
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 27 April 2023
Sec. Pediatric Neurology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1185900
This article is part of the Research Topic Anesthetic Neurotoxicity in Developing Brains: Mechanisms, Biomarkers, and Therapeutic Targets View all 9 articles
Background: The neurotoxicity effects of anesthetic exposure on the developing brain have been one of the current research hotspots and numerous articles were published in the past decades. However, the quality and comparative information of these articles have not been reported. This research aimed to provide a comprehensive overview of the current state of the field by investigating research hotspots and publication trends concerning the neurotoxicity of anesthesia in the developing brain.
Materials and methods: On 15 June 2022, we searched articles on the neurotoxicity of anesthesia in the developing brain through the Science Citation Index databases from 2002 to 2021. Data of the author, title, publication, funding agency, date of publication, abstract, type of literature, country, journal, keywords, number of citations, and research direction were collected for further analysis.
Results: We searched and analyzed 414 articles in English on the field of neurotoxicity of anesthesia in the developing brain from 2002 to 2021. The country with the largest number of publications was The United States (US) (n = 226), which also had the largest total number of citations (10,419). Research in this field reached a small peak in 2017. Furthermore, the largest number of articles were published in three journals, Anesthesiology, Anesthesia and Analgesia, and Pediatric Anesthesia. The top 20 articles that were cited most often were studied. In addition, the top hotspots of this area in clinical investigations and basic research were analyzed separately.
Conclusion: This study provided an overview of the development in the neurotoxicity of anesthesia in the developing brain using bibliometric analysis. Current clinical studies in this area were mainly retrospective; in the future, we should place more emphasis on prospective, multicenter, long-term monitoring clinical studies. More basic research was also needed on the mechanisms of neurotoxicity of anesthesia in the developing brain.
Advances in medical technology have led to a greater number of babies receiving surgery under anesthesia at an early stage after birth. It remains unclear whether anesthesia adversely affects the child's ability to learn and its cognitive function later in life (1).Some fundamental studies have confirmed that anesthesia is harmful to neurons in the developing brain and causes cognitive damage. However, other reports implied that anesthetic exposure is beneficial to brain development (2–4). Many retrospective clinical studies have shown that receiving anesthesia in childhood increases the risk of later studying, awareness, and behavioral abnormalities (5–7). Several studies also demonstrated that receiving anesthesia at an early age had no appreciable impact on neurodevelopment (8).
Although the neurotoxicity of anesthesia in infants and children has received widespread attention in the field of anesthesia, various scientific questions related to the neurotoxicity of anesthesia remain unanswered and require continued research. Therefore, a detailed assessment of the neurotoxicity of anesthesia in the developing brain is necessary to better prepare for further studies.
Bibliometric analysis is the quantitative analysis of the literature on a particular topic using mathematical and statistical methods, preferring to quantify the comprehensive body of knowledge on the topic studied and to guide future research directions by highlighting the latest research trends (9–11). However, there are no relevant bibliometric articles that summarized the latest progress in this field. Our study aimed to provide a thorough overview of the state of the field by examining research hotspots and publication trends related to the neurotoxicity of anesthesia in the developing brain.
We conducted an online literature search using the Science Citation Index databases on 15 June 2022. To reduce the data errors caused by frequent database updates, we completed the search of data in one go. The search strategy was topic = (anesthes* neurotoxic*) and (developing brain*); the publication date = (2002-01-01) to (2022-06-15). We only collected literature in English and limited the article type to “article or review”. Figure 1 illustrates the process of collecting literature. A total of 444 articles emerged from our search strategy, and we excluded Meeting abstract (n = 9), Editorial material (n = 3), Proceedings paper (n = 4), Protocol (n = 2), Survey (n = 2), and 10 articles with little relevance to the study. We ended up with 133 reviews and 281 original articles, which consisted of 247 basic studies and 34 clinical studies. We collected the following bibliometric information: author, title, publication, funding agency, date of publication, abstract, type of literature, country, journal, keywords, number of citations, and research direction. No other exclusion criteria were used.
To carry out a bibliometric study and create graphs, we used CiteSpace software. GraphPad Prism 8.0 (GraphPad, San Diego, CA, USA) was used to conduct the statistical analysis. Numbers or percentages were used to represent data. The two-tailed Pearson correlation method was used to conduct correlation analysis. Statistical significance was set at P < 0.05.
We collected a total of 414 articles by our search criteria. The United States (US) was the country with the highest number of publications in the field of neurotoxicity of anesthesia in the developing brain (n = 226), followed by China Mainland (n = 167). Except for the above two countries, the number of publications in each of the remaining countries was <25. The US had 10,419 citations, the most of any country, followed by China Mainland (3,064), England (2,009), Italy (1,193), and the Netherlands (1,067). Despite the low number of publications produced, some countries had a high number of mean citations per paper. The Netherlands had the highest mean number of citations per paper (152.43), followed by Scotland (129.75) and Sweden (120.60) (Table 1). Next, we compiled the total number of articles published during this time period by each nation and listed the top 10 nations. These 10 countries published <20 articles per year on the neurotoxicity of anesthesia on the developing brain before 2012. From 2012 to 2017, the number of publications per year gradually increased except in 2014. The number of publications reached a peak in 2017 (Figure 2A). We also analyzed the cooperation between countries for each publication (Figure 2B). In Figure 2B, we could visually see that the USA and China were at the center, and they frequently collaborated with other nations, such as Japan, the UK, and Canada.
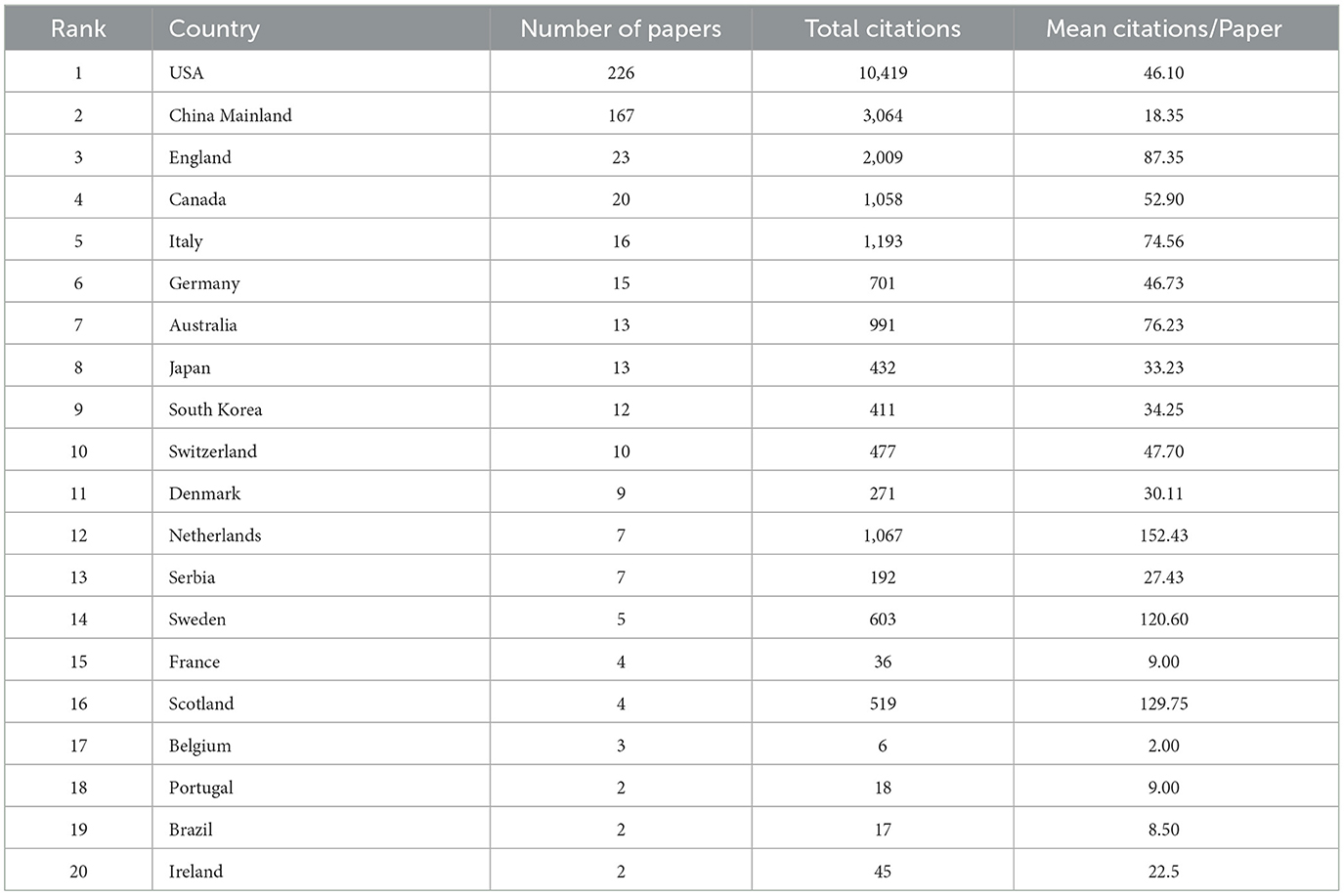
Table 1. The top 20 countries with the highest number of publications on the neurotoxicity of anesthesia in the developing brain.
