
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 17 May 2023
Sec. Stroke
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1181001
This article is part of the Research Topic The Role, Pathophysiology, and Clinical Benefit of Collateral Circulation in Acute and Chronic Ischemic Stroke View all 7 articles
Introduction: Cerebral collateral circulation has a central role in ischemic stroke pathophysiology, and it is considered to correlate with infarct size, the success of reperfusion therapies, and clinical outcomes. Our aim was to study the factors influencing the development of collaterals in patients with acute ischemic stroke eligible for endovascular treatment.
Materials and methods: We enrolled patients with acute ischemic stroke and large vessel occlusion of anterior circulation potentially eligible for endovascular treatment. Included patients performed multiphase CT angiography to assess collaterals that were graded by the Menon Grading Score. We investigated the associations between clinical factors and collaterals and tested independent associations with logistic (good vs. poor collaterals) and ordinal (collateral grade grouped, Menon 0–2, 3, 4–5) regression analysis adjusting for age, sex, stroke severity, and onset to CT time (OCTT).
Results: We included 520 patients, the mean age was 75 (±13.6) years, 215 (41%) were men, and the median (IQR) NIHSS was 17 (11–22). Good collaterals were present in 323 (62%) patients and were associated with lower NIHSS (median 16 vs. 18; p < 0.001) and left hemisphere involvement (60% vs. 45%; p < 0.001), whereas previous stroke/TIA was more frequent in patients with poor collaterals (17 vs. 26%; p = 0.014). These results were confirmed in both logistic and ordinal regression analyses where good collaterals were associated with lower NIHSS (OR = 0.94; 95% CI = 0.91–0.96; cOR = 0.95; 95% CI = 0.92–0.97, respectively) and left hemisphere stroke (OR = 2.24; 95% CI = 1.52–3.28; cOR = 2.11; 95% CI = 1.46–3.05, respectively), while previous stroke/TIA was associated with poor collaterals (OR = 0.57; 95% CI = 0.36–0.90; cOR = 0.61; 95% CI = 0.40–0.94, respectively). Vascular risk factors, demographics, and pre-stroke treatments did not influence the collateral score.
Discussion: The results of our study suggest that risk factors and demographics do not influence the development of collateral circles, except for a negative relation with previous ischemic events. We confirm an already reported observation of a possible protective effect of collaterals on tissue damage assuming NIHSS as its surrogate. The association between left hemispheric stroke and better collaterals deserves to be further explored. Further efforts are needed to identify the factors that favor the development of collaterals.
Endovascular treatment (EVT) of acute ischemic stroke (AIS) proved to be dramatically effective in reducing the disability and mortality of patients. (1) Reperfusion of the ischemic but still viable tissue, namely ischemic penumbra, and reducing the growth of the irreversibly ischemic tissue (i.e., ischemic core) are the objectives of acute stroke therapy. Several metabolic and genetic factors as well as pre-existing characteristics of the cerebral tissue may influence the extent of both the ischemic penumbra and ischemic core (2). In previous studies, leptomeningeal cerebral collaterals (CC) have been associated with lower infarct volume (3) and better clinical outcomes (4).
Cerebral collaterals (CC) have been classified into primary collaterals, which provide fast support to the ischemic area, and secondary collaterals, also constituted by leptomeningeal vessels, which require time to be supportive for the ischemic brain tissue. Pathophysiological factors leading to the development of CC are uncertain (5). The role of age (3, 6, 7), sex, site of occlusion, and cardiovascular risk factors, such as dyslipidemia and statin use as determinants of CC, has been investigated in several studies (3, 6–10). However, the reported results were often conflicting and not conclusive. Understanding determinants of CC might help to develop target therapeutic strategies to increase ischemic brain blood supply in the acute phase of stroke and consequently to improve the early prognosis of patients with AIS (8).
In patients with AIS eligible for EVT, we explored the associations between clinical factors and CC grade.
We conducted a single-center observational retrospective study. We enrolled patients admitted for AIS potentially eligible for EVT at the Stroke Unit of a Comprehensive Stroke Center University Hospital (Florence, Italy) between 2017 and 2022.
