitude areas provides valuable clinical guidance for healthcare professionals assessing the severity of hypoxemia in OSAHS patients. Our study findings are of particular importance for improving OSAHS patient outcomes and quality of life.
The present study has some limitations. Firstly, it was a cross-sectional study conducted at a single center, which may have introduced selection bias. Secondly, conducting only first-night PSGs may have introduced bias due to poor sleep or altered sleep physiology caused by first-night effects. While each PSG report was considered adequate, it’s important to note that sleep efficiency can be influenced by first-night effects, impacting sleep quality and physiology. Thirdly, our patient cohort utilized the Alice-6 LDx, while healthy volunteers used the CONTEC RS01. Unfortunately, we did not directly compare the performance of these kits within the same subjects. Despite inherent variations, our study independently analyzed sleep patterns in each cohort using the specified kits. It is important to note that, as part of our research group, we have previously confirmed RS01’s reported consistency with Alice-6 (16). Fourth, all study subjects were exclusively from high-altitude areas. Additionally, for sea level data, we relied on published information. While this approach allowed us to gather relevant data, it introduces a potential limitation as the sea level data were not directly measured within our study cohort. Lastly, the relatively small number of non-OSAHS study participants led to a significant difference in group sizes between the OSAHS and non-OSAHS groups. Future clinical research is warranted to address these limitations.
This study has revealed important insights into the severity and clinical management of OSAHS. They suggest that patients, who reside in high-altitude areas, with an AHI score of <5/h should not be considered non-urgent, as they may require treatment due to the high risk of nocturnal hypoxia. The ODI has demonstrated that it can accurately differentiate OSAHS severity, making it a valuable diagnostic tool for healthcare professionals. Moreover, the associations between sleep stages and OSAHS severity highlights the importance of monitoring sleep stages in patients with OSAHS. Finally, the evaluation of hypoxemia severity thresholds in OSAHS patients living at mild high altitude provides valuable clinical guidelines for assessing their severity of hypoxemia. Overall, our findings contribute to a better understanding of OSAHS and provide important implications for the clinical management and treatment of this disease.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Qinghai Red Cross Hospital Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
The study was conceived by LH. LH and KP designed the study. LH, KP, QB, SG, CD, LF, ZC, CR, HP, and ZM performed the experiments and data collection. The paper was written by LH. All authors contributed to the article and approved the submitted version.
This work was supported by the Science and Technology Department of Qinghai Province, China (2017-ZJ-Y03 and 2019-HZ-811).
The authors wish to thank all investigators, patients and volunteers who participated in this study. The authors also appreciate the valuable suggestions from Iona J. MacDonald after her careful reading of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Memon, J, and Manganaro, SN. Obstructive sleep-disordered breathing. Treasure Island, FL: StatPearls Publishing (2022).
3. Benjafield, AV, Ayas, NT, Eastwood, PR, Heinzer, R, Ip, MSM, Morrell, MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. (2019) 7:687–98. doi: 10.1016/S2213-2600(19)30198-5
4. Sankri-Tarbichi, AG. Obstructive sleep apnea-hypopnea syndrome: etiology and diagnosis. Avicenna J Med. (2012) 2:3–8. doi: 10.4103/2231-0770.94803
5. Jennum, P, and Riha, RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J. (2009) 33:907–14. doi: 10.1183/09031936.00180108
6. Mbata, G, and Chukwuka, J. Obstructive sleep apnea hypopnea syndrome. Ann Med Health Sci Res. (2012) 2:74–7. doi: 10.4103/2141-9248.96943
7. Rashid, NH, Zaghi, S, Scapuccin, M, Camacho, M, Certal, V, and Capasso, R. The value of oxygen desaturation index for diagnosing obstructive sleep apnea: a systematic review. Laryngoscope. (2021) 131:440–7. doi: 10.1002/lary.28663