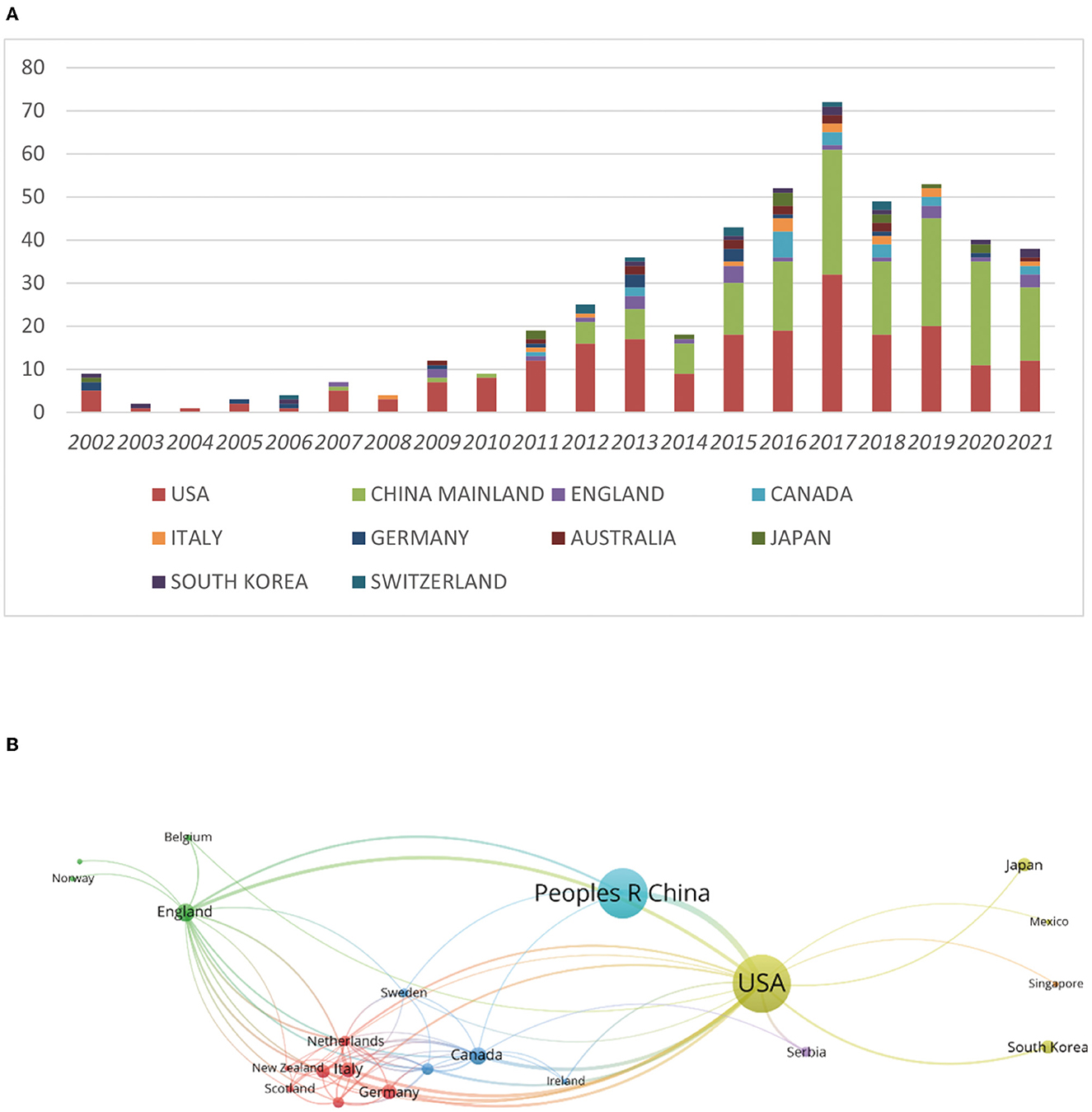
Figure 2. The impact of year and country changes on the number of articles in the field of anesthesia's neurotoxicity to the developing brain. (A) Evolution of the number of publications in the 10 most important countries. (B) Map of the various countries' close cooperation.
We ranked the top 20 corresponding authors and their institutions according to the number of articles published on anesthetic neurotoxicity in the developing brain (Table 2). There were four professors who had all published eight articles and were cited for first place: Loepke Andreas W from Cincinnati Children's Hospital Medical Center; Wang Cheng from US Food & Drug Administration (FDA); Jiang Hong from Shanghai Jiao Tong University; and Liu Hongtao from China Medical University. The H-index was used to evaluate the academic performance of researchers. The authors with high H-index were Andreas W. Loepke (H-index = 8), followed by Wang Cheng (H-index = 7), Jevtovic-Todorovic Vesna (H-index = 6), and Bai Xiaowen (H-index = 6).
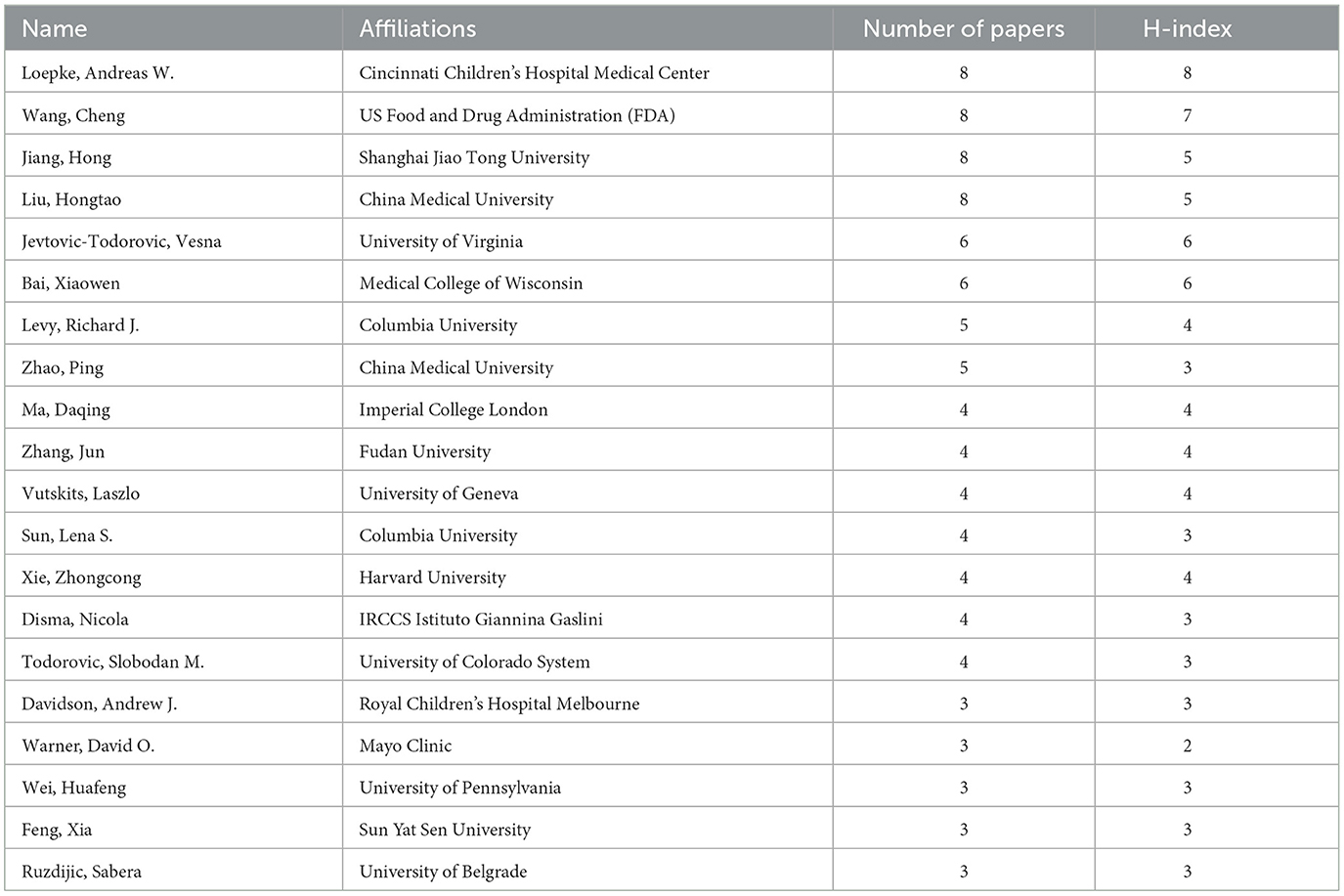
Table 2. The top 20 authors with the highest number of publications on the neurotoxicity of anesthesia in the developing brain.
Next, we analyzed the major institutions. The institution with the largest volume of publications was Shanghai Jiao Tong University (n = 32), followed by Columbia University (n = 31), Harvard University (n = 30), and the League of European Research Universities—LERU (n = 29). In the meanwhile, the Royal Children's Hospital Melbourne came in first place for the average number of citations (n = 103.67), followed by the US Food and Drug Administration (FDA) (n = 101.95), which ranked 19 and six out of 20, respectively.
The H-index for each institution was also analyzed. There were two institutions that were ranked highest with an H-index of 19, namely Harvard University and the League of European Research Universities—LERU, followed by the University of Virginia (H-index = 18) and US Food and Drug Administration (H-index = 16). More detailed data are presented in Table 3, Figure 3A. Figure 3B visualizes the close and extensive collaboration between the various institutions with Shanghai Jiao Tong University, US Food and Drug Administration, and Columbia University acting as connecting Bridges.