We included patients with middle cerebral artery (MCA) occlusion (M1 and/or M2) with (tandem occlusion) or without occlusion of the internal carotid artery (ICA). Only patients with available pre-treatment multiphase CT angiography (mCTA) were included. We excluded patients with hemorrhagic stroke, patients with vertebrobasilar ischemic stroke, and patients with ischemic stroke due to isolated ICA, M3 segment of MCA, or anterior cerebral artery occlusion.
We collected the following clinical data: demographic, anamnestic [hypertension, diabetes, dyslipidemia, smoking habit, previous stroke or transient ischemic attack (TIA), and atrial fibrillation], current medication at the time of stroke (i.e., statin, antithrombotic/anticoagulant, or antihypertensive drugs), and at the time of admission using the National Institutes of Health Stroke Scale (NIHSS). We also collected neuroradiological imaging data such as time from stroke onset (or last time seen well) to brain imaging and side of the involved cerebral hemisphere.
An mCTA with a 0.625 mm slice thickness in a three-time-point acquisition was performed from the aortic arch to the cranial vertex (phase 1) and from the skull base to the vertex (phase 2 and phase 3 after each 8 s delay) for 150–260 mAs radiation dose. A total of 50 ml of iodinated contrast was injected at 5 ml/s, followed by a 30-ml normal saline chase. mCTA provided identification of large vessel occlusion (LVO) and evaluation of CC according to the Menon Grading Score.
The latter was defined by two neuroradiologists (GB and EF) blinded to clinical data and classified according to a six-point ordinal scale described by Menon et al. (11) as reported in Figure 1 and Table 1 (11).
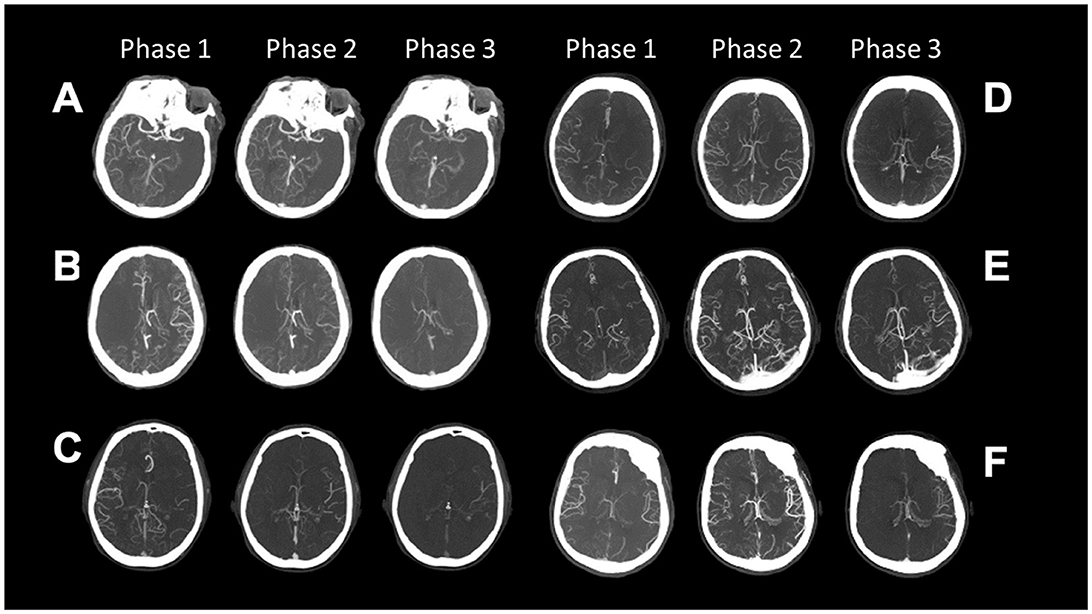
Figure 1. Collaterals on three phases of multiphase CT Angiography classified according to the Menon Grading Score (A–F) is Grade 0–5; (Table 1).
Cerebral collaterals (CCs) were then categorized into good (0–2), intermediate (3), and poor (4, 5). The predictive ability of clinical outcomes was categorized on a cutoff of 0–3 as poor and 4–5 as moderate-to-good CC.