8. Wang, TT, Huang, SX, Zhang, XM, Zhang, XW, and Luo, YX. The relationship between oxygen saturation and related respiratory events in patients with obstructive sleep apnea-hypopnea syndrome. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2017) 31:170–3. doi: 10.13201/j.issn.1001-1781.2017.03.002
9. Wang, L, Wei, DH, Zhang, J, and Cao, J. Time under 90% oxygen saturation and systemic hypertension in patients with obstructive sleep apnea syndrome. Nat Sci Sleep. (2022) 14:2123–32. doi: 10.2147/NSS.S388238
10. Liu, P, Chen, Q, Yuan, F, Zhang, Q, Zhang, X, Xue, C, et al. Clinical predictors of mixed apneas in patients with obstructive sleep apnea (OSA). Nat Sci Sleep. (2022) 14:373–80. doi: 10.2147/NSS.S351946
11. Latshang, TD, Bloch, KE, Lynm, C, and Livingston, EH. Traveling to high altitude when you have sleep apnea. JAMA. (2012) 308:2418. doi: 10.1001/jama.2012.4097
12. Pataka, A, and Riha, RL. The obstructive sleep apnoea/hypopnoea syndrome—an overview. Respir Med CME. (2009) 2:111–7. doi: 10.1016/j.rmedc.2009.03.001
13. Stavrou, VT, Astara, K, Tourlakopoulos, KN, Papayianni, E, Boutlas, S, Vavougios, GD, et al. Obstructive sleep apnea syndrome: the effect of acute and chronic responses of exercise. Front Med. (2021) 8:806924. doi: 10.3389/fmed.2021.806924
14. Myrzaakhmatova, AK. Obstructive sleep apnea at high altitude. Ter Arkh. (2017) 89:103–6. doi: 10.17116/terarkh2017891103-106
15. Huang, L. High altitude medicine in China in the 21st century: opportunities and challenges. Mil Med Res. (2014) 1:17. doi: 10.1186/2054-9369-1-17
16. Pang Huaixia, HL, Zhen, M, Weizhen, S, Xiangrong, H, Chunmei, C, and Baoliang, Y. The diagnostic value of watch sleep monitoring in OSAHS at high altitude. World J Sleep Med. (2019) 6:1667–70. doi: 10.3969/j.issn.2095-7130.2019.12.011
17. Wood, EH. Normal oxygen saturation of arterial blood during inhalation of air and oxygen. J Appl Physiol. (1949) 1:567–74. doi: 10.1152/jappl.1949.1.8.567
18. Radhakrishnan, S, and Nair, SG. Analysis of parameters affecting blood oxygen saturation and modeling of fuzzy logic system for inspired oxygen prediction. Comput Methods Programs Biomed. (2019) 176:43–9. doi: 10.1016/j.cmpb.2019.04.014
19. Muaña, D Oxygen saturation (medicine): National Jewish Health; (2009) Available at: https://www.scribd.com/document/360678527/Oxygen-saturation-medicine-pdf#
20. Gries, RE, and Brooks, LJ. Normal oxyhemoglobin saturation during sleep. How low does it go? Chest. (1996) 110:1489–92. doi: 10.1378/chest.110.6.1489
21. Choi, E, Park, DH, Yu, JH, Ryu, SH, and Ha, JH. The severity of sleep disordered breathing induces different decrease in the oxygen saturation during rapid eye movement and non-rapid eye movement sleep. Psychiatry Investig. (2016) 13:652–8. doi: 10.4306/pi.2016.13.6.652
22. Zhao, MM, and Zhang, XL. Diagnosis and treatment of obstructive sleep apnea hypopnea syndrome. Zhonghua Yi Xue Za Zhi. (2012) 92:1228–30.
23. Chinese Medical Association CMAJ, Chinese Medical Association General Practice Branch, Chinese Medical Association Respiratory Medicine Branch Sleep Respiratory Disorders Group, Chinese Medical Association “Chinese Journal of General Practitioners” Editorial Board, Basic Respiratory System Diagnosis and Treatment Guidelines Writing expert group. Guideline for primary care of adult obstructive sleep apnea. Chin J Gen Pract. (2018) 2019:30–5. doi: 10.3760/cma.j.issn.1671-7368.2019.01.007.
Keywords: obstructive sleep apnea-hypopnea syndrome, apnea, oxygen desaturation index, hyponea treatment, mild high altitude
Citation: Hao L, Peng K, Bian Q, Guo S, Duan C, Feng L, Chen Z, Renzeng C, Pang H and Ma Z (2024) Assessing the contribution of mild high-altitude exposure to obstructive sleep apnea-hypopnea syndrome comorbidities. Front. Neurol. 14:1191233. doi: 10.3389/fneur.2023.1191233
Received: 26 April 2023; Accepted: 20 December 2023;
Published: 08 January 2024.
Edited by:
Ahmed S. BaHammam, King Saud University, Saudi ArabiaReviewed by:
Xiangming Meng, Wuxi Huishan District People’s Hospital, ChinaCopyright © 2024 Hao, Peng, Bian, Guo, Duan, Feng, Chen, Renzeng, Pang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Hao, aGFvbGk1OTlAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.