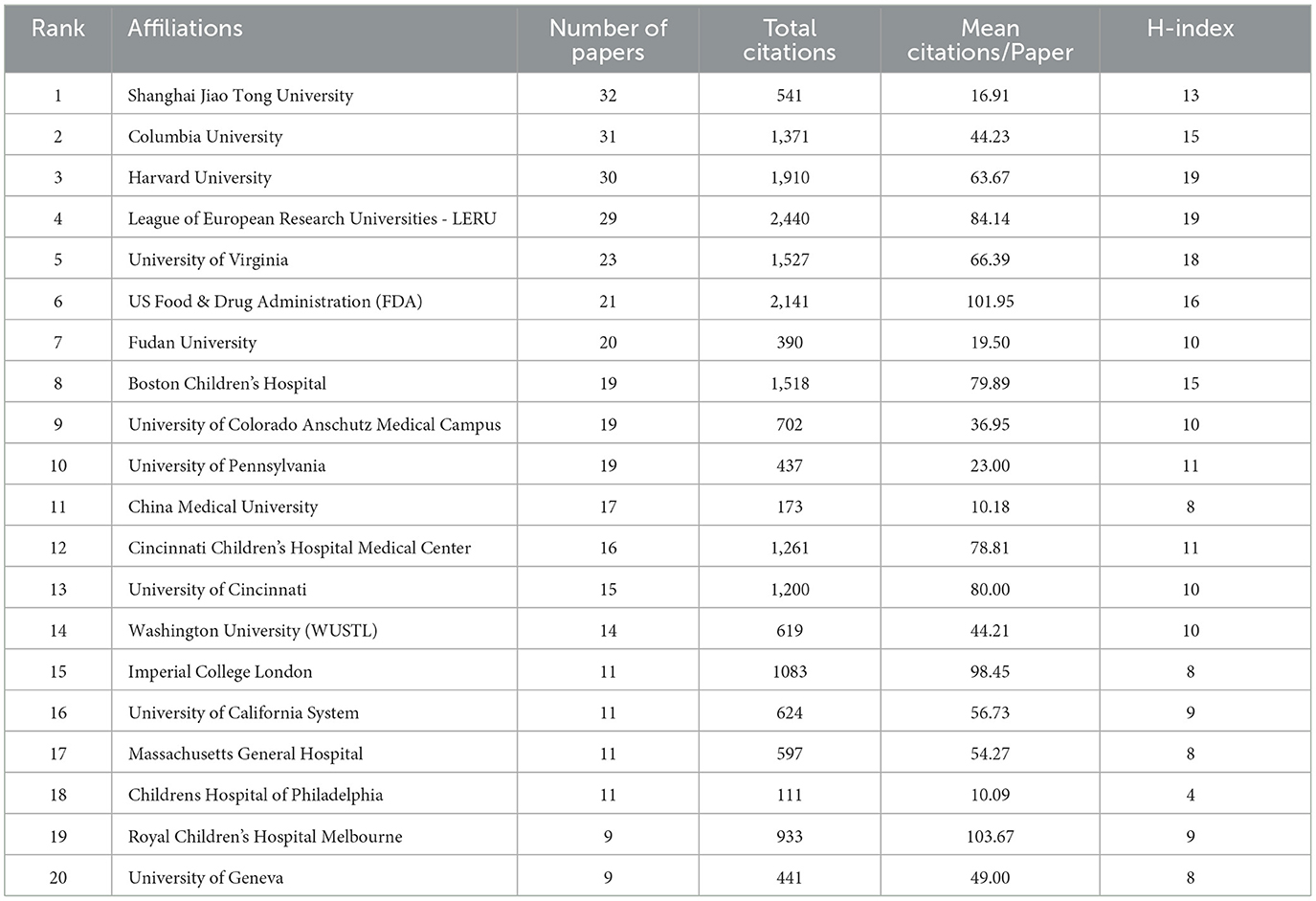
Table 3. The top 20 institutes with the highest number of publications the neurotoxicity of anesthesia in the developing brain.
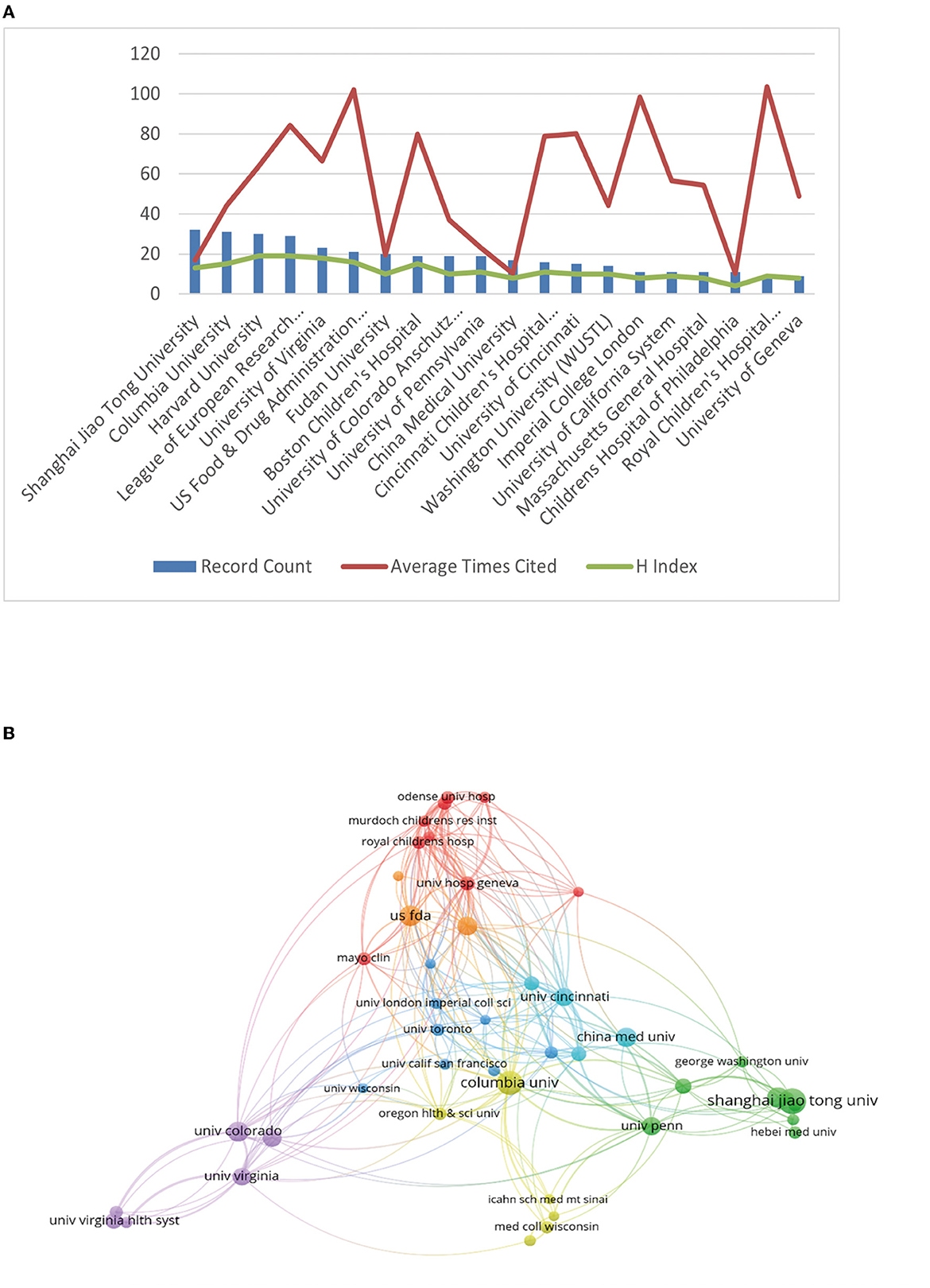
Figure 3. The articles on the neurotoxicity of anesthesia in the developing brain published by different research institutions. (A) The top 20 institutions for productivity. The red line shows the typical number of citations per article, the green line shows the H-index of each institution, and the blue bar graph shows the number of articles published by each institution. (B) The network visualization map displaying institutional collaborations.
Next, the journals that publish articles on the neurotoxicity of anesthesia in the developing brain were surveyed, and they were ranked according to the number of publications they had published. Three journals, Anesthesiology, Anesthesia and Analgesia, and Pediatric Anesthesia topped the list with 22 articles published in this period. It is well known that Anesthesiology and Anesthesia and Analgesia are authoritative journals in the field of anesthesia with a Journal Citation Reports (JCR) classification of Q1 and impact factor (IF) of 7.892 and 5.178, respectively. The highest number of relevant papers published in these two journals indicates that research related to the neurotoxicity of anesthesia in the developing brain is very highly regarded and of great interest in the field of anesthesia. The details are shown in Table 4.