To explore putative factors associated with CCs, we performed two separate analyses. In the first analysis, we divided the population into two groups: good collaterals (Menon grades 4–5) and poor collaterals (Menon grades 0–3). We described the general characteristics of the population and used Student's t-test, Mann–Whitney U-test, and Pearson's chi-square test, as appropriate, to test differences between groups. We examined univariate associations with a p-value of < 0.1 in a multivariable logistic regression model adjusting for age, sex, NIHSS, and onset to CT time (OCTT). In the second analysis, we categorized the population into three groups: poor collaterals (Menon grades 0–2), intermediate collaterals (Menon grade 3), and good collaterals (Menon grades 4–5). We examined univariate associations with a p-value of < 0.1 in a multivariable ordinal regression model adjusting for age, sex, NIHSS, and OCTT. For both multivariable analyses, we considered a p-value of <0.05 statistically significant. Statistical analysis was performed using SPSS for Windows (version 24.0; SPSS, IBM Corp., Armonk, NY). The data that support the findings of this study are available from the corresponding author upon reasonable request.
In the observation period, we included 520 patients with AIS, eligible for EVT. The general characteristics of the study population are shown in Table 2. The mean age was 75 (±13.6) years, and 215 (41%) were men. Median (IQR) NIHSS was 17 (11–22), and median (IQR) OCTT was 240 min (130–420). In total, 45 (8%) patients were treated with intravenous thrombolysis, 226 (44%) with EVT, 220 (42%) with both, and 29 (6%) were not treated with reperfusion therapy.
The distribution of vessel occlusions was as follows: 287 (55%) M1, 92 (18%) M2, 92 (18%) M1–M2, 49 (9%) tandem occlusion, and 283 (54%) patients had a stroke in the left hemisphere.
The distribution of CC status is shown in Figures 2a–c; most of the patients had good CC defined as Menon grades 4–5 (n = 323, 62%).
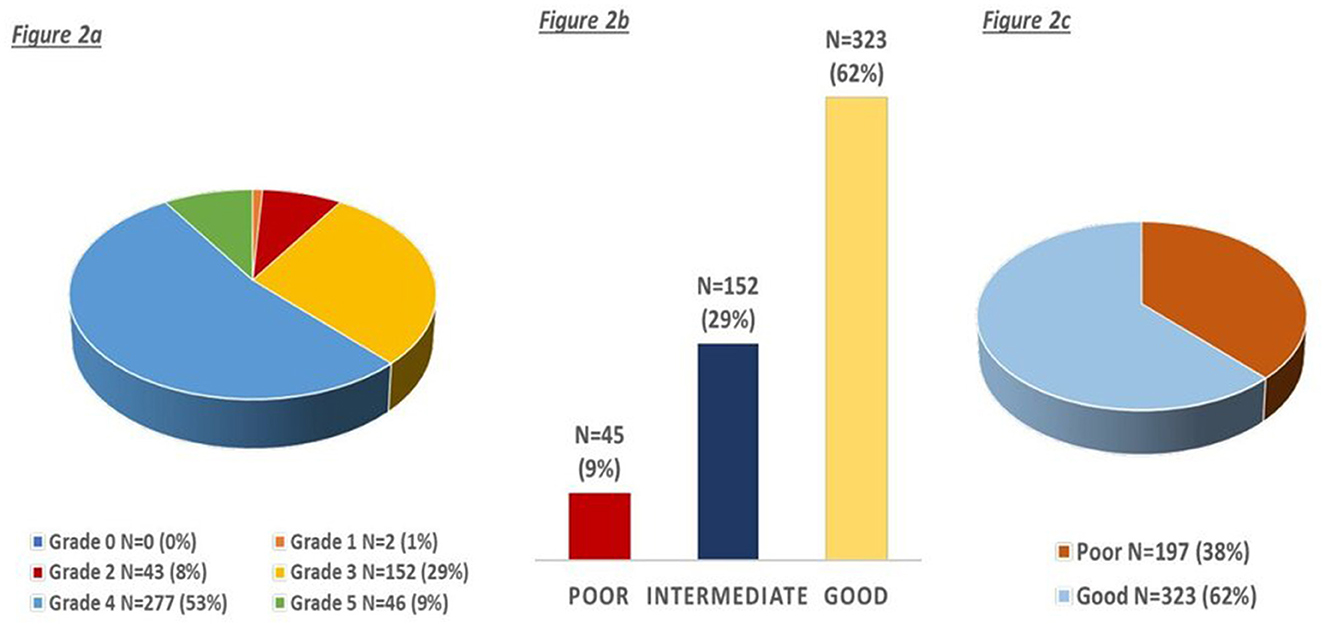
Figure 2. (a–c) Distribution of collaterals according to the ordinal and three-point menon grading score.
Patients with good CC had lower NIHSS (median 16 vs. 18; p < 0.001) and longer OCTT (median 260 min vs. 210 min; p = 0.019), had more frequent ischemia in the left hemisphere (60 vs. 45%; p < 0.001) but less frequently atrial fibrillation (32 vs. 42%; p = 0.036) and past stroke/TIA (17 vs. 26%; p = 0.014). There were no other differences in the general characteristics of the population. In the logistic regression analysis (Table 3), NIHSS (OR = 0.94; 95% CI = 0.91–0.96) and left hemisphere stroke (OR = 2.24; 95% CI = 1.52–3.28) were associated with good collaterals, whereas previous stroke/TIA was associated with reduced odds to have good collaterals (OR = 0.57; 95% CI = 0.36–0.90).
Considering the grouped Menon grade, 45 (9%) had poor collaterals, 152 (29%) had intermediate collaterals, and 323 (62%) had good collaterals (Figure 2b). We observed a trend toward higher age (mean 69, 78, 75; p < 0.001), lower NIHSS (median 19, 18, 16; p < 0.001), and higher OCTT (195, 210, 260; p = 0.05) for poor, intermediate, and good collaterals, respectively. Similarly, patients with left hemisphere stroke (49, 44, 60%; p = 0.004) had more frequently good collaterals, whereas with previous stroke/TIA (21%, 28%, 17%; p = 0.028) they had more frequently poor collaterals (Figure 3). In ordinal regression analysis (Table 4), NIHSS (cOR = 0.95; 95% CI = 0.92–0.97) and left hemisphere stroke (cOR = 2.11; 95% CI = 1.46–3.05) were associated with better odds to have good collaterals as opposed to previous stroke/TIA, which was associated with worse collaterals (cOR = 0.61; 95% CI = 0.40–0.94).
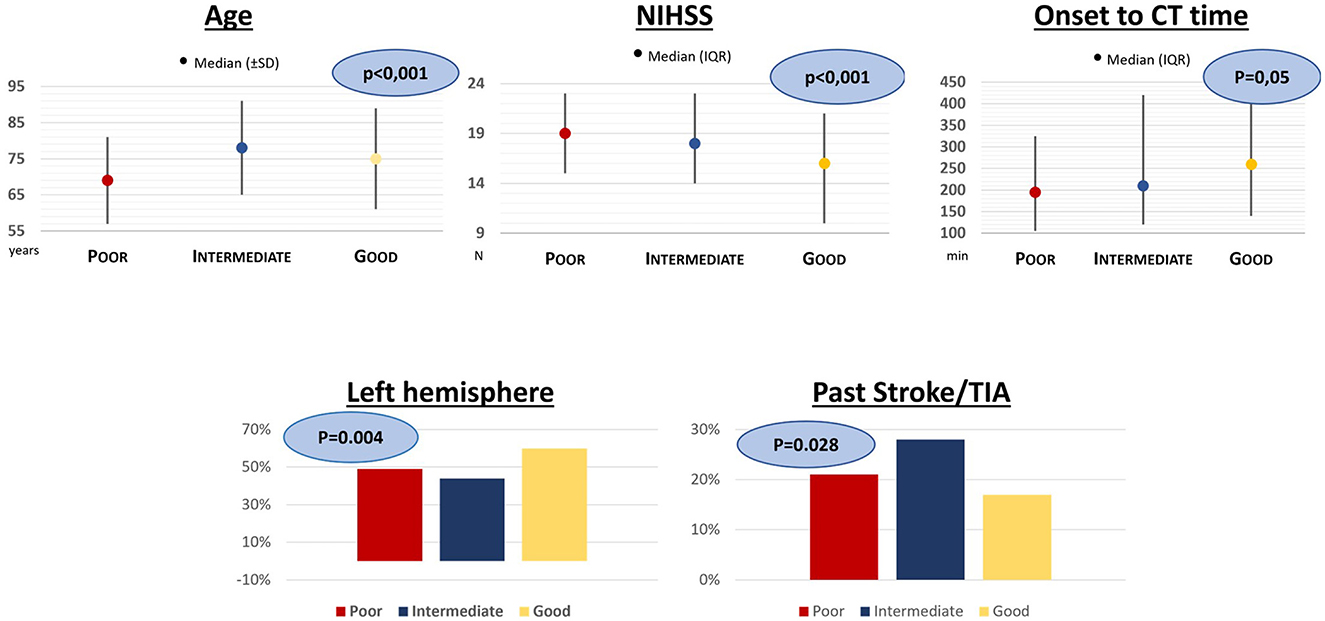
Figure 3. Results of univariate analysis for patients divided into three groups according to the Menon Grading Score.