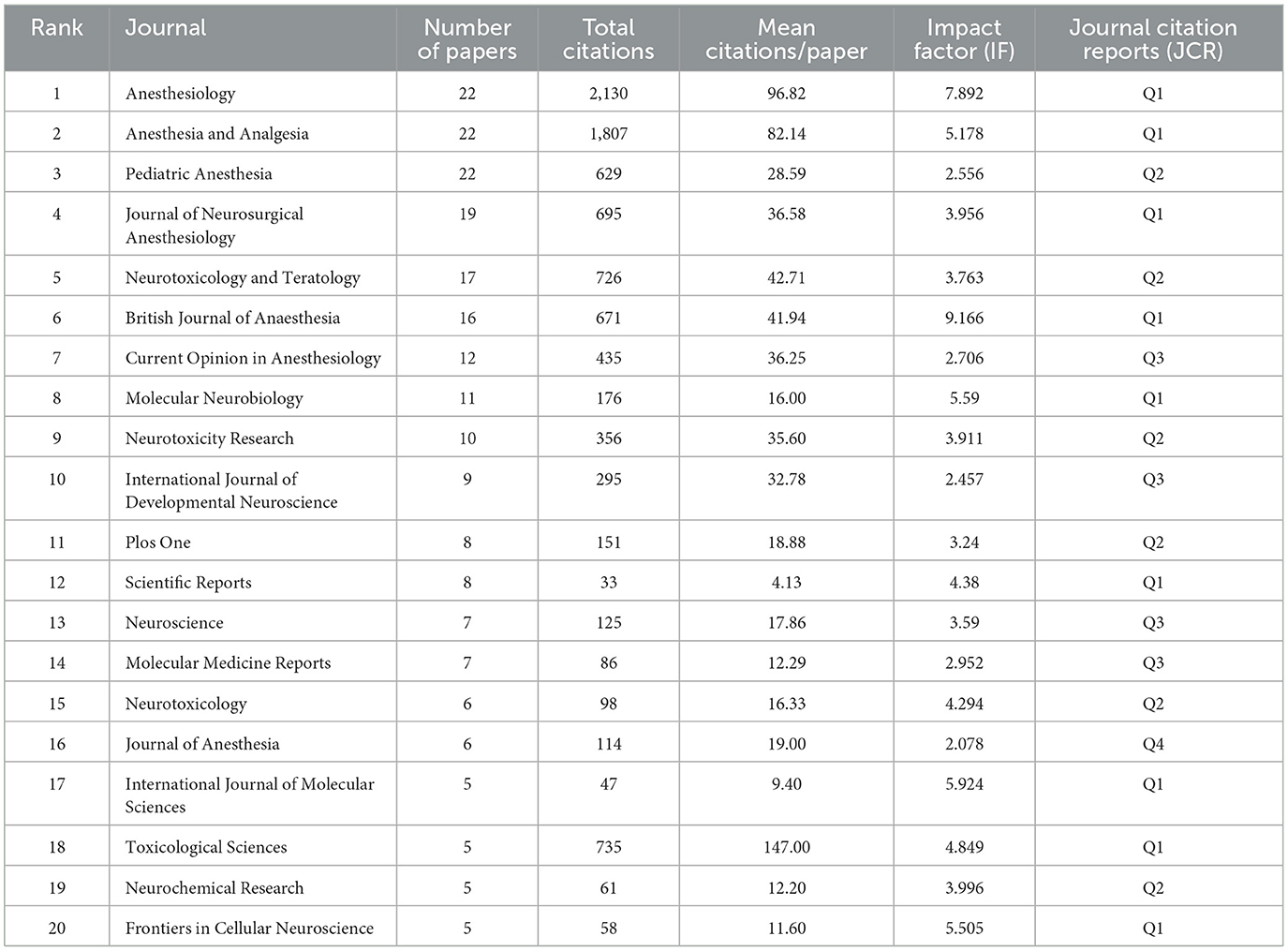
Table 4. The top 20 journals with the highest number of publications on the neurotoxicity of anesthesia in the developing brain.
We calculated the annual number of publications in the top 10 journals. No publications in this field were found in the top 10 journals prior to 2005. The highest number of papers were published in the top 10 journals in 2017, and since then, there has been a general decline in that number (Figure 4).
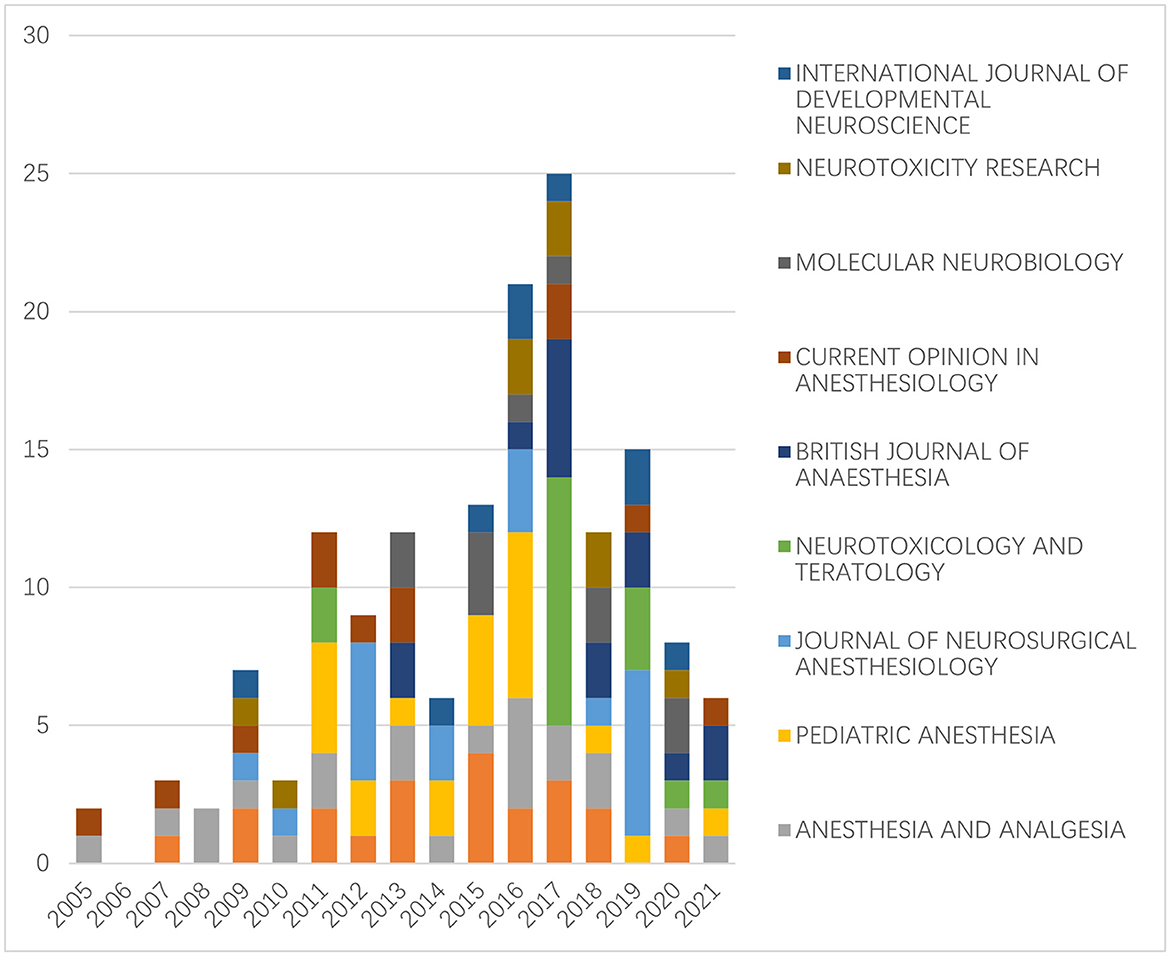
Figure 4. The number of articles on the neurotoxicity of anesthesia in the developing brain published in the top 10 journals each year (no publications in this field in the top 10 journals prior to 2005).
We compiled statistics for each discipline that had articles on anesthetic neurotoxicity in the developing brain after analyzing all disciplines that had such articles. The two disciplines with the largest percentages were, unsurprisingly, neuroscience and anesthesiology, both at 21%, due to our research themes. The above 2 disciplines accounted for nearly half, as shown in Figure 5.
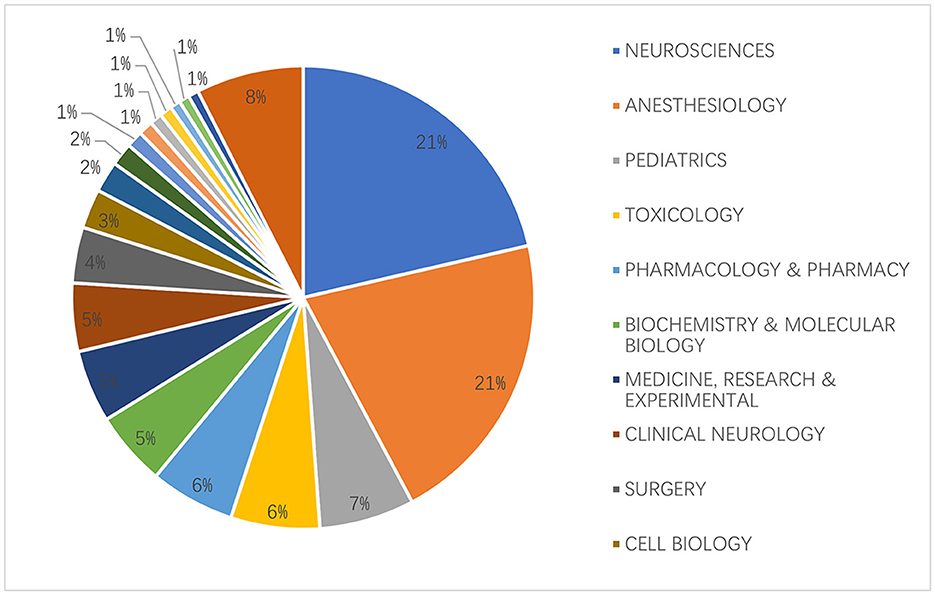
Figure 5. The top 20 subjects related to the neurotoxicity of anesthesia in the developing brain are distributed.