From their first description (5), CCs have been increasingly studied and found to be associated with final infarct size and clinical outcomes after reperfusion therapy (1, 6, 13). The conceptual evolution from “time window” to “tissue window” for acute reperfusion therapy led to the investigation of the role of pre-treatment CC in the selection of patients eligible for therapies.
As previous studies were inconclusive and discordant, the aim of our study was to further explore determinants of CC that could be of help in better understanding their pathophysiology. The next step would have been to plan strategies that can increase CC with the final goal to reduce the extent of the ischemic lesion and widen the therapeutic window. Our study failed to find a relation between vascular risk factors or demographic factors and CC development. Indeed, main modifiable and non-modifiable risk factors such as age, hypertension, diabetes, smoking habit, or dyslipidemia do not seem to influence the development of CC, apart from past stroke/TIA, which was found to be negatively related to CC. The latter is one of the most frequently investigated risk factors in the literature (6, 9, 15–18) again with inconclusive results. A recent study found that previous stroke is associated with slower progression of cerebral infarct, and the authors assume that this could be due to cerebral ischemic preconditioning, an adaptive phenomenon in which brief exposure to ischemia and reperfusion markedly enhances the resilience of the brain to subsequent ischemic insults, inducing arteriogenesis and collateral growth (18). Indeed, the idea that an ischemic insult could reduce infarct growth was explored in preclinical and clinical studies, showing that remote ischemic conditioning could be a strategy for the treatment of acute ischemic stroke. Improvement of cardiac function, increase in CC, the protection of neurovascular units, the formation of gas molecules, and the effect on the function of vascular endothelial cells and the nervous system are among the hypothesized mechanisms (19). Our results are not in line with this hypothesis. However, a previous randomized controlled trial showed an increased risk of symptomatic intracranial hemorrhage in patients treated with r-TPA and CT signs of an old infarct explaining it with the concept of “brain frailty,” and they also demonstrated that this is independently related to poor functional outcome (20). These results were then confirmed by subsequent studies mainly focused on small vessel diseases, showing that “brain frailty”-related CT elements have a dose-dependent relationship with poor collaterals and further led to poor prognosis (21, 22). Moreover, a recent study evidenced that those elements combined with collaterals have a better value to predict the prognosis of patients with acute large artery atherosclerotic stroke (23). These findings led us to hypothesize that all of these elements could be related to determining brain plasticity impairment and consequently the capability of the brain to develop collateral vessels. This points out that it could be worth having more details on the type, site, and size of previous stroke with a properly designed study to better understand the direction of this relationship that could be of help in making therapeutic decisions.
In our study, we also explored the possibility that antihypertensive drugs such as alpha-lithics could reduce the vascular tone and influence CCs but we did not find any association. The same results were obtained considering other pre-stroke treatments (i.e., statins, anticoagulants, or antiplatelets). Of note, the study was not powered to explore such a point so these findings have to be considered as results generating hypothesis.
In keeping with previous studies, we found that lower stroke severity, assessed with NIHSS, was associated with better collateral status (3, 6). Although with some limits due to the ischemic side, higher NIHSS reflects larger ischemic tissue volume (8). Given that CC status has been previously associated with the extent of ischemic core (3), NIHSS may represent a surrogate of it (24). The association of NIHSS with CC seems consistent across studies, and we found a similar magnitude of the effect of NIHSS on collateral status compared with other studies.