Table 5 lists the top 10 funding institutions, with the first four being the US Department of Health Human Services, National Natural Science Foundation of China, NIH National Institute of Medical Sciences, and NIH Eunice Kennedy Shriver National Institute of Child Health Human Development. The top four funding agencies contributed to 82.5% of the publications, and more than 300 articles were funded. Overall, six of these organizations were headquartered in the US, two in Japan, one in China, and one in Europe.
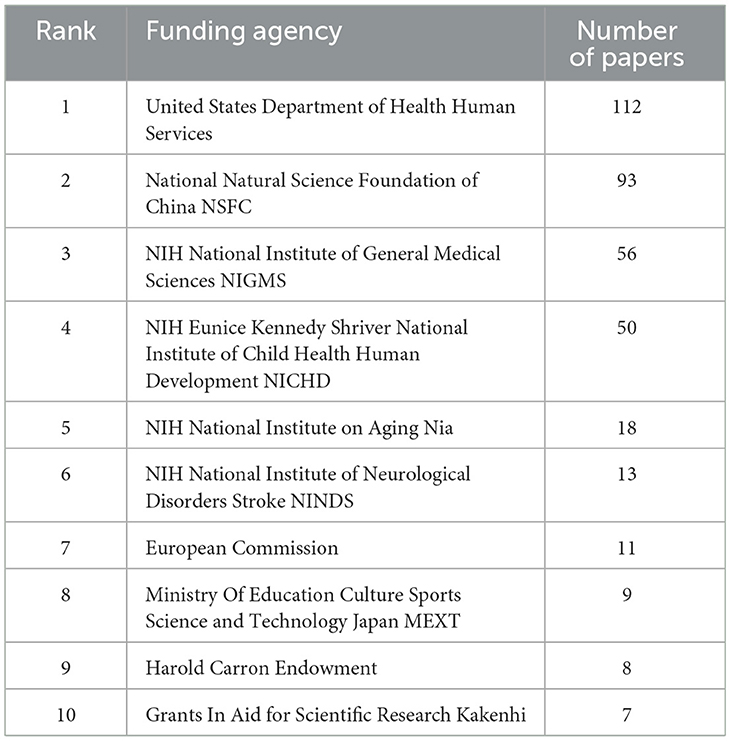
Table 5. The top 10 funding agencies with the highest number of publications on the neurotoxicity of anesthesia in the developing brain.
The top 20 cited publications in the area of neurotoxicity of anesthesia in the developing brain have been listed in Table 6, with quotation counts ranging from 149 to 503. Figures 6A, B make the types of these articles extremely clear. Among these, five were reviews (n = 5, 25%), and the remaining 15 articles were original research (n = 15, 75%). Original research included one conference report, five clinical studies, and nine basic studies. Four of the five clinical studies were retrospective and only one prospective study, which was also the most cited article, written by Davidson AJ et al., published by Lancet-Journal of the Murdoch Childrens Res Inst in 2016, titled “Neurodevelopmental outcome at 2 years of age after general anesthesia and awake-regional anesthesia in infancy (GAS): an international multicentre, randomized controlled trial.” The final conclusion of this trial was that awake regional anesthesia and sevoflurane anesthesia in infancy are equivalent in terms of neurodevelopmental outcomes at 2 years of age. Other retrospective research expressed extraordinary findings; there was an affiliation between early childhood exposure to anesthesia and an elevated danger of bad neurodevelopmental outcomes. While this association was consistent with preclinical animal data, it may also be explained by confounding effects of surgery, pathology, or basic diseases. Four of the nine basic studies discussed ketamine's characteristics. In conclusion, neonatal rhesus monkeys receiving ketamine for 3 h did not exhibit substantial neurotoxic effects; however, intake of the drug for 9 h or longer, such as for 24 h, was linked to brain cell death. The remaining five fundamental studies all mentioned isoflurane. Four investigations showed that exposure to 0.75% isoflurane for 6 h, either alone or in combination with other gases, induced neurotoxicity. Another study impaired memory by providing 1.7% isoflurane for 35 min each day for 4 days.
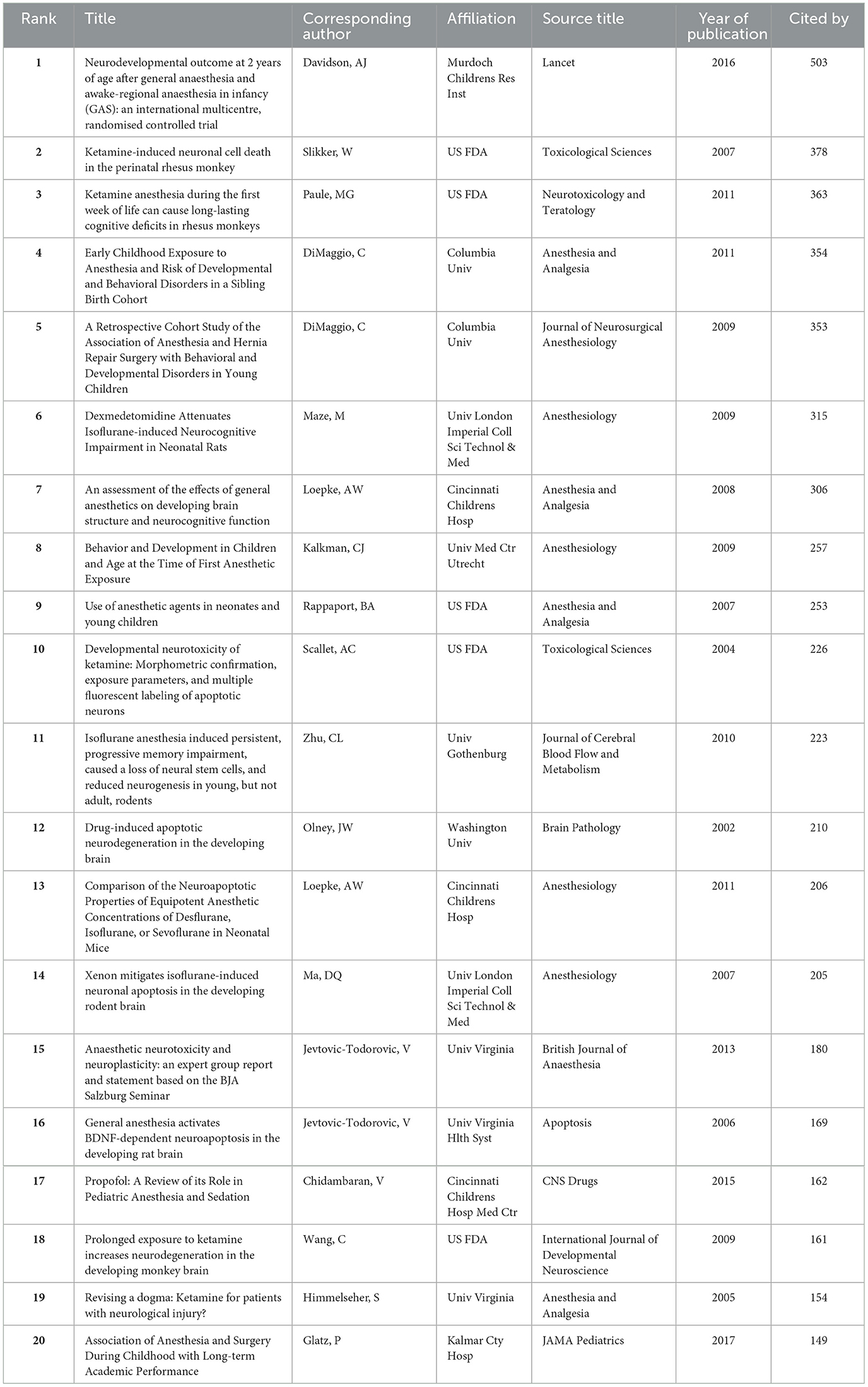
Table 6. The top 20 highest-cited articles on the neurotoxicity of anesthesia in the developing brain.