A recent study found that tissue fate depends on collateral blood flow and that the natural evolution of ischemic injury is irrespective of the time of assessment of the penumbra (25). Similarly, we found that longer OCTT was associated with better CC; however, this result may suffer from a selection bias, as we included in our study patients potentially eligible for EVT, excluding those not eligible (e.g., established large infarct at non-contrast CT, more than 24 h after stroke onset, pre-existing severe disability). However, this result may also reflect that in patients eligible for EVT, good CC may be useful to treat patients in the extended time window, as previously suggested (11, 26). The association between time from stroke onset and CC deserves further investigation to explore whether CC may reflect the tissue viability also in late arrivals.
To the best of our knowledge, the role of the ischemic side in relation to CC has never been investigated. We found that acute ischemia on the left hemisphere was independently associated with better collateral status. This could be explained assuming that a dominant hemisphere has to be more preserved and also requires a higher blood support for its elevated metabolic activity. A recent study with functional magnetic resonance on stroke patients showed that ischemia in the dominant hemisphere was associated with lower activation of the supplementary motor area on neural network reorganization, suggesting a difference in lateralization of this specific process in brain ischemia (27). Moreover, a previous study found a predilection for cerebrovascular disease due to large vessel occlusion at the left side possibly related to greater hemodynamic stress and intimal damage in the left carotid artery; further studies could be conducted in order to investigate whether these hemodynamic differences would also explain a different CC recruitment in two hemispheres (28, 29). A study conducted on infants showed that the left hemisphere has greater metabolic demands than the right and higher neonatal cerebral blood flow velocity on the left hemisphere is positively related to neurobehavioral maturation (30). Our findings about side and CC seem to suggest a better capacity of the dominant hemisphere in the recruitment of CC. This result deserves further examination, possibly with functional magnetic resonance studies.
Our study has certain limitations. First, the retrospective design did not allow a causal link between factors and CC; rather, we described associations that could be explored and confirmed in future prospective studies. Again, data were collected from a single academic center with a hub function for EVT in a large Metropolitan area; thus, we cannot exclude a selection bias toward higher stroke severity in our cohort. Furthermore, protocols for eligible patients for EVT may slightly differ from clinical trials and guidelines, as our population represents a real-world sample of stroke patients. Future studies should have a multicenter design to reduce any bias in this regard. However, in our study, CCs were examined with a validated and reproducible acquisition protocol (11), reducing the probability of assessment bias. We have little information about patients not eligible for reperfusion therapy, and this restricts our results to a limited population.
Half of our patients were imaged after 6 h from symptoms onset, so our results may be translated also to patients eligible for EVT in the extended time window.
In conclusion, we did not find any significant clinical or demographic determinant of CCs, except for a negative association with previous cerebrovascular events. Considering the paramount importance of the topic, it is mandatory to go on applying our efforts to this research, which also focuses on other possible factors that may contribute to CC development. Our study generates a hypothesis that deserves to be further explored.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MS: substantially contributed to the acquisition of data, interpretation of data, and drafting of the manuscript. FA: substantially contributed to the analysis, interpretation of data, and critical revision of the manuscript for important intellectual content. AA: substantially contributed to the acquisition of data. GB and EF: substantially contributed to the acquisition and interpretation of data. CS: substantially contributed to the conception and design of the study, interpretation of data, and critical revision of the manuscript for important intellectual content. All the authors provide approval for publication of the content and agree to be accountable for all aspects of the study in ensuring that questions related to the accuracy or integrity of any part of the study are appropriately investigated and resolved.