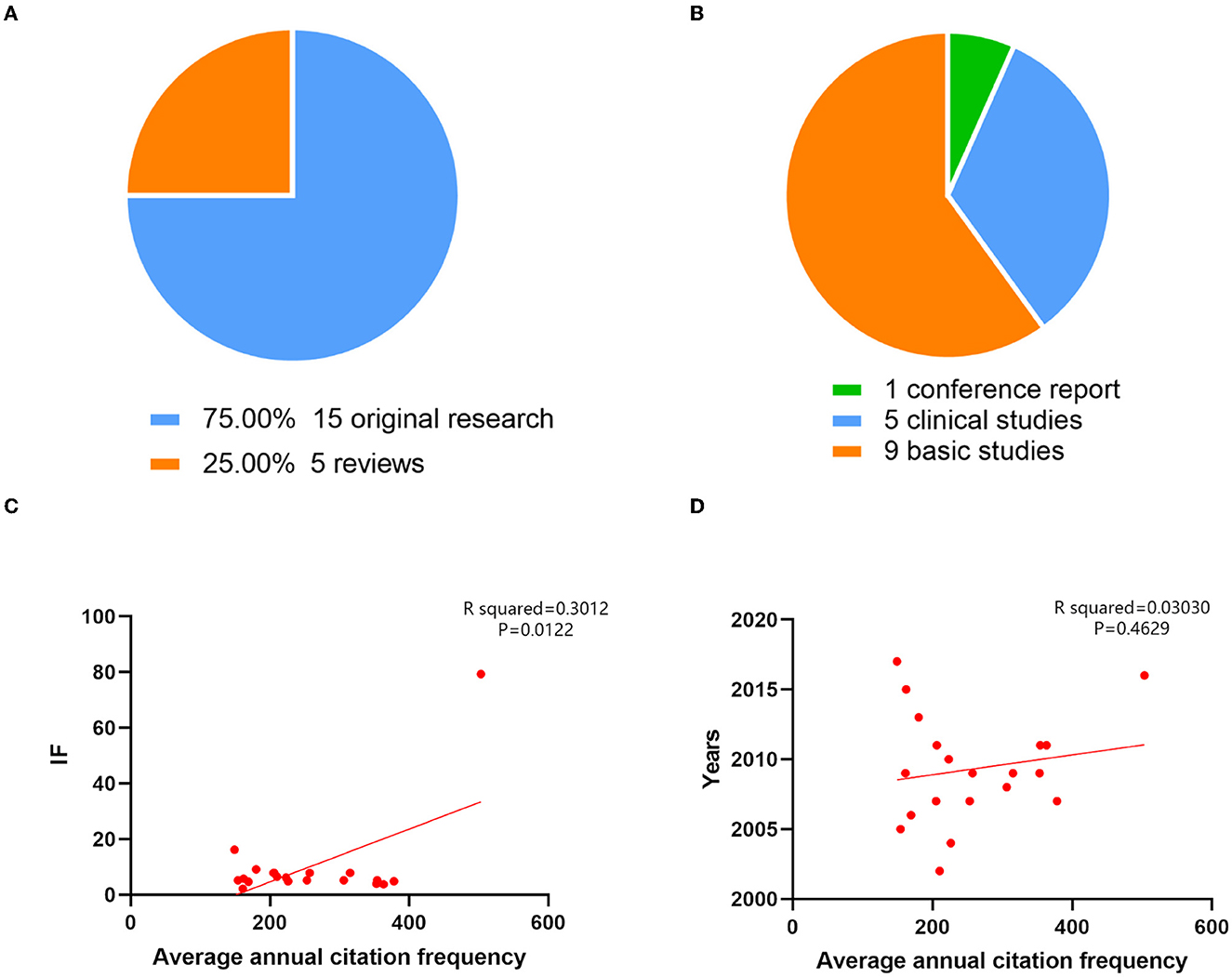
Figure 6. The top 20 cited articles on the neurotoxicity of anesthesia in the developing brain were categorized, and their correlations were examined. (A) The article types of the top 20 cited articles on the neurotoxicity of anesthesia in the developing brain. (B) The proportion of different article types in original research on the neurotoxicity of anesthesia in the developing brain. (C) Based on the correlation analysis, there was a high relationship between the average annual number of citations and the impact factor (R squared = 0.3012; P = 0.0122). (D) The average annual number of citations and the year of the analysis were unrelated (R squared = 0.03030; P = 0.4629).
Additionally, we examined the relationship between the average number of citations, year of publication, and impact factors of the 20 most cited journals. There was no connection between the average number of citations and the year of publication (R2= 0.03030, P = 0.4629). The mean number of citations and the impact factor did, however, significantly correlate with one another (R2 = 0.3012, P = 0.0122) (Figures 6C, D).
Keywords are the core of an article and reflect the topic of the article; by analyzing the keywords, you can learn about the current hotspots being researched in the field. We generated a keyword co-occurrence network through CiteSpace, in which the larger the node, the more frequently the node co-occurs with the central node, which additionally suggests a nearer relationship between the two. We anticipated that the research hotspots on anesthetic neurotoxicity in the developing brain would differ depending on clinical investigations and basic research; therefore, we analyzed them individually. In Figure 7A, we learned that clinical studies were more focused on the end result: “cognition,” “attention,” “neurocognitive outcomes,” “attention-deficit/hyperact,” and “cognitive impairment” were the keywords that appear more frequently. The keywords for basic research were more complex relatively: “synaptogenesis,” “apoptosis,” “signaling pathway,” “NMDA,” “inflammation,” and “autophagy” appeared more frequently in Figure 7B, indicating that the basic trials focused mainly on the exploration of mechanisms.
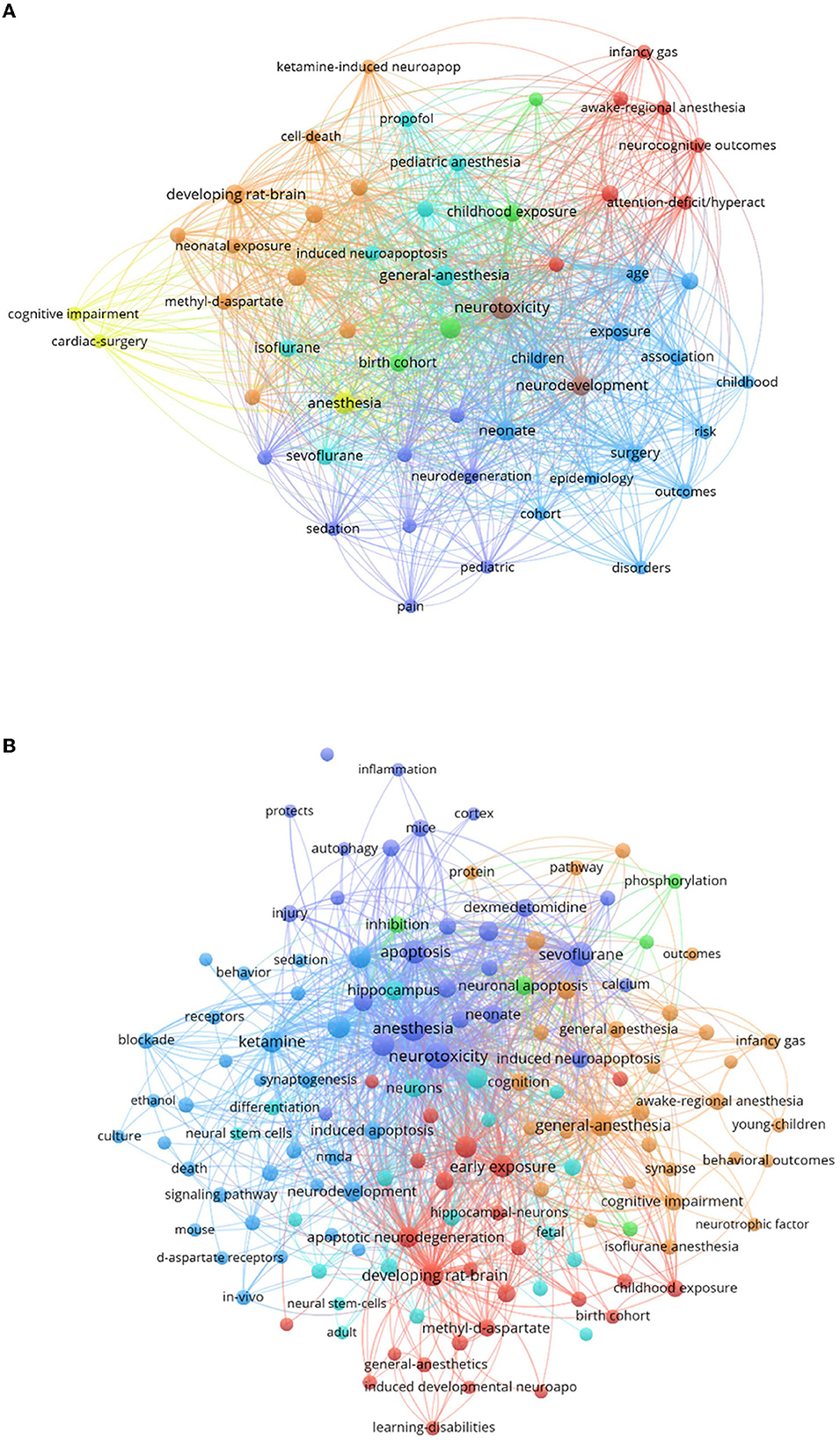
Figure 7. A summary of the hotspot trends of articles on the neurotoxicity of anesthesia in the developing brain. The diameter of the circles and the thickness of the lines show how frequently certain keywords occur together. (A) Clinical research hotspots of articles published on the neurotoxicity of anesthesia in the developing brain. (B) Basic research hotspots of articles published on the neurotoxicity of anesthesia in the developing brain.
The neurotoxicity consequences of anesthetic exposure on the developing brain have been one of the current research hotspots and numerous articles were published in the past decades. Several preclinical studies on the neurotoxicity of anesthesia indicated that exposure to general anesthesia may lead to structural and functional abnormalities in the brain (12). Most general anesthetics have these adverse effects, particularly ketamine and isoflurane, as reflected in this article. As a result, serious questions about the security of young children undergoing frequent or prolonged anesthesia have been raised and the US Food and Drug Administration has released a safety notification in this regard (13, 14). On the applicability of the findings of basic research trials to humans, however, expert opinion is still divided. As anesthesia may adversely affect millions of children requiring surgery every year, more research is required to fully understand the dangers of anesthetic neurotoxicity. Here, 414 articles were solicited from the Science Citation Index extended database, and through a bibliometric analysis, we gave a brief overview of developments in the study of the neurotoxicity of anesthesia on the developing brain.
Overall, research in the area of neurotoxicity of anesthesia to the developing brain culminated in 2017, with a decreasing trend thereafter. This may be due to the fact that as research into anesthetics continues, drugs that are clearly neurotoxic are no longer clinically used in anesthesia and are, therefore, no longer studied in this area.
The US has the most publications and citations among these nations, with China ranking second after the US. The United States has become recognized as an authority in the field thanks to the study of experts such as Andreas W. Loepke, Wang Cheng, Jevtovic-Todorovic Vesna, Bai Xiaowen, and Richard J. Levy. Andreas W. Loepke appears to have undergone the most extensive research, as evidenced by the highest H-index. The position of China is mainly supported by experts, such as Jiang Hong, Liu Hongtao, Zhao Ping, and Zhang Jun.
In terms of journals, most of the articles in the field of neurotoxicity of anesthesia on brain development are published in leading journals in the field of anesthesia, such as Anesthesiology and Anesthesia and Analgesia. This shows that the topic we are studying is of great importance in the field of anesthesia. The results of the hotspot analysis probably reflect the fact that clinical studies have focused on whether early exposure to anesthesia affects later cognition, behavior, and attention, whereas basic research has focused more on how anesthesia causes neurotoxicity. There are currently no uniform findings on any of these. We discovered through statistical analysis that the fields of neuroscience and anesthesiology publish more articles on the neurotoxicity of anesthesia in the developing brain. It could be the cause that the majority of fundamental studies are published in journals connected to neuroscience, while many clinical studies are published in journals linked to anesthesia.
A large number of clinical and preclinical studies have been motivated by the FDA's warnings. Conducting prospective, randomized studies on human infants and children without confounding factors is challenging due to ethical and technical issues. Three clinical investigations are currently more well-known than the others. One of these is the General and Spinal Anesthesia (GAS) study, which is the study conducted by the most cited article of the last 20 years mentioned in this article. The study concluded that there was no proof that early single exposure of kiddies to sevoflurane for <1 h elevated the hazard of altered neurodevelopmental consequences at 2 years of age in contrast with awake regional anesthesia (15). The GAS study reported in 2019 increased the age of the study to 5 years and still found no neurodevelopmental alterations (16). Another trial called the Pediatric Anesthesia and Neurodevelopmental Assessment (PANDA) similarly concluded that young children who received one anesthesia before 36 months were not significantly different from those who did not receive anesthesia in terms of intelligence (4). The primary outcome of the significant study called Mayo Anesthesia Safety in Kids (MASK) also showed that exposure to general anesthesia before the age of 3 years was not associated with significant differences in general cognitive ability as quantified by Full Scale IQ score, relative to unexposed children. However, the secondary outcomes of this study showed that multiple, but not single, exposures were associated with modest decreases in processing speed and fine motor coordination (17). Our focus has changed since the MASK study from “single exposure” to “multiple exposure,” which is a significant advancement. These three studies demonstrate that there is no proof in favor of the hypothesis that one or more anesthetic exposures during early infancy lower intelligence quotient levels in adulthood. However, a recent national population-based cohort study, comparing 11,457 children under 2 years of age who received general anesthesia with 22,914 children who did not, showed that receiving anesthesia early (before 2 years of age) may increase developmental delay; both increased anesthesia frequency and longer overall anesthesia duration increased this risk (18). It also reminds us that not only the type of anesthesia but also the frequency and duration of anesthesia are crucial. Recent clinical research outcomes are still contradictory.
Preclinical studies were a major player in general anesthetic toxicity studies, which have mostly been conducted on rodent models. There is uncertainty as to whether the mechanisms observed in rodent models are clinically relevant (19). Rodents are genetically more distant from humans than non-human primates (NHPs) (20). However, studies using NHPs are limited due to their small numbers and high cost. From the analysis for basic study in this field, we could observe apoptosis, calcium, phosphorylation, synaptogenesis, neurotrophic factor, NMDA, d-aspartate receptors, methyl-d-aspartate, and neural stem cells were research hotspots from 2002 to 2021. Currently, commonly used anesthetics are thought to act through NMDA and GABA receptors, but the specific mechanisms by which anesthesia causes neurodevelopmental disorders are highly complex (21). The keywords that appear in the analysis of basic research hotspots are neuroinflammation, apoptosis, synaptogenesis, and autophagy, all of which are mechanisms of anesthesia-induced neurotoxicity. Many studies have explored specific signaling pathways. The mechanism most frequently cited when discussing anesthesia-induced neurodevelopmental disorders is neuroinflammation. Microglia are the main mediators of neuroinflammation in the brain, and they can polarize into M1 types, which are harmful to neurons, and M2 types, which contribute to tissue repair (22). Sevoflurane may enhance M1 polarization while inhibiting M2 activation (23). It has been suggested that sevoflurane and isoflurane may increase interleukin-6 (IL-6) levels through the nuclear factor-κB (NF-κB) signaling pathway, thereby inducing neuroinflammation and cognitive impairment, and that inhibition of the NF-κB signaling pathway may rescue sevoflurane-induced inflammation and reduce cognitive dysfunction in adulthood (24, 25). Apoptosis is the active, orderly death of cells under certain physiological or pathological conditions, controlled by genes (26). It has been demonstrated that general anesthesia may cause widespread apoptotic neuronal death in the developing brain of baby mice, rats, and rhesus monkeys (27, 28). Early exposure to general anesthetics may impair neuronal plasticity, according to several animal studies (29–33). Autophagy is a double-edged sword, with some studies showing that autophagy causes neurotoxicity, while others confirm its neuroprotective effects. The specific effects of autophagy need to be further investigated (34). Other mechanisms include oxidative stress and iron death (34), which are not represented in the basic research hotspot analysis map, probably due to their low number of studies at present. For specific signaling pathways, Jiaojiao Wang et al. have reviewed the current signaling pathways for postoperative neurotoxicity in neonates due to general anesthesia, such as HIPK2/Akt/mTOR, PI3K/Akt, and JAK/STAT, with a view to providing an important reference for the treatment of clinical disease (35).
In particular, the large circle for “sevoflurane” in the base hotspot clustering diagram indicates that there have been a lot of preclinical studies carried out on it. This may be due to the low solubility of sevoflurane in blood gas and its relatively rapid onset of action and the fact that it is currently the most commonly used inhalation anesthetic in clinical pediatrics and researchers are anxious to know its safety. Repeated or prolonged exposure to sevoflurane has been shown in several animal studies to cause neurotoxicity in the developing brain (28, 36, 37), but the results of clinical studies are not always consistent. As with other anesthetics, the potential mechanisms by which sevoflurane causes neurotoxicity are controversial. A recent review specifically summarized its possible mechanisms, including nerve cell damage and death, tau protein phosphorylation, and neuroendocrine abnormalities (38).
On the one hand, it is known from current studies that the probability of a single, short exposure to anesthesia causing neurotoxicity is particularly small, and future clinical research will be interested in the dose, duration, and frequency of anesthetic exposure. On the other hand, animal experiments are relatively easy to carry out, but it will be worth thinking about how to select animal models, whether the mechanisms observed in animal models are clinically relevant, and how to translate preclinical results into clinical practice.
Our bibliometric analysis also had some limitations. First, our search was conducted in June 2022 and relevant articles published after that date were not included in this study. Second, the earlier the article was published, the more citations it received. The “top 20 most cited articles” did not include all authoritative articles that have been published more recently. This is a result of insufficient citations. Although the correlation between the years of publication and the number of citations of the top 20 articles shows that there is no correlation between the two, time is still a factor to be considered. Finally, there is no doubt that journals with high-impact factors are more favored and that articles published in them are more likely to be cited by other articles, suggesting that high-impact factors inherently lead to bias.
We looked through and evaluated 414 English-language articles on the subject of the neurotoxicity of anesthesia in the developing brain. Although our study has some limitations, it is still meaningful. Our study found that research in this area peaked in 2017. Various countries, led by the United States and China, are exploring and researching this area. Current clinical studies are mainly retrospective, and in the future, we should place more emphasis on prospective, multicenter, long-term follow-up clinical studies. More basic research is also needed on the neurotoxic mechanisms of anesthesia in the developing brain.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
TL and HM conceived and designed the structure of this manuscript and revised the manuscript. YC, YS, KY, XL, DG, and JY analyzed and wrote the manuscript. All authors contributed to the article and approved the submitted version.