The authors acknowledge all the physicians of the Stroke Unit, the Neuroradiology Department, and the Interventional Neuroradiology Department of Careggi University Hospital, Florence.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Goyal M, Menon BK, Van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
2. Henninger N, Lin E, Haussen DC, Lehman LL, Takhtani D, Selim M, et al. Leukoaraiosis and sex predict the hyperacute ischemic core volume. Stroke. (2013) 44:61–7. doi: 10.1161/STROKEAHA.112.679084
3. Nannoni S, Sirimarco G, Cereda CW, Lambrou D, Strambo D, Eskandari A, et al. Determining factors of better leptomeningeal collaterals: a study of 857 consecutive acute ischemic stroke patients. J Neurol. (2019) 266:582–8. doi: 10.1007/s00415-018-09170-3
4. Mohamed A, Shuaib A, Saqqur M, Fatima N. The impact of leptomeningeal collaterals in acute ischemic stroke: a systematic review and meta-analysis. Neurol Sci. (2023) 44:471–89. doi: 10.1007/s10072-022-06437-6
5. Liebeskind DS. Collateral circulation. Stroke. (2003) 34:2279–84. doi: 10.1161/01.STR.0000086465.41263.06
6. Wiegers EJA, Mulder MJHL, Jansen IGH, Venema E, Compagne KCJ, Berkhemer OA, et al. Clinical and imaging determinants of collateral status in patients with acute ischemic stroke in MR clean trial and registry. Stroke. (2020) 51:1493–502. doi: 10.1161/STROKEAHA.119.027483
7. Agarwal S, Scoffings DJ, Simon Jones P, Tulasi Marrapu S, Barry PJ, O'Brien EW, et al. Interaction of age with the ischaemic penumbra, leptomeningeal collateral circulation and haemodynamic variables in acute stroke: a pilot study. J Neurol Neurosurg Psychiatry. (2013) 84:271–6. doi: 10.1136/jnnp-2012-303258
8. Jung S, Wiest R, Gralla J, McKinley R, Mattle H, Liebeskind D. Relevance of the cerebral collateral circulation in ischaemic stroke: time is brain, but collaterals set the pace. Swiss Med Wkly. (2017) 147:w14538. doi: 10.4414/smw.2017.14538
9. Menon BK, Smith EE, Coutts SB, Welsh DG, Faber JE, Goyal M, et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann Neurol. (2013) 74:241–8. doi: 10.1002/ana.23906
10. Malhotra K, Safouris A, Goyal N, Arthur A, Liebeskind DS, Katsanos AH, et al. Association of statin pretreatment with collateral circulation and final infarct volume in acute ischemic stroke patients: a meta-analysis. Atherosclerosis. (2019) 282:75–9. doi: 10.1016/j.atherosclerosis.2019.01.006
11. Menon BK. Neuroimaging in acute stroke. Continuum. (2020) 26:287–309. doi: 10.1212/CON.0000000000000839
12. Rusanen H, Saarinen JT, Sillanpää N. Collateral circulation predicts the size of the infarct core and the proportion of salvageable penumbra in hyperacute ischemic stroke patients treated with intravenous thrombolysis. Cerebrovasc Dis. (2015) 40:182–90. doi: 10.1159/000439064
13. Zhang H, Rzechorzek W, Aghajanian A, Faber JE. Hypoxia induces de novo formation of cerebral collaterals and lessens the severity of ischemic stroke. J Cereb Blood Flow Metab. (2020) 40:1806–22. doi: 10.1177/0271678X20924107
14. Gerber JC, Petrova M, Krukowski P, Kuhn M, Abramyuk A, Bodechtel U, et al. Collateral state and the effect of endovascular reperfusion therapy on clinical outcome in ischemic stroke patients. Brain Behav. (2016) 6:1–9. doi: 10.1002/brb3.513
15. Rocha M, Desai S, Son J, Tonetti DA, Jovin T, Jadhav AP. Clinical characteristics of fast and slow progressors of infarct growth in anterior circulation large vessel occlusion stroke. J Cereb Blood Flow Metab. (2021) 41:1517–22. doi: 10.1177/0271678X211015068
16. Rebchuk AD, Field TS, Hill MD, Goyal M, Demchuk A, Holodinsky JK, et al. Determinants of leptomeningeal collateral status variability in ischemic stroke patients. Can J Neurol Sci. (2022) 49:767–73. doi: 10.1017/cjn.2021.226
17. Pan H, Lin C, Chen L, Qiao Y, Huang P, Liu B, et al. Multiple-factor analyses of futile recanalization in acute ischemic stroke patients treated with mechanical thrombectomy. Front Neurol. (2021) 12:1–11. doi: 10.3389/fneur.2021.704088