HM received grants from the Natural Science Foundation of Beijing (7212023) and the National Natural Science Foundation of China (82071180). TL received grants from the National Natural Science Foundation of China (82271206).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Yang F, Zhao H, Zhang K, Wu X, Liu H. Research progress and treatment strategies for anesthetic neurotoxicity. Brain Res Bull. (2020) 164:37–44. doi: 10.1016/j.brainresbull.2020.08.003
2. Chen C, Shen FY, Zhao X, Zhou T, Xu DJ, Wang ZR, et al. Low-dose sevoflurane promotes hippocampal neurogenesis and facilitates the development of dentate gyrus-dependent learning in neonatal rats. ASN Neuro. (2015) 7:2. doi: 10.1177/1759091415575845
3. Hansen TG. Anesthesia-related neurotoxicity and the developing animal brain is not a significant problem in children. Paediatr Anaesth. (2015) 25:65–72. doi: 10.1111/pan.12548
4. Sun LS Li G, Miller TL, Salorio C, Byrne MW, Bellinger DC, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. (2016) 315:2312–20. doi: 10.1001/jama.2016.6967
5. Hu D, Flick RP, Zaccariello MJ, Colligan RC, Katusic SK, Schroeder DR, et al. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology. (2017) 127:227–40. doi: 10.1097/ALN.0000000000001735
6. Ing C, Ma X, Sun M, Lu Y, Wall MM, Olfson M, et al. Exposure to surgery and anesthesia in early childhood and subsequent use of attention deficit hyperactivity disorder medications. Anesth Analg. (2020) 131:723–33. doi: 10.1213/ANE.0000000000004619
7. Zaccariello MJ, Frank RD, Lee M, Kirsch AC, Schroeder DR, Hanson AC, et al. Patterns of neuropsychological changes after general anaesthesia in young children: secondary analysis of the Mayo Anesthesia Safety in Kids study. Br J Anaesth. (2019) 122:671–81. doi: 10.1016/j.bja.2019.01.022
8. Li R, Wang B, Cao X, Li C, Hu Y, Yan D, et al. Sevoflurane exposure in the developing brain induces hyperactivity, anxiety-free, and enhancement of memory consolidation in mice. Front Aging Neurosci. (2022) 14:934230. doi: 10.3389/fnagi.2022.934230
9. Liao Y, Wang L, Liu F, Zhou Y, Lin X, Zhao Z, et al. Emerging trends and hotspots in metabolic dysfunction-associated fatty liver disease (MAFLD) research from 2012 to 2021: A bibliometric analysis. Front Endocrinol (Lausanne). (2023) 14:1078149. doi: 10.3389/fendo.2023.1078149
10. Mao M, Zhou Y, Jiao Y, Yin S, Cheung C, Yu W, et al. Bibliometric and visual analysis of research on the links between the gut microbiota and pain from 2002 to (2021). Front Med (Lausanne). (2022) 9:975376. doi: 10.3389/fmed.2022.975376
11. Zhou Y, Lin X, Yin S, Zhu L, Yang Y, Li Y, et al. Emerging trends and hot spots in hepatic glycolipid metabolism research from 2002 to 2021: a bibliometric analysis. Front Nutr. (2022) 9:933211. doi: 10.3389/fnut.2022.933211
12. Lin EP, Lee JR, Lee CS, Deng M, Loepke AW. Do anesthetics harm the developing human brain? An integrative analysis of animal and human studies. Neurotoxicol Teratol. (2017) 60:117–28. doi: 10.1016/j.ntt.2016.10.008
13. Communication, FDS. FDA Review Results in New Warnings About Using General Anesthetics and Sedation Drugs in Young Children and Pregnant Women. Available online at: https://www.fda.gov/Drugs/DrugSafety/ucm532356.htm (accessed January 2, 2018).
14. Communication. FDS, FDA Approves Label Changes for Use of General Anesthetic and Sedation Drugs in Young Children. Available online at: https://www.fda.gov/Drugs/DrugSafety/ucm554634.htm (accessed January 2, 2018).
15. Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. (2016) 387:239–50. doi: 10.1016/S0140-6736(15)00608-X
16. McCann ME, de Graaff JC, Dorris L, Disma N, Withington D, Bell G, et al. Neurodevelopmental outcome at 5 years of age after general anaesthesia or awake-regional anaesthesia in infancy (GAS): an international, multicentre, randomised, controlled equivalence trial. Lancet. (2019) 393:664–77. doi: 10.1016/S0140-6736(18)32485-1
17. Warner DO, Zaccariello MJ, Katusic SK, Schroeder DR, Hanson AC, Schulte PJ, et al. Neuropsychological and behavioral outcomes after exposure of young children to procedures requiring general anesthesia: the mayo anesthesia safety in kids (MASK) study. Anesthesiology. (2018) 129:89–105. doi: 10.1097/ALN.0000000000002232
18. Feng YP, Yang TS, Chung CH, Chien WC, Wong CS. Early childhood general anesthesia exposure associated with later developmental delay: A national population-based cohort study. PLoS ONE. (2020) 15:e0238289. doi: 10.1371/journal.pone.0238289
19. Salaun JP, Poirel N, Dahmani S, Chagnot A, Gakuba C, Ali C, et al. Preventing the long-term effects of general anesthesia on the developing brain: how translational research can contribute. Neuroscience. (2021) 461:172–9. doi: 10.1016/j.neuroscience.2021.02.029
20. Wang C, Zhang X, Liu F. Application of advanced preclinical models and methods in anesthetic neurotoxicity research. Neurotoxicol Teratol. (2017) 61:1–6. doi: 10.1016/j.ntt.2017.04.001
21. Rappaport B, Mellon RD, Simone A, Woodcock J. Defining safe use of anesthesia in children. N Engl J Med. (2011) 364:1387–90. doi: 10.1056/NEJMp1102155
22. Tang Y, Le W. Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol. (2016) 53:1181–94. doi: 10.1007/s12035-014-9070-5
23. Pei Z, Wang S, Li Q. Sevoflurane suppresses microglial M2 polarization. Neurosci Lett. (2017) 655:160–5. doi: 10.1016/j.neulet.2017.07.001
24. Dai J, Li X, Wang C, Gu S, Dai L, Zhang J, et al. Repeated neonatal sevoflurane induced neurocognitive impairment through NF-kappaB-mediated pyroptosis. J Neuroinflammation. (2021) 18:180. doi: 10.1186/s12974-021-02233-9
25. Zhang L, Zhang J, Yang L, Dong Y, Zhang Y, Xie Z. Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factor-kappa B pathway in neuroglioma cells. Br J Anaesth. (2013) 110:i82–91. doi: 10.1093/bja/aet115
26. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. (2007) 35:495–516. doi: 10.1080/01926230701320337
27. Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. (2010) 112:834–41. doi: 10.1097/ALN.0b013e3181d049cd
28. Raper J, De Biasio JC, Murphy KL, Alvarado MC, Baxter MG. Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. (2018) 120:761–7. doi: 10.1016/j.bja.2018.01.014
29. Li H, Dai CL, Gu JH, Peng S, Li J, Yu Q, et al. Intranasal administration of insulin reduces chronic behavioral abnormality and neuronal apoptosis induced by general anesthesia in neonatal mice. Front Neurosci. (2019) 13:706. doi: 10.3389/fnins.2019.00706
30. Pallas-Bazarra N, Jurado-Arjona J, Navarrete M, Esteban JA, Hernandez F, Avila J, et al. Novel function of Tau in regulating the effects of external stimuli on adult hippocampal neurogenesis. EMBO J. (2016) 35:1417–36. doi: 10.15252/embj.201593518
31. Sasaki Russell JM, Chinn GA, Maharjan D, Eichbaum Y, Sall JW. Female rats are more vulnerable to lasting cognitive impairment after isoflurane exposure on postnatal day 4 than 7. Br J Anaesth. (2019) 122:490–9. doi: 10.1016/j.bja.2018.12.008
32. Schaefer ML, Perez PJ, Wang M, Gray C, Krall C, Sun X, et al. Neonatal isoflurane anesthesia or disruption of postsynaptic density-95 protein interactions change dendritic spine densities and cognitive function in juvenile mice. Anesthesiology. (2020) 133:812–23. doi: 10.1097/ALN.0000000000003482
33. Wan J, Shen CM, Wang Y, Wu QZ, Wang YL, et al. Repeated exposure to propofol in the neonatal period impairs hippocampal synaptic plasticity and the recognition function of rats in adulthood. Brain Res Bull. (2021) 169:63–72. doi: 10.1016/j.brainresbull.2021.01.007
34. Niu Y, Yan J, Jiang H. Anesthesia and developing brain: what have we learned from recent studies. Front Mol Neurosci. (2022) 15:1017578. doi: 10.3389/fnmol.2022.1017578
35. Wang J, Liu Z. Research progress on molecular mechanisms of general anesthetic-induced neurotoxicity and cognitive impairment in the developing brain. Front Neurol. (2022) 13:1065976. doi: 10.3389/fneur.2022.1065976
36. Dai CL Li H, Hu X, Zhang J, Liu F, Iqbal K, et al. Neonatal exposure to anesthesia leads to cognitive deficits in old age: prevention with intranasal administration of insulin in mice. Neurotox Res. (2020) 38:299–311. doi: 10.1007/s12640-020-00223-y
37. Xie L, Liu Y, Hu Y, Wang B, Zhu Z, Jiang Y, et al. Neonatal sevoflurane exposure induces impulsive behavioral deficit through disrupting excitatory neurons in the medial prefrontal cortex in mice. Transl Psychiatry. (2020) 10:202. doi: 10.1038/s41398-020-00884-5
Keywords: neurotoxicity, anesthesia, developing brain, bibliometric analysis, hotspots
Citation: Cao Y, Sun Y, Liu X, Yu K, Gao D, Yang J, Miao H and Li T (2023) A bibliometric analysis of the neurotoxicity of anesthesia in the developing brain from 2002 to 2021. Front. Neurol. 14:1185900. doi: 10.3389/fneur.2023.1185900
Received: 14 March 2023; Accepted: 04 April 2023;
Published: 27 April 2023.
Edited by:
Hong Ni, Children's Hospital of Soochow University, ChinaReviewed by:
Huang Shiqian, Huazhong University of Science and Technology, ChinaCopyright © 2023 Cao, Sun, Liu, Yu, Gao, Yang, Miao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianzuo Li, bGl0ekBianNqdGguY24=; Huihui Miao, aXZlcnltaGhAaG90bWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.