18. Seo WK, Liebeskind DS, Yoo B, Sharma L, Jahan R, Duckwiler G, et al. Predictors and functional outcomes of fast, intermediate, and slow progression among patients with acute ischemic stroke. Stroke. (2020) 51:2553–7. doi: 10.1161/STROKEAHA.120.030010
19. Qin C, Yan X, Jin H, Zhang R, He Y, Sun X, et al. Effects of remote ischemic conditioning on cerebral hemodynamics in ischemic stroke. Neuropsychiatr Dis Treat. (2020) 16:283–99. doi: 10.2147/NDT.S231944
20. Wardlaw JM, Sandercock P, Cohen G, Farrall A, Lindley RI, von Kummer R, et al. Association between brain imaging signs, early and late outcomes, and response to intravenous alteplase after acute ischaemic stroke in the third international stroke trial (IST-3): Secondary analysis of a randomised controlled trial. Lancet Neurol. (2015) 14:485–96. doi: 10.1016/S1474-4422(15)00012-5
21. Appleton JP, Woodhouse LJ, Adami A, Becker JL, Berge E, Cala LA, et al. Imaging markers of small vessel disease and brain frailty, and outcomes in acute stroke. Neurology. (2020) 94:E439–52. doi: 10.1212/WNL.0000000000008881
22. Delcourt C, Wang X, Zhou Z, Wardlaw JM, Mair G, Robinson TG, et al. Brain imaging abnormalities and outcome after acute ischaemic stroke: the ENCHANTED trial. J Neurol Neurosurg Psychiatry. (2020) 91:1290–6. doi: 10.1136/jnnp-2020-323015
23. Wei C, Shen T, Tang X, Gao Y, Yu X, Chen X. Cerebral small vessel disease combined with cerebral collaterals to predict the prognosis of patients with acute large artery atherosclerotic stroke. Front Neurol. (2022) 13: 969637. doi: 10.3389/fneur.2022.969637
24. Alexandre AM, Colò F, Brunetti V, Valente I, Frisullo G, Pedicelli A, et al. Mechanical thrombectomy in minor stroke due to isolated M2 occlusion: a multicenter retrospective matched analysis. J Neurointerv Surg. (2022) 4:1–6. doi: 10.1136/jnis-2022-019557
25. Bivard A, Spratt N, Miteff F, Levi C, Parsons MW. Tissue is more important than time in stroke patients being assessed for thrombolysis. Front Neurol. (2018) 9:1–7. doi: 10.3389/fneur.2018.00041
26. Boers AMM, Jansen IGH, Berkhemer OA, Yoo AJ, Lingsma HF, Slump CH, et al. Collateral status and tissue outcome after intra-arterial therapy for patients with acute ischemic stroke. J Cereb Blood Flow Metab. (2017) 37:3589–98. doi: 10.1177/0271678X16678874
27. Gao J, Yang C, Li Q, Chen L, Jiang Y, Liu S, et al. Hemispheric difference of regional brain function exists in patients with acute stroke in different cerebral hemispheres: a resting-state fMRI study. Front Aging Neurosci. (2021) 13:1–12. doi: 10.3389/fnagi.2021.691518
28. Rodríguez Hernández SA, Kroon AA, Van Boxtel MPJ, Mess WH, Lodder J, Jolles J, et al. Is there a side predilection for cerebrovascular disease? Hypertension. (2003) 42:56–60. doi: 10.1161/01.HYP.0000077983.66161.6F
29. Hedna VS, Bodhit AN, Ansari S, Falchook AD, Stead L, Heilman KM, et al. Hemispheric differences in ischemic stroke: is left-hemisphere stroke more common? J Clin Neurol. (2013) 9:97–102. doi: 10.3988/jcn.2013.9.2.97
Keywords: ischemic stroke, large vessels occlusion, anterior circulation, cerebral collateral circles, cerebral collateral vessels, leptomeningeal vessels, multiphase CTA, stroke prognosis
Citation: Sperti M, Arba F, Acerbi A, Busto G, Fainardi E and Sarti C (2023) Determinants of cerebral collateral circulation in acute ischemic stroke due to large vessel occlusion. Front. Neurol. 14:1181001. doi: 10.3389/fneur.2023.1181001
Received: 06 March 2023; Accepted: 11 April 2023;
Published: 17 May 2023.
Edited by:
Alessandro Pedicelli, Agostino Gemelli University Polyclinic (IRCCS), ItalyReviewed by:
Luca Scarcia, Hôpital Pitié-Salpêtrière, FranceCopyright © 2023 Sperti, Arba, Acerbi, Busto, Fainardi and Sarti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Sarti, Y3Jpc3RpbmEuc2FydGlAdW5pZmkuaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.